Abstract
Tongue squamous cell carcinoma (SCC) is a most common type of oral cancer. Due to its highly invasive nature and poor survival rate, the development of effective pharmacological therapeutic agents is urgently required. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a polyphenolic flavonoid found in plants and is an active component of Chinese herbal medicine. The present study investigated the pharmacological effects and possible mechanisms of quercetin on apoptosis of the tongue SCC-derived SAS cell line. Following treatment with quercetin, cell viability was assessed via the MTT assay. Apoptotic and necrotic cells, mitochondrial transmembrane potential and caspase-3/7 activity were analyzed via flow cytometric analyses. A caspase-3 activity assay kit was used to detect the expression of caspase-3 activity. Western blot analysis was performed to examine the expression levels of proteins associated with the MAPKs, AMPKα, GSK3-α/β and caspase-related signaling pathways. The results revealed that quercetin induced morphological alterations and decreased the viability of SAS cells. Quercetin also increased apoptosis-related Annexin V-FITC fluorescence and caspase-3 activity, and induced mitochondria-dependent apoptotic signals, including a decrease in mitochondrial transmembrane potential and Bcl-2 protein expression, and an increase in cytosolic cytochrome c, Bax, Bak, cleaved caspase-3, cleaved caspase-7 and cleaved poly (ADP-ribose) polymerase protein expression. Furthermore, quercetin significantly increased the protein expression levels of phosphorylated (p)-ERK, p-JNK1/2 and p-GSK3-α/β, but not p-p38 or p-AMPKα in SAS cells. Pretreatment with the pharmacological JNK inhibitor SP600125 effectively reduced the quercetin-induced apoptosis-related signals, as well as p-ERK1/2 and p-GSK3-α/β protein expression. Both ERK1/2 and GSK3-α/β inhibitors, PD98059 and LiCl, respectively, could significantly prevent the quercetin-induced phosphorylation of ERK1/2 and GSK3-α/β, but not JNK activation. Taken together, these results suggested that quercetin may induce tongue SCC cell apoptosis via the JNK-activation-regulated ERK1/2 and GSK3-α/β-mediated mitochondria-dependent apoptotic signaling pathway.
Keywords: tongue squamous cell carcinoma, quercetin, apoptosis, mitochondria, JNK/ERK/GSK3
Introduction
Oral cancer has been reported to have an increased prevalence in males and in older individuals; >90% of patients with oral cancer are diagnosed with oral squamous cell carcinoma (SCC) (1–3). The risk factors for oral cancer include smoking, alcohol intake and betel nut chewing (4–6). In 2017, the Global Burden of Disease Study estimated the oral disease to affect 3.5 billion population worldwide. Furthermore, oral cancer has been reported was top 15 most common cancers worldwide and caused ~180,000 deaths each year, according to the International Agency for Research (7). It has also been reported that >600 new cases of oral cancer are diagnosed every year in Australia (6,8). Furthermore, the incidence rate of oral cavity SCC has been reported to be 146.2 cases per 100,000 person-years for areca/betel quid chewers in Taiwan (3,9). The five-year survival rate reported was poor in a Taiwanese patient study (10). In a previous study of oral cavity SCC, it was reported that tongue SCC was more prevalent compared with cancer of other oral cavity sites (by ~39%) in a young population (11). In order to increase the survival rate, the development of therapeutic agents for tongue SCC is ongoing.
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is categorized as a flavonol and is an important polyphenol of the flavonoid family, which has been detected in numerous fruits and vegetables, such as apples, cranberries, blueberries and onions (12–14). Quercetin is also an active component in several Chinese herbal medicines, such as Flos Sophorae Immaturus, Hypericum japonicum Thunb. and Yang-Yin-Qing-Fei-Tang (15,16). A number of biological effects of quercetin have been reported, including antiviral, anti-inflammatory and antioxidant effects (17,18). Furthermore, quercetin has been reported to possess anticancer potential (19,20). However, the underlying molecular mechanisms of quercetin in tongue SCC have remained to be fully elucidated.
Apoptosis is a well-known anticancer mechanism. The induction of apoptotic pathways to trigger cancer cell death has been explored in anticancer drug investigations in which damaged cells were removed through extrinsic or intrinsic pathways (21,22). MAPK has been indicated to be involved in cancer cell apoptosis. Furthermore, numerous studies have reported that the activation of JNK is a key signaling process for cancer cell apoptosis via various stimulators (3,23–27). However, to the best of our knowledge, the molecular mechanisms of quercetin in tongue SCC cell apoptosis remain to be clarified. Therefore, the present study investigated the pharmacological effects and possible mechanisms of quercetin on the apoptosis of tongue SCC-derived SAS cells. The roles of MAPK and GSK3-α/β signals and the involvement of mitochondrial dysfunction in quercetin-induced tongue SCC cell apoptosis and death were examined and clarified.
Materials and methods
Materials
Quercetin and other chemicals (including SP600125, PD98059 and LiCl), unless specified otherwise, were purchased from MilliporeSigma. Quercetin (to prepare the stock solution) was dissolved and diluted in DMSO. To the control wells, the maximum volume of DMSO used in the experiments was added, which was <1% per well and did not induce any cytotoxicity. Laboratory plasticware was obtained from Falcon (Corning Life Sciences). Mouse and rabbit monoclonal antibodies specific for caspase-3 (cat. no. 9661), caspase-7 (cat. no. 9491), poly(ADP-ribose) polymerase (PARP) (cat. no. 9542), phosphorylated (p)-JNK (cat. no. 9255), p-ERK1/2 (cat. no. 4377), p-p38 (cat. no. 9216), p-AMPKα (cat. no. 4188), p-GSK3-α/β (cat. no. 9331), cytochrome c (cat. no. 11940), Bcl-2 (cat. no. 15071), Bax (cat. no. 89477), Bak (cat. no. 12105), JNK-1 (cat. no. 3708), ERK1/2 (cat. no. 9102), p38 (cat. no. 8690), AMPKα (cat. no. 2532), GSK3-α/β (cat. no. 5676), β-actin (cat. no. 8457) and secondary antibodies [horseradish peroxidase (HRP)-conjugated anti-mouse IgG (cat. no. 7076) or anti-rabbit IgG (cat. no. 7074)] were purchased from Cell Signaling Technology, Inc.
Cell culture
The human tongue SCC-derived cell line SAS (JCRB0260) was purchased from Japanese Collection of Research Bioresources Cell Bank. SAS cells were cultured in a humidified chamber containing a 5% CO2−95% air mixture at 37°C. All cells were maintained in culture medium containing 45% Dulbecco's modified Eagle's medium, 45% Ham's F12 medium and 10% fetal calf serum (all from Gibco; Thermo Fisher Scientific, Inc.). Cells were seeded to 6- or 24-well culture plates for each experiment and allowed to grow for 12–18 h (the recover overnight), and then treated with quercetin (10–300 µM) for different time intervals in the absence or presence of the inhibitors of SP600125 (20 µM), PD98059 (20 µM) or LiCl (100 µM) for 30 min at 37°C prior to treatment with quercetin.
Morphological analysis
The changes in cell morphology were detected according to a previous study (28). The cells were cultured on a glass slide at a density of 1×106 cells/well at 37°C. After 24 h, a photomicrograph was obtained with a 20× objective lens using a cooled CCD camera attached to a Zeiss Axiovert 135-TV Inverted Fluorescence Phase Microscope (Zeiss AG).
Cell viability
An MTT assay was used to determine the effect of quercetin on the viability of SAS cells. The cells were cultured in a 24-well plate at a density of 2×105 cells/well. After recovery overnight, the culture medium was removed and the cells were washed twice with PBS. Fresh medium with quercetin (10–300 µM) or cisplatin (10 µg/ml; as a positive control) was then added. After 24 h at 37°C, the cells were washed twice with PBS and fresh medium was added with 30 µl MTT (2 mg/ml; MilliporeSigma) at 37°C for 4 h. The medium was then removed and cells were washed twice with PBS. DMSO was added to dissolve the blue formazan crystals in cells and the absorbance of cells was detected at a wavelength of 570 nm to determine cell viability using an ELISA reader (model 550; Bio-Rad Laboratories, Inc.).
Annexin V-FITC/propidium iodide (PI) staining for apoptosis detection
The externalization of phosphatidylserine residues on the outer plasma membrane of cells is an early event during apoptosis, which may be detected by annexin V (29). To assess quercetin-induced apoptosis and necrosis, flow cytometry was performed using the Annexin V-FITC-PI assay kit (BioVision, Inc.). SAS cells were seeded at 2×105 cells/well in a 24-well plate and incubated with quercetin (10–100 µM) at 37°C for 24 h. Subsequently, the cells were incubated using 0.05% trypsin/EDTA for 1 min to detach the cells, which were then centrifuged (200 × g at 4°C for 5 min), re-suspended in 100 µl binding buffer, transferred to a 5-ml fluorescence-activated cell sorting tube and combined with 5 µl Annexin V-FITC and 10 µl PI (50 µg/ml). After incubation for 30 min at room temperature in the dark, 400 µl binding buffer was added to each tube and the samples were immediately analyzed using flow cytometry (FACScalibur; BD Biosciences) using CellQuest software (version 5.1; BD Biosciences). Four cell populations were identified as follows: the viable cell population was in the lower-left quadrant (low FITC and PI signals), the early apoptotic population was in the lower-right quadrant (high FITC and low PI signals), the late apoptotic population was in the upper-right quadrant (high FITC and PI signals), and the necrotic population was in the upper-left quadrant (low FITC and high PI signals).
Caspase-3 activity assay
Caspase-3 activity was assessed as previously described by Lee et al (28) and a Caspase-3 Activity Assay Kit (cat. no. 5723) was used (Cell Signaling Technology, Inc.). The cells were cultured in a 24-well plate at a density of 2×105 cells/well. After treatment with quercetin (10, 30 and 50 µM) or cisplatin (10 µg/ml; as a positive control) at 37°C for 24 h, the cells were lysed and the caspase-3/CPP32 substrate (Ac-DEVD-AMC; 10 µM) was added for 1 h at 37°C. The fluorescence of cleaved substrates was determined using a spectrofluorometer (Spectramax; Molecular Devices, LLC) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm.
Determination of mitochondrial transmembrane potential (MMP)
The alteration of MMP was monitored by flow cytometry as previously described by Chen et al (30). The cells were harvested and washed twice with PBS. All samples were stained with DiOC6 (40 nM) for 30 min at 37°C and the fluorescence of DiOC6 was analyzed by FACSscan flow cytometry (BD Biosciences), using CellQuest software (version 5.1; BD Biosciences).
Detection of caspase 3/7 activity
Caspase 3/7 is widely accepted as a reliable indicator of apoptosis (31). A FLICA DEVD-FMK Caspase 3/7 Assay Kit (cat. no. 94; ImmunoChemistry Technologies, LLC) was used to determine apoptosis by flow cytometry. SAS cells were seeded at 2×105 cells/well in a 24-well plate and incubated with quercetin (50 µM) in the absence or presence of SP600125 (20 µM), PD98059 (20 µM), or LiCl (100 µM) at 37°C for 24 h. Subsequently, the cells were collected in 1.5-ml Eppendorf tubes, centrifuged at 200 × g for 5 min at 4°C, washed twice with PBS and stained with fluorescent probes for 10 min in a dark environment at room temperature. Caspase 3/7 activity was determined based on the fluorescence intensity in cells using flow cytometry (FACScalibur; BD Biosciences) using CellQuest software (version 5.1; BD Biosciences).
Western blot analysis
Western blot analysis was performed according to a previously described protocol (28). In brief, the cells were lysed using Protein Extraction Solution (cat. no. 17081; iNtRON Biotechnology, Inc.) and the protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein samples (50 µg) were resolved by SDS-PAGE (a 13.5% gel for caspase-3, −7, cytochrome c, Bcl-2, Bax, Bak; a 9% gel for p-JNK, JNK-1. P-ERK1/2, ERK1/2, p-p38, p38, p-AMPKα, AMPKα, p-GSK3-α/β, GSK3-α/β and β-actin) and transferred to polyvinylidene difluoride membranes. PBS-0.05% Tween-20 (PBST) containing 5% nonfat dry milk was used to block the membranes for 1 h at room temperature. The blots were then probed with primary antibodies (1:1,000 dilution) for 12–16 h at 4°C. The blots were washed twice with 0.1% PBST and were then probed with HRP-conjugated secondary antibodies (1:5,000 dilution) for 1 h at 4°C. The antibody-reactive bands were revealed using an Immobilon® Western Chemiluminescent HRP substrate kit (MilliporeSigma) and analyzed using a luminescent image analyzer (ImageQuant™ LAS-4000; GE Healthcare Bio-Sciences). The bands underwent densitometric analysis using ImageJ version 1.50d software (National Institutes of Health). For the detection of cytosolic cytochrome c expression levels, the cells were detached, washed twice with PBS, and then homogenized with a pestle and mortar in the extraction buffer [0.4 M mannitol, 25 mM MOPS (pH 7.8), 1 mM EGTA, 8 mM cysteine, and 0.1% (w/v) bovine serum albumin]. The cell debris was removed via centrifugation at 6,000 × g for 2 min. The supernatant was centrifuged at 12,000 × g for 15 min to pellet the mitochondria. The cytochrome c levels in the supernatants (cytosolic fraction) were detected using western blot analysis. For the detection of the phosphorylated proteins and total proteins, the membrane was first probed with the p-protein antibody and analyzed. Subsequently, the same membrane was washed and stripped with western blot stripping buffer (cat. no. ab270550; Abcam) according to the manufacturer's instructions. After stripping, the membrane was probed with the total protein antibody and re-analyzed.
Reverse transcription-quantitative PCT (RT-qPCR) analysis
The mRNA expression levels of Bcl-2, Bax, Bak, caspase-3, caspase-7, caspase-9 and PARP were analyzed using RT-qPCR as described in a previous study (32). Cells were cultured in a 10-cm2 dish and incubated with quercetin (50 µM) in the absence or presence of SP600125 (20 µM), PD98059 (20 µM) or LiCl (100 µM) at 37°C for different time intervals (2–16 h). Subsequently, total intracellular RNA was extracted using an RNeasy kit (Qiagen GmbH) according to the manufacturer's protocol. To eliminate genomic DNA (gDNA), the sample was added to DNase I. Subsequently, the sample was incubated at room temperature for 15 min and heated to 70°C for 10 min to denature DNase I, and then rapidly placed on ice. To reverse transcribe RNA into cDNA, 5 µg RNA was added to a reaction buffer: 2.5 mM deoxynucleotide mix, 40 U/µl RNAase inhibitor (Promega Corporation), 100 nmol random hexamer primers, 1X reverse transcriptase (RTase) buffer (which was supplied with the RTase enzyme) and 30 units AMV RTase enzyme, to which nuclease-free water was added to reach a final volume of 20 µl. The temperature steps of the RT reaction consisted of 10 min at room temperature, 15 min at 42°C and 5 min at 95°C and then the sample was placed on ice. The real-time SYBR Green primers for human caspase-3, caspase-7, caspase-9, PARP, Bcl-2, Bax, Bak and β-actin used in the present study are shown in Table I. Each sample (2 µl) was assessed using Real-Time SYBR Green PCR reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and transgene-specific primers in a 25-µl reaction volume, and amplification was performed using an ABI StepOnePlus™ Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling conditions consisted of 2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 30 sec and 60°C for 1 min. Real-time fluorescence detection was performed during the 60°C annealing/extension step of each cycle. Melt-curve analysis was performed on each primer set to ensure that no primer dimers or nonspecific amplifications were present under the optimized cycling conditions. After 40 cycles, data analysis was performed using StepOne™ software (version 2.1; Applied Biosystems; Thermo Fisher Scientific, Inc.). The fold differences in mRNA expression between the treatment and control groups were determined using the relative quantification method, which utilizes qPCR efficiencies and normalizes them to a housekeeping gene (β-actin was used in the present study), thus comparing relative Cq changes (ΔCq) between the control and experimental samples. The fold change values were calculated using the expression 2−ΔΔCq, where ΔΔC represents ΔCqcondition of interest-ΔCqcontrol (33). Prior to conducting the statistical analyses, the fold change from the mean of the control group was calculated for each individual sample (including the individual control samples to assess the variability in this group).
Table I.
Primer sequences used for reverse transcription-quantitative PCR analysis.
| Gene | Sequence | (Refs.) |
|---|---|---|
| Human caspase-3 | F: 5′-GACTCTAGACGGCATCCAGC-3′ | (57) |
| R: 5′-TGACAGCCAGTGAGACTTGG-3′ | ||
| Human caspase-7 | F: 5-AGTGACAGGTATGGGCGTTC-3 | (57) |
| R: 5-CGGCATTTGTATGGTCCTCT-3 | ||
| Human caspase-9 | F: 5-CTGAGCCAGATGCTGTCCCAT-3 | (58) |
| R: 5-CTGAGCCAGATGCTGTCCCAT-3 | ||
| Human PARP | F: 5-GCAGAGTATGCCAAGTCCAACAG-3 | (59) |
| R: 5-ATCCACCTCATCGCCTTTTC-3 | ||
| Human Bcl-2 | F: 5-TTAGATCTATGGCGCACGCTGGGAGAAC-3 | (60) |
| R: 5-CGAATTCTCACTTGTGGCTCAGATAGG-3 | ||
| Human Bax | F: 5-CTTTTGCTTCAGGGTTTCATCC-3 | (61) |
| R: 5-TTGAGACACTCGCTCAGCTTCT-3 | ||
| Human Bak | F: 5-ATGGTCACCTTACCTCTGCAA-3 | (62) |
| R: 5-TCATAGCGTCGGTTGATGTCG-3 | ||
| Human β-actin | F: 5-GGCGACGAGGCCCAGA-3 | (63) |
| R: 5-CGATTTCCCGCTCGGC-3 |
F, forward; PARP, poly(ADP-ribose) polymerase; R, reverse.
Statistical analysis
Data are presented as the mean ± standard deviation of at least four independent experiments. All data analyses were performed using SPSS software version 12.0 (SPSS, Inc.). Significant differences among the groups were assessed by one-way analysis of variance followed by Tukey's post-hoc test to identify group differences. P<0.05 was considered to indicate a statistically significant difference.
Results
Quercetin induces apoptosis in human tongue SCC SAS cells
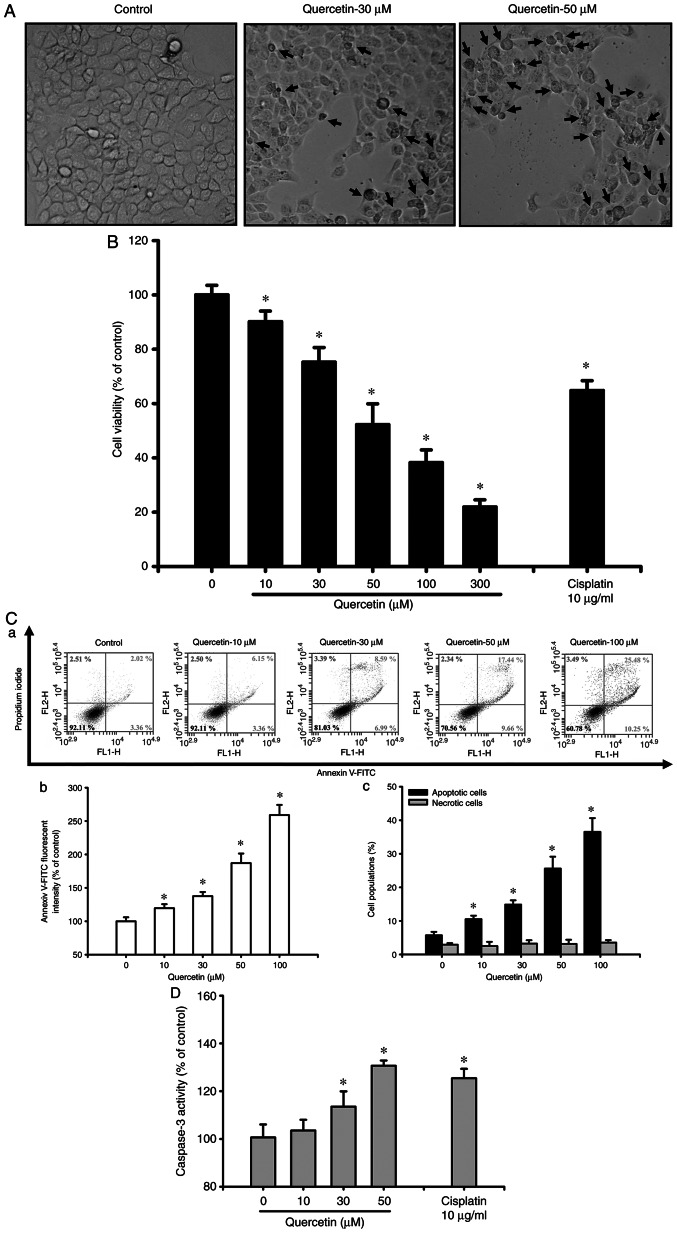
The present study first investigated whether quercetin had an apoptotic effect on tongue SCC cells. Cells were treated with quercetin (10–300 µM) for 24 h. As shown in Fig. 1A, quercetin (30 and 50 µM) markedly induced morphological changes (as indicated by the arrows), including cell shrinkage, as observed by an inverted phase-contrast microscope. Furthermore, cell viability was significantly decreased following treatment with quercetin (10–300 µM; Fig. 1B). The 50% lethal concentration (LC50) in SAS cells was ~50 µM quercetin (Fig. 1B). As determined by flow cytometry, the fluorescence intensity of Annexin V-FITC (Fig. 1C-a and b) and the percentage of apoptotic cells (as indicated by high Annexin V-FITC + low PI plus high Annexin V-FITC + high PI signals; Fig. 1C-c) were significantly increased in quercetin-treated cells in a dose-dependent manner. However, quercetin did not induce cell necrosis in quercetin-treated ASA cells (Fig. 1C). Furthermore, caspase-3 activity was also significantly and dose-dependently increased in cells treated with quercetin (Fig. 1D). Cisplatin (10 µg/ml), as a positive control, was used to induce cytotoxicity (Fig. 1B) and apoptosis (Fig. 1D) in SAS cells. These results indicated that quercetin may be capable of inducing SAS cell apoptosis and death.
Figure 1.
Effects of quercetin on cell morphology and viability in human tongue squamous cell carcinoma SAS cells. (A) Cells were treated with quercetin (30 and 50 µM) for 24 h. The morphological changes of cells were observed under an inverted phase-contrast microscope (magnification, ×400). (B) Cells were treated with quercetin (10–300 µM) for 24 h. Cell viability was determined using the MTT assay. (C-a) Apoptosis was determined by staining with the Annexin V-FITC apoptosis assay kit and analyzed using flow cytometry, and quantitative analysis of the (C-b) percentage of phosphatidylserine exposure on the outer cellular membrane and (C-c) early and late apoptotic cells (Annexin V+/PI− and Annexin V+/PI+) was detected. (D) Cells were treated with quercetin (10–50 µM) for 24 h. Caspase-3 activity was examined by a Caspase-3 Activity Assay Kit. Cisplatin (10 µg/ml) was used as a positive control. In B-D, data are presented as the mean ± standard deviation of four independent experiments with triplicate determination. *P<0.05 vs. vehicle control.
Quercetin induces mitochondrial damage in human tongue SCC SAS cells
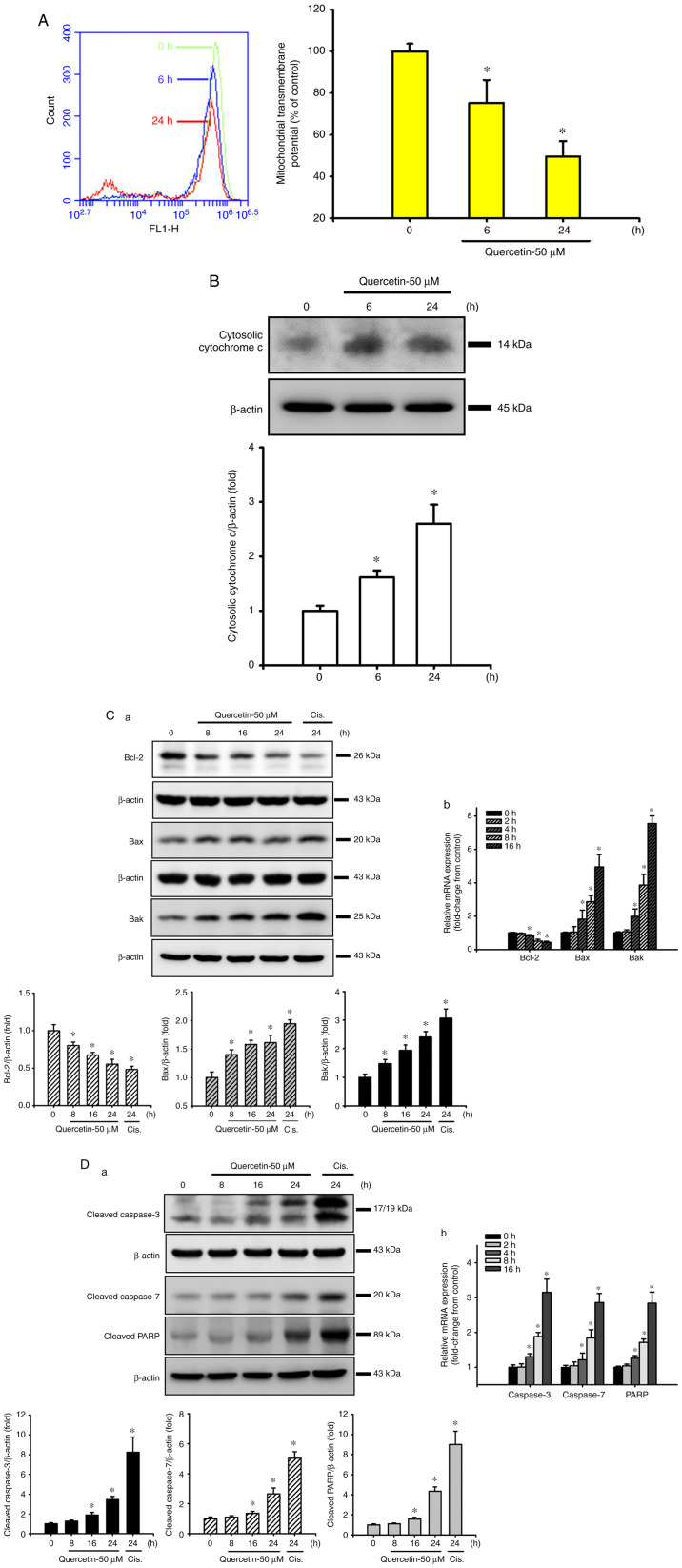
The present study next examined whether the mitochondrial pathway was involved in quercetin-induced SAS cell apoptosis. Cells were treated with quercetin (50 µM) for 6 or 24 h. As shown in Fig. 2A and B, the MMP was significantly decreased and cytosolic cytochrome c protein expression was effectively increased in quercetin-treated cells. Furthermore, the expression levels of mitochondrial apoptosis-related proteins were also detected. Both Bax and Bak protein expression levels were time-dependently increased, whereas Bcl-2 protein expression was time-dependently decreased in response to quercetin (Fig. 2C-a). Cisplatin (10 µg/ml), as a positive control, was used to induce apoptosis-related protein expression in SAS cells (Fig. 2C-a and D-a). In addition, the protein expression levels of cleaved caspase-3, caspase-7 and PARP were time-dependently increased in SAS cells following quercetin exposure (Fig. 2D-a). Moreover, quercetin decreased Bcl-2, and increased Bax, Bak, caspase-3, caspase-7 and PARP mRNA expression levels in SAS cells (Fig. 2C-b and D-b). These results suggested that the mitochondrial pathway may be involved in quercetin-induced apoptosis in human tongue SCC SAS cell cells.
Figure 2.
Effects of quercetin on the signals of mitochondria-dependent apoptosis in human tongue squamous carcinoma SAS cells. (A) Cells were treated with quercetin (50 µM) for 6 or 24 h. The mitochondrial transmembrane potential was determined by flow cytometry. (B) Cells were treated with quercetin (50 µM) for 6 or 24 h. The cytosolic cytochrome c protein expression was analyzed by western blotting. (C-a) Cells were treated with quercetin (50 µM) for 8–24 h. The cytosolic Bcl-2, Bax and Bak protein expression levels were analyzed by western blotting. (C-b) mRNA expression levels of Bcl-2, Bax and Bak were analyzed by RT-qPCR. (D-a) Cells were treated with quercetin (50 µM) for 8–24 h. The cleaved caspase-3, caspase-7 and PARP protein expression levels were analyzed by western blotting. Cisplatin (10 µg/ml) was used as a positive control. (D-b) mRNA expression levels of caspase-3, caspase-7 and PARP were analyzed by RT-qPCR. In A, C-b and D-b data are presented as the mean ± standard deviation of four independent experiments with triplicate determination. In B, C-a and D-a, images are representative of at least three independent experiments. Semi-quantification was performed by densitometric analysis. Bar graphs are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. vehicle control. PARP, poly(ADP-ribose) polymerase; RT-qPCR, reverse transcription-quantitative PCR.
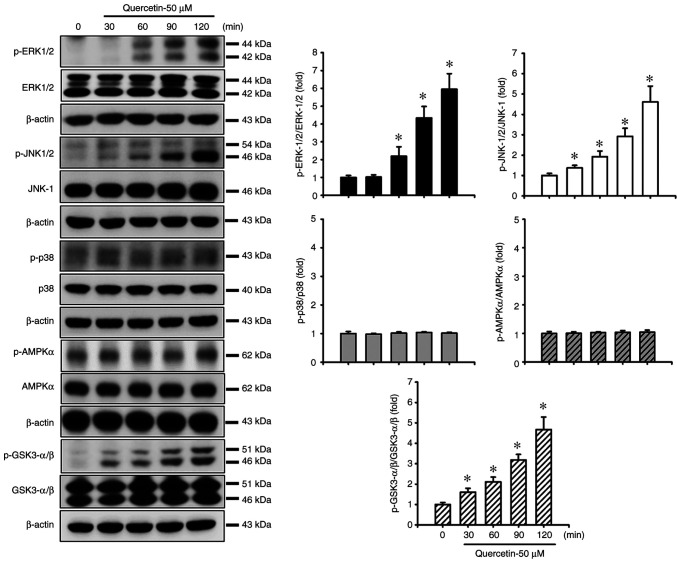
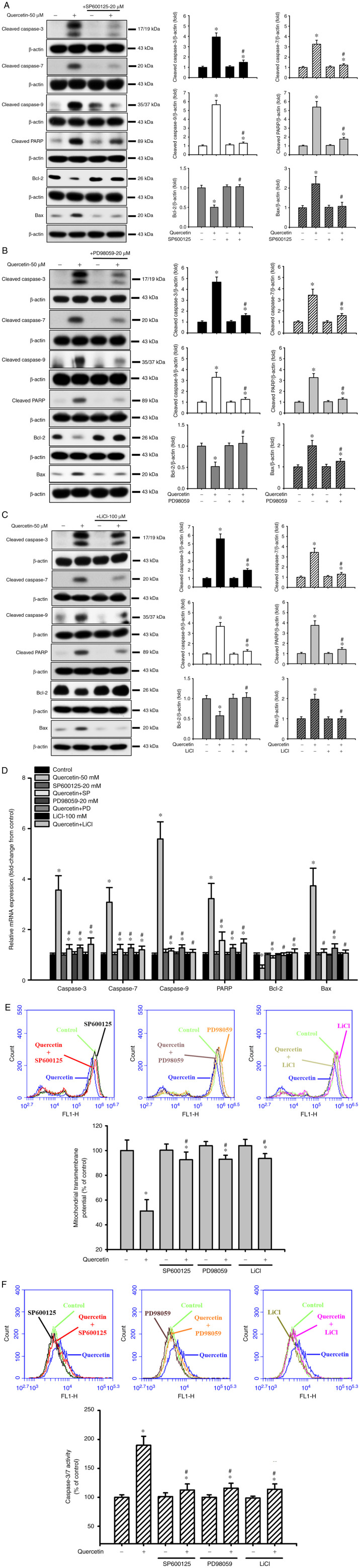
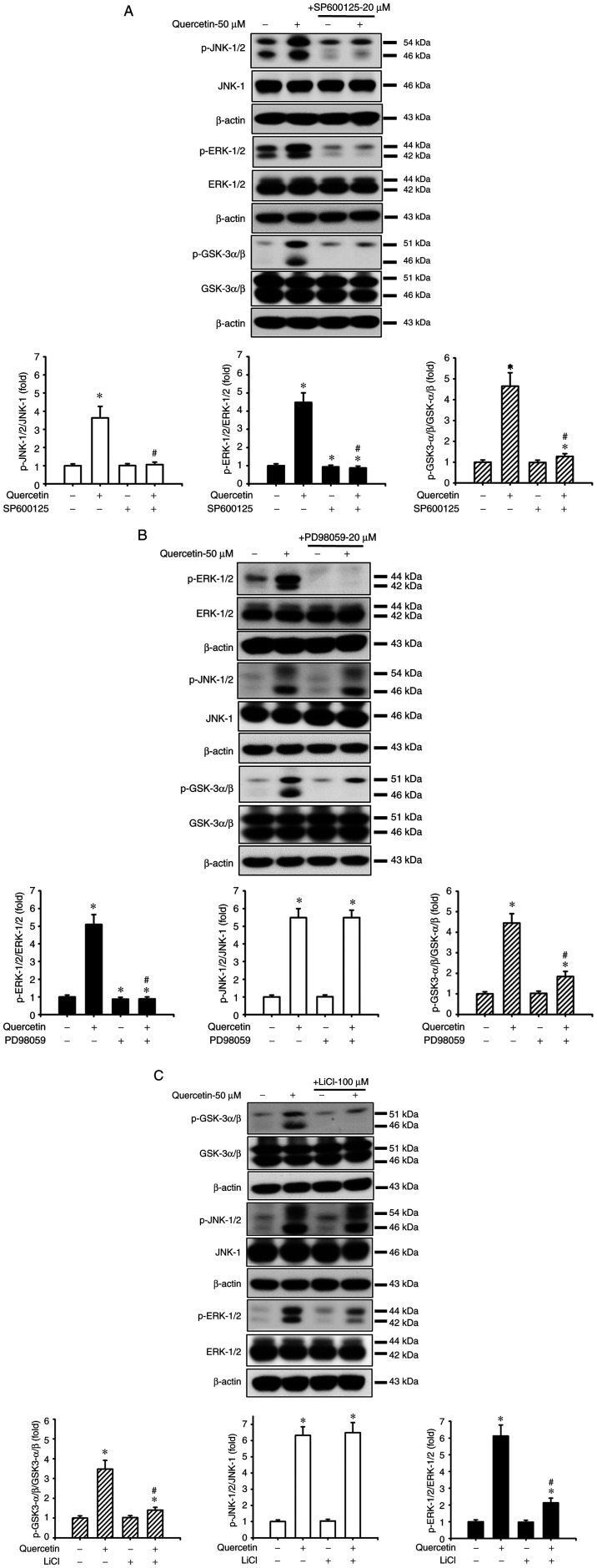
Quercetin induces apoptosis through the JNK, ERK1/2 and GSK3-α/β signaling pathways in human tongue SCC SAS cells. The present study also investigated the upstream signals for quercetin-induced mitochondrial apoptosis in SAS cells. Cells were treated with quercetin (50 µM) for 30–120 min. As shown in Fig. 3, quercetin significantly and time-dependently increased the protein expression levels of p-ERK1/2, p-JNK1/2 and p-GSK3-α/β, but not those of p-p38 and p-AMPKα in SAS cells. Furthermore, the roles of JNK and ERK in quercetin-induced apoptosis were investigated (Fig. 4). Cells were treated with or without a JNK inhibitor (SP600125, 20 µM) or an ERK inhibitor (PD98059, 20 µM) for 30 min, and were then treated with quercetin (50 µM) for 24 h. As shown in Fig. 4A, B and D, SP600125 and PD98059 effectively inhibited the quercetin-induced increase in protein and mRNA expression levels of caspase-3, caspase-7, caspase-9, PARP and Bax, and reversed the quercetin-induced decrease in the protein and mRNA expression levels of Bcl-2 in SAS cells. Furthermore, the role of GSK3 in quercetin-induced apoptosis was assessed. The cells were treated with or without the GSK3 inhibitor LiCl (100 µM) for 30 min and were then treated with quercetin (50 µM) for 16 or 24 h. As shown in Fig. 4C and D, LiCl effectively inhibited the increased protein and mRNA expression levels of caspase-3, caspase-7, caspase-9, PARP and Bax, and decreased the protein and mRNA expression levels of Bcl-2 in quercetin-treated cells. These inhibitors (SP600125, PD98059, and LiCl) could also significantly reverse the decreased MMP (Fig. 4E) and the increased caspase-3/7 activity (Fig. 4F) in quercetin-treated cells. These results indicated that quercetin-induced mitochondrial apoptosis may be regulated by JNK1/2, ERK1/2 and GSK3 signaling pathways in SAS cells.
Figure 3.
Effects of quercetin on MAPK, AMPKα and GSK3-α/β signals in human tongue squamous carcinoma SAS cells. Cells were treated with quercetin (50 µM) for 30–120 min. The protein expression levels of p-ERK1/2, p-JNK1/2, p-p38, p-AMPKα and p-GSK3-α/β were determined by western blot analysis. Images are representative of at least three independent experiments. Semi-quantification was determined by densitometric analysis. Values are expressed as the mean ± standard deviation of three independent experiments. *P<0.05 vs. vehicle control. AMPK, adenosine monophosphate-activated protein kinase; GSK, glycogen synthase kinase; p-, phosphorylated.
Figure 4.
Effects of selective inhibitors of JNK, ERK1/2 and GSK3 on mitochondria-dependent apoptotic signals induced by quercetin in human tongue squamous carcinoma SAS cells. Cells were treated with or without (A) JNK inhibitor-SP600125 (20 µM), (B) ERK inhibitor-PD98059 (20 µM) or (C) GSK3 inhibitor-LiCl (100 µM) for 30 min, and were then treated with quercetin (50 µM) for 24 h. The protein expression levels of cleaved caspase-3, caspase-7, caspase-9 and PARP, Bcl-2 and Bax were determined by western blotting. (D) Cells were treated with or without SP600125 (20 µM), PD98059 (20 µM) or LiCl (100 µM) for 30 min, and were then treated with quercetin (50 µM) for 16 h. The mRNA expression levels of caspase-3, caspase-7, caspase-9, PARP, Bcl-2 and Bax were analyzed by reverse transcription-quantitative PCR. (E and F) In addition, cells were treated with or without SP600125 (20 µM), PD98059 (20 µM) or LiCl (100 µM) for 30 min and were then treated with quercetin (50 µM) for 24 h. (E) Mitochondrial transmembrane potential was determined by flow cytometry. (F) Caspase-3/7 activity was analyzed by measuring fluorescence intensity using flow cytometry. In A-C, images are representative of at least three independent experiments. Semi-quantification was determined by densitometric analysis. Bar graphs are presented as the mean ± standard deviation of three independent experiments. In D-F, data are presented as the mean ± standard deviation of four independent experiments with triplicate determination. *P<0.05 vs. vehicle control; #P<0.05 vs. quercetin treatment alone. GSK, glycogen synthase kinase; PARP, poly(ADP-ribose) polymerase.
The present study examined the relationship between JNK, ERK and GSK3-α/β signals in SAS cells in the presence or absence of quercetin. As shown in Fig. 5A, SP600125 effectively reduced the quercetin-increased protein expression levels of p-JNK1/2, p-ERK1/2 and p-GSK3-α/β. Moreover, PD98059 treatment effectively reduced the quercetin-increased protein expression levels of p-ERK/12 and p-GSK3-α/β but not p-JNK1/2 (Fig. 5B). Similarly, LiCl treatment effectively reduced the quercetin-increased protein expression levels of p-GSK3-α/β and p-ERK1/2 but not p-JNK1/2 (Fig. 5C). These results suggested that JNK1/2 activation downstream-regulated ERK1/2- and GSK3-α/β-mediated apoptotic signaling pathways may have important roles in quercetin-induced tongue SCC SAS cell apoptosis.
Figure 5.
Effects of selective inhibitors of JNK, ERK1/2 and GSK3 on the quercetin-induced phosphorylation of JNK, ERK and GSK3-α/β in human tongue squamous carcinoma SAS cells. Cells were treated with or without (A) JNK inhibitor-SP600125 (20 µM), (B) ERK1/2 inhibitor-PD98059 (20 µM) or (C) GSK3 inhibitor-LiCl (100 µM) for 30 min, and were then treated with quercetin (50 µM) for 2 h. The protein expression levels of p-JNK1/2, p-ERK1/2 and p-GSK3-α/β were determined by western blotting. Images are representative of at least three independent experiments. Semi-quantification was determined by densitometric analysis. Bar graphs are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. vehicle control; #P<0.05 vs. quercetin treatment alone. GSK, glycogen synthase kinase; p-, phosphorylated.
Discussion
Oral cavity SCC has an increased prevalence in males and in older individuals, and has had a poor survival rate over the past two decades (21). The incidence of tongue SCC is higher than other types of oral SCC. Among 2,262 cases of oral cavity SCC, in patients aged 20 to 44 years, the incidence of tongue SCC was 39%, whereas the incidence was 23% in patients ≥45 years of age (11). The main treatment methods of tongue SCC include radiotherapy, chemotherapy and surgical resection; however, these clinical therapies can induce adverse effects, such as mucositis, skin reaction and dysphagia. Moreover, the prognosis and survival rates for tongue SCC remain poor, and development of more effective methods is required for prognosis and therapy (3,21,34–36). Therefore, the development of therapeutic drugs for improving the prognosis and survival rates of tongue SCC is required.
Quercetin is a pentahydroxyflavone that occurs in several natural food products, particularly fruits, vegetables, black tea and red wine (13,14). The safe oral dose of quercetin is 1 g/day and absorption is up to 60% (14,37). Furthermore, quercetin has been determined to be an active component in a number of Chinese herbal medicines (15,16). Quercetin has been reported to be a lipophilic compound that can cross the cellular membrane and exert cytotoxic effects on numerous types of cancer, including ovarian, lung, breast, nasopharyngeal, kidney, colorectal, prostate and pancreatic cancer (37–41). A previous study revealed that quercetin, at a concentration of 300 µM, could induce cytotoxicity in human lung embryonic fibroblasts, and could induce cell death in umbilical vein endothelial cells at concentrations between 61 and 303 µM (42). The present study demonstrated that quercetin, at concentrations of 30 and 50 µM, markedly induced morphological changes, including cell shrinkage, in SAS cells. The LC50 of quercetin in SAS cells was ~50 µM; notably, this concentration did not induce cytotoxicity to human normal cells according to the findings of Matsuo et al (42).
During apoptosis, cascade protease activities are induced to cleave apoptosis-related proteins, leading to rapid cell death (43). It has been reported that most of the stimuli that induce apoptosis occur through the mitochondria-dependent pathway, which is affected by the mitochondrial outer membrane permeabilization (44). Following MMP disruption and cytochrome c release from the mitochondria to the cytosol, cytosolic cytochrome c binds to the adaptor molecule apoptotic protease-activating factor 1 to form the apoptosome and to activate caspases, including caspase-3, caspase-7 and caspase-9 (44,45). A JNK-regulated mitochondria-dependent signaling pathway has been reported to participate in cantharidin-induced oral SCC apoptosis and death (3). Furthermore, quercetin has been revealed to induce cytotoxicity and oxidative stress, leading to mitochondria-related apoptosis in tumor cells (37,46,47). Notably, quercetin has been shown to induce reactive oxygen species generation, to decrease the MMP, to increase the expression of proapoptotic proteins Bad, Bax and Bid, and to decrease the expression of antiapoptotic proteins Mcl-1, Bcl-2 and Bcl-x, in gastric cancer cells (48). A previous study has also shown that quercetin may increase the cytosolic calcium concentration, protein expression of Bax, release of apoptosis-inducing factors, and activation of caspase-3, caspase-8 and caspase-9, and decrease the MMP and protein expression of Bcl-2 in human breast cancer cells (49). An in vitro study has shown that quercetin alters the ratio of anti/proapoptotic proteins, decreases the MMP, and increases the release of cytochrome c, apoptosis-inducing factor and Endo G from the mitochondria, leading to apoptosis-triggered cell destruction in oral SCC SAS cells (50). The present study also demonstrated that quercetin induced apoptosis and triggered apoptosis-related signals, including the activation of caspase-3/7, cleavage of caspase-3/7 and PARP, decrease in MMP, increase in cytosolic cytochrome c, increase in Bax and Bak protein and mRNA expression, and decrease in Bcl-2 protein and mRNA expression in SAS cells. These previous findings and the present results indicated the pharmacological efficiency of quercetin in inducing apoptosis of SAS cells. Nevertheless, a further understanding of the molecular mechanisms underlying the effects of quercetin on SAS cell apoptosis is required.
MAPKs, including ERK1/2, JNK and p38 kinase, have been reported to be involved in apoptosis signaling pathways. Several stimuli, including DNA damage, hydrogen peroxide, cell surface receptor Fas, UV irradiation, heat, osmotic shock and chemotherapeutic drugs, can induce apoptosis-related cell death (51–53). Both JNK and p38 kinase activation have been shown to induce mitochondria-related apoptosis in HepG2 cells (53). It has also been reported that ERK1/2 activation is involved in stress-induced neuronal cell apoptosis (54). In the present study, quercetin increased the phosphorylation of both JNK and ERK, but not p38, in SAS cells. Thus, the activation of both JNK and ERK signals may be involved in the quercetin-induced apoptosis of SAS cells.
GSK3 is a serine/threonine kinase and has two isoforms, GSK3-α and GSK3-β (55). Phorbol esters, the tumor-promoting reagent, have been shown to inhibit GSK3 signaling through the classical MAPK cascade (56). However, the distinct relationship between GSK3 and MAPK signals involved in the inhibitory effects of quercetin on SAS cell survival still requires further clarification. In the present study, quercetin significantly increased GSK3-α/β phosphorylation in SAS cells. Pretreatment of the SAS cells with the specific inhibitors SP600125 (JNK inhibitor), PD98059 (ERK inhibitor) and LiCl (GSK3 inhibitor) effectively abrogated the quercetin-induced apoptosis. Moreover, it was revealed that SP600125 effectively inhibited the phosphorylation of JNK, ERK and GSK3-α/β, and PD98059 and LiCl effectively inhibited both ERK and GSK3-α/β phosphorylation, but not JNK phosphorylation, in quercetin-treated SAS cells. These results suggested that JNK activation-mediated ERK/GSK3-α/β signaling may serve a critical role in the downstream regulation of mitochondria-dependent apoptosis in quercetin-induced tongue SCC cell death.
In conclusion, the present study demonstrated that quercetin induced cell death in tongue SCC cells via the JNK activation-regulated ERK1/2 and GSK3-α/β downstream-mediated mitochondria-dependent apoptotic signaling pathway. Future in vivo research is still required to clarify the therapeutic potential of quercetin in tongue SCC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- SCC
squamous cell carcinoma
- MMP
mitochondrial transmembrane potential
- PARP
poly(ADP-ribose) polymerase
Funding Statement
This study was supported by grants from the Changhua Christian Hospital, Changhua, Taiwan (grant no. 110-CCH-IRP-057), the Taichung Tzu chi Hospital, The Buddhist Tzuchi Medical Foundation (grant no. TTCRD 109-13), the Far Eastern Memorial Hospital, New Taipei City, Taiwan (grant no. FEMH-2013-C-028) and the China Medical University, Taichung, Taiwan (grant no. CMU109-S-64).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Study conception and design: CFH and CCS. Implementation of the experiments: CFH, SHL, KMF, JML and CCW. Acquisition of data and original draft preparation: CFH, SHL, TJH, KIL and KMF. Analysis and interpretation of data: CFH, KIL, KMF and WCL. CFH and CCS confirm the authenticity of all the raw data. Writing, reviewing and editing the manuscript: SHL and CCS. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: Epidemiology and aetiology. Periodontol 2000. 2011;57:19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 2.Hung CM, Chang CC, Lin CW, Ko SY, Hsu YC. Cucurbitacin E as inducer of cell death and apoptosis in human oral squamous cell carcinoma cell line SAS. Int J Mol Sci. 2013;14:17147–17156. doi: 10.3390/ijms140817147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su CC, Lee KI, Chen MK, Kuo CY, Tang CH, Liu SH. Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLoS One. 2016;11:e0168095. doi: 10.1371/journal.pone.0168095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF., Jr Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 5.Koo K, Barrowman R, McCullough M, Iseli T, Wiesenfeld D. Non-smoking non-drinking elderly females: A clinically distinct subgroup of oral squamous cell carcinoma patients. Int J Oral Maxillofac Surg. 2013;42:929–933. doi: 10.1016/j.ijom.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Wong T, Wiesenfeld D. Oral cancer. Aust Dent J. 2018;63((Suppl 1)):S91–S99. doi: 10.1111/adj.12594. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization, (WHO), corp-author https://www.who.int/news-room/fact-sheets/detail/oral-health. [ March 25; 2020 ];Oral health. Available from. [Google Scholar]
- 8.Australian Institute of Health, Welfare (AIHW), corp-author Australian Cancer Incidence and Mortality (ACIM) books: Head and neck including lip. http://www.aihw.gov.au/acim-books. 2017 July; Available from. [Google Scholar]
- 9.Yang YH, Chen CH, Chang JS, Lin CC, Cheng TC, Shieh TY. Incidence rates of oral cancer and oral pre-cancerous lesions in a 6-year follow-up study of a Taiwanese aboriginal community. J Oral Pathol Med. 2005;34:596–601. doi: 10.1111/j.1600-0714.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin NC, Hsien SI, Hsu JT, Chen MY. Impact on patients with oral squamous cell carcinoma in different anatomical subsites: A single-center study in Taiwan. Sci Rep. 2021;11:15446. doi: 10.1038/s41598-021-95007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: Increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 12.Day AJ, Williamson G. Gross GG, Hemingway RW, Yoshida T. Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology. Basic Life Sciences. Vol 66. Kluwer Academic/Plenum Publishers; New York, NY: 1999. Human metabolism of dietary quercetin glycosides; pp. 415–434. [DOI] [PubMed] [Google Scholar]
- 13.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of US fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 14.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Tan L, Zhang JW, Chen H, Liang B, Qiu T, Li QS, Cai M, Zhang QH. Quercetin is the active component of Yang-Yin-Qing-Fei-Tang to induce apoptosis in non-small cell lung cancer. Am J Chin Med. 2019;47:879–893. doi: 10.1142/S0192415X19500460. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Chen M, Fan Y, Elsebaei F, Zhu Y. Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence detection. Talanta. 2012;88:222–229. doi: 10.1016/j.talanta.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Lee HH, Shin YS, Kang H, Cho H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch Pharm Res. 2017;40:623–630. doi: 10.1007/s12272-017-0898-x. [DOI] [PubMed] [Google Scholar]
- 18.Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients. 2016;8:529. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, An JM, Chung WY, Park KK, Hwang JK, Kim du S, Seo SR, Seo JT. Xanthorrhizol induces apoptosis through ROS-mediated MAPK activation in human oral squamous cell carcinoma cells and inhibits DMBA-induced oral carcinogenesis in hamsters. Phytother Res. 2013;27:493–498. doi: 10.1002/ptr.4746. [DOI] [PubMed] [Google Scholar]
- 22.Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzo PI, Saatcioglu F. Inhibition of apoptosis in prostate cancer cells by androgens is mediated through downregulation of c-Jun N-terminal kinase activation. Neoplasia. 2008;10:418–428. doi: 10.1593/neo.07985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DM, Won M, Chung CS, Kim S, Yim HJ, Jung SH, Jeong S. JNK-mediated transcriptional upregulation of RhoB is critical for apoptosis of HCT-116 colon cancer cells by a novel diarylsulfonylurea derivative. Apoptosis. 2010;15:1540–1548. doi: 10.1007/s10495-010-0531-7. [DOI] [PubMed] [Google Scholar]
- 25.Wei G, Wang M, Carr BI. Sorafenib combined vitamin K induces apoptosis in human pancreatic cancer cell lines through RAF/MEK/ERK and c-Jun NH2-terminal kinase pathways. J Cell Physiol. 2010;224:112–119. doi: 10.1002/jcp.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalan A, Yu W, Sanders BG, Kline K. Simvastatin inhibition of mevalonate pathway induces apoptosis in human breast cancer cells via activation of JNK/CHOP/DR5 signaling pathway. Cancer Lett. 2013;329:9–16. doi: 10.1016/j.canlet.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Ryu MJ, Chung HS. [10]-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim. 2015;51:92–101. doi: 10.1007/s11626-014-9806-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee KI, Lin JW, Su CC, Fang KM, Yang CY, Kuo CY, Wu CC, Wu CT, Chen YW. Silica nanoparticles induce caspase-dependent apoptosis through reactive oxygen species-activated endoplasmic reticulum stress pathway in neuronal cells. Toxicol In Vitro. 2020;63:104739. doi: 10.1016/j.tiv.2019.104739. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Meng XW, Flatten KS, Loegering DA, Kaufmann SH. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2013;20:64–76. doi: 10.1038/cdd.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YW, Yang YT, Hung DZ, Su CC, Chen KL. Paraquat induces lung alveolar epithelial cell apoptosis via Nrf-2-regulated mitochondrial dysfunction and ER stress. Arch Toxicol. 2012;86:1547–1558. doi: 10.1007/s00204-012-0873-8. [DOI] [PubMed] [Google Scholar]
- 31.Lu TH, Su CC, Tang FC, Chen CH, Yen CC, Fang KM, Lee KI, Hung DZ, Chen YW. Chloroacetic acid triggers apoptosis in neuronal cells via a reactive oxygen species-induced endoplasmic reticulum stress signaling pathway. Chem Biol Interact. 2015;225:1–12. doi: 10.1016/j.cbi.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Chung YP, Yen CC, Tang FC, Lee KI, Liu SH, Wu CC, Hsieh SS, Su CC, Kuo CY, Chen YW. Methylmercury exposure induces ROS/Akt inactivation-triggered endoplasmic reticulum stress-regulated neuronal cell apoptosis. Toxicology. 2019;425:152245. doi: 10.1016/j.tox.2019.152245. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Simon C, Plinkert PK. Combined modality approaches in the treatment of head and neck cancer patients. HNO. 2008;56:575–584. doi: 10.1007/s00106-008-1718-x. (In German) [DOI] [PubMed] [Google Scholar]
- 35.Wu JY, Yi C, Chung HR, Wang DJ, Chang WC, Lee SY, Lin CT, Yang YC, Yang WC. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. 2010;46:226–231. doi: 10.1016/j.oraloncology.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Sayáns M, Suárez-Peñaranda JM, Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM, García-García A. Tissue inhibitor of metalloproteinases in oral squamous cell carcinomas-a therapeutic target? Cancer Lett. 2012;323:11–19. doi: 10.1016/j.canlet.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Shafabakhsh R, Asemi Z. Quercetin: A natural compound for ovarian cancer treatment. J Ovarian Res. 2019;12:55. doi: 10.1186/s13048-019-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–844. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 39.Kuo PC, Liu HF, Chao JI. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem. 2004;279:55875–55885. doi: 10.1074/jbc.M407985200. [DOI] [PubMed] [Google Scholar]
- 40.Ong CS, Tran E, Nguyen TT, Ong CK, Lee SK, Lee JJ, Ng CP, Leong C, Huynh H. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol Rep. 2004;11:727–733. [PubMed] [Google Scholar]
- 41.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, Arunakaran J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin Nutr. 2014;33:718–726. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull. 2005;28:253–259. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RC, Cullen SP, Martin SJ. Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 44.Lopez J, Tait SW. Mitochondrial apoptosis: Killing cancer using the enemy within. Br J Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Muñoz-Pinedo C, Green DR. Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev Cell. 2010;18:802–813. doi: 10.1016/j.devcel.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metodiewa D, Jaiswal AK, Cenas N, Dickancaité E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med. 1999;26:107–116. doi: 10.1016/S0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 47.Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch Biochem Biophys. 2000;378:224–233. doi: 10.1006/abbi.2000.1832. [DOI] [PubMed] [Google Scholar]
- 48.Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ Toxicol. 2018;33:1168–1181. doi: 10.1002/tox.22623. [DOI] [PubMed] [Google Scholar]
- 49.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, Wood WG, Kuo SJ, Chen DR. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp Toxicol. 2009;28:493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 50.Ma YS, Yao CN, Liu HC, Yu FS, Lin JJ, Lu KW, Liao CL, Chueh FS, Chung JG. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol Lett. 2018;15:9663–9672. doi: 10.3892/ol.2018.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 52.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245–19256. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 53.Yuan L, Wang J, Xiao H, Wu W, Wang Y, Liu X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem Toxicol. 2013;53:62–68. doi: 10.1016/j.fct.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Song H, Kim W, Choi JH, Kim SH, Lee D, Park CH, Kim S, Kim DY, Kim KT. Stress-induced nuclear translocation of CDK5 suppresses neuronal death by downregulating ERK activation via VRK3 phosphorylation. Sci Rep. 2016;6:28634. doi: 10.1038/srep28634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:4629495. doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh BY, Kim KH, Chung SS, Lee RA. Silencing the livin gene enhances the cytotoxic effects of anticancer drugs on colon cancer cells. Ann Surg Treat Res. 2016;91:273–277. doi: 10.4174/astr.2016.91.6.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu JH, Zheng GB, Liu CY, Zhang LY, Gao HM, Zhang YH, Dai CY, Huang L, Meng XY, Zhang WY, Yu XF. Dracorhodin perchlorate induced human breast cancer MCF-7 apoptosis through mitochondrial pathways. Int J Med Sci. 2013;10:1149–1156. doi: 10.7150/ijms.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchiumi F, Watanabe T, Ohta R, Abe H, Tanuma S. PARP1 gene expression is downregulated by knockdown of PARG gene. Oncol Rep. 2013;29:1683–1688. doi: 10.3892/or.2013.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nencioni L, De Chiara G, Sgarbanti R, Amatore D, Aquilano K, Marcocci ME, Serafino A, Torcia M, Cozzolino F, Ciriolo MR, et al. Bcl-2 expression and p38MAPK activity in cells infected with influenza A virus: impact on virally induced apoptosis and viral replication. J Biol Chem. 2009;284:16004–16015. doi: 10.1074/jbc.M900146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotsafti A, Farinati F, Cardin R, Cillo U, Nitti D, Bortolami M. Autophagy and apoptosis-related genes in chronic liver disease and hepatocellular carcinoma. BMC Gastroenterol. 2012;12:118. doi: 10.1186/1471-230X-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasan Z, Ashraf M, Tayyebi A, Hussain R. M. leprae inhibits apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 gene expression. BMC Microbiol. 2006;6:78. doi: 10.1186/1471-2180-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goidin D, Mamessier A, Staquet MJ, Schmitt D, Berthier-Vergnes O. Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal Biochem. 2001;295:17–21. doi: 10.1006/abio.2001.5171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.