Abstract
Introduction: Smoking affects the occurrence and development of many diseases. We attempt to study the structure of intestinal flora in the middle-aged and elderly population as well as how smoking affects the intestinal flora. Methods: We collected population information, biochemical indicators, and patient feces from 188 middle-aged and elderly male patients, and their feces were tested for the 16S rRNA gene of intestinal flora. Results: We performed a cluster analysis on the intestinal structure of the included population and found that there was a significant difference in the number of smokers between each group (p = 0.011). Subsequently, the microbiological diversity analysis of current smokers and nonsmokers was carried out. The results indicated that there was a significant difference in species composition between the two groups (p = 0.029). Through the analysis on LEfSe differential bacteria, it was found that in current smoking patients, the abundances of the genus Bifidobacterium and the genus Coprobacillus were less, while the abundances of the genera Shigella, Paraprevotella, Burkholderia, Sutterella, Megamonas, and p-75-a5 under the family level of Erysipelotrichaceae were slightly high. We analyzed the correlation between the abundances of these eight different bacteria and clinical indicators. The results revealed the following: the abundance of the genus Bifidobacterium was negatively correlated with fasting blood glucose (r = −0.198, p = 0.006) and positively correlated with uric acid (r = 0.207, p = 0.004) and total bilirubin (r = 0.175, p = 0.017); Shigella bacteria were positively correlated with fasting blood glucose (r = 0.160, p = 0.028) and uric acid (r = 0.153, p = 0.036) levels; the genus Paraprevotella and BMI (r = −0.172, p = 0.018) are negatively correlated; the abundance of the genus Burkholderia was positively correlated with γ-glutamyltransferase (r = 0.146, p = 0.045) levels; Sutterella was correlated with fasting blood glucose (r = 0.143, p = 0.05) and creatinine level (r = −0.16, p = 0.027), which was positively correlated with fasting blood glucose and negatively correlated with creatinine. Conclusions: In middle-aged and elderly patients with cardiovascular disease, smoking can reduce the abundance of Bifidobacterium, while the abundances of some negative bacteria such as Burkholderia, Sutterella, and Megamonas increase.
Introduction
The intestine is not only an important place for human digestion and absorption but also the largest immune organ, which plays an extremely important role in maintaining the body’s normal immune defense functions. In addition to the traditional mechanical barriers and immune barriers, intestinal microbes also play an important role. The human intestine provides a good habitat for microbes. Meanwhile, intestinal microorganisms can rely on the host’s intestinal life to assist the host to complete a variety of physiological and biochemical functions, including metabolic functions that the human body does not possess.1 As early as the early 20th century, some scholars proposed after research that there were about 100 trillion bacteria in the intestinal flora of a normal person, the total number of which was equivalent to the total number of human cells. The enormous genome contained in these microorganisms is also called the human being’s “Second Genome”.2−4 For such a huge genome, the technical problems of its utilization and mining have always plagued us. Most of the intestinal microbes are obligate anaerobes, and they are numerous and difficult to isolate and cultivate. Currently, with the development of microbial gene sequencing technology, this technical problem has been bypassed. By comparing different patients by group, people apply statistical principles to infer the role of microorganisms. As a result, increasingly more studies have been conducted on the cognition of microorganisms. Previous studies have reported that in comparison with the intestinal microorganism between cardiovascular patients and healthy people, it has been found that the intestinal flora of cardiovascular patients will undergo characteristic changes, such as an increase in the number of Lactobacillus and a decrease in the number of Bacteroides.5 Studies have also reported that some bacterial genes and specific bacteria can also be applied for the diagnosis of cardiovascular diseases,6,7 and there may even be some potential therapeutic values for the occurrence and development of diseases.8,9 However, there are many factors influencing intestinal microbes. In addition to the diseases and other factors, there are reports that different exercise methods, psychological factors, and bad living habits can also affect intestinal microorganisms.10,11
Smoking is universal among populations in various countries, and its harm is great, resulting in the occurrence and development of many diseases. Besides, smoking, a risk factor, is easily overlooked in scientific research, so it is a major interference factor in scientific analysis. Therefore, it is significant to conduct research on smoking. At present, in the study of the intestinal flora, it has been reported that smoking can affect the composition of intestinal flora, while the conclusions are not unified.12,13 Recently, a prospective study compared smoking and nonsmoking 100 healthy soldiers to explore the effect of smoking on the intestinal flora. The study concluded that there were significant differences in the microbial composition between the two groups; the difference between the former and the latter is mainly due to the relatively low abundance of bifidobacteria species and the high abundance of negative bacteria in smokers.14 Since the population included in this study is healthy male soldiers, whether the conclusions are universal and whether it is applicable to all middle-aged and elderly cardiovascular patients is not yet known. Based on the research significance of smoking in specific populations, we included 188 male patients with cardiovascular disease to study the structure of their intestinal flora and explore the effect of smoking on the intestinal flora.
Results
Basic Population Information and Intestinal Microorganism Composition
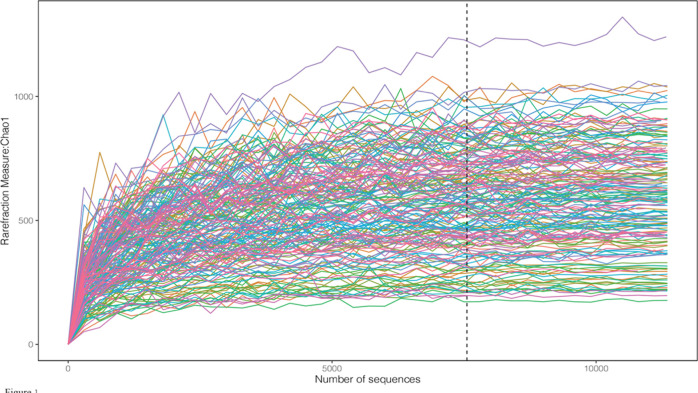
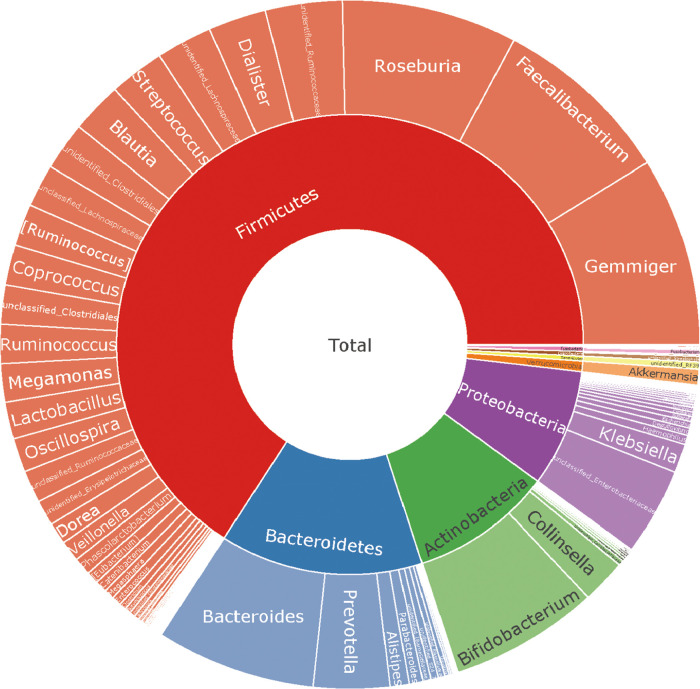
We included 188 male patients with cardiovascular disease. The baseline characteristics of the patients are shown in Table 1. The average age of the enrolled patients was 56 years. The population of coronary heart disease accounted for 70.74% of the total population, the hypertension population accounted for 49.47%, and the diabetes population accounted for 12.77% of the total population. The current proportion of smokers in the included patients is 39.36%, and that of alcohol drinkers is 43.62%. The proportion of this data is similar to previous research reports.21 We recorded the patient’s long-term oral medication status and biochemical examination indicators. 16S rRNA sequencing was performed on 188 patients. To determine whether the sample size was sufficient and to estimate the community abundance, we applied the species accumulation curve (see Figure 1). From the curve, we could see that all items were stable, suggesting that the included sample size was sufficient for statistical analysis. To display the microbial species composition of the included sample population, we utilized the double-layer pie chart to display (see Figure 2). At the phylum level, the relative abundances of Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria were 63.93, 9.56, 13.65, and 8.01%, respectively. At the genus level, the relative abundances of Faecalibacterium, Gemmiger, Bacteroides, Roseburia, and Bifidobacterium were 8.12, 8.58, 7.16, 7.86, and 6.70%, respectively. In addition, we calculated the standard deviation variations of various bacteria Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria as 0.166, 0.126, 0.122, and 0.11, respectively. At the genus level, the variations of Faecalibacterium, Gemmiger, Bacteroides, Roseburia, and Bifidobacterium were 0.083, 0.086, 0.082, 0.086, and 0.107, respectively.
Table 1. Baseline Characteristics of 188 Male Patientsabc.
| variable | value |
|---|---|
| age, year | 56 ± 10 |
| body mass index, kg/m2 | 26(25–29) |
| systolic blood pressure, mm Hg | 126 ± 17 |
| smoking status | |
| never | 50(26.60%) |
| past | 64(34.04%) |
| current | 74(39.36%) |
| drinking status, standard drinks/wk | |
| never | 106(56.38%) |
| 1–4 | 52(27.66%) |
| 5–8 | 18(9.57%) |
| >8 | 12(6.38%) |
| basic diseases | |
| coronary heart disease | 133(70.74%) |
| hypertension | 93(49.47%) |
| diabetes | 24(12.77%) |
| long-term oral medication | |
| aspirin | 132(70.21%) |
| clopidogrel | 89(47.34%) |
| stain | 129(68.62%) |
| β-block | 94(50.00%) |
| calcium channel blockers | 39(20.74%) |
| ACEI or ARB | 102(54.26%) |
| diuretic | 12(6.38%) |
| laboratory results | |
| white blood cells, ×109/L | 6.8(5,62–8.4) |
| neutrophilicgranulocyte,% | 58.75(52.53–66.68) |
| hemoglobin, ×1012/L | 4.74(4.52–5.07) |
| platelets, ×109/L | 220.5(186–256.75) |
| blood urea nitrogen, mmol/L | 5.4(4.3–6.2) |
| creatinine, μmol/L | 71(62–79.25) |
| uric acid, μmol/L | 338(298–392) |
| glucose, mmol/L | 4.83(4.42–5.7) |
| total cholesterol, mmol/L | 3.53(2.84–4.19) |
| triglyceride, mmol/L | 1.45(1.06–2.11) |
| high density lipoprotein,mmol/L | 0.98(0.84–1.2) |
| total bilirubin, μmol/L | 11.3(8.1–14.3) |
| γ-glutamyl transpeptidase, U/L | 24(18–37) |
Data are presented as median (interquartile range), mean ± SD, or number (%).
ACEI: angiotensin-converting enzyme inhibitor.
ARB: angiotensin ii receptor antagonist.
Figure 1.
Chao1 curves of each sample; the estimated OTU richness basically approached saturation in all samples.
Figure 2.
Taxonomic composition of the gut microbiome of 188 male patients; double pie chart (inner circle: phylum level, outer circle: genus level).
Analysis of Factors Affecting Intestinal Flora
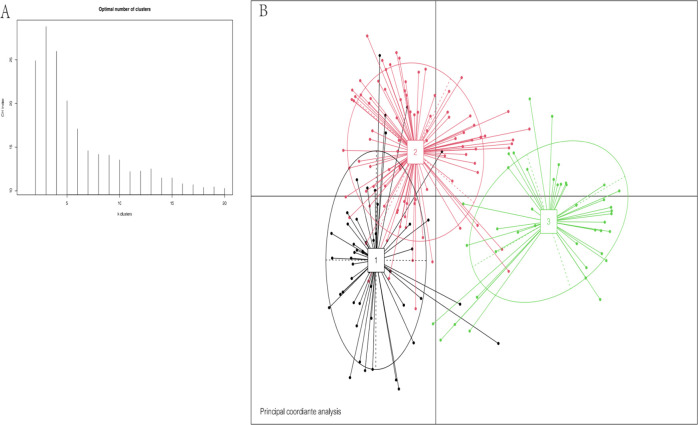
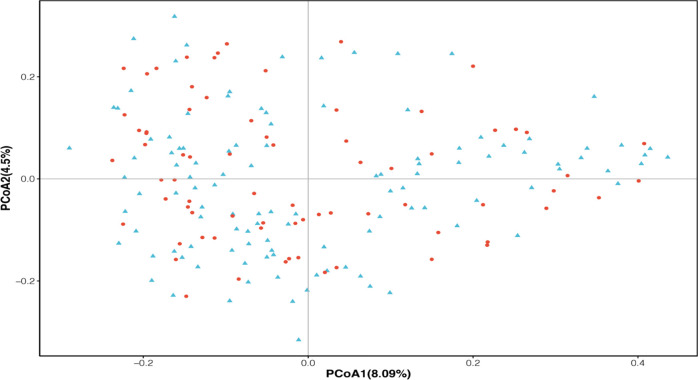
Using cluster analysis, we found that we could divide the subjects into three enterotypes based on bacterial species compositions (see Figure 3). Enterotype 1, enterotype 2, and enterotype 3 are Prevotella, Gemmiger, and Bifidobacterium, respectively. We compared the basic data and clinical indicators in different groups of patients between the three intestinal types (see Table 2). There were no significant differences in age, BMI, and admission blood pressure between each group. There was no significant difference in the basic diseases (heart disease, hypertension, diabetes) between the two groups. There was no significant difference in the types of long-term oral drugs between the two groups. Laboratory test indicators were evaluated for the patients in each group, indicating that there were no significant differences. However, unexpectedly, among the three intestinal types, we found that there was a significant difference in the number of patients smoking, and the P value was 0.011. We considered that smoking would affect the composition of the intestinal flora. This result is similar to a previous study reported in South Korea.14
Figure 3.
Cluster analysis based on the bacterial species compositions of 188 male patients((1) enterotype 1, (2) enterotype 2, (3) enterotype 3). (A) The number of Enterotype was calculated according to CH index. (B) Jensen–Shannon-based principal coordinates analysis.
Table 2. Clinical Characteristics of Male Patients according to Enterotypea,b.
| variable | enterotype 1 (n = 49) | enterotype 2 (n = 96) | enterotype 3 (n = 43) | P value |
|---|---|---|---|---|
| age, year | 56 ± 9 | 57 ± 10 | 55 ± 10 | 0.377 |
| body mass index, kg/m2 | 28(25–30) | 26(24–29) | 26(24–28) | 0.094 |
| systolic blood pressure, mm Hg | 129 ± 17 | 125 ± 17 | 127 ± 17 | 0.272 |
| smoking status | 0.011 | |||
| never or past | 21 | 63 | 30 | |
| current | 28 | 33 | 13 | |
| drinking status | 0.122 | |||
| nonheavy drinker | 17 | 41 | 12 | |
| heavy drinker* | 3 | 6 | 3 | |
| basic diseases | ||||
| coronary heart disease | 37 | 62 | 33 | 0.171 |
| hypertension | 29 | 42 | 22 | 0.206 |
| diabetes | 9 | 8 | 7 | 0.169 |
| long-term oral medication | ||||
| aspirin | 37 | 66 | 29 | 0.633 |
| clopidogrel | 27 | 43 | 19 | 0.448 |
| stain | 35 | 67 | 27 | 0.632 |
| β-block | 29 | 43 | 22 | 0.257 |
| calcium channel blockers | 14 | 18 | 7 | 0.275 |
| ACEI or ARB | 31 | 46 | 25 | 0.181 |
| diuretic | 5 | 3 | 4 | 0.172 |
| laboratory results | ||||
| white blood cells, ×109/L | 6.69(5.86–8,8) | 6.83(5.51–8.41) | 6.91(6–8.39) | 0.661 |
| neutrophilicgranulocyte, % | 56.5(51.35–62.45) | 59.9(53.5–67.3) | 55.6(47.9–67) | 0.171 |
| hemoglobin, ×1012/L | 5(4.8–5.21) | 4.71(4.52–5.06) | 4.68(4.42–4.97) | 0.179 |
| platelets, ×109/L | 223(187.52–279.25) | 217(188–257) | 233(182–253) | 0.821 |
| blood urea nitrogen, mmol/L | 5.25(4–6.23) | 5.4(4.5–6.2) | 5.1(4.2–6.2) | 0.546 |
| creatinine, μmol/L | 72.5(56.56–82) | 69(63–79) | 68.98(61–77) | 0.955 |
| uric acid, μmol/L | 340.5(308.75–412) | 344(310–389.4) | 347(313–392) | 0.88 |
| glucose, mmol/L | 4.75(4.46–5.69) | 4.84(4.4–5.52) | 4.75(4.32–5.77) | 0.617 |
| total cholesterol, mmol/L | 3.44(2.54–4.02) | 3.58(2.89–4.32) | 3.43(2.74–4.18) | 0.88 |
| triglyceride, mmol/L | 1.58(1.12–2.03) | 1.33(1.05–2.01) | 1.77(0.99–2.35) | 0.735 |
| high density lipoprotein, mmol/L | 0.92(0.84–1.19) | 0.96(0.84–1.19) | 1(0.83–1.29) | 0.594 |
| total bilirubin, μmol/L | 11.55(9.13–14.88) | 11.5(7.8–13.8) | 10.6(8.2–15.9) | 0.985 |
| γ-glutamyl transpeptidase, U/L | 23.45(18–34.75) | 25(18–34.5) | 29(18–42) | 0.867 |
Data are presented as median (interquartile range), mean ± SD, or number (%). * Drinking status, more than 8 standard drinks/wk.
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin ii receptor antagonist.
Smoking Affects the Distribution of Intestinal Flora
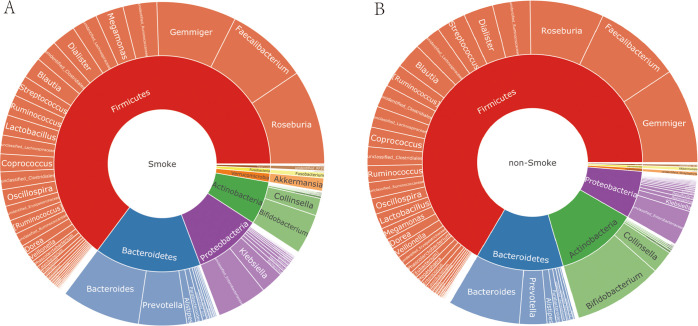
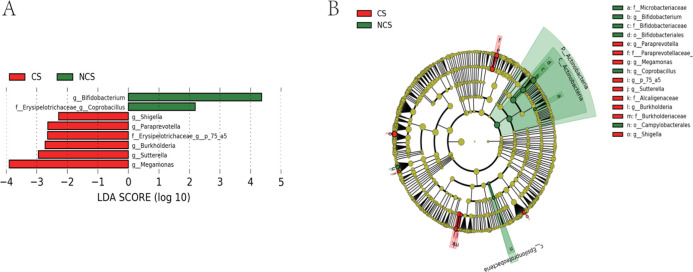
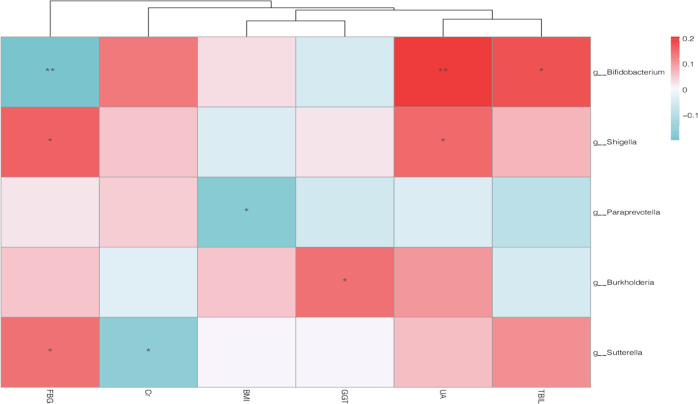
To understand how smoking affected the intestinal flora of patients, we drew a two-layer pie chart of smokers and nonsmokers. We can see from Figure 4 and Table 3 that for smoking patients, at the phylum level, the relative abundances of Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria were 62.61, 6.24, 15.41, and 9.75%, respectively. At the genus level, the relative abundances of Faecalibacterium, Gemmiger, Bacteroides, Roseburia, and Bifidobacterium were 7.87, 7.67, 7.59, 9.13, and 3.86%, respectively. For nonsmokers, at the phylum level, the relative abundances of Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria were 64.79, 11.71, 12.51, and 6.89%, respectively. At the genus level, the relative abundances of Faecalibacterium, Gemmiger, Bacteroides, Roseburia, and Bifidobacterium were 8.29, 9.18, 6.88, 7.05, and 6.88%, respectively. We detected no significant difference between smokers and nonsmokers with respect to α-diversity evaluated in terms of species richness and diversity indices. For analysis of β-diversity, we used Bray–Curtis distance to analyze smokers and nonsmokers. Figure 5 shows the results of Bray–Curtis-based principal coordinates analysis of gut microbiome according to smoking status, which revealed a significant difference in microbial composition between current and noncurrent smokers. In addition, we used Permanova to analyze the differences between groups, and the results showed that P value was equal to 0.029. There were significant differences in species diversity between the two groups. On the basis of LEfSe analysis, we found that there are differences in the abundance of some bacteria in the intestinal flora of smoking patients and nonsmoker. We could see from Figure 6 that for the genus-level bacteria, in the current smoking patients, the abundances of the genus Bifidobacterium and the genus Coprobacillus were less, while the abundances of the genera Shigella, Paraprevotella, Burkholderia, Sutterella, Megamonas, and p-75-a5 under the family level of Erysipelotrichaceae were slightly high. We analyzed the correlation between the abundance of these eight different bacteria and clinical indicators and found that the abundances of these differential bacteria were significantly correlated with some clinical indicators. As shown in Figure 7, the heatmap of the correlation between the bacterial abundance and the clinical indicators revealed that the abundance of the genus Bifidobacterium was related to fasting blood glucose, uric acid, and total bilirubin levels. Specifically, the genus Bifidobacterium was negatively correlated with fasting blood glucose (r = −0.198, p = 0.006) and positively correlated with uric acid (r = 0.207, p = 0.004) and the total bilirubin (r = 0.175, p = 0.017). Shigella bacteria was related with and positively correlated with and fasting blood glucose (r = 0.160, p = 0.028) and uric acid (r = 0.153, p = 0.036) levels. The genus Paraprevotella was related with and negatively correlated with BMI (r = −0.172, p = 0.018). The abundance of the genus Burkholderia was positively correlated with the level of γ-glutamyltransferase (r = 0.146, p = 0.045). The genus Sutterella was related to fasting blood glucose (r = 0.143, p = 0.05) and creatinine (r = −0.16, p = 0.027) levels, which was positively correlated with fasting blood glucose, and negatively correlated with Creatinine.
Figure 4.
Taxonomic composition of the gut microbiome according to the smoking status ((A) current smokers, (B) noncurrent smokers); double pie chart (inner circle: phylum level, outer circle: genus level).
Table 3. Comparison of the Proportions of Bacterial Genera between the Two Groups.
| phylum | genus | smoke (%) | nonsmoke (%) |
|---|---|---|---|
| Firmicutes | Faecalibacterium | 7.87 | 8.29 |
| Firmicutes | Gemmiger | 7.67 | 9.18 |
| Bacteroidetes | Bacteroides | 7.59 | 8.54 |
| Firmicutes | Roseburia | 9.13 | 7.05 |
| Actinobacteria | Bifidobacterium | 3.86 | 6.88 |
| Proteobacteria | Klebsiella | 2.20 | 0.75 |
Figure 5.
Bray–Curtis-based principal coordinates analysis of the gut microbiome of 188 male patients according to smoking status. Y: current smokers; N: noncurrent smokers.
Figure 6.
(A) Linear discriminant analysis (LDA) effect size and (B) cladogram of the gut microbiome of 188 male patients according to smoking status. CS: current smoking; NCS: noncurrent smoking.
Figure 7.
Spearman correlation heatmap analysis performed at the representative differential bacteria and clinical indicators. (FBG: fasting blood glucose; Cr: creatinine; BML: body mass index; GGT: γ-glutamyl transpeptidase; UA: uric acid; TBIL: total bilirubin). *:p < 0.05; **:p < 0.01.
Discussion
In our study, the phylum-level Firmicutes and Bacteroidetes bacteria accounted for about 80% of the overall population, and the phylum-level Proteobacteria and Actinobacteria bacteria accounted for 8 and 9%, respectively. The previous research reported that Firmicutes and Bacteroidetes typically comprised more than 90% of the adult human gut microbiome and that the average abundances of Proteobacteria and Actinobacteria were approximately 4 and 8%, respectively.15−17 The phyla Firmicutes and Bacteroidetes belong to the dominant bacterial group in the human body. Firmicutes and Bacteroidetes are relatively small in our research population, which may be related to the fact that most of the samples we included are middle-aged and elderly and have cardiovascular diseases. Subsequently, we divided the intestinal types based on the species composition as well as cluster analysis. Comparing the basic data of three groups in each intestinal type, we found that there was only a difference in smoking among the three intestinal types, speculating that smoking affected the intestinal bacteria. Therefore, we further analyzed the influence of smoking on the host’s intestinal flora. In the comparison of 74 current smokers and 104 current nonsmokers, there were differences in intestinal microbial diversity between the two groups (see Figure 5, permanova: p = 0.029), Then, we further look for which bacteria are different abundance.
Among smoking patients, the abundance of the genus Bifidobacterium is slightly low. Current studies have confirmed that Bifidobacterium is an important intestinal beneficial microorganism, which has many important physiological functions, including providing a biological barrier to human health, enhancing immunity,22,23 resisting tumor,24 improving gastrointestinal function,25 resisting aging,26 etc. Bifidobacterium can inhibit the growth of harmful bacteria in the human body; resist the infection of pathogenic bacteria; synthesize vitamins needed by the human body; promote the absorption of minerals by the human body; and produce organic acids such as acetic acid, propionic acid, butyric acid, and lactic acid to stimulate intestinal peristalsis, promote defecation, prevent constipation, inhibit intestinal decay, purify the intestinal environment, decompose carcinogens, and stimulate the human immune system, so as to improve disease resistance. This result also explains the harm of smoking in the intestinal flora, and the reduction of the bacterial abundance in the smoking population has also been reported by previous studies.14 In further studies on Bifidobacterium, through the correlation between bacterial abundance and clinical indicators, it was found that the genus Bifidobacterium was negatively correlated with fasting blood glucose and positively correlated with uric acid and the total bilirubin level. This conclusion is consistent with existing studies. In a recent experiment, the function of Bifidobacterium was finally confirmed by giving probiotics treatment to diabetic mice.27 This conclusion has also been confirmed in clinical studies.28 The relationship between Bifidobacterium bacteria, uric acid and total bilirubin has also been reported. In one report, chicory (a plant) improves high uric acid. In a study reporting that chicory (a plant) improves hyperuricemia, basic experiments confirmed that chicory could increase the abundance of Bifidobacterium bacteria and improve uric acid levels.29 The total bilirubin is a significant indicator of liver function evaluation, and the treatment of Bifidobacterium in liver disease has also been confirmed.27,30 It is the first report that the low abundance of Coprobacillus bacteria in smokers is reported. The existing studies have confirmed that the abundance of this bacterium is abnormal in diabetic people.31,32 In this study, the LDA of the differential bacteria Bifidobacterium in the nonsmokers was greater than 4. It is considered that the main bacterial flora in the smoking population is Bifidobacterium.
In addition to the lower abundance of the intestinal microorganisms in smokers than nonsmokers, this study also found an increase in negative flora in smokers, such as Shigella, Paraprevotella, Burkholderia, Sutterella, and Megamonas. The genus Shigella dysentery is the pathogen causing typical bacillary dysentery. The increased abundance of Shigella bacteria in smokers also suggests that smoking may endanger human health through changes in the composition of the intestinal flora. In the correlation study with clinical indicators, it was found that the abundance of Shigella bacteria was positively correlated with the abundance of uric acid and fasting blood glucose in patients. This result is similar to previous research reports.33 It is considered that smoking can lead to the increase of this bacterium, which will lead to the occurrence and development of gastrointestinal diseases and even kidney disease. The abundance of Paraprevotella bacteria in smokers increases, which is consistent with recent research reports.34 In previous studies of intestinal microorganisms, the abundance of the genus Paraprevotella is mostly increased in different disease groups.35,36 For this phenomenon, it is considered that this bacterium can damage the intestinal mucosa.36 In this study, the genus Paraprevotella was negatively correlated with the BMI index. This conclusion has also been confirmed by other research reports recently.37 In addition to the increase in the abundance of the above-mentioned bacteria in smokers, the abundance of the genus Burkholderia in smokers also increased. The relationship between this bacterium and cigarettes has been reported in 2010.38,39 The conclusion of this study is also to further verify and confirm that this bacterium is related to host smoking. The genus Burkholderia is reported to be less in human intestinal microbes, most of which are soil and insects. Interestingly, the genus Burkholderia seems to have higher abundance in soils where pesticides have been applied. Studies have shown that although the genus Burkholderia can decompose pesticides, it will eventually cause many potential harms to the human body. In combination with the positive correlation between the abundance of this bacterium and the concentration of host γ-glutamyltransferase in this study, we hold that the relationship between pesticides, soil, and Burkholderia may be similar to the relationship between smoking, host, and Burkholderia. Therefore, we speculate that smoking may increase the abundance of these bacteria, and the concentration of chemicals derived from tabacco in a small range can stimulate the increase of these bacteria and reduce the damage of smoking, while it may cause further harm to the human body if it exceeds a certain threshold. The relationship between this bacterium and γ-glutamyltransferase has also been reported in previous studies.40 In a previous clinical study, some scholars suggested that Sutterella was related to smoking. The conclusions of this study have also further confirmed this.41 In the case of long-term fasting42 and severe diarrhea,43 the abundance of this bacterium increases. In the correlation study, this bacterium is negatively correlated with creatinine and positively correlated with fasting blood sugar. It seems that this bacterium has a certain correlation with metabolic nephropathy. This conclusion has been similarly reported in some studies.44 The study of the increase in the population of the genus Megamonas in smoking has also been reported. A recent Italian study adopted the same method to prove that Megamonas was related to bad habits such as smoking, and the conclusion was consistent with this study: smoking could increase the abundance of Megamonas.45 This study elaborated the differences of intestinal microbiota between smoking and nonsmoking patients. The definitions of smoking and nonsmoking in this study were adopted by WHO and are widely used.14 However, whether this definition can fully meet the scientific research needs further verification. In addition to the time limit, further animal experiments and clinical studies are needed to add a more practical quantitative indicator to the smoking population, such as the number of cigarettes inhaled per day. This is also the deficiency of this study.
To sum up, in the middle-aged and elderly patients with cardiovascular disease, smoking will reduce the abundance of Bifidobacterium, while the abundances of some negative bacteria such as Burkholderia, Sutterella, and Megamonas will increase. After dividing the population differently, we found that there was a significant difference between the intestinal microorganisms of smoking and nonsmokers. Moreover, in smokers, there are more passive bacteria in the intestinal tract, which has a greater impact on clinical indicators, while the intestinal microorganisms in nonsmokers have a positive impact. It is consistent with the current research results, suggesting that these different bacteria have a certain impact on the occurrence and development of liver and kidney diseases and chronic metabolic diseases.
Methods
Study Design and Population
We included 188 male patients with cardiovascular disease who were hospitalized in the Department of Cardiology, the First Affiliated Hospital of Xinjiang Medical University from January 2019 to May 2019. All patients recruited in this study underwent coronary angiography. According to the coronary angiography, coronary heart disease was diagnosed in patients when at least one main coronary artery stenosis of them was larger than 50%, and there were totally 133 cases of coronary heart disease. 93 patients were diagnosed with hypertension and 24 patients with diabetes.
We collected clinical data of patients, including the basic population information and laboratory data of the enrolled population. We excluded the following patients: 1. Patients diagnosed with heart failure, structural heart disease, and pulmonary heart disease. 2. Patients with a history of using antibiotic or probiotic within 3 months. 3. Patients with severe liver and kidney dysfunction, such as patients whose creatinines were not less than 2-fold of the normal upper limit, patients whose aspartate transaminases or alanine transaminases were not less than 3-fold of the normal upper limit, etc. 4. Patients with abnormal stool morphology such as diarrhea and dry stools. The study design complied with the Declaration of Helsinki, and it was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. Before the recruitment, the informed consent of eligible patients was obtained.
Past History and Clinical Data
For alcohol intake, subjects who drank more than eight standard drinks per week were classified as heavy drinkers.15 For smoking, we adopted the World Health Organization (WHO) definition. Smoking every day or intermittently within 6 months was defined as a smoker (Current Smoking status). At the time of the survey, smokers had stopped smoking for more than 6 months that difinited as past smokers. 74 people were defined as current smokers, 64 people as past smokers, and 50 people as non-smokers. Peripheral venous blood (5 mL) was taken from each patient after 12 h fasting. The testing laboratory data included blood routine testing parameters, blood biochemical analysis results, renal function parameters, liver function parameters, and blood lipid analysis results.
Fecal Specimen Collection, DNA Extraction, and Sequencing
We provided a stool sampler for each participant for sample collection. All of the participants underwent a formal training on how to collect the sample before recruitment. The stool sample freshly collected from each participant was divided into five aliquots of 200 mg and immediately transported to the laboratory and frozen at −80 °C. Bead-beating method was utilized to isolate the bacterial DNA from facal samples as described previously.16 Polymerase chain reaction was used to amplify the V3-V4 region of 16S rRNA genes using the extracted DNA from each sample as the template.
PCR amplification of the bacterial 16S rRNA genes V3–V4 region was performed using the forward primer 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The PCR components contained 5 μl of buffer (5×), 0.25 μL of Fast pfu DNA Polymerase (5U/μL), 2 μl (2.5 mM) of dNTPs, 1 μL (10 uM) of each Forward and Reverse primer, 1 μL of DNA Template, and 14.75 μL of ddH2O. Thermal cycling consisted of initial denaturation at 98 °C for 5 min, followed by 25 cycles consisting of denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 45 s, with a final extension of 5 min at 72 °C. PCR amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and pair-end 2 × 250 bp sequencing was performed using the Illlumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). The sequencing data were processed using the Quantitative Insights into Microbial Ecology (v1.8.0) pipeline, as described previously.17
Microbiome Data Analysis
Cluster analysis based on bacterial species composition was performed to classify the subjects into enterotypes. According to the abundance of genus level, the results are obtained by Jensen-Shannon Divergence distance and Partition Around Medoids clustering algorithm.18 For analysis of α-diversity, the richness and diversity of samples were determined by abundance-based coverage estimators, Chao1 and Jackknife estimation. In addition, the Simpson and Shannon diversity indices at a 3% distance were calculated using the CL community program (ChunLab, Inc.). Good’s method was applied to calculate sequencing coverage. For comparisons of the composition of a selected taxon, we used the Wilcoxon rank-sum test. For analysis of β-diversity, the overall phylogenetic distance between communities was estimated and visualized using Bray–Curtis distance-based principal coordinates analysis. For species difference analysis, we applied LEfSe (LDA effect size) analysis, which combined the nonparametric Kruskal–Wallis and Wilcoxon rank-sum tests with the effect size of the linear discriminant analysis (LDA).19,20
Statistical Analysis
The SPSS version 22 (SPSS, Inc., Chicago, IL) and R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria) were applied for the statistical analysis of clinical data. For LEfSe (LDA effect size) analysis, we applied the Galaxy online analysis platform (http://huttenhower.sph.harvard.edu/galaxy/), and continuous variables were analyzed through using Student’s t-test. The chi-square test or Fisher exact test was adopted to analyze categorical variables. All results were considered statistically significant when p-values were less than 0.05.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (81770235) and the Xinjiang Science and Technology Aid Project (2019E0278). H.-T.Y. and J.-K.L. made substantial contributions to the study conception and design and to the drafting and critical revision of the manuscript for important intellectual content.
Author Contributions
# H.-T.Y. and W.-J.X. contributed equally to this article.
The authors declare no competing financial interest.
References
- Sender R.; Fuchs S.; Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Gill S. R.; Pop M.; Deboy R. T.; Eckburg P. B.; Turnbaugh P. J.; Samuel B. S.; Gordon J. I.; Relman D. A.; Fraser-Liggett C. M.; Nelson K. E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.; Li R.; Raes J.; Arumugam M.; Burgdorf K. S.; Manichanh C.; Nielsen T.; Pons N.; Levenez F.; Yamada T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K.; Holmes E.; Wilson I. D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- Emoto T.; Yamashita T.; Sasaki N.; Hirota Y.; Hayashi T.; So A.; Kasahara K.; Yodoi K.; Matsumoto T.; Mizoguchi T.; et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 908–921. 10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Y.; Wu T. T.; Liu Z. Q.; Li A.; Guo Q. Q.; Ma Y. Y.; Zhang Z. L.; Xun Y. L.; Zhang J. C.; Wang W. R.; et al. Gut Microbiome-Based Diagnostic Model to Predict Coronary Artery Disease. J. Agric. Food Chem. 2020, 68, 3548–3557. 10.1021/acs.jafc.0c00225. [DOI] [PubMed] [Google Scholar]
- Xu J.; Yang Y. Implications of gut microbiome on coronary artery disease. Cardiovasc. Diagn. Ther. 2020, 10, 869–880. 10.21037/cdt-20-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Lin S.; Vanhoutte P. M.; Woo C. W.; Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016, 133, 2434–2446. 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- Keirns B. H.; Koemel N. A.; Sciarrillo C. M.; Anderson K. L.; Emerson S. R. Exercise and intestinal permeability: another form of exercise-induced hormesis?. Am. J. Physiol.: Gastrointest. Liver Physiol. 2020, 319, G512–G518. 10.1152/ajpgi.00232.2020. [DOI] [PubMed] [Google Scholar]
- Aatsinki A. K.; Kataja E. L.; Munukka E.; Lahti L.; Keskitalo A.; Korja R.; Nolvi S.; Häikiö T.; Tarro S.; Karlsson H.; Karlsson L. Infant fecal microbiota composition and attention to emotional faces. Emotion 2020, 10.1037/emo0000924. [DOI] [PubMed] [Google Scholar]
- Nolan-Kenney R.; Wu F.; Hu J.; Yang L.; Kelly D.; Li H.; Jasmine F.; Kibriya M. G.; Parvez F.; Shaheen I.; et al. The Association Between Smoking and Gut Microbiome in Bangladesh. Nicotine Tob. Res. 2020, 22, 1339–1346. 10.1093/ntr/ntz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurso G.; Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best Pract. Res., Clin. Gastroenterol. 2017, 31, 579–588. 10.1016/j.bpg.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Yoon H.; Lee D. H.; Lee J. H.; Kwon J. E.; Shin C. M.; Yang S. J.; Park S. H.; Lee J. H.; Kang S. W.; Lee J. S.; et al. Characteristics of the Gut Microbiome of Healthy Young Male Soldiers in South Korea: The Effects of Smoking. Gut Liver 2021, 15, 243–252. 10.5009/gnl19354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. C.; Kim J. S.; Jung J. G.; Kim S. S.; Yoon S. J.; Ryu J. S. Effect of alcohol consumption on risk of hyperhomocysteinemia based on alcohol-related facial flushing response. Korean J. Fam. Med. 2013, 34, 250–257. 10.4082/kjfm.2013.34.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Chen X.; Hu X.; Niu H.; Tian R.; Wang H.; Pang H.; Jiang L.; Qiu B.; Chen X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68 10.1186/s40168-019-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Yang S.; Zhang X.; Huang S.; Wang N.; Wang M.; Long M.; He J. Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit. Biomed. Res. Int. 2018, 2018, 3623274 10.1155/2018/3623274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M.; Raes J.; Pelletier E.; Le Paslier D.; Yamada T.; Mende D. R.; Fernandes G. R.; Tap J.; Bruls T.; Batto J. M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh S.; Angelakis E.; Karamitros T.; Bachar D.; Bahijri S.; Ajabnoor G.; Alfadul S. M.; Farraj S. A.; Al Amri T.; Al-Hejin A.; et al. Impact of smoking cessation, coffee and bread consumption on the intestinal microbial composition among Saudis: A cross-sectional study. PLoS One 2020, 15, e0230895 10.1371/journal.pone.0230895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N.; Izard J.; Waldron L.; Gevers D.; Miropolsky L.; Garrett W. S.; Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z.; Wei H.; Yang L.; Yao T.; Mao Z.; Sun Q. Catastrophic health expenditure: A comparative analysis of smoking and non-smoking households in China. PLoS One 2020, 15, e0233749 10.1371/journal.pone.0233749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer F.; Enrique M.; Llopis S.; Barrena M.; Navarro V.; Álvarez B.; Chenoll E.; Ramón D.; Tortajada M.; Martorell P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: a novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2021, 10.1111/1751-7915.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.; Zhang Y.; Xu D.; Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J. Clin. Lab. Anal. 2021, 35, e23650 10.1002/jcla.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky J.; Haruki K.; Lau M. C.; Dias Costa A.; Väyrynen J. P.; Ugai T.; Arima K.; da Silva A.; Felt K. D.; Zhao M.; et al. Association of Fusobacterium nucleatum with Specific T-cell Subsets in the Colorectal Carcinoma Microenvironment. Clin. Cancer Res. 2021, 27, 2816–2826. 10.1158/1078-0432.CCR-20-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi T. B.; Devadas K.; George M.; Gandhimathi A.; Chouhan D.; Retnakumar R. J.; Alexander S. M.; Varghese J.; Dharmaseelan S.; Chandrika S. K.; et al. Low Bifidobacterium Abundance in the Lower Gut Microbiota Is Associated With Helicobacter pylori-Related Gastric Ulcer and Gastric Cancer. Front. Microbiol. 2021, 12, 631140 10.3389/fmicb.2021.631140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.; Nguyen S. M.; Yang Y.; Xu W.; Cai H.; Wu J.; Cai Q.; Long J.; Zheng W.; Shu X. O. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am. J. Clin. Nutr. 2021, 113, 684–694. 10.1093/ajcn/nqaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.; Zhu H.; Gao F.; Qian Z.; Mao W.; Yin Y.; Tan J.; Chen D. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020, 11, 6528–6541. 10.1039/D0FO00180E. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Zhang Y.; Xu D.; Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J. Clin. Lab. Anal. 2021, 35, e23650 10.1002/jcla.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian M.; Wang J.; Wang Y.; Nie A.; Zhu C.; Sun Z.; Zhou Z.; Zhang B. Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomed. Pharmacother. 2020, 131, 110719 10.1016/j.biopha.2020.110719. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Wang Z.; He F.; Qiu H.; Wang Y.; Wang H.; Zhou J.; Zhou J.; Cheng G.; Zhou W.; et al. Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis. J. Nutr. Biochem. 2019, 63, 54–61. 10.1016/j.jnutbio.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Mrozinska S.; Kapusta P.; Gosiewski T.; Sroka-Oleksiak A.; Ludwig-SnapmczynaΓka A. H.; Matejko B.; Kiec-Wilk B.; Bulanda M.; Malecki M. T.; Wolkow P. P.; et al. The Gut Microbiota Profile According to Glycemic Control in Type 1 Diabetes Patients Treated with Personal Insulin Pumps. Microorganisms 2021, 9, 155 10.3390/microorganisms9010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Yu X.; Xu X.; Ming J.; Wang Z.; Gao B.; Xing Y.; Zhou J.; Fu J.; Liu T.; et al. The Fecal Microbiota Is Already Altered in Normoglycemic Individuals Who Go on to Have Type 2 Diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 598672 10.3389/fcimb.2021.598672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S. M.; Mei L.; Huang H.; Cao S. F.; Zhao R. H.; Zheng P. Y. [Correlation analysis of gut microbiota and biochemical indexes in patients with non-alcoholic fatty liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2019, 27, 369–375. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Ma C.; Zhang B.; Bi L. Dynamic changes in gut microbiota under the influence of smoking and TNF-a-blocker in patients with ankylosing spondylitis. Clin. Rheumatol. 2020, 39, 2653–2661. 10.1007/s10067-020-05032-4. [DOI] [PubMed] [Google Scholar]
- Hattori S.; Nakamura M.; Yamamura T.; Maeda K.; Sawada T.; Mizutani Y.; Yamamoto K.; Ishikawa T.; Furukawa K.; Ohno E.; et al. The microbiome can predict mucosal healing in small intestine in patients with Crohn’s disease. J. Gastroenterol. 2020, 55, 1138–1149. 10.1007/s00535-020-01728-1. [DOI] [PubMed] [Google Scholar]
- Deng Y.; Tang D.; Hou P.; Shen W.; Li H.; Wang T.; Liu R. Dysbiosis of gut microbiota in patients with esophageal cancer. Microb. Pathog. 2021, 150, 104709 10.1016/j.micpath.2020.104709. [DOI] [PubMed] [Google Scholar]
- Gallè F.; Valeriani F.; Cattaruzza M. S.; Gianfranceschi G.; Liguori R.; Antinozzi M.; Mederer B.; Liguori G.; Romano Spica V. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164 10.3390/nu12072164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A. R.; Berger S.; Vogel T. M. Human pathogens abundant in the bacterial metagenome of cigarettes. Environ. Health Perspect. 2010, 118, 351–356. 10.1289/ehp.0901201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago K.; Kikuchi Y.; Nakaoka S.; Katsuyama C.; Hayatsu M. Insecticide applications to soil contribute to the development of Burkholderia mediating insecticide resistance in stinkbugs. Mol. Ecol. 2015, 24, 3766–3778. 10.1111/mec.13265. [DOI] [PubMed] [Google Scholar]
- Siegel M. J.; Freeman A. J.; Ye W.; Palermo J. J.; Molleston J. P.; Paranjape S. M.; Stoll J.; Leung D. H.; Masand P.; Karmazyn B.; et al. CFLD Network. Heterogeneous Liver on Research Ultrasound Identifies Children with Cystic Fibrosis at High Risk of Advanced Liver Disease: Interim Results of a Prospective Observational Case-Controlled Study. J. Pediatr. 2020, 219, 62–69.e4. 10.1016/j.jpeds.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler A. D.; Knox N.; Kabakchiev B.; Milgrom R.; Kirsch R.; Cohen Z.; McLeod R. S.; Guttman D. S.; Krause D. O.; Silverberg M. S. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One 2013, 8, e66934 10.1371/journal.pone.0066934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I.; Liu K.; Long D.; Faisal S.; Hilal M. G.; Ali I.; Huang X.; Long R. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Front. Microbiol. 2021, 12, 642999 10.3389/fmicb.2021.642999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. D.; Liu B. N.; Zhao S. H.; Zhou Y. L.; Bai L.; Liu E. Q. Changes in gut microbiota composition and diversity associated with post-cholecystectomy diarrhea. World J. Gastroenterol. 2021, 27, 391–403. 10.3748/wjg.v27.i5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Zhang H.; Liu H.; Zhang H.; Bao Y.; Di J.; Hu C. The genus Sutterella is a potential contributor to glucose metabolism improvement after Roux-en-Y gastric bypass surgery in T2D. Diabetes Res. Clin. Pract. 2020, 162, 108116 10.1016/j.diabres.2020.108116. [DOI] [PubMed] [Google Scholar]
- Palmas V.; Pisanu S.; Madau V.; Casula E.; Deledda A.; Cusano R.; Uva P.; Vascellari S.; Loviselli A.; Manzin A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532 10.1038/s41598-021-84928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]