ABSTRACT
Our study aimed to describe the population pharmacokinetics (PK) of vancomycin in critically ill patients receiving extracorporeal membrane oxygenation (ECMO), including those receiving concomitant renal replacement therapy (RRT). Dosing simulations were used to recommend maximally effective and safe dosing regimens. Serial vancomycin plasma concentrations were measured and analyzed using a population PK approach on Pmetrics. The final model was used to identify dosing regimens that achieved target exposures of area under the curve (AUC0-24) of 400–700 mg · h/liter at steady state. Twenty-two patients were enrolled, of which 11 patients received concomitant RRT. In the non-RRT patients, the median creatinine clearance (CrCL) was 75 ml/min and the mean daily dose of vancomycin was 25.5 mg/kg. Vancomycin was well described in a two-compartment model with CrCL, the presence of RRT, and total body weight found as significant predictors of clearance and central volume of distribution (Vc). The mean vancomycin renal clearance and Vc were 3.20 liters/h and 29.7 liters respectively, while the clearance for patients on RRT was 0.15 liters/h. ECMO variables did not improve the final covariate model. We found that recommended dosing regimens for critically ill adult patients not on ECMO can be safely and effectively used in those on ECMO. Loading doses of at least 25 mg/kg followed by maintenance doses of 12.5–20 mg/kg every 12 h are associated with a 97–98% probability of efficacy and 11–12% probability of toxicity, in patients with normal renal function. Therapeutic drug monitoring along with reductions in dosing are warranted for patients with renal impairment and those with concomitant RRT. (This study is registered with the Australian New Zealand Clinical Trials Registry [ANZCTR] under number ACTRN12612000559819.)
KEYWORDS: dosing, ECMO, pharmacokinetics, glycopeptides, antibiotics, methicillin-resistant Staphylococcus aureus, renal replacement therapy, MRSA
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is being increasingly used in critically ill adult patients with acute cardiorespiratory failure refractory to maximal medical management. ECMO patients often present with infections and have a greater risk of developing nosocomial infections during their hospital stay (1). Therefore, effective antimicrobial therapy is a vital intervention to ensure therapeutic success for such patients (2). However, this goal is often challenging in critical illness and may be complicated by extreme physiological derangements leading to altered pharmacokinetics (PK) and, consequently, antimicrobial exposure (3). The altered PK phenomenon has been well described in critically ill patients. and the introduction of ECMO is hypothesized to further exacerbate these alterations and may impact therapeutic outcomes (4). ECMO is hypothesized to alter the PK of antimicrobials predominantly by circuit sequestration, leading to increased volume of distribution (Vd) and altered drug clearance (CL) (5). Previous ex vivo and neonatal PK data have demonstrated significant alterations in the primary PK parameters of some drugs, and it has been suggested that lipophilic and highly protein bound drugs would be affected the most (6–8). However, significant physiological differences exist between neonatal and adult patients, and therefore specific adult PK data are needed to guide effective antimicrobial therapy.
Vancomycin is a glycopeptide antibiotic that is relatively hydrophilic (logP −3.1) (9), with a plasma protein binding of ∼50% (10). It is predominantly cleared via renal elimination with 75–80% of administered doses cleared via glomerular filtration (11). Vancomycin exhibits optimal antimicrobial activity when the area under the concentration-time curve during a 24-h period (AUC0-24) to MIC (AUC0-24/MIC) is greater than 400 mg · h/liter (12, 13). However, data suggest that the risk of vancomycin-associated nephrotoxicity is significantly increased at AUC0-24 greater than 600–700 mg · h/liter (13, 14).
Previous population PK studies of vancomycin in critically ill ECMO patients have provided conflicting findings (4, 15, 16). Wide variations in the estimated PK parameters were also observed in these studies and, importantly, with the influence of ECMO on vancomycin PK and dosing requirements still unclear. The Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation (ASAP ECMO) study was designed to describe the PK of commonly used drugs in critically ill adult patients receiving ECMO (17). Vancomycin has a molecular weight of 1447 Da, moderate protein binding, a relatively small Vd, and a reported sieving coefficient of 0.7 ± 0.1 (18); all these factors contribute to a clinically significant removal of vancomycin by RRT. The concomitant use of ECMO and RRT is therefore common scenario where clinicians have little guidance to support optimal dosing in this complex presentation. The aims of this paper were to describe the population PK of vancomycin in critically ill adults receiving ECMO with and without RRT, and to provide dosing recommendations for this group of patients.
RESULTS
Population characteristics.
Twenty-two patients were enrolled and contributed 205 plasma samples for analysis. Table 1 summarizes the baseline characteristics, clinical information of the patients, and the relevant ECMO data. The median age was 41 years, with the patients predominantly male. Eleven (50%) patients were on concomitant RRT at the time of sampling. In the non-RRT group, the median CrCL was 75 ml/min (19), and the mean (standard deviation, SD) daily dose of vancomycin was 25.5 mg/kg (6.9). In the RRT group, the mean (SD) vancomycin daily dose was 21.4 mg/kg (8.3), and the mean (SD) effluent and blood flow rate were 20.8 ml/kg/h (19.4) and 218.8 ml/min (25.9), respectively. Vancomycin was administered as an intermittent infusion over a mean (SD) rate of 948.9 mg/h (337.3).
TABLE 1.
| Demographic data | Resultc (n = 22) |
|---|---|
| Male, n (%) | 14 (63%) |
| Age (yr) | 41 (36-53) |
| wt (kg) at admission | 76 (70-90) |
| Body max index (kg/m2) | 26 (23-29) |
| No. of patients on RRT | 11 (50%) |
| CVVHDF | 5 (45%) |
| CVVHD | 3 (27%) |
| EDD | 2 (18%) |
| SLED | 1 (9%) |
| No. of patients by indications for ECMO | |
| Pneumonia | 5 (23%) |
| Cardiogenic shock | 4 (18%) |
| Heart transplant | 3 (14%) |
| ARDS | 2 (9%) |
| Sickle cell crisis | 1 (5%) |
| Myocarditis | 1 (5%) |
| Valve replacement | 1 (5%) |
| Cardiomyopathy | 1 (5%) |
| Cardiac arrest | 1 (5%) |
| Lung transplant | 1 (5%) |
| TRALI | 1 (5%) |
| Illness severity score | |
| APACHE II (on admission to ICU) | 17 (14-26) |
| SOFA (on sampling day) | 10 (8-12) |
| Native CrCL on day of sampling (ml/min)d | 75 (60-82) |
| Albumin (g/liter) | 24 (23-31) |
| Bilirubin (μmol/liter) | 28 (16-51) |
| Total protein (g/liter) | 51 (49-57) |
| Urea (mmol/liter) | 10 (8-12) |
| No. of patients by daily vancomycin dosing regimen | |
| Below 10 mg/kg | 1 (5%)e |
| 10-15 mg/kg | 2 (9%) |
| 15.1-20 mg/kg | 6 (27%) |
| 20.1-25 mg/kg | 4 (18%) |
| 25.1-30 mg/kg | 5 (23%) |
| 30.1-35 mg/kg | 3 (14%) |
| Above 35 mg/kg | 1 (5%) |
| ECMO circuit data | Result |
| No. of veno-venous/no. of veno-arterial | 9/13 (41%/59%) |
| Type of ECMO pump, n (%) | |
| Jostra Rotaflow | 18 (82%) |
| CardioHelp | 2 (9%) |
| Levitronix Centrimag | 2 (9%) |
| Type of ECMO oxygenator, n (%) | |
| Maquet Quadrox | 16 (73%) |
| Medos Hilite | 4 (18%) |
| Medtronic Affinity | 2 (9%) |
| Flow rate (liters/min) | 4.1 (3.5-5.4) |
| Days on ECMO before sampling | 8 (3-10) |
Data for 22 patients.
RRT, renal replacement therapy; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous haemodiafiltration; SLED, sustained low efficiency dialysis; EDD, extended daily diafiltration; ARDS, acute respiratory distress syndrome; TRALI, transfusion-related acute lung injury; APACHE II, acute physiology and chronic health evaluation II score; SOFA, sequential organ failure assessment.
Data displayed in median (IQR, interquartile range) or n (%) as appropriate.
Estimated creatinine clearance only included those patients not on renal replacement therapy (n = 11).
Loading dose.
PK model building.
The final PK model describing the data was a two-compartment model with total body weight included as a covariate on the central volume of distribution (Vc) (normalized to the population mean value of 80 kg) and CrCL (normalized to the population mean value of 77 ml/min), and the presence of RRT was used as binary covariate on CL. The model produced acceptable fits on the diagnostic plots (Fig. 1). The final error model was a gamma (multiplicative) of 3.
| (1) |
| (2) |
OR if patient is on RRT then:
Vcw represents the scaled Vc with body weight with allometric scaling (20), and CLr represent the renal clearance scaled with CrCL to produce total clearance in non-RRT patients. CLrrt represents the CL of vancomycin in the presence of RRT.
FIG 1.
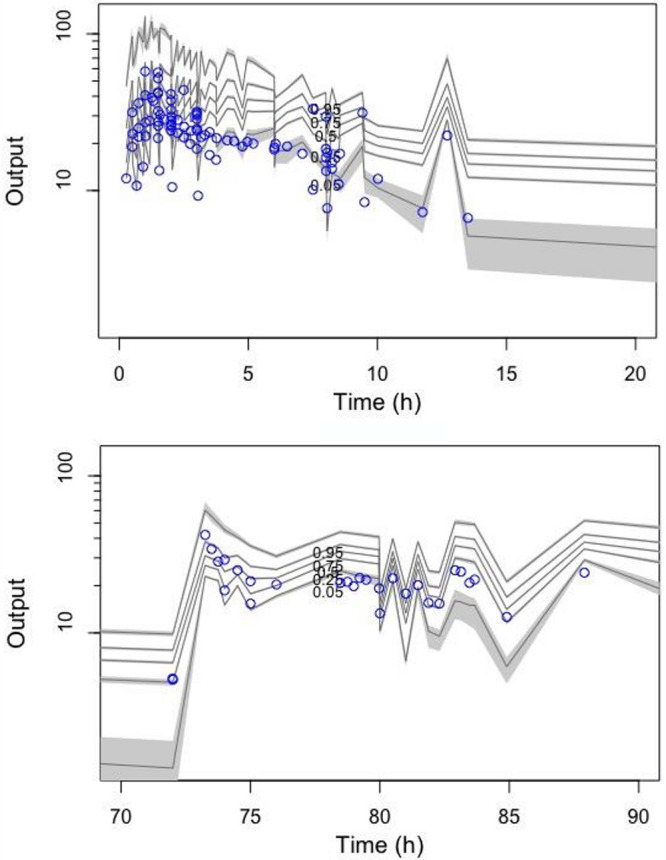
Visual Predictive Check for patients with trough concentrations (top) and patients without trough concentrations (bottom). n = 1,000 simulations of vancomycin plasma concentration data (open circles represent observed data and the lines represent the 5th, 25th, 50th, 75th, and 95% percentiles based on simulations of the pharmacokinetic model).
Table 2 outlines the PK parameter estimates for the final PK model.
TABLE 2.
Pharmacokinetic parameter estimates for final vancomycin PK modela
| Parameter | Mean | SD | Coefficient of variation (%) | Median | Shrinkage (%) |
|---|---|---|---|---|---|
| CL (liters/h) | 3.20 | 0.56 | 17.64 | 3.49 | 4.38 |
| CLrrt (liters/h) | 0.15 | 0.11 | 72.43 | 0.21 | 8.28 |
| Vc (liters) | 29.73 | 14.76 | 49.67 | 30.86 | 10.47 |
| kCP (h−1) | 0.94 | 1.15 | 122.14 | 0.31 | 13.69 |
| kPC (h−1) | 2.11 | 4.46 | 211.47 | 0.08 | 13.90 |
| Error model | Gamma = 3 (multiplicative) | ||||
CL, vancomycin clearance; CLrrt, vancomycin clearance while a patient is on renal replacement therapy; Vc, volume of distribution of the central compartment; kCP, constant for distribution of vancomycin from the central to the peripheral compartment; kPC, constant for distribution of vancomycin from the peripheral to the central compartment.
Fig. 1 and the supplemental material file provide the diagnostic tools used for model selection.
Dosing simulations.
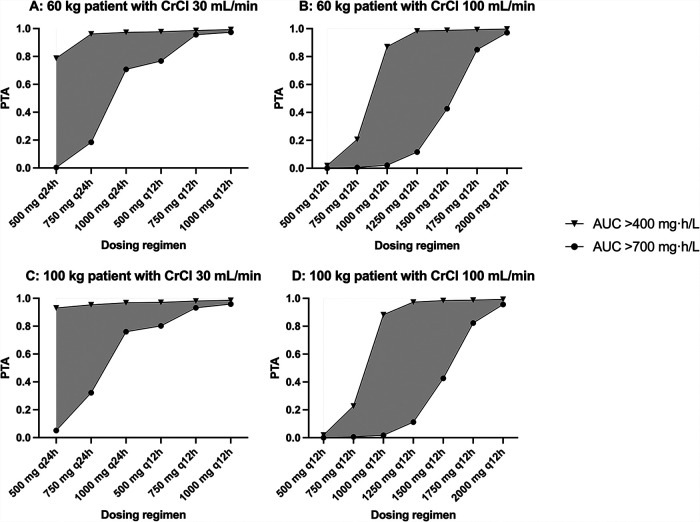
Fig. 2 shows the probability of target attainment (PTA) for efficacy and toxicity across various total body weights (60 kg and 100 kg) and degrees of native renal function (CrCL of 30 ml/min and 100 ml/min) at steady state with various vancomycin dosing regimens. Based on our dosing simulations, for patients with a CrCL of 100 ml/min, a dose of 1250 mg administered every 12 h (12-hourly) generated the largest therapeutic window with 97–98% probability of efficacy and 11–12% probability of toxicity. For patients with a CrCL of 30 ml/min, a dose of 500 mg 24-hourly produced a 79–93% probability of efficacy and 5% probability of toxicity. Patients on concomitant RRT were at significant risks of toxicity with regimens with probabilities of toxicity exceeding 92% for 750 mg 72-hourly regimens following a loading dose of 8.3–10 mg/kg. Therapeutic drug monitoring is highly recommended in this group of patients.
FIG 2.
Probability of target attainment (PTA) at targets AUC0-24 ≥ 400 mg · h/liter and toxicity at AUC0-24 > 700 mg · h/liter for a patient with various weights (60 kg and 100 kg) and various degrees of renal function (CrCL of 30 ml/min and 100 ml/min) at steady state with various intermittent vancomycin dosing regimens. Gray area demonstrates the therapeutic window, the difference in the probability of attaining concentrations of efficacy and toxicity.
DISCUSSION
This paper substantially advances our understanding of dosing needs of vancomycin in critically ill adult ECMO patients by recommending optimized dosing regimens that are maximally effective and safe for this patient population. Our study generated comparable PK parameters to the previous ECMO studies, further supporting the conclusion that ECMO-related variables are not significant predictors of vancomycin PK (Vc or CL). Subsequently, recommended doses for the critically ill population not on ECMO can be used in this subset of patients.
Vancomycin exhibits a 50% plasma protein binding affinity, which has been identified in previous literature (8, 10) as a physicochemical property associated with ECMO circuit sequestration. However, our study described a large clinical effect that was not supportive of the findings from the ex vivo studies. The PK parameter estimates generated in our study were comparable to other published vancomycin PK models of critically ill patients on ECMO summarized in Table 3 (15, 21–23). These findings were in line with the conclusions from these papers, suggesting that ECMO itself was not associated with significant changes in vancomycin Vd and CL (21). In a single-center ECMO population PK study, Jung et al. developed a three-compartment model and described significant effects of ECMO modality (VV vs VA) on vancomycin intercompartmental clearance between the Vc and one of the peripheral compartments. We tested for the same effect during our stepwise model-building approach but did not observe a significant relationship. Jung et al. reported a significantly smaller Vc (0.11 liters/kg vs 0.39 liters/kg) but a similar CL (4.01 liters/h vs 3.20 liters/h) in comparison to our study. The difference in these estimates may be due to dissimilarities in model structure, and/or our inclusion of more patients on VV ECMO (13% vs 41%) and/or the inclusion of RRT patients. Another study reported an ECMO-induced enlarged Vd (4), however this was also tested and no significant relationship was observed in our study.
TABLE 3.
Comparison of vancomycin population PK in critically ill adult patients receiving ECMO and/or RRTa
| Study | Population [n] | No. of compartments | Vc (liters/kg)b | Vp (liters/kg) | Vss (liters/kg) | Q (liters/h) | CL (liters/h) | CLrrt (liters/h) |
|---|---|---|---|---|---|---|---|---|
| Current study | Critically ill adults, ECMO with and without RRT [22] | Two | 0.39 |
Kpc 2.11
Kcp 0.94 |
3.20 | 0.15 | ||
| Jung et al. (37) | Critically ill adults, ECMO without RRT [22] | Three | 0.11 |
V2 0.29 V3 0.08 |
0.40 |
Q2VV 6.23
Q2VA 4.95 Q3 9.09 |
4.01 | |
| Lim et al. (24) | Critically ill adults [20] | Two | 0.52 | 0.77 | 1.29 | 6.99 | 3.96 | |
| Donadello et al. (21) | Critically ill adults, ECMO and non-ECMO, include RRT [11] | Two | 0.45 | 0.82 | 1.27 | 3.60 | 3.70 | 0.6*3.7 = 2.22 |
| Wu et al. (22) | Critically ill adults, ECMO [22] | One | 0.83 | 4.31 | ||||
| Mulla & Pooboni (23) | Critically ill neonates, children and adults, ECMO [45] | Two | 0.37 for age > 4000 days | 0.25 | 0.62 | 0.64 | 0.04*79 = 3.16 | |
| Moore et al. (15) | Critically ill adults, ECMO [14] | Two | 0.25 | 0.34 | 0.59 | 11.2 | 2.83 | |
| Mahmoud et al. (38) | Critically ill adults, ECMO [8] | Three | 0.16 |
Vp 0.39 Vecmo 0.002 |
0.56 | 9.1 | 6.89 | |
| Economou et al. (30) | Critically ill adults, PIRRT [11] | Two | 0.45 |
Kpc 5.29
Kcp 5.97 |
2.15 | ClPIRRT 3.47 | ||
| Deldot et al. (18) | Critically ill adults, CVVHDF [10] | NCA | 0.62 | - | 1.8 |
Vc, central volume of distribution; Vp, peripheral volume of distribution; Vss, steady state volume of distribution; Q, intercompartmental rate constant; CL, clearance; CLrrt, dialytic clearance; ECMO, extracorporeal membrane oxygenation; RRT, renal replacement therapy; PIRRT, prolonged intermittent renal replacement therapy; CVVHDF, continuous venovenous haemodiafiltration; NCA, noncompartmental analysis.
Liters/kg calculations were made based on reported mean weight from the respective study and assumes a linear relationship.
Our data are further informed by very comparable parameters generated from critically ill patients not on ECMO (21, 24). When comparing our parameters with other critically ill population PK studies, Lim et al. generated very similar PK parameters (0.52 liters/kg vs 0.39 liters/kg for Vc, and 3.96 liters/h vs 3.20 liters/h for CL) (24). This is in line with the findings in a matched cohort study where the authors concluded that the introduction of ECMO treatment did not significantly alter vancomycin PK (21).
A common dosing recommendation, of a 25 mg/kg loading dose followed by a 12.5–20 mg/kg maintenance dose administered 12-hourly, provided an acceptable probability of efficacy and toxicity for patients with CrCL 100 ml/min. This is not dissimilar to the Infectious Diseases Society of America (IDSA) clinical practice guidelines for critically ill adult patients, recommending the use of a 25 mg/kg loading dose followed by a 15–20 mg/kg 12-hourly maintenance therapy (25). Patients with CrCL of 30 ml/min benefit from a reduction of maintenance dosing frequency to 48-hourly to avoid drug accumulation. Patients receiving concomitant RRT are exposed to high risks of toxicity; therefore, therapeutic drug monitoring (TDM) should be used to guide ongoing maintenance therapy, and should be standard practice for therapy with this drug in critically ill patients.
An observational study of vancomycin TDM data in ECMO patients by Marella et al. recently proposed that patients with mild-moderate acute kidney injury (AKI) were at risk of underdosing while patients with established AKI on RRT may experience supratherapeutic concentrations (26). The authors’ observation that concomitant use of ECMO and RRT exposes patients to a high risk of supratherapeutic concentration was consistent with our findings. Furthermore, our CLrrt estimate of 0.15 liters/h was smaller than estimates previously reported of vancomycin CL with CVVHDF (18) and other modes of continuous RRT (27). This is likely due to the lower effluent rates used in our patients (mean rate of 1.5 liters/h). It is important to note that Marella et al. utilized a trough concentration (Cmin) as a surrogate for AUC0-24, consequently potentially underestimating the actual vancomycin exposure (28). Therefore, it is pertinent to recognize that the frequency of supratherapeutic/toxic exposure identified in the Marella et al. study may be under-represented.
Overall, our study recommends the use of 25 mg/kg loading dose followed by a maintenance dose of 12.5–20 mg/kg 12-hourly for patients with normal renal function and a 1250 mg (12.5–20 mg/kg) 48-hourly regimen for patients with an AKI (∼CrCL 30 ml/min). These dosing regimens provide the largest therapeutic window between the probability of efficacy and toxicity, 97–98% probability of efficacy and 11–12% probability of toxicity, for patients with intact renal function.
A key limitation to our study was that all types of RRT were included as a binary covariate. Although our study found that patients with concomitant use of ECMO and RRT showed significant variability in drug exposure, no empirical dosage regimen was found to be completely safe and effective, a function of the narrow therapeutic window of this drug. The result may also be contributed to by the small sample size of 11 in the RRT group, which was insufficient to study the relationships among RRT intensity, duration, modality, and drug clearance. It is important to note that the average RRT effluent rate was at the lower end of effluent ranges and therefore interpretations from this study should be made accordingly. Future studies designed to investigate this specific relationship with the collection of dialysis effluent could shed more light into this relationship in the context of ECMO. The use of TDM is highly recommended in this specific subset of ECMO patients. Nevertheless, our results support previously published population PK studies showing that the total CL of vancomycin during RRT was lower than in patients without RRT (29). Further investigations are required to better describe the CL in the presence of prolonged intermittent RRT (PIRRT) and off-PIRRT (30). Prospective validation of the findings from this study is required to improve the robustness of the recommendations made. Another limitation of this study was the absence of non-ECMO critically ill patient population acting as the control group for the ECMO patient cohort. Therefore, this study was unable to conclusively describe the PK differences or similarities between critically ill ECMO and non-ECMO patient. Lastly, the potential effects of concurrent medications and subsequent PK changes were not studied as part of this research. However, based on data available, concurrent drugs used in this study should not affect vancomycin PK.
This multicentre PK study, conducted across four countries, did not identify any ECMO-related variables that statistically improve the final population PK model of vancomycin and does not support dose alterations based on the presence of ECMO alone. The PK parameters generated from our study cohort were similar to those found in the literature of critically ill patients not on ECMO. The current recommendation of a 25 mg/kg loading dose in critically ill patients not on ECMO appears to also be appropriate for patients on ECMO. An empirical maintenance dose of 12.5–20 mg/kg 12-hourly for patients with good renal function appears to provide the largest therapeutic window for common pathogens with MIC = 1 mg/liter. Patients with impaired renal function and those requiring RRT require much lower doses, and these patients should be dosed with therapeutic drug monitoring to avoid drug accumulation and potential toxicity.
MATERIALS AND METHODS
Setting.
The ASAP ECMO study was a prospective, open-labeled, multi-center PK study that was conducted at five intensive care units (ICU) across Australia, New Zealand, South Korea, and Switzerland from November 2012 to November 2019. A detailed study protocol and general results have been published elsewhere and are only discussed briefly here (17). Ethical approval was provided by the lead site (The Prince Charles Hospital, Brisbane, Australia – HREC/11/QPCH/121) with individual institutional approvals obtained according to local protocols: Auckland City Hospital, Auckland, New Zealand – LRS/12/06/020/AM08; The Alfred Hospital, Melbourne, Australia – 541/12; Bern University Hospital, Bern, Switzerland – 2017-01315; Seoul National University Bundang Hospital, Seoul, Republic of Korea – B-1804/465-305; St Vincent’s Hospital, Sydney, Australia – HREC/11/SVH/97. Written informed consent was acquired from the participant’s next of kin.
Study population.
ICU patients aged between 18 and 90 years old who were receiving vancomycin while undergoing ECMO for respiratory and/or cardiac dysfunction were eligible for inclusion. Patients who had a known allergy to the study drug, who were pregnant, had bilirubin >150 μmol/liter, received ongoing massive blood transfusion (>50% blood volume) in the preceding 8 h, or received therapeutic plasma exchange in the preceding 24 h were excluded.
Vancomycin dosing and administration.
Vancomycin was dosed, reconstituted and administered via intermittent infusion according to local hospital protocol.
Study procedures/protocol.
Pharmacokinetic sampling was performed on one or two dosing occasions after the patient was established on ECMO. Blood samples were drawn from an existing arterial line and were collected into 2 ml lithium-heparinised tubes at 0, 15, 30, 45, 60, 90, 120, 180, 360, 480 min post commencement of infusion.
Blood samples were centrifuged at 3000 g for 10 min to separate plasma. Plasma samples were frozen at the study site at −80°C. All frozen plasma samples were then couriered and assayed at the central bioanalysis laboratory at the University of Queensland Centre of Clinical Research (UQCCR), Brisbane, Australia.
Clinical and demographic data were collected and de-identified by trained research staff. Each study site maintained an electronic database for their participants, which was subsequently consolidated into a single database.
Vancomycin assay.
Total concentrations of vancomycin in plasma were measured by a validated high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS) method on a Shimadzu Nexera2 UHPLC system coupled to a Shimadzu 8030+ triple quadrupole mass spectrometer (Shimadzu Corp., Kyoto, Japan). Clinical samples were assayed in batches alongside calibrators and quality controls, and results were subject to batch acceptance criteria (US FDA 2018). Vancomycin detection was monitored at 746.1 → 144.2 nm. Linearity was validated over the concentrations 1 to 100 μg/ml. Precision and accuracy were within 14.0%, and the lowest limit of quantification was 3 μg/ml.
Population pharmacokinetic analysis.
One-, two- and three-compartment models were tested using the Nonparametric Adaptive Grid algorithm within Pmetrics package for R (Los Angeles, CA, USA) (31). Both Lambda (additive) and Gamma (multiplicative) error models were tested for inclusion. Biologically plausible variables tested were actual body weight, body mass index (BMI), age, serum creatinine, creatinine clearance (CrCL), ECMO mode and flow rate, and Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores. Biologically plausible variables were added as a linear and power function to the central volume of distribution (Vc) and CL in a forward stepwise manner. CrCL was calculated via the Cockcroft-Gault equation (32) for patients not receiving RRT:
If the inclusion of a covariate resulted in an increase in the coefficient of determination of the linear regression (R2) and in the reduction of the bias of the goodness-of-fit plots, as well as in a statistically significant reduction in the log-likelihood (P < 0.05), the covariate was supported for inclusion.
(i) Population pharmacokinetic model diagnostics.
The R2 and the bias of the observed versus predicted plots as well as the log-likelihood of each run were considered for the goodness-of-fit evaluation. Predictive performance evaluation was based on mean predicted error (bias) and the mean bias-adjusted prediction error (imprecision) of the population and individual prediction models. The visual predictive check plot and the normalized prediction distribution errors were used to test the suitability of the final covariate model.
(ii) Dosing simulations.
Different vancomycin dosing regimens in patients with various body weights (60 kg and 100 kg) and degrees of native renal function (CrCL of 0 ml/min on RRT, 30 ml/min and 100 ml/min) were simulated using Monte Carlo dosing simulation in Pmetrics (n = 1000). Simulations for patients on RRT were assumed to be anuric (native CrCL of 0 ml/min). Simulated dosing regimens were 500–2000 mg 12–72-hourly administered over a rate of 1000 mg/h. Simulations were performed to determine the PTA to achieve AUC0-24/MIC ratio ≥400 (MIC of 1 mg/liter) for efficacy (33) and AUC0-24 ≥700 mg · h/liter for toxicity. These concentrations were selected from various reported target and toxicity thresholds due to a high number of studies reporting improved outcomes within these limits (13, 34–36).
For each dosing regimen, the PTA was calculated as the percentage of patients achieving AUC0-24 of 400–700 mg · h/liter, at 72-h of therapy after a loading dose of 25 mg/kg for non-RRT, and at 144-h of therapy after a loading dose of 8.3–10 mg/kg for RRT simulations. The optimal dosing regimen was defined as the regimen that provided the highest probability of achieving efficacious exposures while achieving the lowest probability of toxicity.
ACKNOWLEDGMENTS
This work was supported by The Prince Charles Hospital Research Foundation, Australian and New Zealand Intensive Care Foundation, Australia and New Zealand College of Anaesthetists (S12/001; S13/021), Society of Hospital Pharmacists of Australia, National Health and Medical Research Council of Australia (APP1079421; APP1099452). Jason A. Roberts receives funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065), as well as an Advancing Queensland Clinical Fellowship.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Juthani BK, Macfarlan J, Wu J, Misselbeck TS. 2018. Incidence of nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Heart & Lung 47:626–630. 10.1016/j.hrtlng.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Jamal JA, Economou CJ, Lipman J, Roberts JA. 2012. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care 18:460–471. 10.1097/MCC.0b013e32835685ad. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JA, De Waele JJ, Dimopoulos G, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J. 2012. DALI: Defining Antibiotic Levels in Intensive care unit patients: a multi-centre point of prevalence study to determine whether contemporary antibiotic dosing for critically ill patients is therapeutic. BMC Infect Dis 12:152. 10.1186/1471-2334-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekar K, Fraser JF, Smith MT, Roberts JA. 2012. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 27:741. 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Cheng V, Abdul-Aziz MH, Roberts JA, Shekar K. 2019. Overcoming barriers to optimal drug dosing during ECMO in critically ill adult patients. Expert Opin Drug Metab Toxicol 15:103–112. 10.1080/17425255.2019.1563596. [DOI] [PubMed] [Google Scholar]
- 6.Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL, Fraser JF. 2015. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care 19:437. 10.1186/s13054-015-1151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekar K, Roberts JA, McDonald CI, Fisquet S, Barnett AG, Mullany DV, Ghassabian S, Wallis SC, Fung YL, Smith MT, Fraser JF. 2012. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 16:R194. 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekar K, Roberts JA, McDonald CI, Ghassabian S, Anstey C, Wallis SC, Mullany DV, Fung YL, Fraser JF. 2015. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 19:164. 10.1186/s13054-015-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. 2006. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34:D668–72. 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterfield J, Patel N, Pai M, Rosano T, Drusano G, Lodise T. 2011. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob Agents Chemother 55:4277–4282. 10.1128/AAC.01674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzke GR, Zhanel GG, Guay DRP. 1986. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet 11:257–282. 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 12.Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva JA, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC) . 2020. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 46:1127–1153. 10.1007/s00134-020-06050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77:835–864. 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 14.Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. 2019. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 69:1881–1887. 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore JN, Healy JR, Thoma BN, Peahota MM, Ahamadi M, Schmidt L, Cavarocchi NC, Kraft WK. 2016. A population pharmacokinetic model for vancomycin in adult patients receiving extracorporeal membrane oxygenation therapy. CPT Pharmacometrics Syst Pharmacol 5:495–502. 10.1002/psp4.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aljutayli A, Marsot A, Nekka F. 2020. An update on population pharmacokinetic analyses of vancomycin, Part I: in adults. Clin Pharmacokinet 59:671–698. 10.1007/s40262-020-00866-2. [DOI] [PubMed] [Google Scholar]
- 17.Shekar K, Roberts JA, Welch S, Buscher H, Rudham S, Burrows F, Ghassabian S, Wallis SC, Levkovich B, Pellegrino V, McGuinness S, Parke R, Gilder E, Barnett AG, Walsham J, Mullany DV, Fung YL, Smith MT, Fraser JF. 2012. ASAP ECMO: antibiotic, sedative and analgesic pharmacokinetics during extracorporeal membrane oxygenation: a multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol 12:29. 10.1186/1471-2253-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DelDot ME, Lipman J, Tett SE. 2004. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br J Clin Pharmacol 58:259–268. 10.1111/j.1365-2125.2004.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Holford NHG, Anderson BJ. 2017. Allometric size: the scientific theory and extension to normal fat mass. European J Pharmaceutical Sciences 109:S59–S64. 10.1016/j.ejps.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, Belhaj A, Vincent JL, de Backer D, Taccone FS. 2014. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care 18:632. 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FL. 2016. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc 115:560–570. 10.1016/j.jfma.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Mulla H, Pooboni S. 2005. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol 60:265–275. 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim H-S, Chong YP, Noh Y-H, Jung J-A, Kim YS. 2014. Exploration of optimal dosing regimens of vancomycin in patients infected with methicillin-resistant Staphylococcus aureus by modeling and simulation. J Clin Pharm Ther 39:196–203. 10.1111/jcpt.12123. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF, Infectious Diseases Society of America . 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18-55–e55. 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 26.Marella P, Roberts J, Hay K, Shekar K. 2020. Effectiveness of therapeutic drug monitoring guided vancomycin dosing in adult patients receiving extra corporeal membrane oxygenation. Antimicrob Agents Chemother 64:e01179-20. 10.1128/AAC.01179-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boereboom FT, Ververs FF, Blankestijn PJ, Savelkoul TJ, van Dijk A. 1999. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med 25:1100–1104. 10.1007/s001340051018. [DOI] [PubMed] [Google Scholar]
- 28.Neely MN, Kato L, Youn G, Kraler L, Bayard D, Van Guilder M, Schumitzky A, Yamada W, Jones B, Minejima E. 2018. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother 62:e02042-17. 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, De Backer D, Vincent JL, Taccone FS. 2013. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrobial Agents 41:261–266. 10.1016/j.ijantimicag.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Economou CJP, Kielstein JT, Czock D, Xie J, Field J, Richards B, Tallot M, Visser A, Koenig C, Hafer C, Schmidt JJ, Lipman J, Roberts JA. 2018. Population pharmacokinetics of vancomycin in critically ill patients receiving prolonged intermittent renal replacement therapy. Int J Antimicrob Agents 52:151–157. 10.1016/j.ijantimicag.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuck SK, Raber SR, Rodvold KA, Areff D. 2000. National survey of extended-interval aminoglycoside dosing. Clin Infect Dis 30:433–439. 10.1086/313692. [DOI] [PubMed] [Google Scholar]
- 33.European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs and zone diameters. https://mic.eucast.org/Eucast2/. Accessed 10 August 2021.
- 34.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, Rybak MJ. 2018. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 62:e01684-17.[PMC]. 10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. 2018. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 52:805–810. 10.1016/j.ijantimicag.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Jung Y, Lee DH, Kim HS. 2021. Prospective cohort study of population pharmacokinetics and pharmacodynamic target attainment of vancomycin in adults on extracorporeal membrane oxygenation. Antimicrob Agents Chemother 65:e02408-20. 10.1128/AAC.02408-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoud AA, Avedissian SN, Al-Qamari A, Bohling T, Pham M, Scheetz MH. 2020. Pharmacokinetic assessment of pre- and post-oxygenator vancomycin concentrations in extracorporeal membrane oxygenation: a prospective observational study. Clin Pharmacokinet 59:1575–1587. 10.1007/s40262-020-00902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01377-21-s0001.pdf, PDF file, 0.1 MB (144.7KB, pdf)