Abstract
Background:
Loud noises in the neonatal intensive care unit (NICU) exacerbate patient cardiac and respiratory activity, disrupt sleep, and may contribute to hearing deficits, speech and language disorders, and neurodevelopmental delays among NICU graduates.
Aims:
This study evaluated infant-patient tolerance and nurse ease of use of a novel frequency-selective hearing protection device, DREAMIES (NEATCap Medical, LLC).
Study Design and Subjects:
Fifty neonates receiving care in a Level III NICU participated in a 2-phase prospective study. In Phase 1, 25 infants (mean 36.6 wks GA) wore DREAMIES for two consecutive 30-min periods. In Phase 2, 25 infants (mean 34.8 wks GA) wore DREAMIES between care and feeding times during an 8-hr Device-On period followed by an 8-hr Device-Off period for three consecutive days.
Outcome Measures:
Subject tolerance was defined by device-related skin irritation, vital sign measurements, and behavioral state. Device fit and ease of use were also evaluated by NICU nurses.
Results:
No skin breakdown was reported in any infant in either phase. Only transient skin erythema was observed. Periods when infants wore DREAMIES resulted in lower heart and respiratory rates and increased sleep (P<0.001). Nurses reported little to no difficulty in applying or removing the device.
Conclusion:
Findings suggest DREAMIES are a safe, easy to use, and effective device that reduces exposure to NICU noise, and may improve cardio-respiratory activity and promote sleep among neonatal patients. Further studies are warranted to examine longer term use and potential benefits of DREAMIES for improving outcomes in infants receiving NICU care.
Keywords: NICU, noise reduction, premature infant, infant developmental, infant sleep, nursing
INTRODUCTION
The adverse acoustic environment of the neonatal intensive care unit (NICU) has been associated with increased pathophysiologic events (e.g., apnea, bradycardia) and disrupted sleep in hospitalized infants [1–4], and is a suspected contributing factor in hearing deficits, speech and language disorders, and neurodevelopmental delays in NICU graduates.[5] Multiple approaches to reduce noise exposure in the NICU have been pursued, including hearing protection devices [6–8], modifications to incubator design [9–12], adjustments in caregiver activities [13–15], and architectural renovations ranging from alterations of existing NICUs to new construction of single-family, single-bed rooms.[16–20] Each of these approaches are characterized by different complexity, effectiveness, and cost. In particular, the architectural renovation approach has complications associated with design, work flow implications, and high cost of over $200,000 per infant station.[21] None of the previous approaches have attempted to preferentially block high-frequency NICU noise commonly emitted from the cardio-respiratory monitor alarms, life-support machines and other medical equipment at the infant’s bedside.[22]
The optimal acoustic milieu for language acquisition and overall neurodevelopment for NICU patients would, in theory, be a re-creation of the in-utero environment in which the developing fetus is protected from noxious high volume/high frequency sounds while still exposed to the low frequency sounds emanating from the mother, including the human voice.[5,23,24] Not yet studied in neonates, the NEATCAP® (neurosensory, environmental, adaptive, technology) DREAMIES® device (NEATCap Medical, LLC, Bethlehem, PA), herein referred to as DREAMIES, is an innovative hearing protection device designed to mimic the sound-filtering characteristics observed in the gravid uterus.[25,26] Excessive high frequency noise (like alarms of NICU bedside equipment) are blocked while some low frequency sound (like that of a parent’s voice) pass through, consistent with intrauterine sounds recorded during pregnancy (see Figure 1).[23,24]
Figure 1.
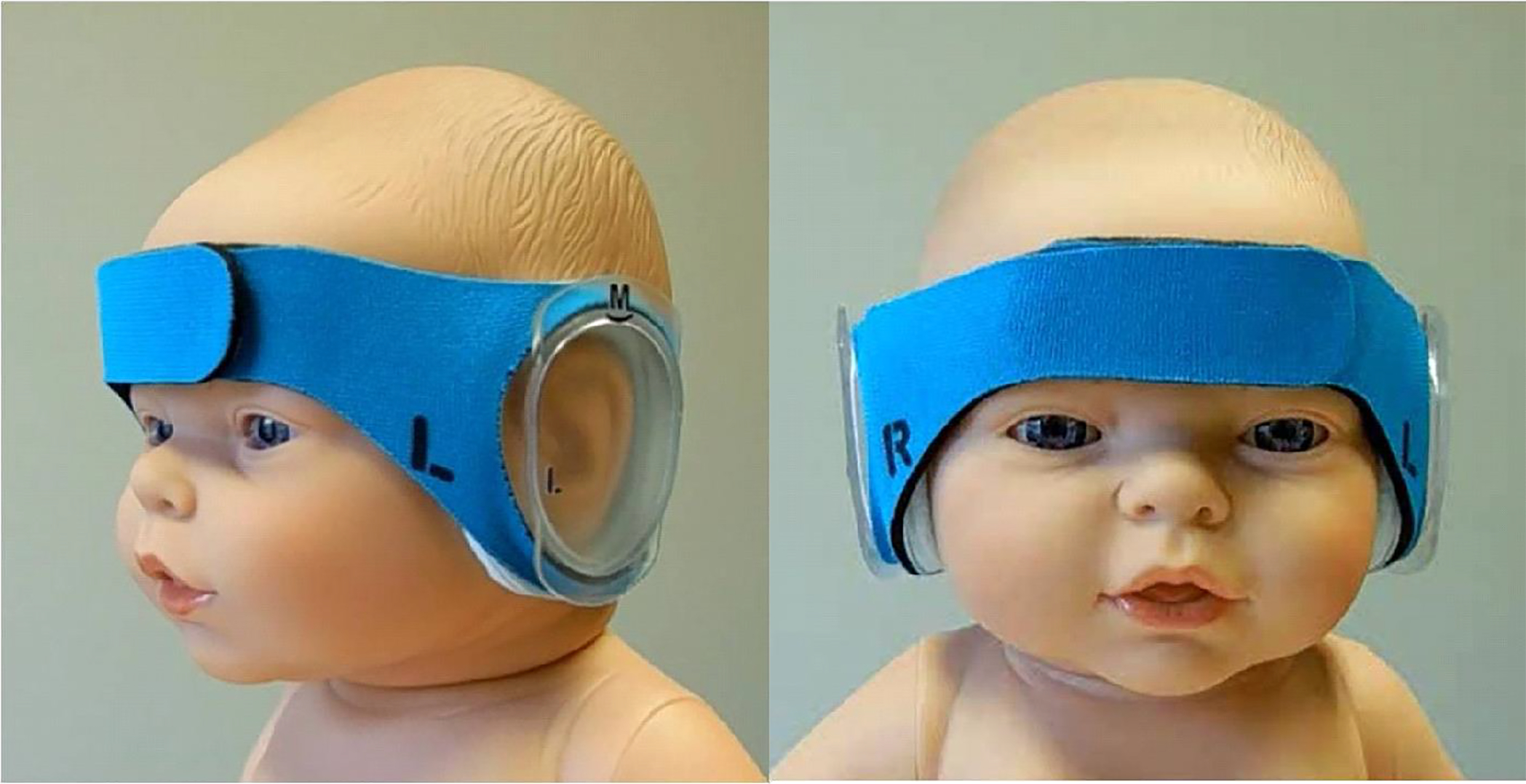
Photo of the DREAMIES device on preterm infant doll model.
The purpose of this 2-phase pilot study was to evaluate NICU patient tolerance and nursing ease of use of DREAMIES. In Phase 1, we studied effects of short-term wear on infant changes in skin condition and routine vital signs associated with the device. Based on the results of the first phase, in Phase 2 we assessed effects of extended wear over the course of three days on infant skin condition and hypothesized that infants would have improved cardiac and respiratory activity and increased sleep while wearing the hearing protection device compared to when the hearing device was off.
MATERIAL AND METHODS
Human Subjects
Neonates were recruited using consecutive admissions from the level III NICU at Magee-Womens, UPMC Hamot Hospital, Erie, PA. The goal was to enroll a heterogeneous cohort of infants representative of the NICU population in a confined timeframe, with focus on whether nurses could easily apply the device to any infant in the NICU (e.g., very small, fragile premature infants to larger full-term newborns) and tolerance of the device, particularly skin irritation, among the cohort. In Phase 1, 25 infants were enrolled over an approximately 1 month period between February and March 2017. In Phase 2, a separate cohort of 30 infants were enrolled over an approximately 4.5 month period between May and October 2017.
Inclusion criteria were infants hospitalized since birth and treated in the NICU for prematurity (< 37 weeks GA) or full-term newborns treated for Neonatal Abstinence Syndrome (NAS) or other diagnoses including respiratory distress, hypoglycemia and/or possible sepsis. Enrollment criteria required that infants were at least >12 hrs old and within the first two weeks of NICU hospitalization. Exclusion criteria included significant cranial trauma noted on admission, congenital anomalies of the head and/or neck, hemodynamic instability requiring pharmacologic intervention or a recommendation by the attending neonatologist not to enroll the patient.
Written informed consent was obtained from the parents of each infant participant. The study was approved by the Institutional Review Boards for Human Subjects at the Univerity of Pittsburgh and at UPMC Hamot Hospital.
Study Device
DREAMIES is a circumaural hearing protector (“ear muffs”) designed for repetitive applications with a single infant (see Figure 2).[25,26] The disposable device employs a soft neoprene-nylon headband that holds position-adjustable, removable, transparent ear cups in place. DREAMIES were provided in four sizes of the ear cups and headbands to accommodate a range of infant ear span and head circumference from 23 cm to 39 cm.
Figure 2.
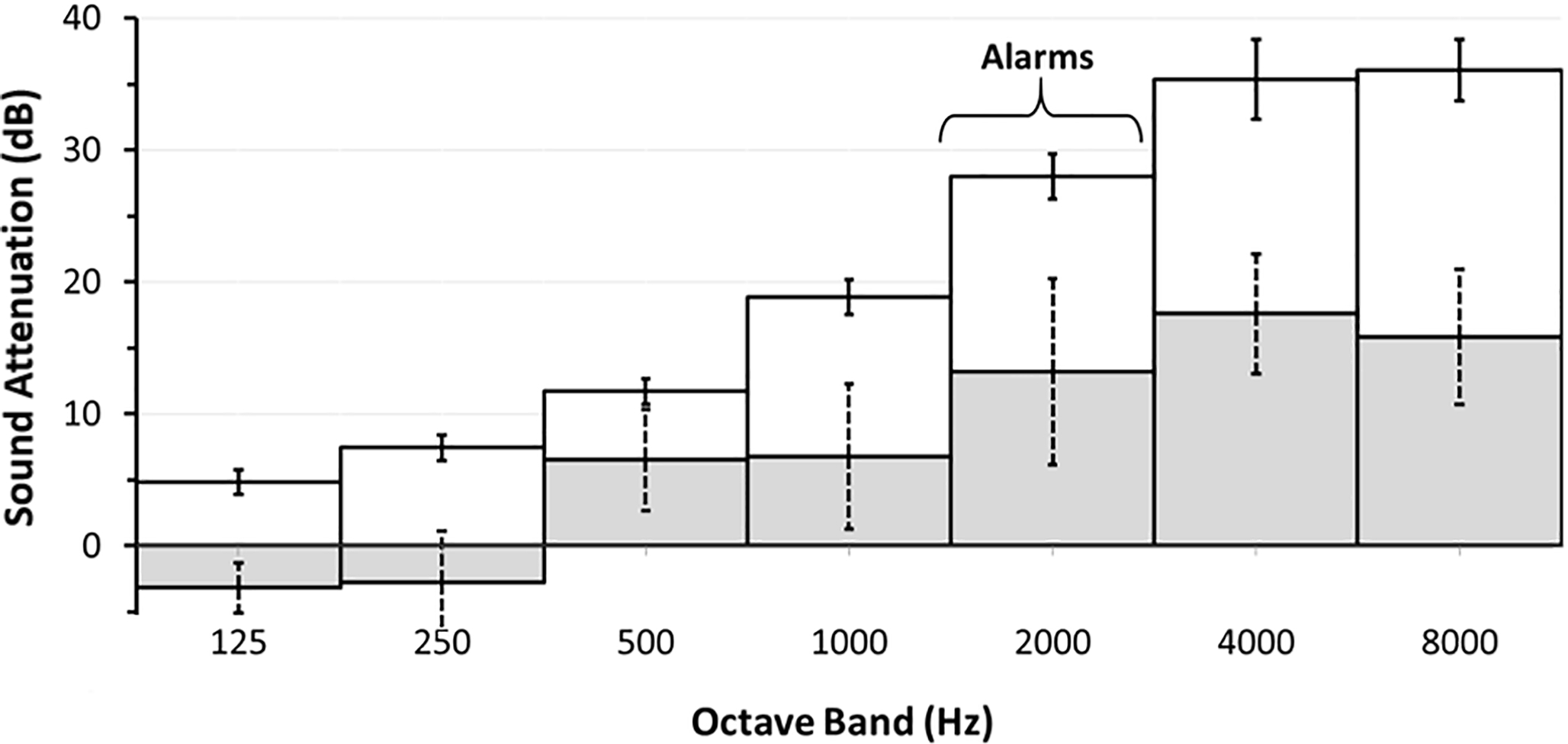
Sound attenuation of DREAMIES device. White bars with solid SD = DREAMIES device; Shaded bars with dotted SD = adapted from animal model [23]). Note, sound filtering of the DREAMIES is similar to that of the sheep gravid uterus (commonly used animal model of maternal-fetal interactions, adapted from Gerhardt [23]). DREAMIES preferentially block high-frequency sounds (e.g., bedside monitor alarms [22]) and allows transmission of some low-frequency sounds (e.g., human voice [38]) as observed in womb during gestation.[23,24].
Study Design
This study was divided into two separate phases. Each phase employed a within-subject design and included two independent groups of NICU infants.
Phase 1.
Infants participated in two consecutive 30-min sessions separated by approximately 30 min during the day while receiving standard of care in their hospital crib or isolette. Immediately preceding routine nursing cares (e.g., assessments, diaper changing, feeds), baseline vital signs (Baseline) were obtained from the infant’s bedside monitor (Drager Infinity Delta, Teleford, PA). Following nursing cares, DREAMIES were applied by a single senior-level NICU nurse clinician (JB) trained to place DREAMIES on fragile neonates. The nurse obtained occipital-frontal head circumference measurements, applied the device and confirmed proper fit. Each infant wore DREAMIES for two consecutive 30-min intervals while supine (DEV1-ON30; DEV1-ON60). At the end of each DREAMIES interval, vital signs (heart rate, respiratory rate, temperature, arterial blood-oxygen saturation (SaO2), and mean arterial blood pressure (MAP) were obtained from the bedside monitor. After obtaining vital signs, the device was removed to assess the underlying skin area. A fourth set of vital signs was obtained 30 min after final device removal (Post). Data were recorded at the bedside by the NICU clinician. The primary endpoints of Phase 1 were: 1) Presence or absence of skin breakdown and/or irritation at the site of device application after each of two successive 30-min periods of wear (DEV1-ON30 and DEV1-ON60); 2) Changes in vital signs between Baseline and Post conditions and DEV1-ON30 and DEV1-ON60 periods.
Phase 2.
A separate cohort of neonates participated in a subsequent assessment to examine prolonged use of the DREAMIES device. As part of standard care, all NICU nurses (n=45) were trained before infant recruitment on the different aspects of the study protocol including device placement and study charting. Nurses participated in a demonstration session and were provided verbal and written instruction on how and when to apply and remove the DREAMIES device and when and what to observe for skin irritation. Photos of proper and improper positioning of the device and examples of potential skin irritation, along with timing and events to record, were kept in a research study binder for nurses assigned to an enrolled study participant. Clinical-care nursing assignment to infants enrolled in the study followed standard NICU-assignment practice. Thus, any NICU nurse assigned to an enrolled subject was trained and responsible for carrying out the study protocol for any given session period.
Devices used in Phase 2 incorporated revisions to the manufacturing process to round the edges of the skin-contacting components [25] in an effort to eliminate transient, linear demarcation erythema that was observed in Phase 1. Infants wore the revised DREAMIES device between care and feeding times during an 8-hr period (DEV2-ON) followed by an 8-hr period of device off (DEV2-OFF) for three consecutive days, starting at the same time of day within an infant for each of the study days (see Figure 3, Study Protocol). The start time among subjects varied based on nursing assignment over three work shifts.
Figure 3.
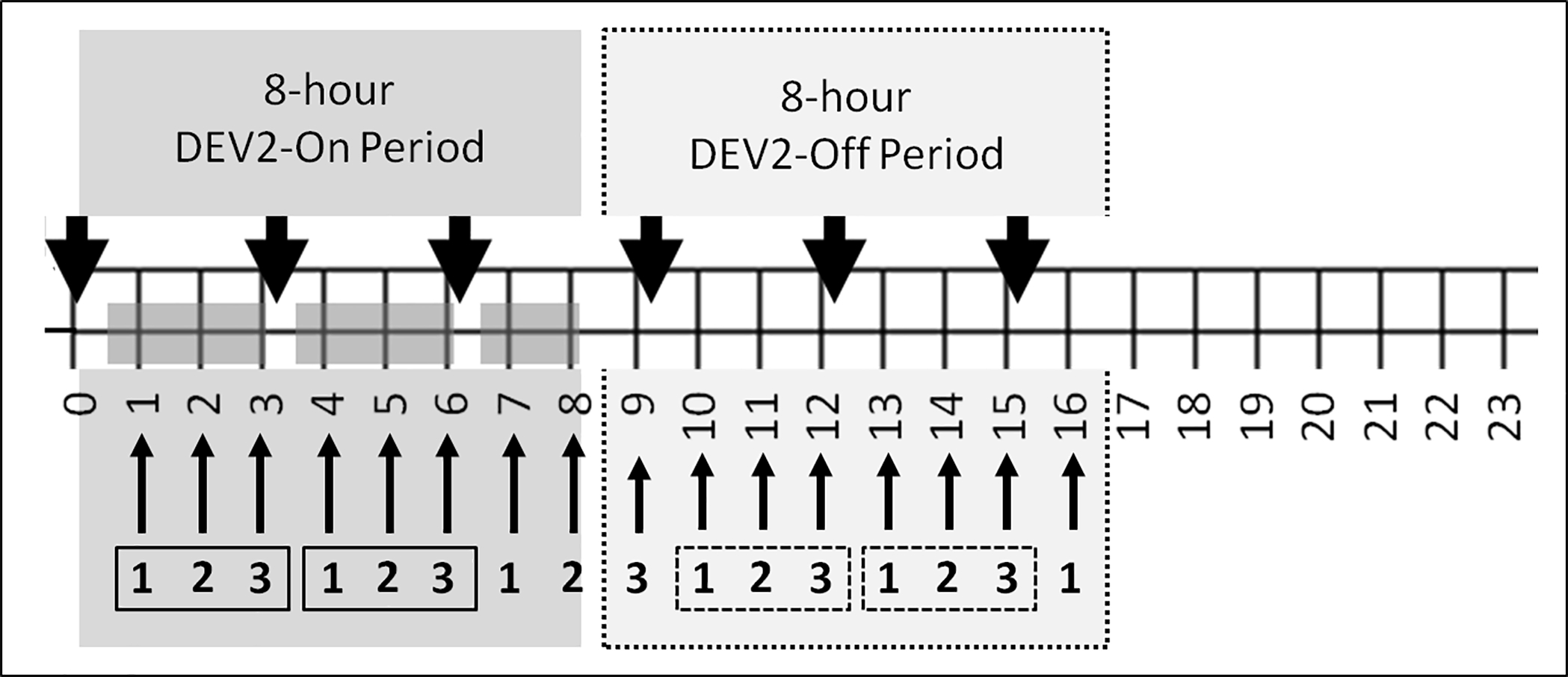
Phase 2 study protocol: Data recording times during DEV2-ON and DEV2-OFF periods. Thick black arrows denote the start of nursing cares (every 3 hours). Thin arrows indicate hourly vital signs and sleep assessments. Dark gray regions on the 24-hour timeline indicate when the device was in place on the baby. White regions on the 24-hour timeline indicate when the device was not on the baby. Boxes with 1 2 3 enclosed with solid line indicate the 3-hour periods when DREAMIES was on the infant between nursing cares (DEV2-ON). Boxes with 1 2 3 enclosed with a dotted line indicate 3-hour periods when DREAMIES was not on the infant between nursing cares (DEV2-OFF).
For each consecutive session day, respiratory rate, heart rate, SaO2 and behavioral sleep state were recorded hourly during the 8-hr period the device was worn (DEV2-ON, hrs 1–8) and for 8 hrs following device removal (DEV2-OFF, hrs 9–16; Figure 3). During each 8-hr interval, DREAMIES were removed every three hrs for nursing cares (duration ~20–40 min) and replaced after feeds and routine care. Underlying skin assessments, temperature, and MAP were obtained at nursing cares (i.e., after 3 and 6 hrs, DEV2-ON; after 1, 4, and 7 hrs DEV2-OFF). Respiratory and heart rate were also obtained retrospectively from the bedside monitor for 8 hrs for the day prior to the first DEV2-ON period (Pre-Study) and for the day after the last DREAMIES session (Post-Study) for the equivalent 8-hr DEV2-ON period. Figure 3 depicts the protocol timing for the daily sessions. The primary endpoints for Phase 2 were: 1) Presence or absence of skin breakdown and/or irritation at the site of device application; 2) Changes in vital signs and behavioral state of alertness (6-category Neonatal Behavioral State of Alertness Scale [27] between Pre-Study, DEV2-ON, DEV2-OFF, and Post-Study conditions; and 3) Nursing assessments of device fit and ease of use.
Study Measures
Nursing assessment of infant skin.
For Phase 1 and Phase 2, nurses assessed integrity of skin underlying DREAMIES using a yes/no score for breakdown (tearing of skin) and a categorical scale for erythema (redness in the form of circles or lines from the device): None; Mild (erythema dissipated within 3–10 min); Moderate erythema (dissipated within 10–30 min); Severe (did not dissipate within 30 min). Location of the breakdown and erythema were also noted accordingly: forehead, ears, and/or back of head. Figure 4 shows the nursing survey.
Figure 4.
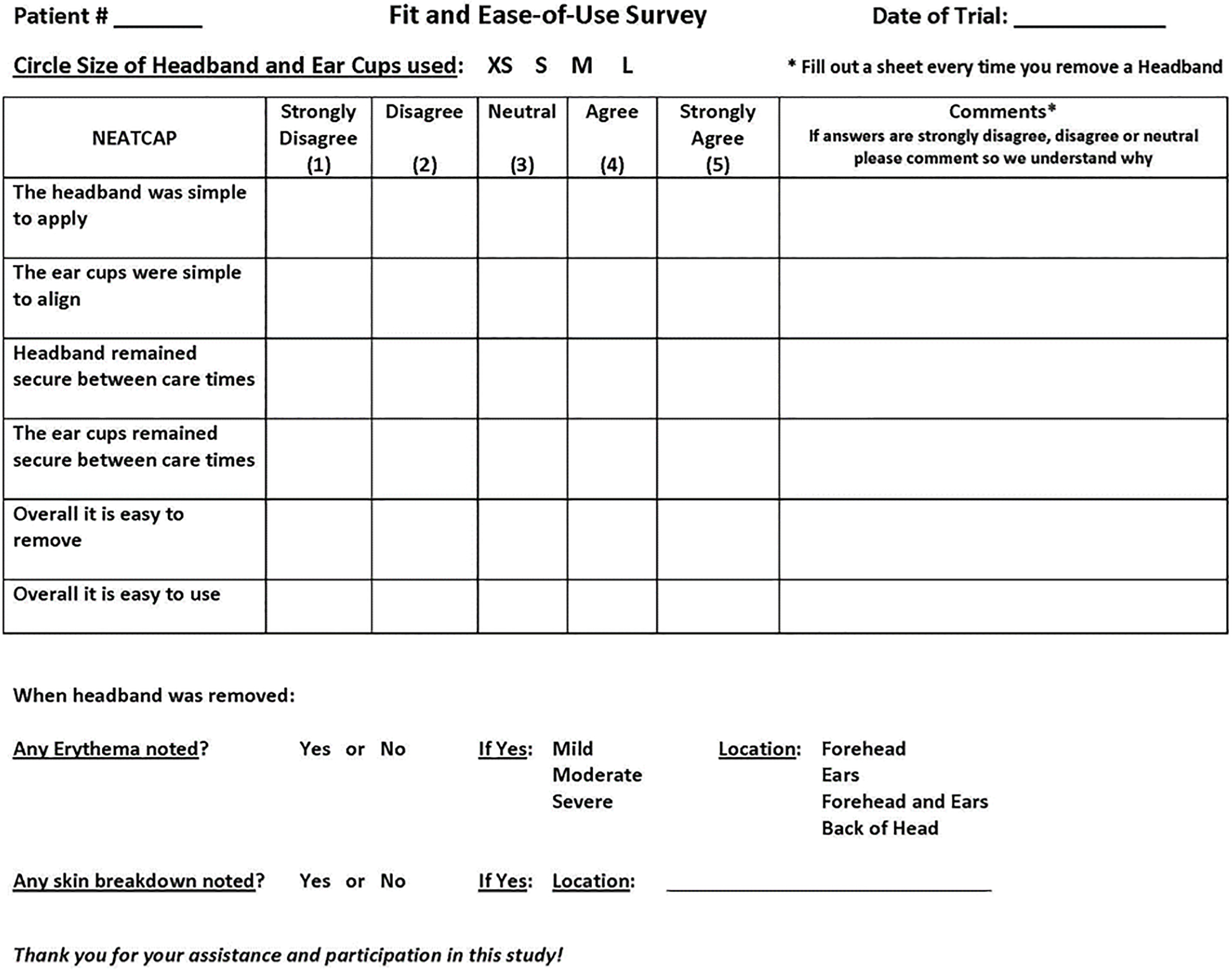
The nursing fit and ease of use survey.
Physiological assessments.
For Phase 1 and Phase 2, heart rate derived from photoplethysmography, respiratory rate derived from thoracic impedance electrocardiography, axillary temperature, SaO2, and MAP were recorded from standard of care clinical sensors. Physiological measures were retrieved from electronic medical records obtained from hourly recordings from the infants’ NICU bedside monitor (Drager Infinity Delta, Teleford, PA) and from standard of care nursing assessments performed every three hrs (nursing cares). During the Device-ON periods, physiological assessments were obtained from when the device was in place on the baby, prior to nursing cares at which time the device was removed.
Behavioral State.
For Phase 2, nurse observations of infant’s behavioral state were recorded hourly at the bedside according to standard of care charting practices using the 6-category Neonatal Behavioral State of Alertness Scale adapted from Brazelton.[27] Observation of behavioral states at routine and study interventions were obtained immediately preceding the start of cares and prior to DREAMIES removal to help ensure the infant was not provoked to an arousal/wake state by nurses touching the infant.
Nursing evaluation of DREAMIES fit and ease-of-use.
For Phase 2, at the end of the 8-hr DEV2-ON period for each session day bedside nurses completed a 6-question survey (see Figure 4) that assessed fit and ease-of-use of the DREAMIES device, including application and removal of the headband and ear cups.
Statistical Analyses
Statistical calculations were performed using commercially available software (SPSS version 25, Chicago, IL). Non-parametric Friedman’s and Wilcoxon signed-rank tests were used for analyses of scaled-ordinal data sets. Parametric tests were used for analyses of continuous variables. For Phase 1, separate repeated measures analyses of variance (ANOVA) were used to assess effects of Condition (4 levels: Baseline, DEV1-ON30, DEV1-ON60, and Post) on heart rate, respiratory rate, temperature, SaO2 and MAP. For Phase 2, separate repeated measures ANOVA were used to assess effects of Condition (2 levels: ON and OFF), Time (8 levels: 8-hours) and Day (3 levels: 1–3 days) on each vital (heart rate, respiratory rate, temperature, and SaO2 and MAP). The Greenhouse-Geisser correction was used and epsilon (ε) with unadjusted degrees of freedom was reported. Where a main effect was observed for factors with more than two levels, post-hoc pair-wise t-tests were used to compare differences. Two-tailed P values with Bonferroni adjustments were reported for post-hoc tests with multiple comparisons. Pearson product-moment correlation coefficient analysis was used to establish the association between device size and infant head circumference. Parametric values were expressed as means and SD. Non-parametric values were expressed as median and interquartile range (IQR). P values <0.05 were considered statistically significant.
RESULTS
Subject Demographics and Clinical Characteristics
Pertinent demographics and clinical characteristics of the subject populations in Phase 1 and Phase 2 are provided in Table 1. Twenty-five parents were approached to participate their infant in Phase 1, and 56 parents were approached in Phase 2; zero and 26 declined, respectively. Notably, while we did not assess why parents declined to participate, unsolicited anecdotal reports were consistent with reasons commonly observed in NICU research among vulnerable infants.[28] In Phase 1, all 25 infants completed the study; 11 were premature infants (mean 33.48 wks, SD 2.03) and 14 were full-term infants including four with Neonatal Abstinence Syndrome (mean 39.07 wks, SD 1.41). In Phase 2, 30 infants were enrolled; 1 infant was discharged home prior to initiation of testing; 3 infants were discharged home prior to completion of the study; 1 mother withdrew consent. Phase 2 analyses include data from the 25 infants who completed the protocol; 16 were premature infants (mean GA 32.5 wks, SD 2.3) and 9 were full-term infants including three with Neonatal Abstinence Syndrome (mean GA 39.1 wks, SD 1.6). A total of thirty-three nurses were assigned to care for the 25 infants across the different study periods in Phase 2. Given no nursing shift exceeded 12 hours, 2–4 nurses typically cared for and participated in the study assessments for any one infant over the 3-day study session.
Table 1.
Infant demographics for Phase 1 and Phase 2
| Characteristic | Phase 1 (n=25) | Phase 2 (n=25) |
|---|---|---|
|
| ||
| Gender Male: number (%) | 15 (60) | 17 (68) |
| Premature Infants: number (%) | 11 (44) | 16 (64) |
| Mean Gestational Age: weeks (SD) | 35.6 (3.4) | 34.1 (3.9) |
| Mean Study Age: Post Menstrual Age (SD): | 36.7 (3.3) | 35.2 (3.9)* |
| Mean Study Age: Day of Life (SD) | 7.4 (2.9) | 7.8 (4.3)* |
| Mean Gestation Weight: kg (SD) | 2.7 (0.9) | 2.3 (0.9) |
| Mean Study Weight: kg (SD) | 2.6 (0.8) | 2.3 (0.9)* |
| Mean Occipital-Frontal Circumference: cm (SD) | 32.3 (3.0) | 31.4 (3.6)* |
Phase 2 data reported are from the first study day.
Device size and infant head circumference.
The measured occipital-frontal head circumference for each infant in each phase guided the selection of the headband size employed based on the following device sizing guidelines: Extra-Small (23–27cm), Small (27–31cm), Medium (31–35cm), Large (35–39cm). In Phase 1, all but four subjects wore the matched-size band and ear cup; 4% infants wore extra small, 28% wore small, 36% wore medium, and 32% wore large headbands. In Phase 2, all but two subjects wore the matched-size band and ear cover for all three DREAMIES session days; 8% infants wore extra-small, 28% small, 36% medium and 28% large. One subject was switched to a larger size band after the first session and one subject was switched to a smaller headband and ear cover for their third session. Infant occipital-frontal head circumference was positively correlated with device size (r=0.965; P<0.001).
Phase 1
Skin irritation.
There was no skin breakdown or severe erythema reported after either DEV1-ON30 or DEV1-ON60. No erythema was observed in 5 infants for DEV1-ON30 and in 4 infants for DEV1-ON60. Mild erythema was reported around the ears and/or forehead in 18/25 infants for DEV1-ON30 and 21/25 infants for DEV1-ON60; 2/25 infants had moderate erythema for DEV1-ON30, which was not observed at the subsequent DEV1-ON60. In Phase 1, erythema presented mostly as a transient linear demarcation on the skin.
Physiologic activity.
There was a statistically significant main effect of Condition on heart rate (F(3,72)=23.71, ε=0.892; P<0.001), respiratory rate F(3,72)=32.43, ε=0.959; P<0.001), and MAP (F(3,72)=4.58, ε=0.922; P=0.007). Paired comparisons within each of these outcomes revealed: 1) Heart rate for DEV1-ON30 (mean 136.68 bpm; SD 16.16) and for DEV1-ON60 (mean 136.76 bpm; SD 16.16) were each significantly lower compared to Baseline (mean 155.68 bpm; SD 17.93; P<0.001) and to Post-DREAMIES (mean 153.52 bpm; SD 14.24; P<0.001). No heart rate differences were observed between DEV1-ON30 and DEV1-ON60, or between Baseline and Post-DEV1. 2) Respiratory rate for DEV1-ON30 (mean 44.92 breaths/min, SD 8.89) and for DREAMIES-ON60 (mean 44.88 breaths/min, SD 11.10) were each significantly lower compared to Baseline (mean 64.44 breaths/min, SD 12.16; P<0.001) and to Post-DEV1 (57.20, SD 12.34; P<0.001). No respiratory rate differences were observed between DEV1-ON30 and DEV1-ON60. Post-DEV1 respiratory rate was lower than Baseline respiratory rate (P=0.040); 3). The only significant difference in MAP was between DEV1-ON30 (mean 55.56 mmHg, SD 9.49) and Post-DEV1 (mean 62.12 mmHg, SD 11.98; P=0.008). No other differences in MAP were observed among Baseline (mean 59.44 mmHg, SD 14.35), DEV1-ON30, DEV1-ON60 (mean 58.36 mmHg, SD 10.14) or Post-DEV1. There was no effect of condition on temperature (mean 36.98° C, SD 0.21) or SaO2 (mean 97.93%, SD 2.28).
Phase 2
Skin irritation.
There was no skin breakdown or severe erythema reported at any nursing cares period during the 8-hour DEV2-ON period (i.e., 3rd hour, 6th hour, or 8th hour upon device removal) on any of the three study days. In contrast to the linear demarcation observed in Phase 1, erythema in Phase 2 presented as a general diffuse redness. No differences in erythema were observed for these three time periods across the 3 days. Of a total 225 assessments of erythema (25 subjects × 3 times/day × 3 days), 95% of the time nurses observed little to no erythema around the forehead and/or ears. In summary, there were 85 reports of no erythema (38%), 125 mild (57%), 9 moderate (4%), and no reports of severe erythema; 3 instances had no report (1%). Notably, 6 of the 9 moderate erythema observations were within the same subject; each of the other 3 moderate reports of erythema were a one-time observation in 3 different subjects. There were no indications of erythema persisting for more than 30 min after device removal in any infant. The device was re-applied per protocol in all infants as any observed erythema dissipated by the end of routine cares.
Physiologic activity.
There was a statistically significant main effect of Condition (F(1,21)=11.52, ε=1.0; P=0.003) and Day (F(2,42)=7.57, ε=0.993; P=0.002) and a Condition × Day interaction (F(2,42=3.81, ε=0.851; P=0.038) on heart rate; there was no effect of Time or any other interactions. Post-hoc analyses revealed for Day 2 and Day 3 heart rate was significantly lower for DEV2-ON (mean 150.66 beats/min, SD 9.98) than DEV2-OFF (mean 155.42 beats/min, SD 9.40; P=0.001), whereas there was no difference in heart rate between conditions on Day 1 (mean 149.06, SD 9.78). The Pre-Study heart rate was also significantly lower (mean 146.79 beats/min, SD 9.12) than the Post Study heart rate (mean 155.07 beats/min, SD 10.77; P<0.001).
Respiratory rate was significantly lower for DEV2-ON (mean 54.17 breaths/min, SD 7.39) than DEV2-OFF (58.92, SD 8.18; F(1,21)=21.54,ε =1.0; P<0.001); Day or Time did not affect respiratory rate. There were no differences in respiratory rate between the Pre-Study and Post-Study days (mean 57.59 breaths/min, SD 9.46; P=0.102).
Temperature (mean 37.05°C, SD 0.21) and SaO2 (mean=97.53%, SD 2.00) were not affected by Condition, Day or Time. There was inadequate data collected for MAP for the DEV2-OFF periods to assess differences between DEV2-ON periods.
Behavioral State.
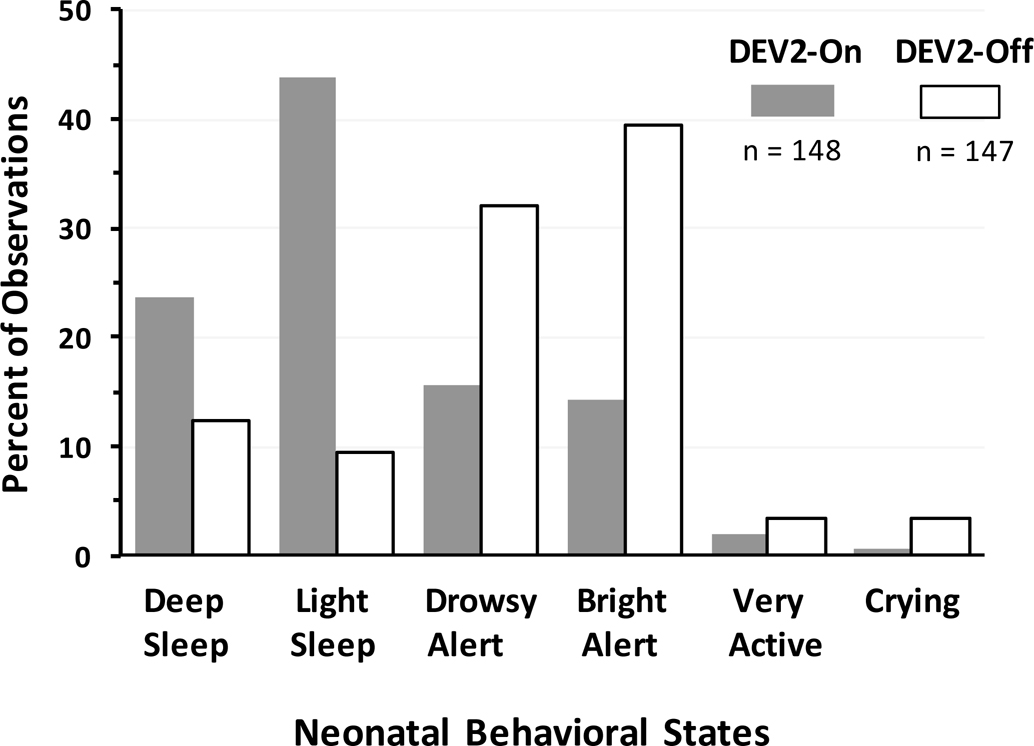
Figure 5 shows the percent of observations for each behavioral state at the end of the 3-hour interval prior to nursing cares for equivalent periods of DEV2-ON (hours 3 and 6 collectively) and DEV2-OFF (hours 12 and 15 collectively). On average, nurses reported infants had approximately 15% more sleep (quiet and active behavioral sleep states) and less wake (drowsy alert, active alert, active, crying states) for DEV2-ON (86% sleep; 14% wake) than DEV2-OFF (71% sleep; 29% wake). Within each condition, there was no difference between behavioral state scores at equivalent 3-hour periods preceding nursing cares; i.e., between DEV2-ON at 3 and 6 hours or between DEV2-OFF at 12 and 15 hours (see Figure 3 for protocol hours). Paired comparisons between conditions combined for equivalent 3-hr time periods revealed a significantly lower behavioral state score, indicative of sleep, for DEV2-ON (median=2.0; IQR 2.0–3.0) than DEV2-OFF (median=3.0; IQR 3.0–4.0; P<0.001).
Figure 5.
Increased sleep and decreased wake with DEV2-ON compared to DEV2-OFF. Closed bar = DEV2-ON (hours 3 and 6); Open bar = DEV2-OFF (hours 12 and 15).
Nursing evaluation of DREAMIES.
Thirty-three nurses participated in the DREAMIES fit and ease of use survey (Figure 4). A total of 75 evaluations (25 subjects × 3 days) were obtained for each of six survey questions, which included 4 missing responses for the ease of use question. On average, 93% (419/450) of the total nursing evaluations were positive for headband and ear cover placement and device removal. There were 16 “neutral” reports, 10 reports of difficulty applying or securing the band and 1 report of difficulty securing the ear covers.
DISCUSSION
This two-phase pilot study found DREAMIES improved physiologic activity and promoted sleep among hospitalized NICU infants. Short, consecutive 30-min periods up to 1 hour (Phase 1), and long, consecutive ~3-hour periods up to 8 hours for 3 consecutive days (Phase 2) with DREAMIES on resulted in significant decreases in heart and respiratory rates compared to DREAMIES off in two separate cohorts of neonates treated in the NICU. No skin breakdown was reported in any infant in either phase. Predominantly little to no erythema was observed, with only a few reports of moderate, transient redness around infants’ forehead and ears following DREAMIES removal. Nurses chiefly reported little to no difficulty in applying or removing the device.
A primary aim of this study was to assess skin integrity after short (1-hour session) and long (3 consecutive 8-hour session days) periods of DREAMIES device wear. In Phase 1, mild though quickly dissipating linear demarcation erythema (typical to that commonly observed with removal of standard of care clinical skin sensors) in most subjects led us to modify the headband and ear cups to further minimize redness while still allowing a good fit.[25] Phase 2 demonstrated that the revised DREAMIES continued to provide minimal erythema despite prolonged wear. Although there were instances where some infants were observed to have moderate erythema, the redness always dissipated within 30 min of device removal. There were no instances where a subject had to be discontinued because erythema persisted beyond the time needed to complete routine cares. Further, within one 8-hr DEV2-ON period, the erythema dissipation time did not increase from first to second to third care Intervals.
In both Phases, nurses were able to fit the device on all infants (see Table 1 for demographics) using the four available ear cover and band sizes designed to accommodate head circumferences ranging between 23 and 39 cm. Nearly all nurses found the device easy to use. There were a couple of instances in which the band size was switched to better fit the infant and some anecdotal reports associated with the headband sliding down the forehead to the eyes. Future quality improvement studies that incorporate additional in-service training may provide insight to further improve band fit and/or placement.
Another important aim of this pilot study was to assess changes in physiologic activity with device use. Consistent with our hypothesis, autonomic function improved during both short (Phase 1) and long (Phase 2) intervals when DREAMIES were worn compared to periods when DREAMIES were off. Clinical data consistently show that high sound levels are harmful to the general health of premature infants, mimicking acute-pain stress response.[1,3,29–32] We observed significant reductions in elevated heart and respiratory rates (Phase 1 and Phase 2) and reduced MAP (Phase 1). Temperature and blood-oxygenation, which were within normative values at the start of each study session remained stable throughout study conditions. These findings suggest that DREAMIES, a hearing protection device that filters out noxious loud noise common in NICU environments, helps improve physiologic activity critical for ensuring healthy neurosensory development in vulnerable NICU neonates.
Findings from Phase 2 also support our hypothesis that by masking loud, noxious NICU noise, DREAMIES promote sleep and reduce wakefulness (see Figure 5). Nurses assessed behavioral state [27] at hourly intervals when the infant should have been sleeping and observations were purposely conducted preceding interventions (i.e., before removal of DREAMIES) and nursing cares so not to provoke arousal. Although these assessments were only a small snapshot in time, it is promising that on average for DEV2-ON infants were reportedly asleep 85% of the time compared to 71% for DEV2-OFF periods. It is unclear whether the increase in wake-alert periods observed for DEV2-OFF was due to carry-over effects inherent to the within-subject design (i.e., increased sleep during DEV2-ON resulted in more alert wake periods during DEV2-OFF), response bias (see limitation below), or indeed due to environmental noise in the NICU. Additional studies using polysomnography are needed to systematically examine whether DREAMIES reduce sleep fragmentation associated with noxious NICU noise and promote sleep/wake patterning conducive to healthy infant development and neurobehavioral outcomes.[33–35]
As neonatal clinicians have become aware of the potential adverse effects of noise in the NICU, many interventions have been studied to manage high levels of noise in the NICU, with little promise of being easy to integrate with best-practice care protocols, and/or effective, including silicone ear plugs [8], adhesively-bonded ear muffs [6,7], and acoustic monitors that visually alert staff to high levels of noise in the NICU. [13] Another approach has been multi-million dollar redesign of NICU’s throughout the country in order to provide single patient rooms.[21] There is controversial evidence that such an approach may lead to an increase in developmental delays in the speech center of the brain, potentially related to relative “auditory sensory deprivation”.[19] More recently, studies have suggested the importance of promoting environments that reflect sounds of the womb, including mother’s voice, heartbeat and bowel sounds.[24,36,37] Aligning with this idea, DREAMIES selectively dampens high-frequency noxious noise that can result in hearing loss and histologic damage, and passes through important low-frequency sound levels (Figure 2) analogous to intrauterine sounds recorded during pregnancy.[23,24] Importantly, the DREAMIES device does not fully mask all sounds, which could lead to sensory deprivation. Further study is needed to assess whether acoustic filters (such as the DREAMIES device) that optimally mimic the sound attenuation of the gravid mother’s body affect autonomic nervous system patterning to promote healthy acoustic, speech and language development among infants born prematurely and other vulnerable patients who require prolonged care in NICU environments.
Study Limitations
A limitation to this pilot study is that bedside noises were not recorded during the study periods. However, the robust and consistent improvements in autonomic function we observed during the time periods when subjects wore the DREAMIES was noted in both study phases, and support our conclusion that the observed changes in HR, RR and state effects were due to DREAMIES use rather than coincidental changes in ambient noise. The variable start periods of Phase 2 DREAMIES use make a coincidental, environmental origin of the observed effects extremely unlikely.
Another limitation was that vital signs were recorded hourly. Continuous assessment of vitals may provide a more quantitative evaluation of the effect over time. However, we found significant changes in vital signs with DREAMIES despite minimal data points. Future studies that simultaneously record continuous bedside noise levels and vitals will help confirm and identify specific noise events that are associated with tachycardia, tachypnea, and increased alertness (decreased sleep), and also assess if there is adaptation to specific noises over time.
A major limitation to the sleep assessment results was that nurses were not masked to use of the DREAMIES device. The investigators were well aware of this issue. All attempts to fabricate a sham device similar in appearance to DREAMIES, but without any attenuation or modification of external sound, were unsuccessful when tested in a laboratory setting. While study patient vital signs data likely were not affected by lack of masking, as they were retrieved via time-stamp from the bedside monitors, it is certainly possible that the NICU nurses participating in the study had some degree of response bias in their sleep assessments given that they were aware when infants had the DREAMIES device on. Future studies that employ full polysomnography with EEG to objectively score sleep state, using sleep raters masked to study patient condition will be needed to corroborate the observed sleep findings.
A final constraint of our study was its limitation to a single-center NICU caring for a relatively small number of study infants with wide-ranging gestational ages. Larger studies that allow stratification across age ranges and disorders will help determine if there are specific patient populations that may maximally benefit from the use of the DREAMIES device. Longer duration studies that include outpatient follow-up also are needed to determine if there are persistent benefits to controlling noise exposure in the NICU.
Conclusions
In this two-phase pilot study we demonstrated that DREAMIES can easily be applied by NICU nurses and safely provide high-frequency noise protection to fragile neonates without skin damage. Our preliminary findings of improved cardiac and respiratory activity and enhanced sleep during DREAMIES use need to be confirmed in other NICU’s. In addition, larger, longer duration studies, stratified among patient populations are needed to assess the benefits of such noise protection in the NICU and to determine if there are optimal developmental periods or infant cohorts in which the DREAMIES are most effective for improving long-term neurodevelopmental outcomes.
Highlights.
NICU noise protection
Infant tolerance
Ease-of-use
Cardio-respiratory; sleep
Acknowledgments
We gratefully acknowledge the assistance of the nursing staff of the NICU at NICU at Magee-Womens, UPMC Hamot Hospital for their assistance in the performance of this study. We thank Mr. Timothy Cooney, MS, for help with initial statistical evaluation of the data. Preliminary findings from this study were presented as a poster at the 31st Annual Gravens Conference on the Environment of Care for High Risk Newborns , 2018. The study has been registered with ClinicalTrials.gov NCT0274066.
Funding support
This study was supported by a grant awarded under the Pitt Innovation Challenge (PInCh®) through the Clinical and Translational Science Institute, University of Pittsburgh, and the National Institutes of Health Grant UL1TR001857 (MB). Dr. Bloch-Salisbury’s effort on this project was supported by the National Heart, Lung, and Blood Institute (NHLBI) and the National Center for Complementary & Integrative Health (NCCIH), National Institutes of Health, Grant 1U54HL143541. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- NICU
Neonatal Intensive Care Unit
- DREAMIES
NEATCAP® DREAMIES® (NEATCap Medical, LLC) circumaural hearing protector device
- DEV1-ON30
DREAMIES Phase 1 device worn for first 30-min period
- DEV1-ON60
DREAMIES Phase 1 device worn for second 30-min period
- DEV2-ON
Condition periods when DREAMIES Phase 2 device were worn
- DEV2-OFF
Condition periods when DREAMIES Phase 2 were not worn
- SaO2
arterial blood-oxygen saturation
- MAP
mean arterial blood pressure
Footnotes
Declaration of Interest:
Fred Kimock, PhD, and Andrew Unger, MD, have a financial interest in NEATCap Medical, LLC, the developer and manufacturer of the study device. Dr. Balsan, Ms. Burns, Ms. Hirsch, and Dr. Bloch-Salisbury were supported in part by NIH funding and otherwise have no other conflict of interest. Dr.Telesco declares no conflict of interest.
This trial is registered on clinicaltrials.gov NCT02744066.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wachman EM, Lahav A, The effects of noise on preterm infants in the NICU, Arch. Dis. Child. Fetal Neonatal Ed. 96 (2011) 305–310. 10.1136/adc.2009.182014. [DOI] [PubMed] [Google Scholar]
- [2].Bremmer P, Byers JF, Kiehl E, Noise and the premature infant: physiological effects and practice implications., J. Obstet. Gynecol. Neonatal Nurs. 32 (2003) 447–454. 10.1177/0884217503255009. [DOI] [PubMed] [Google Scholar]
- [3].Brown G, NICU Noise, Neonatal Netw. 28 (2008) 165–173. [DOI] [PubMed] [Google Scholar]
- [4].Monsén MG, Edéll-Gustafsson UM, Noise and sleep disturbance factors before and after implementation of a behavioural modification programme, Intensive Crit. Care Nurs. 21 (2005) 208–219. 10.1016/j.iccn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [5].Graven SN, The full-term and premature newborn: Sound and the developing infant in the NICU: Conclusions and recommendations for care, J. Perinatol. 20 (2000) S88–S93. 10.1080/19338244.2017.1304883. [DOI] [PubMed] [Google Scholar]
- [6].Zahr LK, de Traversay J, Premature infant responses to noise reduction by earmuffs: Effects on behavioral and physiologic measures, J Perinatol. 15 (1995) 448–455. [PubMed] [Google Scholar]
- [7].Abujarir R, Salama H, Greer W, Al Thani M, Visda F, The impact of earmuffs on vital signs in the neonatal intensive care unit, J. Neonatal. Perinatal. Med. 5 (2012) 249–259. 10.3233/NPM-2012-57511. [DOI] [Google Scholar]
- [8].Turk CA, Williams AL, Lasky RE, A randomized clinical trial evaluating silicone earplugs for very low birth weight newborns in intensive care, J. Perinatol. 29 (2009) 358–363. 10.1038/jp.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu L, Gujjula S, Kuo SM, Multi-channel real time active noise control system for infant incubators, Proc. 31st Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. Eng. Futur. Biomed. EMBC 2009. (2009) 935–938. 10.1109/IEMBS.2009.5333780. [DOI] [PubMed] [Google Scholar]
- [10].Liu L, Gujjula S, Thanigai P, Kuo SM, Still in Womb: Intrauterine Acoustic Embedded Active Noise Control for Infant Incubators, Adv. Acoust. Vib. 2008 (2008) 1–9. 10.1155/2008/495317. [DOI] [Google Scholar]
- [11].Thanigai P, Kuo SM, Yenduri R, Nonlinear active noise control for infant incubators in neo-natal intensive care units, ICASSP, IEEE Int. Conf. Acoust. Speech Signal Process. - Proc. 1 (2007) 109–112. 10.1109/ICASSP.2007.366628. [DOI] [Google Scholar]
- [12].Beemanpally K, Pottim KR, Kuo SM, Multi-channel hybrid active noise control systems for infant incubators, in: 41st Int. Congr. Expo. Noise Control Eng. 2012, INTER-NOISE 2012, 2012: pp. 9806–9811. [Google Scholar]
- [13].Liu WF, The impact of a noise reduction quality improvement project upon sound levels in the open-unit-design neonatal intensive care unit, J. Perinatol. 30 (2010) 489–496. 10.1038/jp.2009.188. [DOI] [PubMed] [Google Scholar]
- [14].Milette I, Decreasing noise level in our NICU: The impact of a noise awareness educational program, Adv. Neonatal Care. 10 (2010) 343–351. 10.1097/ANC.0b013e3181fc8108. [DOI] [PubMed] [Google Scholar]
- [15].Walsh-Sukys M, Reitenbach A, Hudson-Barr D, DePompei P, Reducing light and sound in the Neonatal Intensive Care Unit : An evaluation of patient safety, staff satisfactino and costs, J Perinatol. 21 (2001) 230–235. [DOI] [PubMed] [Google Scholar]
- [16].Liu WF, Comparing sound measurements in the single-family room with open-unit design neonatal intensive care unit: The impact of equipment noise, J. Perinatol. 32 (2012) 368–373. 10.1038/jp.2011.103. [DOI] [PubMed] [Google Scholar]
- [17].Krueger C, Schue S, Parker L, Neonatal intensive care unit: Sound levels before and after structural reconstruction, MCN. Am. J. Matern. Child Nurs. 32 (2007). [DOI] [PubMed] [Google Scholar]
- [18].Stevens DC, Akram Khan M, Munson DP, Reid EJ, Helseth CC, Buggy J, The impact of architectural design upon the environmental sound and light exposure of neonates who require intensive care: An evaluation of the boekelheide neonatal intensive care nursery, J. Perinatol. 27 (2007) S20–S28. 10.1038/sj.jp.7211838. [DOI] [PubMed] [Google Scholar]
- [19].Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, Van Essen DC, Inder T, Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments, J. Pediatr. 164 (2014). 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Byers JF, Waugh WR, Lowman LB, Sound level exposure of high-risk infants in different environmental conditions., Neonatal Netw. 25 (2006) 25–32. 10.1891/0730-0832.25.1.25. [DOI] [PubMed] [Google Scholar]
- [21].Hardy NP, Cost and design analyses of neonatal intensive care units: Comparings single family room, double-occupancy, open-bay, and combination setting for best design practices (Unpublished Masters thesis), University of Florida, 2005. [Google Scholar]
- [22].Darbyshire JL, Müller-Trapet M, Cheer J, Fazi FM, Young JD, Mapping sources of noise in an intensive care unit, Anaesthesia. 74 (2019) 1018–1025. 10.1111/anae.14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gerhardt KJ, Abrams RM, Oliver CC, Sound environment of the fetal sheep, Am. J. Obstet. Gynecol. 162 (1990) 282–287. 10.1016/0002-9378(90)90866-6. [DOI] [PubMed] [Google Scholar]
- [24].Parga JJ, Daland R, Kesavan K, Macey PM, Zeltzer L, Harper RM, A description of externally recorded womb sounds in human subjects during gestation, PLoS One. 13 (2018) 1–15. 10.1371/journal.pone.0197045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kimock FM, Rambo Z, Thear EG, Thear GH, Whalen J, Medical Headgear., US Pat. Appl. 15/881,111, 2018.
- [26].Kimock FM, Rambo Z, Kimock BJ, Unger A, Thear EG, Thear GH, Medical Headgear, US Patent 10,413,696, 2019.
- [27].McGrath JM, Vittner D, Behavioral assessment, in: Tappero E, Honeyfield M (Eds.), Phys. Assess. Newborn A Compr. Approach to Art Phys. Examnation, 5th ed., Petualuma, 2015: pp. 83–100. 10.4135/9781506352671.n14. [DOI] [Google Scholar]
- [28].Ward FR, Parents’ views of invovlement inconcurrent research with their neonates, BoneJ Empir Res Hum Res Ethics. 5 (2010) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Peng NH, Bachman J, Jenkins R, Chen CH, Chang YC, Chang YS, Wang TM, Relationships between environmental stressors and stress biobehavioral responses of preterm infants in NICU, Adv. Neonatal Care. 13 (2013). 10.1097/ANC.0000000000000023. [DOI] [PubMed] [Google Scholar]
- [30].Salavitabar A, Haidet KK, Adkins CS, Susman EJ, Palmer C, Storm H, Preterm infants’ sympathetic arousal and associated behavioral responses to sound stimuli in the neonatal intensive care unit, Adv. Neonatal Care. 10 (2010) 158–166. 10.1097/ANC.0b013e3181dd6dea. [DOI] [PubMed] [Google Scholar]
- [31].Zimmerman E, Keunen K, Norton M, Lahav A, Weight gain velocity in very low-birth-weight infants: Effects of exposure to biological maternal sounds, Am. J. Perinatol. 30 (2013) 863–870. 10.1055/s-0033-1333669. [DOI] [PubMed] [Google Scholar]
- [32].Catlett AT, Holditch-Davis D, Environmental stimulation of the acutely ill preterm: Physiological effects and nursing implications, Neonatal Netw. 8 (1990) 19–26. [PubMed] [Google Scholar]
- [33].Bertelle V, Sevestre a, Laou-Hap K, Nagahapitiye MC, Sizun J, Sleep in the neonatal intensive care unit, J. Perinat. Neonatal Nurs. 21 (2007) 140–150. 10.1097/01.JPN.0000270631.96864.d3. [DOI] [PubMed] [Google Scholar]
- [34].Allen KA, Promoting and protecting infant sleep, Adv. Neonatal Care. 12 (2012) 288–291. 10.1097/ANC.0b013e3182653899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weisman O, Magori-Cohen R, Louzoun Y, Eidelman A, Feldman R, Sleep-wake transitions in premature neonates predict early development, Pediatrics. 128 (2011) 706–714. [DOI] [PubMed] [Google Scholar]
- [36].Webb AR, Heller HT, Benson CB, Lahav A, Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation, Proc. Natl. Acad. Sci. U. S. A. 112 (2015) 3152–3157. 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zimmerman E, McMahon E, Doheny L, Levine P, Lahav A, Transmission of biological maternal sounds does not interfere with routine NICU care: assessment of dose variability in very low birth weight infants, J. Pediatr. Neonatal Individ. Med. 1 (2012) 73–80. 10.7363/010107. [DOI] [Google Scholar]
- [38].Albertini G, Giaquinto S, Mignano M, Spectral analysis of the human voice: A potentially useful tool in rehabilitation, Eur. J. Phys. Rehabil. Med. 45 (2009) 537–545. [PubMed] [Google Scholar]