Abstract
We have recently demonstrated that most strains of Campylobacter jejuni produce capsular polysaccharide (CPS), which can be detected by immunoblotting with homologous Penner antisera on polyvinylidene difluoride membranes (A. V. Karlyshev, D. Linton, N. A. Gregson, A. J. Lastovica, and B. W. Wren, Mol. Microbiol. 35:529–541, 2000). In this report, we describe a universal and rapid staining procedure using Alcian blue for C. jejuni CPS, which does not rely on the availability of antisera and identifies CPS in untypeable strains. Furthermore, Alcian blue staining identified CPS in its lipid-free form directly on Tricine gels, and we demonstrate that CPS is thermostable and is accumulated in the culture supernatant in a lipid-free form. The identification of a newly described CPS and its lipid-free form in C. jejuni should prove invaluable in studying the pathogenesis and epidemiology of this important pathogen.
Campylobacter jejuni is the principal cause of food poisoning in the United States and other developed countries (reviewed in reference 23). C. jejuni is also increasingly recognized for its association with the serious postinfection neurological complications of Guillain-Barré syndrome (26). Significantly, prior infections with C. jejuni Penner serotypes O:19 and O:41 are more commonly associated with these devastating neuropathies (11). The Penner serotyping system has been widely adopted for the epidemiological analysis of C. jejuni and is based on passive hemagglutination using heat-stable antigens, which were shown previously to consist of lipopolysaccharide (LPS) (14). C. jejuni produces lipooligosaccharide (LOS) and in some cases also high-molecular-weight polysaccharide, which in most cases could not be detected using the silver staining technique (18). We have recently demonstrated that, in contrast to previous reports, the high-molecular-weight polysaccharide is ubiquitous in C. jejuni and is genetically and biochemically similar to capsular polysaccharides (CPSs) in other gram-negative bacteria (12). This was a surprising finding, as a capsule had not previously been identified in C. jejuni using traditional staining techniques such as ruthenium red. C. jejuni CPSs from a range of strains could be detected by blotting with homologous Penner antiserum. However, some C. jejuni strains, especially fresh clinical isolates, do not interact with available Penner antisera and are untypeable, thus making detection of CPS in these strains extremely difficult. Moreover, the structure of the monomers of CPS can vary between different strains (4), presumably due to the presence of at least five hypervariable genes in the polysaccharide biosynthesis locus (12, 16).
Alcian blue has been used previously to stain and identify CPSs from several bacterial species (1). We report that, in all nine C. jejuni strains tested, the newly identified CPS was stained with Alcian blue dye. This rapid and reliable staining procedure does not depend on the availability of homologous antisera and, in addition, allows detection in culture supernatant of the lipid-free form of CPS. We demonstrate that, in contrast to the cell-bound CPS, the secreted molecules lack a phospholipid moiety. These data and the finding that deoxycholate (DOC) can inhibit conversion of the CPS into a lipid-free form suggest the presence of a phospholipase distinct from that of outer membrane-bound detergent-resistant phospholipase PldA. This was further supported by the analysis of CPS production in defined C. jejuni pldA mutants.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni strain X is a recent isolate from a patient with enteritis; strain G1 is an isolate from a Guillain-Barré syndrome patient. Other strains are described in the legend to Fig. 1. The strains were grown at 37°C on blood agar plates (Columbia agar [Oxoid, Basingstoke, United Kingdom], supplemented with 7% horse blood) or on a minimal broth medium (MEM Alpha medium; Gibco BRL, Life Technologies, Paisley, United Kingdom) with a growth supplement (Oxoid) in a microaerobic atmosphere for 2 days. Medium for growth of the mutant strain G1 kpsM::Kanr was supplemented with kanamycin at a concentration of 50 μg ml−1.
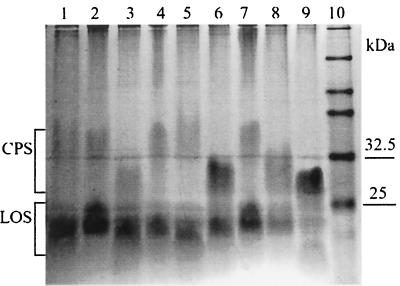
FIG. 1.
Detection of CPS using Alcian blue in different C. jejuni strains. Lanes: 1, NCTC 12501 (HS:2); 2, NCTC 12502 (HS:3); 3, NCTC 12561 (HS:4); 4, NCTC 12509 (HS:10); 5, NCTC 12517 (HS:19); 6, 81116 (NCTC 11828, HS:6, 7); 7, NCTC 11168 (HS:2); 8, G1 (HS:1); 9, × (untypeable); 10, prestained markers.
Preparation of CPS samples.
Crude extracts of C. jejuni CPS were prepared from all strains essentially as described previously (10). Bacteria from 2-day blood agar plates were directly solubilized in 100 μl of lysis buffer containing 31.25 mM Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate, 0.025% bromophenol blue, and 20% glycerol. Samples were heated at 100°C for 5 min. Twenty-microliter aliquots of the supernatant were removed to a fresh tube, to which 1 μl of a 20-mg/ml solution of proteinase K (Sigma) was added, and the mixture was incubated at 50°C for 1 h. Cell extracts were prepared in a similar way, except that no boiling was used and 1 volume of the lysis buffer was added to the samples prior to proteinase K treatment.
In order to prepare fractions, bacteria were treated subsequently with saline at room temperature and at 50°C. One loop of bacteria from 2-day blood agar plates was washed with 0.5 ml of saline; supernatant was collected (F1); the pellet was resuspended in 0.5 ml of saline, incubated at 50°C for 1 h, and centrifuged, and supernatant (F2) and pellet (P) were collected. Supernatant obtained after growth in minimal medium (10 ml) was filter sterilized using 0.2-μm-pore-size membranes (Pall, Portsmouth, United Kingdom), concentrated using the Centriplus 3 system (Millipore, Watford, United Kingdom) to 300 μl, diluted to 5 ml, and concentrated again to the final volume of 250 μl followed by dialysis against distilled water.
Electrophoresis and Western blotting.
The samples were fractionated on 12.5% Tricine–sodium dodecyl sulfate–polyacrylamide gels (20) using a Hoefer minigel system (Pharmacia Biotech, St. Albans, United Kingdom). Prestained protein markers (New England BioLabs) were used. Following electrophoresis, the gels were stained with either Alcian blue (0.1% Alcian blue in 40% ethanol–5% acetic acid) or silver (22). In the combined Alcian blue-silver staining procedure, the gels were initially stained with Alcian blue.
CPS and LOS were detected in Western blotting experiments using a polyvinylidene difluoride (PVDF) membrane (Millipore) and a semidry electroblotting apparatus (Hoefer; Pharmacia Biotech). Blots were blocked for 1 h at ambient temperature in Tris-buffered saline containing 0.01% Tween 20 (TBST) and 3% skimmed milk, followed by incubation for 1 h with Penner antiserum at a dilution of 1:100 in TBST containing 1% bovine serum albumin, washed three times in TBST, and incubated for a further hour with peroxidase-labeled anti-rabbit immunoglobulin G (Sigma-Genosys, Pampisford, United Kingdom) diluted 1:1,000 in TBST-bovine serum albumin. Following washing as described above, blots were developed using the diaminobenzidine staining kit with nickel enhancement according to the manufacturer's instructions (Vector Laboratories, Burlingame, Calif.).
Western blotting for the detection of lipid A and 3-deoxy-d-manno-octulosonic acid (Kdo) moieties was performed essentially as described above according to the protocol described in reference 15. For the detection of lipid A, the membranes were incubated in 1% acetic acid for 1 h. After treatment with the corresponding monoclonal antibodies (MAbs), the membranes were incubated with a 1:1,000 dilution of peroxidase-labeled anti-mouse immunoglobulin G (Sigma-Genosys), and the blots were developed as described above.
Construction of defined pldA mutants.
PCRs were performed using 1 U of Taq polymerase (Gibco BRL, Life Technologies) in 20-μl volumes containing 0.1 μg of primers and DNA at 94°C for 1 min and 25 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 2 min, followed by 7 min of extension at 72°C. The PCR products were purified using S300 microspin columns (Bio-Rad, Hemel Hempstead, United Kingdom) and/or analyzed on agarose gels. A 0.9-kb fragment of the NCTC 11168 pldA gene was PCR amplified using primers ak113 (GGC TAG TGA TTT ACA ACA AGC) and ak114 (CCA GTG GAA AGT CTT TGC AAG TG) and cloned into vector pGEM-T Easy (Promega, Southampton, United Kingdom). DNA extracted from one of the recombinant clones was digested with BglII enzyme and ligated with a BamHI fragment containing a Kanr cassette (21). After transformation into Escherichia coli XL2-Blue (Stratagene, Amsterdam, The Netherlands), Kanr clones were selected and analyzed for the presence of the inserts using PCR with primers ak113 and a Kanr cassette-derived primer, DL4 (TGT TGC TGT CTC CCA GGT CG). DNA recovered from clones with a Kanr cassette oriented in the same direction as the pldA gene was used for electroporation of strains X and G1 as described previously (24). Allelic replacement in the transformants was confirmed using PCR with ak113 and ak114 primers.
Treatment with phospholipases.
Treatment with a mixture of phospholipase C (type IX from Bacillus cereus [Sigma-Genosys]), phospholipase A2 (from bee venom [Sigma-Genosys]), and phospholipase D (type I from cabbage [Sigma-Genosys]) was performed as described previously (12). One unit of each phospholipase was added to the samples adjusted to 50 mM Tris-HCl (pH 8.0) and incubated for 1 h at 30°C followed by incubation at 37°C for 12 to 14 h.
RESULTS
Detection of C. jejuni CPS using Alcian blue dye.
Figure 1 shows the use of Alcian blue to detect CPS in nine C. jejuni strains, including the untypeable strain X (Fig. 1, lane 9). The size and intensity of the CPS band vary between the strains. For example, the intensity of the CPS band of strain NCTC 11168 (Fig. 1, lane 7) is usually much weaker than that of strain 81116 (lane 6) and G1 (lane 8). Strain G1, a clinical isolate from a patient with Guillain-Barré syndrome, was chosen for further analysis.
CPS is thermostable and can be selectively extracted after incubation of cells at 50°C.
Production of C. jejuni extracellular proteins was found to be stimulated by the addition of normal horse serum (19). As at least some extracellular proteins are essential for bacterial pathogenesis (13), and due to a possible biological role of CPSs, we hypothesized that these molecules could be coinduced with other virulence factors. The effects of growth temperature and the addition of horse blood, horse serum, and lysed blood to Columbia base agar were tested. None of the additives had any stimulatory effect on the synthesis of CPS (Fig. 2A). Similar results were obtained for cultures grown at 42°C (data not shown). In subsequent experiments, bacteria were routinely grown at 37°C on Columbia agar supplemented with horse blood.
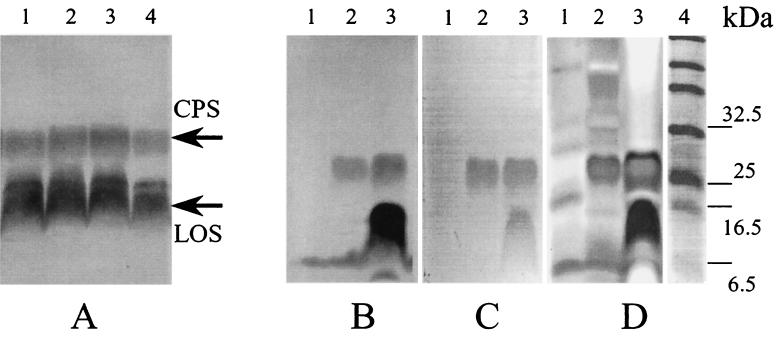
FIG. 2.
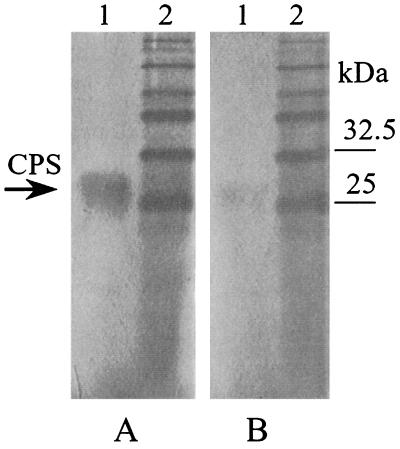
Analysis of CPS extracted from C. jejuni strain G1. (A) Blotting with Penner 1 antiserum. Shown are effects of supplements to the Columbia agar base medium on CPS production at 37°C. Lane 1, no supplements; lane 2, normal horse serum; lane 3, horse blood; lane 4, lysed horse blood. (B to D) Efficiency of CPS staining using blotting with Penner 1 antiserum (B), Alcian blue (C), and Alcian blue followed by silver staining according to the method of Tsai and Frasch (22) (D). Lanes 1, supernatant of cells washed with saline; lanes 2, 50°C extract; lanes 3, lysate; lane 4, prestained markers.
CPS was found to be relatively tightly bound to the bacteria and could not be released by vortexing in saline at room temperature (Fig. 2B, lane 1). However, after incubation of bacterial suspension at 50°C in saline, the polysaccharide appeared in the supernatant with very little contamination from LOS (Fig. 2B, lane 2), the latter being mainly associated with the bacteria (Fig. 2B, lane 3). This fraction is designated F2 (fraction 2). The results demonstrate that the highest yield and purity of CPS can be achieved by saline extraction at 50°C and are in agreement with previously reported data (6). Filter sterilization of the extracts using 0.2-μm-pore-size membranes significantly reduced contamination with LOS without an effect on CPS (data not shown). This is probably due to the tendency of LOS to form micelles. Although both CPS and LOS can be stained with Alcian blue, the CPS/LOS signal ratio is much higher with the Alcian blue staining procedure (Fig. 2C) than with Western blotting (Fig. 2B).
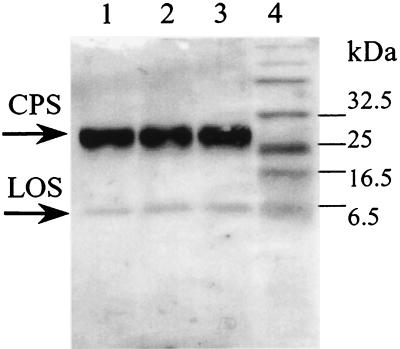
Alcian blue staining followed by prolonged silver staining using the Tsai-Frasch procedure (22) can increase the intensity of the CPS band with significant increase of the background level (Fig. 2D). No CPS could be detected with silver staining alone (data not shown). No changes in the intensity of the CPS band and no accumulation of a possible lipid-free product of hydrolysis could be detected after incubation of samples at 100°C (Fig. 3), confirming that these molecules are thermostable, as one would expect given the heat treatment used in the Penner serotyping procedure (17).
FIG. 3.
Thermal stability of the C. jejuni G1 CPS. Western blotting with Penner 1 antisera of fraction F2 (see text) after incubation at 100°C for 0 (lane 1), 5 (lane 2), and 10 (lane 3) min. Lane 4 shows prestained markers.
Phospholipid moiety, but not polysaccharide chain, is responsible for membrane binding.
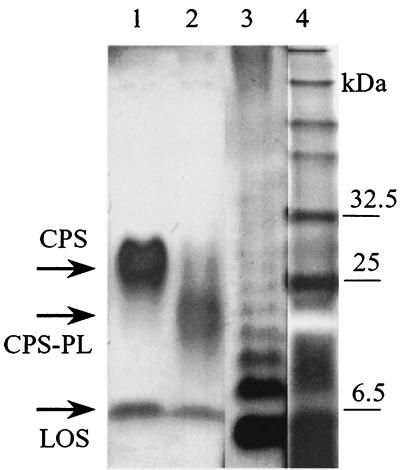
We previously reported that after treatment with phospholipases CPS could not be detected by Western blotting (12). Removal of the phospholipid could have prevented the molecules from either entering the Tricine gel or binding to PVDF membranes. To determine whether lipid-free CPS (CPS-PL) enters the gel, we stained membranes with Alcian blue. As shown in Fig. 4, both CPS (lane 1) and CPS-PL (lane 2) can be visualized, indicating that the CPS-PL does migrate into Tricine gels but seems to have lost its ability to bind hydrophobic PVDF membranes, probably due to the absence of a hydrophobic lipid moiety.
FIG. 4.
Gel detection of C. jejuni G1 CPS (lane 1) and its lipid-free form CPS-PL (lane 2) by a combination of Alcian blue-silver staining procedures. S. enterica serovar Typhimurium LPS is present in lane 3 for comparison. Lane 4 shows prestained markers.
The staining properties of Alcian blue are considered to be associated with the interaction of this cationic dye with negatively charged macromolecules (5). The data presented here demonstrate that the polysaccharide moiety of CPS binds Alcian blue, suggesting that these molecules are also negatively charged. A polysaccharide extraction procedure based on reversible binding of negatively charged polysaccharides, containing sulfate groups, to positively charged membranes has been described previously (1). To determine if this method could also be used for purification of C. jejuni CPS, we investigated binding of these molecules to positively charged Hybond N membranes.
Hybond N can effectively bind CPS (Fig. 5A, lane 1). However, it was not possible to extract bound polysaccharide even in the presence of as high a concentration of sodium chloride as 5 M. Interestingly, no binding of lipid-free CPS to the membrane could be detected (Fig. 5A, lane 2). The results suggest that hydrophobic rather than electrostatic interaction is a major factor for binding CPS to the membrane and that the repeating units do not contain strongly acidic groups such as sulfates and phosphates which are required for electrostatic interaction with the positively charged membrane.
FIG. 5.
Effect of phospholipase treatment on binding of CPS to a Hybond N membrane. After blotting, the membrane was stained with Alcian blue as described in the text. (A) Lane 1, CPS; lane 2, prestained markers. (B) Lane 1, CPS-PL; lane 2, prestained markers.
CPS is accumulated in the culture medium in a lipid-free form.
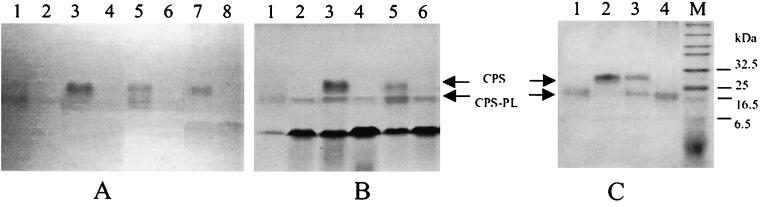
It was observed that CPS recovered from culture supernatant of strain G1 grown in the absence of DOC migrates faster (Fig. 6A, lane 1) than cell-associated CPS (Fig. 6A, lane 7). However, in the presence of DOC in the medium extracellular and cell-bound CPSs have similar sizes (Fig. 6A, lanes 3 and 7). Brief treatment of the culture with DOC also results in the accumulation of the full-sized CPS (Fig. 6A, lane 5). Treatment of the CPS from the culture supernatant after growth in the presence of DOC (Fig. 6C, lane 2) results in the formation of a form similar to that produced in the absence of DOC (Fig. 6C, lanes 1 and 4). This experiment suggests that a smaller size of extracellular CPS is the result of removal of a phospholipid moiety by a bacterial phospholipase.
FIG. 6.
Production of extracellular and cell-bound CPSs by C. jejuni grown on minimal medium. (A and B) The gel was stained with either Alcian blue (A) or Alcian blue and silver (B). Lanes 1, 3, 5, and 7, wild-type strain; lanes 2, 4, 6, and 8, kpsM mutant (control); lanes 1 to 6, culture supernatant; lanes 7 and 8, total cell lysate; lanes 1 and 2, no DOC present; lanes 3 and 4, DOC present in the growth medium; lanes 4 and 5, DOC added 3 h before the end of incubation. A sharp ∼18-kDa band is present in all lanes in panel B and comigrates with CPS-PL, corresponding to proteinase K. (C) Effect of phospholipases on the product (CPS) accumulated in the supernatant in the presence of DOC (from panel A, lane 3). Lane 1, CPS-PL (control); lane 2, CPS; lanes 3 and 4, limited and complete hydrolysis with PL, respectively; lane M, prestained markers.
An outer membrane phospholipase, PldA, is not involved in the conversion of the CPS to its lipid-free form in liquid culture.
A phospholipase A (PldA), belonging to a family of bacterial enzymes found in several bacteria, has been genetically and biochemically characterized for the related species Campylobacter coli (9). However, no biological function could be assigned to this enzyme (7). The gene was also found in C. jejuni strain NCTC 11168 (16). To determine if this enzyme is responsible for processing CPS, we constructed defined pldA mutants for strains G1 and X. Interestingly, all extracellular CPSs accumulated in the supernatant of both mutants grown in liquid medium were present in the lipid-free form, as in the wild-type strains (data not shown), indicating that PldA is not involved in the processing of CPS.
DISCUSSION
We recently demonstrated that the previously described O antigen in many C. jejuni strains is CPS and is a major component of the Penner antigen used for serotyping C. jejuni (12). In this study, Alcian blue dye was used to stain the newly identified CPS in all C. jejuni strains tested. This rapid and reliable staining procedure, which does not depend on the availability of homologous antisera, identified CPS in untypeable strains and allowed detection of the lipid-free form of CPS.
The Penner serotyping procedure involves passive hemagglutination based on extracts of bacterial suspensions heated at 100°C (14). The phosphodiether linkage in many CPSs was found to be heat labile (25). Our finding demonstrates that this is not the case for C. jejuni CPS. Resistance of the CPS to heat treatment implies that the sugar residue linking polysaccharide to the phospholipid moiety is different from those present in other CPSs. The reason for the heat stability could be the lack of Kdo linkage to the phospholipid moiety, which would make the molecule unstable. The thermal stability of C. jejuni CPS makes it an unusual representative of type II or type III CPSs, which are invariably thermolabile (25).
MAbs against E. coli lipid A (MAb 43) and branched-terminal Kdo residues (MAb 20) failed to cross-react with C. jejuni CPS (data not shown). By contrast, C. jejuni intermediates of LOS containing a terminal Kdo cross-reacted with MAb 20, as well as LPS of Salmonella enterica serovar Typhimurium, generating a typical ladderlike pattern after interaction with MAb 43. Although these results were inconclusive regarding the presence or absence of a lipid A in CPS, the ability to detect a lipid-free product after phospholipase treatment using Alcian blue dye is a further demonstration that CPS is substituted with a phospholipid, rather than with lipid A.
The present study suggests that C. jejuni CPS can be released from the cell surface presumably due to the action of a phospholipase which is independent of PldA, suggesting the presence of a further phospholipase in C. jejuni. Production of a second phospholipase by the related species C. coli has been postulated by Grant et al. (9). In contrast to strains G1 and X, no extracellular form of CPS could be detected in strain NCTC 11168 (data not shown), suggesting the absence of an additional phospholipase in this strain. This is consistent with the absence of a second identifiable phospholipase gene in the NCTC 11168 genome sequence (16).
Our data demonstrate that the CPS can be released from the cell surface in either native or lipid-free form depending on the presence or absence of DOC. DOC is a component of bile salts found in large quantities in the intestine of birds and mammals where C. jejuni resides. DOC and bile salts have been reported previously to regulate the production of surface appendages in Campylobacter species and may have an effect on the expression and processing of CPS in vivo (8). The role of the released extracellular polysaccharide is unclear, but it may be important for the survival and pathogenesis of enteric Campylobacter in the host environment.
It has been shown previously that the binding of the cationic dye Alcian blue to polysaccharides is due to the presence of negative charges on the macromolecules (5). In some cases such as HS:3 (2) and HS:19 (3), high-molecular-weight LPSs of C. jejuni were found to contain no negatively charged sugar residues. However, we were able to demonstrate CPS in these strains using Alcian blue (Fig. 1, lanes 2 and 5). A lipid-free form of CPS in strain G1 could not be detected after blotting on a Hybond N membrane even after gentle staining with Alcian blue at a higher pH to prevent desorption from the membrane (data not shown). The absence of binding to the membrane after the removal of the lipid moiety may indicate that the repeating unit of the CPS lacks strongly charged acidic groups (e.g., phosphate and sulfate) required for electrostatic interaction with a positively charged membrane. On the other hand, the presence of weakly charged acidic groups, such as carboxyl, would ensure interaction with the Alcian blue dye. Sugars containing carboxyl groups (e.g., uronic acids and their derivatives) are common components of CPSs from different pathogenic bacteria.
Despite the apparent variability in the intensity of CPS bands among different C. jejuni strains (probably the result of variable affinities of the dye for different CPSs due to the differences in their chemical structures), the present study demonstrates that the Alcian blue staining procedure is an efficient way of detecting C. jejuni CPS and will be invaluable for structural and epidemiological investigations of this newly identified polysaccharide molecule.
ACKNOWLEDGMENTS
This work was supported by the BBSRC and the Wellcome Trust, United Kingdom.
We are grateful to Ben Appelmelk for a gift of monoclonal anti-lipid A and anti-Kdo antibodies, Normal Gregson for antisera, and Dennis Linton for useful discussions.
REFERENCES
- 1.Al-Hakim A, Linhardt R J. Isolation and recovery of acidic oligosaccharides from polyacrylamide gels by semi-dry electrotransfer. Electrophoresis. 1990;11:23–28. doi: 10.1002/elps.1150110106. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Pang H. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of O antigen chains from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:250–255. doi: 10.1021/bi00167a033. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Pang H. Structures of the O chains from lipopolysaccharides of Campylobacter jejuni serotypes O:23 and O:36. Carbohydr Res. 1992;231:13–30. doi: 10.1016/0008-6215(92)84003-b. [DOI] [PubMed] [Google Scholar]
- 5.Bonnell B S, Smith S G, Hedrick J L. Dichromatic staining of electrophoretically separated extracellular matrix macromolecules. Anal Biochem. 1999;271:91–93. doi: 10.1006/abio.1999.4121. [DOI] [PubMed] [Google Scholar]
- 6.Chart H, Frost J A, Oza A, Thwaites R, Gillanders S, Rowe B. Heat-stable serotyping antigens expressed by strains of Campylobacter jejuni are probably capsular and not long-chain lipopolysaccharide. J Appl Bacteriol. 1996;81:635–640. doi: 10.1111/j.1365-2672.1996.tb03558.x. [DOI] [PubMed] [Google Scholar]
- 7.Dekker N. Outer-membrane phospholipase A: known structure, unknown biological function. Mol Microbiol. 2000;35:711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- 8.Doig P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 9.Grant K A, Belandia I U, Dekker N, Richardson P T, Park S F. Molecular characterization of pldA, the structural gene for a phospholipase A from Campylobacter coli, and its contribution to cell-associated hemolysis. Infect Immun. 1997;65:1172–1180. doi: 10.1128/iai.65.4.1172-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes R A, Hadden R D, Gregson N A, Smith K J. Pathogenesis of Guillain-Barre syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 12.Karlyshev A V, Linton D, Gregson N A, Lastovica A J, Wren B W. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 13.Konkel M E, Kim B J, Rivera-Amill V, Garvis S G. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol Microbiol. 1999;32:691–701. doi: 10.1046/j.1365-2958.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 14.Moran A P, Penner J L. Serotyping of Campylobacter jejuni based on heat-stable antigens: relevance, molecular basis and implications in pathogenesis. J Appl Microbiol. 1999;86:361–377. doi: 10.1046/j.1365-2672.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- 15.Pantophlet R, Brade L, Brade H. Detection of lipid A by monoclonal antibodies in S-form lipopoly-saccharide after acidic treatment of immobilized LPS on western blot. J Endotoxin Res. 1997;4:89–95. [Google Scholar]
- 16.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 17.Penner J L, Hennessy J N, Congi R V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983;2:378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- 18.Preston M A, Penner J L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987;55:1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera-Amill V, Konkel M E. Secretion of Campylobacter jejuni Cia proteins is contact dependent. Adv Exp Med Biol. 1999;473:225–229. doi: 10.1007/978-1-4615-4143-1_23. [DOI] [PubMed] [Google Scholar]
- 20.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 21.Trieu-Cuot P, Gerbaud G, Lambert T, Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985;4:3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 23.Wassenaar T M, Blaser M J. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999;1:1023–1033. doi: 10.1016/s1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- 24.Wassenaar T M, Fry B N, van der Zeijst B A. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield C, Roberts I S. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol. 1999;31:1307–1319. doi: 10.1046/j.1365-2958.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome. J Infect Dis. 1997;176(Suppl. 2):S150–S153. doi: 10.1086/513800. [DOI] [PubMed] [Google Scholar]