Abstract
An epidemic of pneumonia with fibrinous polyserositis and multifocal arthritis emerged in captive American alligators (Alligator mississippiensis) in Florida, United States, in 1995. Mycoplasma alligatoris sp. nov. was cultured from multiple organs, peripheral blood, synovial fluid, and cerebrospinal fluid of affected alligators. In a subsequent experimental inoculation study, the Henle-Koch-Evans postulates were fulfilled for M. alligatoris as the etiological agent of fatal mycoplasmosis of alligators. That finding was remarkable because mycoplasmal disease is rarely fatal in animals. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies produced by alligators in response to M. alligatoris exposure was developed by using plasma obtained from naturally infected alligators during the original epidemic. The assay was validated by using plasma obtained during an experimental dose-response study and applied to analyze plasma obtained from captive and wild crocodilian species. The ELISA reliably detected alligator seroconversion (P < 0.05) beginning 6 weeks after inoculation. The ELISA also detected seroconversion (P < 0.05) in the relatively closely related broad-nosed caiman Caiman latirostris and the relatively distantly related Siamese crocodile Crocodylus siamensis following experimental inoculation with M. alligatoris. The ELISA may be used to monitor exposure to the lethal pathogen M. alligatoris among captive, repatriated, and wild crocodilian species.
An epidemic of mycoplasmosis emerged in a collection of American alligators (Alligator mississippiensis) in Florida in 1995 (4, 8). Thirty-three 200- to 300-kg adult male alligators died, and 13 moribund alligators were euthanatized within 1 month of the index case. Lesions observed at necropsy ranged from mild interstitial and peribronchiolar pneumonia to fibronecrotic pneumonia. Extrapulmonary complications included pericarditis, myocarditis, and multifocal arthritis. Mycoplasma alligatoris proposed sp. nov. was isolated from multiple tissues, blood, synovial fluid, and cerebrospinal fluid of affected alligators. In a pilot experimental inoculation study (5, 6), healthy alligators were inoculated with M. alligatoris strain A21JP2T (ATCC 700619) to reproduce the disease and fulfill the Henle-Koch-Evans postulates (11) for M. alligatoris as the etiological agent of synovitis, polyserositis, and pneumonia of alligators. The results were remarkable because, except for bovine and caprine pleuropneumonia, mycoplasmal disease is rarely fatal in animals.
The origin of the 1995 epidemic remains unknown, but the disease may emerge under conditions of captivity. Crocodilians are frequently sold or traded among collections, exhibits, zoos, and ranches. Many collections include multiple species of crocodilians. Some crocodilians from commercial ranches are repatriated after “head-starting” hatchlings from eggs collected in the wild, a potential vector for catastrophic infection of wild populations if lethal disease emerges in captivity. A validated serodiagnostic tool could be used to test captive crocodilians for exposure to M. alligatoris and prevent spread of the pathogen. The objective is to ensure that crocodilians used for commercial purposes or returned to the wild are free of the pathogen. In this report, we describe the development, validation, and application of an enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to M. alligatoris in alligators, caimans, and crocodiles.
MATERIALS AND METHODS
Antigen preparation.
M. alligatoris strain A21JP2T was cloned from a single colony isolated from limb joint synovial fluid of an American alligator that died during a 1995 epidemic (6). Whole-cell lysate antigen (16) was prepared from a 1-liter culture of third-passage M. alligatoris A21JP2T grown at 30°C in ATCC medium 988 broth (SP4 [40]) supplemented with 20% (vol/vol) fetal bovine serum (Gibco, Grand Island, N.Y.). The protein concentration was determined colorimetrically (Bio-Rad, Hercules, Calif.) and adjusted to 100 μg/ml, and the antigen was stored at −70°C in polypropylene cryovials.
Whole-cell lysate antigen was similarly prepared from Mycoplasma bovis ATCC 25523, Mycoplasma iowae ATCC 33552, Mycoplasma microti ATCC 700935, Mycoplasma pulmonis ATCC 19612, and mycoplasmas isolated from other reptiles, including Mycoplasma agassizii ATCC 700616, Mycoplasma crocodyli ATCC 51981, Mycoplasma testudinis ATCC 43263, two isolates of the unnamed mycoplasma ATCC 700618 from tortoises, and Acholeplasma laidlawii isolated from a tortoise (3). Those mollicutes were grown in SP4 supplemented with fetal bovine serum or Frey's medium (40) supplemented with horse serum (Gibco).
Antigen enriched for lipid-associated membrane proteins (LAMP) was prepared from a 1-liter culture of third-passage M. alligatoris A21JP2T grown at 30°C in SP4 broth supplemented with 20% fetal bovine serum by Triton X-114 phase fractionation (14). The protein concentration was adjusted to 100 μg/ml, and the antigen was stored at −70°C in polypropylene cryovials. A LAMP-enriched antigen was similarly prepared from M. agassizii.
Crocodilian plasma samples.
Blood samples were collected from the supravertebral sinus into glass tubes containing lithium heparin anticoagulant (Becton Dickinson, Rutherford, N.J.). Plasma was separated by low-speed centrifugation. All plasma samples were stored at −70°C in polypropylene cryovials until the analyses were performed.
Plasma samples were obtained from 40 naturally exposed alligators during the 1995 epidemic. In a pilot experimental inoculation study, healthy juvenile (approximately 1 m long) alligators were inoculated by intracoelomic injection (n = 2) or by instillation through the glottis (n = 2) with 106 CFU of M. alligatoris strain A21JP2T in 1 ml of SP4 broth. A control alligator received sterile broth. Plasma samples were collected at weekly intervals until the alligators either died or were euthanatized 14 weeks postinoculation (w.p.i.). In a follow-up experimental dose-response study, healthy young adult (approximately 1.5 to 2 m long) female alligators were inoculated by instillation through the glottis with 102, 104, or 106 CFU (six per treatment) of M. alligatoris strain A21JP2T in 1 ml of SP4 broth. Four controls received sterile SP4 broth or no treatment. Plasma samples were collected at weekly intervals for 4 weeks and then biweekly until the alligators either died or were euthanatized at 12 to 16 w.p.i. In an experimental host range study conducted concurrently with the dose-response study, six each of healthy young adult female broad-nosed caimans (Caiman latirostris) and Siamese crocodiles (Crocodylus siamensis) were inoculated by instillation through the glottis with 106 CFU of M. alligatoris strain A21JP2T in 1 ml of SP4 broth. Controls (two per species) received sterile SP4 broth. Plasma samples were collected at weekly intervals for 4 weeks and then biweekly until the crocodilians either died or were euthanatized at 12 to 16 w.p.i. Plasma samples were also obtained during routine health assessment of 70 American alligators in captivity at the Silver Springs Wildlife Park (Ocala, Fla.); 29 Siamese crocodiles in captivity at the Sriracha Crocodile Farm (Sriracha, Thailand), and 42 wild American crocodiles (Crocodylus acutus) in Belize.
Conjugate.
Proteins were precipitated from a pool of American alligator plasma samples by adding ammonium sulfate to a concentration of 25% (wt/vol) and dissolved in 10 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.5. The proteins were fractionated by ion-exchange chromatography on mixed-resin silica gel (Bakerbond ABX Antibody Exchanger; Mallinckrodt Baker, Phillipsburg, N.J.) using a gradient of 0 to 100% elution buffer (500 mM ammonium sulfate, 20 mM sodium acetate). Column fractions enriched for immunoglobulins (Ig) were identified by predominant Coomassie-stained bands of approximately 28 kDa, consistent with reptile Ig light chain, and approximately 60 kDa, consistent with reptile IgY heavy chain (36, 37), after denaturing 12.5% polyacrylamide gel electrophoresis (PAGE; PhastGel System; Pharmacia, Piscataway, N.J.). The Ig were purified by column filtration on Sephacryl S-300 gel (Pharmacia), and purity was reconfirmed by PAGE. Rabbit antiserum against the purified alligator Ig was prepared by using standard immunization methods (Pel-Freez Biologicals, Rogers, Ark.). Polyclonal anti-alligator Ig antibodies were purified from rabbit serum by affinity column chromatography (HiTrap Protein G; Pharmacia) and then conjugated with biotin (EZ-Link Sulfo-NHS-LC biotin; Pierce, Rockford, Ill.).
To determine the relative avidity of the conjugate, each well of a 96-well flat-bottomed microplate (Nunc-Immuno MaxiSorp; Nunc, Kamstrup, Denmark), excluding the perimeter wells, was coated with 50 μl of healthy American alligator (n = 2), broad-nosed caiman (n = 2), or Siamese crocodile (n = 2) plasma serially diluted from 1:2 to 1:1,024 in PBS/A (sodium phosphate buffer [pH 7.2] containing 0.15 M NaCl and 0.02% NaN3) by incubating overnight at 4°C in a sealed humidified container. The wells were washed four times with 350 μl of PBS/A containing 0.05% Tween 20 (PBS/T) by using an automatic microplate washer (EL 403H; Bio-Tek Instruments, Winooski, Vt.) and then blocked with 250 μl per well of PBS/T containing 5% (wt/vol) nonfat dry milk (PBS/TM) overnight at 4°C. After four more washes, 50 μl of biotinylated polyclonal anti-alligator Ig (approximately 150 ng of protein) diluted in PBS/TM was added. The microplate was incubated at room temperature for 60 min on a nutator and washed as described above, and then 50 μl of alkaline phosphatase-conjugated streptavidin (Roche, Indianapolis, Ind.) diluted 1:1,000 in PBS/TM was added to each well. The microplate was incubated and washed as described above, and then 100 μl of p-nitrophenyl phosphate disodium (1 mg/ml in 0.01 M sodium bicarbonate [pH 9.6], 2 mM MgCl2) was added to each well. The microplate was then incubated in the dark without agitation at room temperature. The A405 of each well was measured after 30 min (Thermomix microplate reader with Softmax Pro version 1.2.0 software; Molecular Devices, Sunnyvale, Calif.). Cross-reactivity with plasma available from Chinese alligator (Alligator sinensis), black caiman (Melanosuchus niger), American crocodile, Morelet's crocodile (Crocodylus moreletii), Nile crocodile (Crocodylus niloticus), Australian saltwater crocodile (Crocodylus porosus), Cuban crocodile (Crocodylus rhombifer), and false gavial (Tomistoma schlegelii) was also evaluated.
Immunoblotting.
The specificities of the polyclonal anti-alligator Ig were demonstrated by Western immunoblotting. Samples of plasma from healthy alligators, caimans and crocodiles were diluted 1:50 in PBS/A and then separated by PAGE under denaturing conditions with a precast 10% (bis-Tris)polyacrylamide gel and MOPS (morpholinepropanesulfonic acid) running buffer (NuPAGE; Novex, San Diego, Calif.). The separated proteins were electroblotted by standard methods from the gel onto a nitrocellulose membrane, which was then blocked with PBS/TM overnight at 4°C. The blocked blot was washed with PBS/T and then incubated in purified but unbiotinylated polyclonal anti-alligator Ig (1 μg/ml in PBS/A) for 60 min at room temperature with agitation. After washing, the membrane was incubated in alkaline phosphatase-conjugated donkey anti-rabbit Ig (diluted 1:10,000 in PBS/TM; Pierce) for 60 min at room temperature with agitation. After the final wash, the blot was developed in substrate buffer (0.1 M Tris-HCl [pH 8.8], 1 mM MgCl2) containing nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (Life Technologies, Grand Island, N.Y.).
Western immunoblots of whole-cell lysate and LAMP-enriched antigen preparations used for ELISA were similarly prepared. Antigens bound by specific antibodies in pooled seropositive alligator, caiman, and crocodile plasma (see below) were detected by using the polyclonal anti-alligator Ig and alkaline phosphatase-conjugated donkey anti-rabbit Ig as described above.
Determination of ELISA parameters.
Plasma samples obtained from the pilot experimental inoculation study served as positive and negative specimens to establish reaction conditions for a preliminary ELISA. The titer (heuristically defined as the highest plasma dilution with an A405 more than twice that of the negative control) of plasma obtained from alligators during the 1995 epidemic was determined by using whole-cell lysate antigen. Selected samples were then pooled to create high-range (titer, 1:5,120), midrange (titer, 1:1,280), and low-range (titer, 1:80) plasma pools used in the final ELISA validation.
ELISA procedure.
The A21JP2T whole-cell lysate or LAMP-enriched antigen was thawed, passed several times through a 30-gauge needle, and then diluted to 15 μg/ml in PBS/A. Each microplate well, excluding the perimeter wells, was coated with 50 μl of antigen and then washed and blocked as described above. After four washes, 50 μl of crocodilian plasma diluted 1:1,000 in PBS/TM was added in duplicate, and the microplate was incubated at room temperature for 60 min on a nutator. From this point forward, the assay was as described above for conjugate validation. On each microplate, the background was the mean A405 of duplicate wells processed identically to the sample wells except without plasma. The background absorbance was subtracted from the measured A405 of each well before data analyses. Each microplate also included duplicate high-range, midrange, and low-range alligator plasma pool standards.
Binding of antibody in alligator plasma to whole-cell lysate or LAMP-enriched antigens prepared from the other mollicutes listed above was also assayed in the ELISA format described. Control wells were coated with culture medium with and without fetal calf or horse serum.
Within-batch and between-batch assay variability was assessed by using the Youden plot graphic method (17). The ELISA values obtained for the high-range, midrange, and low-range plasma pool standards included on each microplate coated with whole-cell lysate were used to establish target values and control limits to be used for monitoring the consistency of the assay (10 batches). The values obtained for each standard at the beginning of a series of assays were plotted against the values obtained for the same standards at the end of the series. Imprecision was shown by random deviations at right angles to the slope formed by the expected values, and drift was indicated if all deviations were in the same direction at right angles to the slope.
Statistical analyses.
Data from the dose-response study and the host range study were combined for statistical analysis. Seroconversion was determined by repeated-measure analysis of variance with main effects of w.p.i., species, and treatment group within species. Fisher's protected least significant difference (LSD) was used for post hoc comparisons if main effects were significant. A P value of < 0.05 was considered significant.
RESULTS
Controlling the spread of mycoplasmal infections among captive animals such as swine, poultry, and laboratory rodents depends primarily on identification of individuals whose exposure to the pathogen is indicated by the presence of specific antibody. The most commonly used serological test to diagnose mycoplasmal infections is the ELISA, which is amenable to automation and stringent quality controls. The rationale of the experiments described was to develop reagents and a protocol for an ELISA which could be used to control the spread of M. alligatoris, the newly recognized lethal mycoplasmal pathogen of alligators, among crocodilians.
Conjugate avidity and specificity.
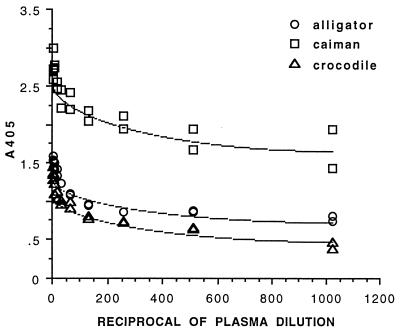
The relative avidity and specificity of the biotinylated polyclonal anti-alligator Ig antibody used in the ELISA was compared among alligator, caiman, and crocodile Ig. The results of conjugate binding to serially diluted plasma from each species coated directly in ELISA microplate wells are shown in Fig. 1. Crocodiles are relatively phylogenetically distant from alligators and caimans but the avidity of the polyclonal antibody was only slightly lower for Siamese crocodile Ig than for alligator Ig. Avidity for broad-nosed caiman Ig was about twofold higher than for alligator Ig. Those results demonstrated that same-species control plasma should be used for diagnostic crocodilian ELISA. The conjugate also cross-reacted (minimum titer, 1:>10) in similar assays with plasma available from the other crocodilian species listed above (data not shown).
FIG. 1.
Relative avidity of polyclonal rabbit anti-alligator Ig antibodies for Ig from healthy crocodilians measured by ELISA. Serially diluted plasma from pools of each species was coated directly in microplate wells, and conjugate binding was measured under conditions used for the diagnostic ELISA. Avidity for crocodile Ig was slightly lower and for caiman Ig was about twofold higher than for alligator Ig.
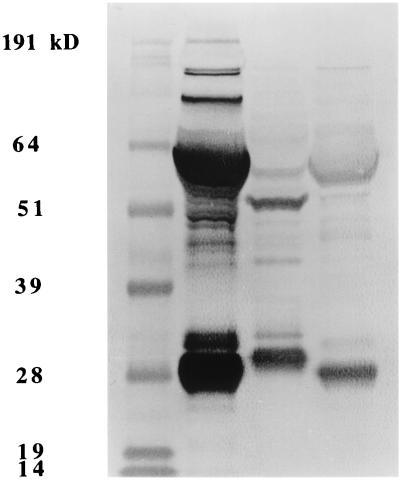
The specificity of the polyclonal anti-alligator Ig antibody for crocodilian plasma proteins separated by denaturing PAGE is shown in Fig. 2. The two predominant bands recognized in alligator and crocodile plasma had apparent sizes of approximately 28 kDa, consistent with reptile Ig light chain, and approximately 55 kDa, consistent with reptile IgY heavy chain (36, 37), on the 10% polyacrylamide gel. Other bands detected may represent incompletely denatured IgY, IgM heavy chain, or antibodies against contaminants remaining in the plasma fraction used for immunization. The two predominant bands recognized in caiman plasma consistently appeared to be slightly larger and smaller, respectively, than those in alligator and crocodile plasma.
FIG. 2.
Western immunoblot of polyclonal rabbit anti-alligator Ig binding to pooled plasma from healthy crocodilians. Lanes (left to right): prestained molecular mass standards, alligator plasma, caiman plasma, and crocodile plasma. The predominant bands recognized by the conjugate, which was used as the secondary antibody in the ELISA, are consistent with reptile 28-kDa Ig light chain and 55-kDa IgY heavy chain separated on a denaturing 10% polyacrylamide gel. The predominant bands recognized in caiman plasma were consistently slightly different in size from those in alligator and crocodile plasma.
ELISA development.
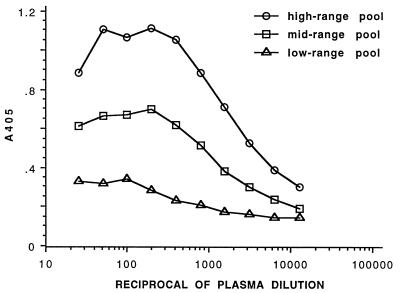
In the pilot experimental inoculation study, three of four inoculated alligators died between 1 and 3 w.p.i. before seroconversion. Plasma obtained at necropsy at 14 w.p.i. from the one surviving inoculated alligator (titer, 1:640) and from the uninoculated control (titer, 1:<10) were the only specimens obtained under controlled conditions which could serve as positive and negative specimens, respectively, to establish the preliminary ELISA conditions. Once those were optimized, it was important to test standards which produced a wide range of absorbance values, to determine what sample dilution should be used for a single-dilution ELISA. A sample dilution of 1:1,000 gave absorbance readings within the linear response to dilution of the high-range, midrange, and low-range plasma pool standards and the microplate reader under the assay conditions described (Fig. 3).
FIG. 3.
Sample dilution validation. Serial dilutions of pools of alligator plasma with high-range (1:5,120), midrange (1:1,280), and low-range (1:80) titers were assayed by ELISA using whole-cell lysate antigen. A dilution of 1:1,000 gave absorbance readings within the linear response to dilution of each pool under the assay conditions described.
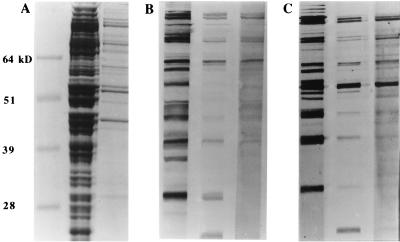
Coomassie staining of antigens separated by sodium dodecyl sulfate-PAGE showed that about 10% of total M. alligatoris protein remained in the LAMP-enriched fraction. That value was consistent with the total protein yields for whole-cell lysate and LAMP preparations from equal volumes of broth cultures. The Western immunoblot patterns of antigens used in the ELISA are shown in Fig. 4. The majority of the antigens recognized on immunoblots of whole-cell lysate were present in the LAMP-enriched fraction. Antigens recognized by caiman and crocodile antibodies were a subset of those recognized by alligator antibodies.
FIG. 4.
(A) PAGE of Coomassie-stained M. alligatoris proteins used as ELISA antigens. Lanes (left to right): prestained molecular mass standards, whole-cell lysate, and LAMP-enriched fraction. The LAMP proteins, prepared by Triton X-114 phase fractionation, were a minor proportion of total cellular protein. (B) Western immunoblot of M. alligatoris whole-cell lysate antigen probed with seropositive (left to right) alligator plasma, caiman plasma, and crocodile plasma. Antigens recognized by caiman and crocodile antibodies were a subset of those recognized by alligator antibodies. (C) Western immunoblot of M. alligatoris LAMP antigen probed with seropositive (left to right) alligator plasma, caiman plasma, and crocodile plasma. The majority of antigens recognized in immunoblots of whole-cell lysate were LAMP antigens.
The between-batch bias control limits were established at 26% above and below the target value for the low-range standard (mean A405 = 0.167 ± 0.030 [standard error (SE)]; coefficent of variation [CV] = 0.181) and 18% above and below the midrange (mean A405 = 0.676 ± SE 0.081, CV = .120) and high-range (mean A405 = 1.493 ± SE 0.159, CV = 0.106) standards. The drift control limits were established at angles 4° (11%) above and below the slope of the target values. The control limits are statistically arbitrary (17).
Detection of crocodilian seroconversion by ELISA.
Three alligators inoculated with 106 CFU and two caimans died within 4 w.p.i. and a third caiman died 10 w.p.i. during the dose-response and host range studies. Mycoplasmas were cultured quantitatively in high numbers from trachea, lung, coelomic cavity, liver, spleen, interior of pericardial sac, heart, blood, limb joints, and brain of those crocodilians. Mycoplasmas were not cultured at any time point from the remaining three highest-dose alligators and caimans, alligators inoculated with 104 or 102 CFU, or any of the negative controls. Mycoplasmas were cultured at necropsy in small numbers from a single site, the tonsils, of three inoculated crocodiles. The identity of the mycoplasmas reisolated from each individual was confirmed by restriction endonuclease analyses of the PCR-amplified mycoplasmal 16S rRNA gene. Only M. alligatoris was recovered. Together, those findings indicated that the culture methods employed in this study did not detect any mycoplasmas among the normal flora of crocodilians.
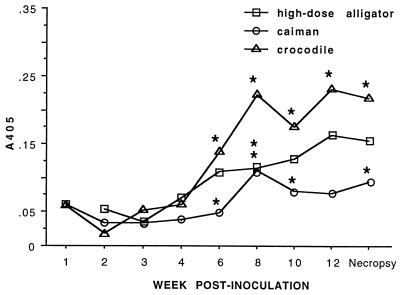
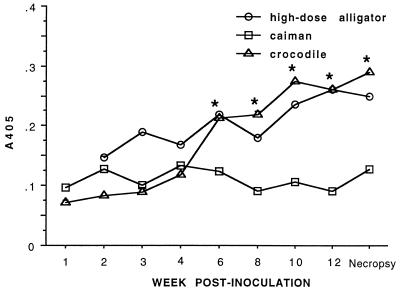
Plasma obtained during the dose-response and host range studies was used to validate the ELISA. Seroconversion was defined as a statistically significant increase in A405 value from the preinoculation group mean and from the same-species negative control mean. In all groups inoculated with 106 CFU of M. alligatoris, seroconversion (P < 0.05) was detectable beginning 6 to 8 w.p.i. when whole-cell lysate antigen was used (Fig. 5). For all groups, the minimum statistically significant increase was to A405 values about two times those of preinoculation or negative control means. That value was in remarkably good agreement with the cutoff between positive and negative results in the only other validated reptile mycoplasmosis ELISA (36) and with the value used heuristically to determine positive-control pool titers. When M. alligatoris LAMP-enriched antigen was used in the ELISA, crocodile seroconversion was also evident by 6 w.p.i., but increases in specific antibody in alligators were not statistically significant, and caiman plasma showed no measurable binding to LAMP-coated plates whatsoever (Fig. 6).
FIG. 5.
Seroconversion assayed by whole-cell lysate antigen-based ELISA. Each species (n = 6) was inoculated with 106 CFU of M. alligatoris. Seroconversion was determined by repeated-measure analysis of variance with main effects of week and species. Fisher's protected LSD was used for post hoc comparisons if main effects were significant. Time points at which species means differed (P < 0.05) from preinoculation and from same-species negative controls (data for control alligators are shown in Fig. 7; two controls per species for caiman and crocodiles [data not shown]) are indicated by asterisks. Statistical power to detect seroconversion of alligators and caimans was reduced at later time points because some individuals in those groups succumbed to mycoplasmosis.
FIG. 6.
Crocodile seroconversion assayed by LAMP antigen-based ELISA. Each species (n = 6) was inoculated with 106 CFU of M. alligatoris. Seroconversion was determined by repeated-measure analysis of variance with main effects of week and species. Fisher's protected LSD was used for post hoc comparisons if main effects were significant. Time points at which the inoculated crocodile group mean differed (P < 0.05) from preinoculation and from the negative control crocodiles (n = 2; data not shown) are indicated by asterisks. Changes from preinoculation and from negative controls (four alligators and two caimans) in alligators and caimans were not significant. Statistical power to detect seroconversion of alligators and caimans was reduced at later time points because some individuals in those groups succumbed to mycoplasmosis.
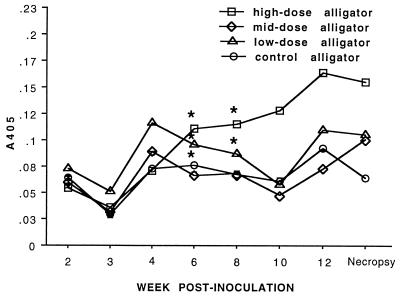
The rate of seroconversion was consistent with the rate of specific antibody response of other reptiles to experimental mycoplasmosis (7) and of alligators to experimental immunization with other antigens (21). However, the magnitude of the specific antibody response of alligators inoculated with M. alligatoris in the dose-response study was lower than that observed in plasma from the 1995 epidemic. The magnitude of response was higher (P < 0.05) for crocodiles than for caimans (Fig. 5), even without adjustment for apparent differences in conjugate avidity. The statistical power to detect seroconversion of alligators inoculated with 106 CFU and of caimans later than 3 w.p.i. was reduced because the specific antibodies developed too slowly to be detected before half of the individuals in those groups died. Trends toward seroconversion in alligators inoculated with 104 or 102 CFU were not statistically significant (Fig. 7).
FIG. 7.
Alligator seroconversion assayed by whole-cell lysate antigen-based ELISA. Alligators (six per group) were inoculated with 102 (low-dose), 104 (middose), or 106 (high-dose) CFU of M. alligatoris. Seroconversion was determined by repeated-measure analysis of variance with main effects of week and dose. Fisher's protected LSD was used for post hoc comparisons if main effects were significant. Time points at which dose group means differed (P < 0.05) from preinoculation and from the negative control alligators (n = 4) are indicated by asterisks. Seroconversion of the high-dose group was evident by 6 w.p.i. (same data shown in Fig. 5), but statistical power at later time points was reduced because some individuals in that group succumbed to mycoplasmosis. No meaningful trend toward seroconversion was detected for the low-dose or middose groups.
Binding to other antigens.
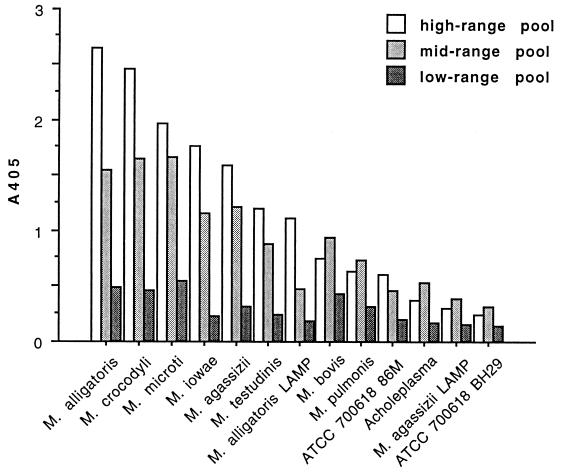
Binding of antibody in standard pools of alligator plasma to whole-cell lysate or LAMP-enriched antigens of other mollicutes in the ELISA format is shown in Fig. 8. A gradient of cross-reactivity was observed, but there was no consistent relationship to the source of the other mollicutes (reptile versus avian or mammalian hosts) or to their position in the phylogeny of mollicutes based on 16S rRNA gene nucleotide sequences (23). The maximum amount of binding that could be attributed to reactivity of alligator plasma or polyclonal rabbit anti-alligator Ig antibody with culture medium components was 0.05 A405 unit.
FIG. 8.
Cross-reactivity of alligator plasma pools with high-range (1:5,120), midrange (1:1,280), and low-range (1:80) anti-M. alligatoris titers with whole-cell lysate or LAMP antigens of other mollicutes assayed by ELISA. No relationship to the mollicutes' usual hosts (reptile, avian, or mammalian) or to their position in the phylogeny of mollicutes based on 16S rRNA gene sequences was observed.
Survey for M. alligatoris-specific antibodies in captive crocodilians.
The validated ELISA was used to analyze plasma obtained during routine health assessments of American alligators in captivity in the United States, American crocodiles being collected in Belize, and Siamese crocodiles in captivity in Thailand. The clinical health status of all of those crocodilians was normal, and there was no known history of mycoplasmosis or unexplained morbidity in those groups. All samples were seronegative (A405 values less than two times that of the same-genus negative control). Those results were further support for the conclusion that mycoplasmas are not commonly found among the normal flora of crocodilians and indicated that the risk of false-positive ELISA results is low.
DISCUSSION
Epidemiology and control of crocodilian diseases are issues of broad significance to protection of many species, alternative commodity production, and wetland conservation. Commercial use of alligators illustrates the potential significance of M. alligatoris pathogenesis and the need for a diagnostic test. Ranching of American alligators is an alternative commodity industry in the U.S. Gulf Coast states. More than 100 ranches annually produce approximately 150,000 hides and 330,000 kg of meat, worth in excess of $75 million to $150 million, from approximately 175,000 alligators. The ranching of crocodilians for commercial production of hides and meat is an international industry, and live alligators are exported from the United States. In the 1990s, there were more than 600 ranches in 47 countries, with annual stock in excess of 1.1 million crocodilians. American alligators are the most commonly ranched species, with about 500,000 held in production facilities. Annual alligator hide production outside the United States exceeds 182,000. The annual world market is approximately 2 million crocodilian hides.
The crocodilian ranching industry is based on collection of eggs and hatchlings from the wild. By deriving an economic value from those animals, individuals and governments are encouraged to protect not only the crocodilian populations but also the wetlands necessary for the continued survival and reproduction of the species. Commercial ranching operations thus are closely tied to the health and productivity of wild crocodilian populations. For that reason, some wild populations are intermittently replenished with juvenile crocodilians repatriated from commercial ranches after “head-starting” hatchlings from eggs collected in the wild. That practice could provide a vector for infectious disease transmission which might be interrupted by appropriate serological screening.
Several features of reptile immunity present special challenges to diagnosis of infection by measuring seroconversion (1). Specific immunity begins with maternal antibodies present in reptile egg yolk (24, 37). Ontogenic maturation of immune system cells progresses slowly in some species, with full immune competence not reached until months after hatching, but other species seem to be immunocompetent at hatching (10). Body temperature can have a direct effect on reptile immune systems, with species-specific optima and compromised immunity at body temperature extremes (9, 39). The spleen, thymus, and gut-associated lymphoid tissues of many reptiles undergo marked seasonal involutions (12, 43). That cycle seems to coincide with neuroendocrine rhythms correlated with mating periods (33, 34), independent of the direct effects of body temperature. Compromised immunity, including inhibited B-cell proliferation and differentiation and low antibody titers, was correlated with seasonal high levels of corticosteroids and sex steroids in several species of reptiles (20, 32, 43). The effects of each of those classes of hormones on immunity may be independent, as has been investigated by administration of exogenous testosterone (34) or hydrocortisone (33) or by pharmacological adrenalectomy (33). Consistent with those observations, sexual dimorphism in reptile immune responses has also been reported, with males having a somewhat delayed and less vigorous response and significantly lower antibody titers than females in response to immunization during seasons when testosterone is significantly higher in males (31, 35). The B-cell inhibition observed may be due in part to lack of T-cell-derived lymphokines, because strong cell-mediated immune responses coincide with seasons during which lymphoid tissue is well developed. However, seasonal effects on responses to immunization with a T-cell-independent antigen have also been observed (11). Thus, potential influences of age, temperature, season, and hormones on immunity should be considered when attempting to interpret the results of serological tests such as ELISA to diagnose infection in reptiles. The majority of the alligators that died during the 1995 epidemic were >200-kg adult males which died in September and October, while the alligators used in the pilot inoculation and dose-response studies were <20-kg young adult females which were inoculated in June. Those age, season, and sex differences may account for the somewhat less pronounced antibody responses observed in those studies.
It is unlikely that the less pronounced antibody response observed in alligators in the pilot inoculation and dose-response studies was because the M. alligatoris had attenuated immunogenicity (without loss of virulence) after clonal isolation and laboratory passage. Many mycoplasmal species exhibit extensive intraspecies phenotypic variability, manifested as antigenic variation in the context of immune recognition by infected hosts, so mycoplasmas are almost always very immunogenic (42). The antigenic variation can be apparent even among successive isolates obtained from an infected individual (22, 28). The variation probably provides the mycoplasmas with flexibility in interacting with their environment and may contribute to host specificity, pathogenicity, and evasion of host defenses (30, 42).
Another feature of reptile immunity that presents special challenges to diagnosis of infection by measuring seroconversion is the slow rate of specific antibody development. In the epidemiological sense (18), the sensitivity of a diagnostic test is the ability of the test to identify individuals with the disease in the group of diseased individuals. Specificity is the ability to identify individuals without disease in a healthy population. The positive predictive value (PPV) is the ability to distinguish true infections in a population of individuals with positive test results, and the negative predictive value (NPV) is the ability to distinguish true negative individuals from diseased individuals that have a negative test result. A reference standard that is independent of the assay being evaluated is required to estimate those parameters. In the dose-response and host range experiments, the reference standard was experimental inoculation with 106 CFU of M. alligatoris. Through 4 w.p.i., the sensitivity of the ELISA was 0, the specificity was 100%, PPV was 0, and NPV was only 31% because specific antibody had not begun to appear. Even at necropsy, the sensitivity was only 67%, the specificity was 100%, PPV was 100%, and NPV was only 57% because half of the alligators and one-third of the caimans died before seroconversion could occur. Although those percentages are influenced by the numbers of experimental animals in the control and inoculated groups, they illustrate that the significance of the ELISA results, especially negative results, can change over time. An acutely lethal pathogen such as M. alligatoris may escape serological detection at necropsy of crocodilians that die abruptly. Testing of paired samples, obtained at least 8 weeks apart, is necessary to minimize the risk of false-negative ELISA results.
Surface-exposed epitopes on integral membrane proteins may be dominant immunogens during mycoplasmal infections (14). The LAMP are anchored in the mycoplasmal membrane by covalent lipid modification or hydrophobic transmembrane domains (2). In some experiments, antibodies to LAMP antigens were highly mycoplasma species specific and did not cross-react with LAMP from other species (41). Therefore, LAMP immunogen-based diagnostics such as ELISA promise high molecular specificity for some mycoplasmal species (15). Although M. alligatoris LAMP were among the prominent reactive antigens on Western immunoblots (Fig. 4), they did not generate a proportionate signal in the ELISA format for each species of crocodilian tested. The difference in antibody binding between formats may have been due to differences in quantity and type of epitopes remaining exposed after denaturation and electrostatic binding to nitrocellulose in the immunoblots versus coating of the native proteins onto hydrophilic polystyrene in the ELISA.
The cross-reactivity with antigens of other mollicutes was in remarkable contrast to the lack of cross-reactivity observed in a similar whole-cell-lysate-based ELISA developed for tortoise antibodies against M. agassizii (36). The difference may reflect different immune system exposure to mycoplasmal antigens during the acute disease caused by highly invasive M. alligatoris, in contrast to the chronic disease caused by mucosa-tropic M. agassizii. The cross-reactivity may have been predominantly against internal mycoplasmal antigens conserved among mollicutes. This idea is supported by the complete lack of serological relationships between M. alligatoris and 71 other species of mollicutes assayed by reciprocal growth inhibition and colony surface immunofluorescence labeling tests (6). It was of concern that the alligators might possess existing antibody against serum proteins or other mollicute culture medium components potentially contaminating the mycoplasma lysate and LAMP preparations as a result of prior dietary exposure to those antigens, which could confound the ELISA. However, cross-reactivity with culture medium components was found to be insignificant.
The results of the experimental inoculation studies described briefly above were remarkable because, except for bovine and caprine pleuropneumonia, mycoplasmosis is rarely fatal in animals (29, 38). One other mycoplasmal disease of crocodilians has been described. Polyarthritis was observed in young ranch-reared Nile crocodiles in Zimbabwe (26). At necropsy, M. crocodyli (19) was isolated from affected joints and lung tissue of the crocodiles. The disease could be reproduced by experimental inoculation of crocodiles with M. crocodyli. Treatment with antibiotics seemed to resolve initial clinical signs, but relapses were common. A preliminary study suggested that a vaccine might afford limited protection in young crocodiles (27). Although M. crocodyli and M. alligatoris are very closely related, as indicated by 98% 16S rRNA gene nucleotide sequence similarity (D. R. Brown, M. B. Brown, and J. G. Tully, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. G-10, 1997), experimental intracoelomic or intrapleural inoculation of Nile crocodiles with 109 CFU of M. crocodyli was not lethal (26, 27). However, a more recent report associated M. crocodyli with a die-off of hundreds of ranched crocodiles (K. Mohan, C. M. Foggin, F. Dziva, and P. Muvavarirwa, Proc. 13th Int. Congr. Int. Org. Mycoplasmol., abstr. J-08, 2000). The susceptibility of alligators and caimans to infection with M. crocodyli remains unknown. An unidentified mycoplasma was isolated from a gharial (Gavialis gangeticus) that died with pneumonia (25). Future study of the responses of the phylogenetically ancient defense systems of crocodilians to M. alligatoris infection may allow unique comparative analyses of host-pathogen interactions in mycoplasmal disease.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture NRICGP grant 9802614, Morris Animal Foundation grant 98ZO-44, and contributions from the St. Augustine Alligator Farm and Zoological Park.
Technical assistance by Diane Duke (Interdisciplinary Center for Biotechnology Research) and Barbara Crenshaw (Department of Pathobiology) is gratefully acknowledged. Stuart Rosenberg (Silver Springs Wildlife Park, Ocala, Fla.), Yos Temsiripong (Sriracha Crocodile Farm, Sriracha, Thailand), and Sharon Deem (Wildlife Conservation Society, Bronx, N.Y.) provided crocodilian plasma.
REFERENCES
- 1.Ambrosius H. Immunoglobulins and antibody production in reptiles. In: Marchalonis J J, editor. Comparative immunology. Oxford, United Kingdom: Blackwell Scientific Publications; 1976. pp. 298–334. [Google Scholar]
- 2.Bricker T M, Boyer M J, Keith J, Watson-McKown R, Wise K S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988;56:295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D R, Crenshaw B C, McLaughlin G S, Schumacher I M, McKenna C E, Klein P A, Jacobson E R, Brown M B. Taxonomic analysis of the tortoise mycoplasmas Mycoplasma agassizii and Mycoplasma testudinis by 16S rRNA gene sequence comparisons. Int J Syst Bacteriol. 1995;45:348–350. doi: 10.1099/00207713-45-2-348. [DOI] [PubMed] [Google Scholar]
- 4.Brown D R, Clippinger T L, Helmick K E, Schumacher I M, Bennett R A, Johnson C M, Vliet K A, Jacobson E R, Brown M B. Mycoplasma isolation during a fatal epizootic of captive alligators (Alligator mississippiensis) in Florida. Int Org Mycoplasmol Lett. 1996;4:42–43. [Google Scholar]
- 5.Brown D R, Richey L J, Johnson J M, Jacobson E R, Vliet K A, Gross T, Tully J G, Brown M B. Virulence and seroprevalence of a mycoplasmal pathogen of American alligators. Int Org Mycoplasmol Lett. 1998;5:103. [Google Scholar]
- 6.Brown, D. R., J. M. Johnson, L. A. Zacher, T. L. Clippinger, J. G. Tully, and M. B. Brown.Mycoplasma alligatoris sp. nov., a new species from American alligators. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 7.Brown M B, McLaughlin G S, Klein P A, Crenshaw B C, Schumacher I M, Brown D R, Jacobson E R. Upper respiratory tract disease in the gopher tortoise is caused by Mycoplasma agassizii. J Clin Microbiol. 1999;37:2262–2269. doi: 10.1128/jcm.37.7.2262-2269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clippinger, T. L., R. A. Bennett, C. M. Johnson, K. A. Vliet, S. L. Deem, J. Oros, E. R. Jacobson, I. M. Schumacher, D. R. Brown, and M. B. Brown. An epidemic of mycoplasmosis in captive alligators (Alligator mississippiensis) and transmission of the etiologic agent. J. Zoo Wildl. Med., in press. [DOI] [PubMed]
- 9.Cone R E, Marchalonis J J. Cellular and humoral aspects of the influence of environmental temperature on the immune response of poikilothermic vertebrates. J Immunol. 1972;108:952–957. [PubMed] [Google Scholar]
- 10.El Deeb S O, Saad A H M. Ontogenic maturation of the immune system in reptiles. Dev Comp Immunol. 1990;14:151–159. doi: 10.1016/0145-305x(90)90087-u. [DOI] [PubMed] [Google Scholar]
- 11.El Ridi R, Badir N, El Rouby S. Effect of seasonal variations on the immune system of the snake, Psammophis schokari. J Exp Zool. 1981;216:357–365. [Google Scholar]
- 12.El Ridi R, Zada S, Afifi A, El Deeb S, El Rouby S, Farag M, Saad A-H. Cyclic changes in the differentiation of lymphoid cells in reptiles. Cell Differ. 1988;24:1–8. doi: 10.1016/0045-6039(88)90081-4. [DOI] [PubMed] [Google Scholar]
- 13.Evans A S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49:175–195. [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S-H, Lo S-C. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect Immun. 1994;62:3916–3921. doi: 10.1128/iai.62.9.3916-3921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurevich V A, Ley D H, Markham J F, Whithear K G, Walker I D. Identification of Mycoplasma synoviae immunogenic surface proteins and their potential use as antigens in the enzyme-linked immunosorbent assay. Avian Dis. 1995;39:465–474. [PubMed] [Google Scholar]
- 16.Horowitz S A, Cassell G H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978;22:161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffcoate S L. Use of Youden plot for internal quality control in the immunoassay laboratory. Ann Clin Biochem. 1982;19:435–437. doi: 10.1177/000456328201900609. [DOI] [PubMed] [Google Scholar]
- 18.Kenny G E. Serodiagnosis. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 505–512. [Google Scholar]
- 19.Kirchhoff H, Mohan K, Schmidt R, Runge M, Brown D R, Brown M B, Foggin C M, Muvavarirwa P, Lehmann H, Flossdorf J. Mycoplasma crocodyli sp. nov., a new species from crocodiles. Int J Syst Bacteriol. 1997;47:742–746. doi: 10.1099/00207713-47-3-742. [DOI] [PubMed] [Google Scholar]
- 20.Leceta J, Zapata A. Seasonal variations in the immune response of the tortoise Mauremys caspica. Immunology. 1986;57:483–487. [PMC free article] [PubMed] [Google Scholar]
- 21.Lerch E G, Huggins S E, Bartel A H. Comparative immunology: active immunization of young alligators with hemocyanin. Proc Soc Exp Biol Med. 1967;124:448–451. doi: 10.3181/00379727-124-31761. [DOI] [PubMed] [Google Scholar]
- 22.Levisohn S, Rosengarten R, Yogev D. In vivo variation of Mycoplasma gallisepticum antigen expression in experimentally infected chickens. Vet Microbiol. 1995;45:219–231. doi: 10.1016/0378-1135(95)00039-d. [DOI] [PubMed] [Google Scholar]
- 23.Maniloff J. Phylogeny of mycoplasmas. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 549–559. [Google Scholar]
- 24.Maung R T. Immunity in the tortoise Testudo ibera. J Pathol Bacteriol. 1963;85:51–66. doi: 10.1002/path.1700850106. [DOI] [PubMed] [Google Scholar]
- 25.Misra P R, Patra S K, Mohapatra H K, Patra K C, Mohapatra S. Aetiopathological findings from young gharial mortality cases. Indian Vet J. 1996;73:888–889. [Google Scholar]
- 26.Mohan K, Foggin C M, Muvavarirwa P, Honeywill J, Pawandiwa A. Mycoplasma-associated polyarthritis in farmed crocodiles (Crocodylus niloticus) in Zimbabwe. Onderstepoort J Vet Res. 1995;62:45–49. [PubMed] [Google Scholar]
- 27.Mohan K, Foggin C M, Muvavarirwa P, Honywill J. Vaccination of farmed crocodiles (Crocodilus niloticus) against Mycoplasma crocodyli infection. Vet Rec. 1997;141:476. doi: 10.1136/vr.141.18.476. [DOI] [PubMed] [Google Scholar]
- 28.Olson L D, Renshaw C A, Shane S W, Barile M F. Successive synovial Mycoplasma hominis isolates exhibit apparent antigenic variation. Infect Immun. 1991;59:3327–3329. doi: 10.1128/iai.59.9.3327-3329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruuth E, Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 31.Saad A H. Sex-associated differences in the mitogenic responsiveness of snake blood lymphocytes. Dev Comp Immunol. 1989;13:225–229. doi: 10.1016/0145-305x(89)90003-7. [DOI] [PubMed] [Google Scholar]
- 32.Saad A H, El Deeb S. Immunological changes during pregnancy in the viviparous lizard, Chalcides ocellatus. Vet Immunol Immunopathol. 1990;25:279–286. doi: 10.1016/0165-2427(90)90051-s. [DOI] [PubMed] [Google Scholar]
- 33.Saad A H, El Ridi R. Endogenous corticosteroids mediate seasonal cyclic changes in immunity of lizards. Immunobiology. 1988;177:390–403. doi: 10.1016/S0171-2985(88)80007-X. [DOI] [PubMed] [Google Scholar]
- 34.Saad A H, Khalek N A, El Ridi R. Blood testosterone level: a season-dependent factor regulating immune reactivity in lizards. Immunobiology. 1990;180:184–194. doi: 10.1016/S0171-2985(11)80327-X. [DOI] [PubMed] [Google Scholar]
- 35.Saad A H, Shoukrey N. Sexual dimorphism on the immune responses of the snake Psammophis sibilans. Immunobiology. 1988;177:404–419. doi: 10.1016/s0171-2985(88)80008-1. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher I M, Brown M B, Jacobson E R, Collins B R, Klein P A. Detection of antibodies to a pathogenic mycoplasma in desert tortoises (Gopherus agassizii) with upper respiratory tract disease. J Clin Microbiol. 1993;31:1454–1460. doi: 10.1128/jcm.31.6.1454-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher I M, Rostal D C, Yates R, Brown D R, Jacobson E R, Klein P A. Transfer and persistence of maternal antibodies against Mycoplasma agassizii in desert tortoise (Gopherus agassizii) hatchlings. Am J Vet Res. 1999;60:826–831. [PubMed] [Google Scholar]
- 38.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–415. [Google Scholar]
- 39.Tait N N. The effect of temperature on the immune response in cold-blooded vertebrates. Physiol Zool. 1969;42:29–35. [Google Scholar]
- 40.Tully J G. Culture medium formulation for primary isolation and maintenance of mollicutes. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. Vol. 1. San Diego, Calif: Academic Press; 1995. pp. 33–39. [Google Scholar]
- 41.Wang R Y-H, Lo S-C. ELISA in human urogenital infections and AIDS. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. Vol. 2. San Diego, Calif: Academic Press; 1996. pp. 115–121. [Google Scholar]
- 42.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–489. [Google Scholar]
- 43.Zapata A G, Varas A, Torroba M. Seasonal variations in the immune system of lower vertebrates. Immunol Today. 1992;13:142–147. doi: 10.1016/0167-5699(92)90112-K. [DOI] [PubMed] [Google Scholar]