Abstract
We describe an outbreak of necrotizing enterocolitis (NEC) that occurred in the neonatal intensive care unit of our hospital. A total of 12 neonates developed NEC in June-July 1998. For two of them, twin brothers, the NEC turned out to be fatal. Enterobacter sakazakii, a known contaminant of powdered milk formula, was isolated from a stomach aspirate, anal swab, and/or blood sample for 6 of the 12 neonates. A review of feeding procedures revealed that 10 of the 12 patients were fed orally with the same brand of powdered milk formula. E. sakazakii was isolated from the implicated prepared formula milk as well as from several unopened cans of a single batch. Molecular typing by arbitrarily primed PCR (AP-PCR) confirmed, although partially, strain similarity between milk and patient isolates. No further cases of NEC were observed after the use of the contaminated milk formula was stopped. With this outbreak we show that intrinsic microbiological contamination of powdered milk formula can be a possible contributive factor in the development of NEC, a condition encountered almost exclusively in formula-fed premature infants. The use of sterilized liquid milk formula in neonatal care could prevent problems with intrinsic and extrinsic contamination of powdered milk formula.
Neonatal necrotizing enterocolitis (NEC), characterized by intestinal necrosis and pneumatosis intestinalis, is the most common gastrointestinal emergency in the newborn. The disease has an incidence rate of 2 to 5% in premature infants. The incidence rate increases to 13% in those weighing <1,500 g at birth. NEC still has a mortality rate of 10 to 55% (26). The triad of neonatal intestinal ischaemia, microbial colonization of the gut, and excess protein substrate in the intestinal lumen associated with oral formula feeding seems to be a prerequisite in the pathogenesis of NEC (17). Geographical and temporal clustering of the disease and the termination of epidemics by standard infection control procedures underline the importance of infectious agents in the development of NEC (5). Outbreaks have been related to pathogens usually absent in the normal intestinal flora of the neonate, such as Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Salmonella spp., Pseudomonas aeruginosa, Clostridium spp., coagulase-negative staphylococci, (methicillin-resistant) Staphylococcus aureus, Candida glabrata, coronavirus, enterovirus, and rotavirus (31). However, the sources of these pathogens were not always identified by environmental sampling.
Enterobacter sakazakii, a rarely isolated microorganism previously classified as a yellow-pigmented Enterobacter cloacae and recognized as a separate species in 1980 (9), has been involved in several cases of neonatal meningitis and sepsis (1, 2, 4, 12–14, 16, 19, 22, 25, 27–28, 30). In most of these cases the infant formula has been suspected to be the source of infection. E. sakazakii has been found to be a frequent contaminant of powdered milk formulas, and it has been cultured from unused formula products in 13 countries (21).
In this report we describe for the first time a cluster of NEC associated with the isolation of E. sakazakii in patients and the use of powdered infant milk formula.
MATERIALS AND METHODS
Background.
An outbreak of NEC occurred during June-July 1998 in our neonatal intensive care unit (NICU). The unit is a 16-bed tertiary referral center. In the two months of the outbreak, a cohort of 50 neonates was admitted at our NICU. Median birth weight was 2,335 g (interquartile range, 1,305 to 3,040 g), median gestational age was 35 weeks (interquartile range, 30 to 39 weeks), and median length of stay was 16 days (interquartile range, 8 to 43 days). Twenty-two (44%) neonates had a birth weight of <2,000 g.
Case definition.
Bell's staging of NEC as modified by Walsh and Kliegman was used (29). Infants with stage I disease (suspected NEC) have suggestive clinical symptoms such as abdominal distention, gastric residual, emesis, and/or hematochezia but nondiagnostic radiographs. Infants with stage II disease (definite NEC) have diagnostic abdominal radiographs showing pneumatosis intestinalis. Infants with stage III disease (advanced NEC) are critically ill with impending or proven intestinal perforation.
Patient cultures.
We reviewed the results of all bacterial cultures taken from the neonates during the outbreak. Surveillance cultures, consisting of an anal swab, a stomach aspirate, and a blood culture, were obtained from each NEC patient, if possible and if ordered by the pediatrician.
Environmental cultures.
Taking into account the properties of the isolated microorganism and the fact that all NEC patients were orally fed, environmental sampling was focused on the milk kitchen. When formula is prepared in our milk kitchen, the powder is weighed on sterilized plates with sterilized spoons. The formula is mixed in a sterilized bowl with a sterilized blender head which is rinsed between preparations in cooked tap water. Milk solutions are prepared with chilled mineral water once a day between 9 and 11 a.m., divided into disposable bottles, and closed with disposable, gamma-irradiated teats. The bottles are stored temporarily on a special cooling table before transportation to the different pediatric wards, where they are placed immediately in the refrigerator. The milk bottles are warmed up with a dry-air bottle heater or in a microwave just before use.
During the outbreak one extra bottle of each milk formula, freshly prepared in our milk kitchen, was set aside for microbiological analysis. Furthermore, we collected samples of the mineral water used in the milk preparations and of the water used to rinse the blender head between preparations.
Microbiological methods.
Anal swabs and stomach aspirates were inoculated on four agar plates: tryptic soy agar (Life Technologies, Paisley, Scotland) supplemented with 5% horse blood, hemin T, and NAD; tryptic soy agar supplemented with 5% horse blood and nalidixic acid; MacConkey agar (Life Technologies,); and mannitol salt agar (Oxoid, Basingstoke, England). Blood samples were cultured with the BBL Septi-Chek system (Becton Dickinson, Cockeysville, Md.) by inoculation of 1 to 3 ml of blood in a 20-ml brain heart infusion broth bottle. Each environmental sample was inoculated directly on tryptic soy agar supplemented with 5% sheep blood, on MacConkey agar, and ±1 ml of the sample was inoculated in fastidious anaerobe broth (Lab M, Bury, England) for enrichment. Agar plates and broth were incubated aerobically at 37°C. All enrichments were reinoculated on four agar plates after 48-h incubation. Isolates were identified as E. sakazakii by standard laboratory methods (10).
Molecular typing.
Molecular typing was performed by arbitrarily primed PCR (AP-PCR). Ten strains of E. sakazakii isolated in our laboratory from nine patients between 1989 and 1996 were used as control isolates. All patient, environmental, and control isolates were examined in a single assay to reduce intertest variability. Briefly, target DNA was prepared from bacteria grown overnight at 37°C on Mueller-Hinton agar (Difco Laboratories, Sparks, Md.) supplemented with 5% sheep blood. A single colony was suspended in 300 μl of distilled water and boiled for 10 min. AP-PCR was performed in 50-μl reaction volumes containing 10 mM Tris HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 0.01% gelatin, 200 μM each deoxynucleoside triphosphate, 1 mM primer ERIC2 (for “enterobacterial repetitive intergenic consensus motif”; 5′-AAGTAAGTGACTGGGGTGAGCG), and 1 U of Taq DNA polymerase.
A Perkin-Elmer Gene AMP 9600 was used for amplification. PCR cycling conditions consisted of four cycles of 4 min at 94°C, 4 min at 40°C, and 4 min at 72°C, followed by 30 cycles of 24 s at 94°C, 22 s at 55°C, and 1 min at 72°C. DNA fragments were fractionated on a 1% agarose gel and visualized by ethidium bromide staining.
The photographs of the AP-PCR fingerprints were scanned and the digitized data were analyzed with GelCompar v4.1 (Applied Maths, Kortrijk, Belgium). Degrees of homology were determined by Dice comparisons, and clustering correlation coefficients were calculated by the unweighted pair group method using arithmetic averages.
RESULTS
Patients.
A total of 12 patients (24%; 55% of the neonates with a birth weight of <2,000 g) were identified to have clinical signs of NEC during June-July 1998 (Table 1). Four patients required operative treatment (stage III). For the twin brothers the NEC turned out to be fatal (patients 3 and 4).
TABLE 1.
Clinical characteristics of neonates with NEC (June-July 1998)
| Patient | Sexb | Date of birth (day/mo) | Gestational age (wk) | Birth wt (g) | Length of stay (days) | Type of feeding | Start of feeding (day/mo) | Date of onset (day/mo) | NEC stagec |
E. sakazakii culturea
|
Date of death (day/mo) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Stomach | Anus | |||||||||||
| 1 | M | 05/05 | 27 | 850 | 120 | Alfaré | 19/06 | 29/06 | I | − | + | − | |
| 2 | F | 21/05 | 31 | 1,930 | 64 | Prématil | 05/06 | 06/06 | II | − | NP | NP | |
| 3 | M | 22/05 | 27 | 995 | 42 | Alfaré | 26/06 | 01/07 | III | NP | NP | NP | 02/07 |
| 4d | M | 22/05 | 27 | 965 | 63 | Alfaré | 21/06 | 24/06 | III | +e | − | + | 23/07 |
| 5 | F | 23/05 | 29 | 815 | 74 | Alfaré | 11/06 | 03/07 | II | NP | NP | NP | |
| 6 | F | 08/06 | 28 | 1,200 | 89 | Alfaré | 12/06 | 30/06 | III | − | − | −f | |
| 7 | M | 10/06 | 28 | 1,100 | 80 | Alfaré | 16/06 | 19/06 | III | − | − | +g | |
| 8 | F | 14/06 | 27 | 590 | 111 | Alfaré | 20/07 | 23/07 | II | − | + | +g | |
| 9 | F | 16/06 | 31 | 1,350 | 47 | Alfaré | 22/06 | 03/07 | I | − | + | +e | |
| 10 | F | 16/06 | 32 | 1,490 | 39 | Alfaré | 19/06 | 25/06 | I | NP | NP | NP | |
| 11 | M | 26/06 | 32 | 1,290 | 43 | Alfaré | 27/06 | 03/07 | I | − | − | + | |
| 12 | M | 03/07 | 30 | 1,550 | 32 | Prématil | 06/07 | 07/07 | I | − | − | − | |
NP, not performed; −, negative; +, positive.
F, female; M, male.
I, suspected disease; II, definite disease; III, advanced disease.
Twin brother of patient 3.
Two morphologically different E. sakazakii isolates were found.
Enterobacter cloacae, Escherichia coli, and Enterococcus faecalis were isolated.
Isolate was not stored and thereby was not available for molecular typing.
All twelve neonates had a birth weight of <2,000 g and had been fed orally with formula milk before the development of NEC. During June-July 1998, 10 of the 12 neonates with NEC received the same semielemental formula with low osmolarity (Alfaré, produced by Nestlé, Nunspeet, The Netherlands), compared to 4 of the 38 without NEC (P < 0.0001 [Fisher's exact test]).
During June-July 1998, 6 of the 12 neonates with NEC had positive cultures for E. sakazakii, compared to 0 of the 38 without NEC (P < 0.0001 [Fisher's exact test]). Furthermore, 6 of the 14 neonates who received Alfaré had positive cultures for E. sakazakii, compared to 0 of the 36 who did not receive the formula (P = 0.0002 [Fisher's exact test]). A total of 11 E. sakazakii strains from the six culture-positive NEC patients were isolated. The anal swab of patient 6 yielded Enterobacter cloacae, Escherichia coli, and Enterococcus faecalis. E. sakazakii was isolated from blood culture (patient 4), from anal swabs (patients 4, 7, 8, 9, and 11), and from stomach aspirates (patients 1, 8, and 9). In the blood culture of patient 4 and the anal swab of patient 9 two morphologically different E. sakazakii isolates were identified.
Environmental samples.
Cultures of the extra prepared milk bottles from our milk kitchen revealed the presence of E. sakazakii in several Alfaré milk preparations. Cultures of milk formulas of other brands were negative or resulted in the isolation of Bacillus spp., coagulase-negative staphylococci, or Acinetobacter spp. Cultures of the mineral water and the rinsing water remained negative.
To exclude the possibility of contamination during preparation and storage, we performed cultures for unopened cans of Alfaré milk. By inoculating 3 g of powder directly in fastidious broth, E. sakazakii could be isolated from several unopened cans of one of the two batches of Alfaré milk present in our kitchen stocks (SPNAV-CT, manufactured January 1998, expiration January 2000). A total of 14 E. sakazakii strains from Alfaré milk were isolated.
Molecular typing.
Molecular typing by AP-PCR was performed for 9 isolates from five patients, 14 milk isolates, and 10 control isolates (Table 2). Two patient isolates, from the anal swabs of patients 7 and 8, were not stored and thereby not available for molecular typing.
TABLE 2.
Results of AP-PCR molecular typing of patient, milk, and control isolates of E. sakazakii
| Isolate | Origina | Collection date (day/mo/yr) | AP-PCR profile |
|---|---|---|---|
| From patients | |||
| P1 | Blood, patient 4 | 06/07/1998 | III |
| P2 | Blood, patient 4 | 06/07/1998 | II |
| P3 | Stomach, patient 9 | 07/07/1998 | Ia |
| P4 | Anus, patient 9 | 07/07/1998 | III |
| P5 | Anus, patient 9 | 07/07/1998 | Ia |
| P6 | Stomach, patient 1 | 07/07/1998 | III |
| P7 | Anus, patient 11 | 07/07/1998 | Ia |
| P8 | Anus, patient 4 | 07/07/1998 | II |
| P9 | Stomach, patient 8 | 23/07/1998 | Ia |
| From milk | |||
| M1 | Alfaré milk | 09/07/1998 | Ia |
| M2 | Alfaré milk | 10/07/1998 | Ia |
| M3 | Alfaré milk | 13/07/1998 | Ia |
| M4 | Alfaré milk | 13/07/1998 | Ia |
| M5 | Alfaré milk | 16/07/1998 | Ia |
| M6 | Alfaré milk | 22/07/1998 | Ia |
| M7 | Alfaré milk | 22/07/1998 | Ia |
| M8 | Alfaré milk | 23/07/1998 | Ia |
| M9 | Alfaré milk | 23/07/1998 | Ia |
| M10 | Alfaré milk | 23/07/1998 | Ia |
| M11 | Alfaré milk | 23/07/1998 | Ia |
| M12 | Alfaré milk | 23/07/1998 | Ia |
| M13 | Alfaré milk | 23/07/1998 | Ia |
| M14 | Alfaré milk | 24/07/1998 | Ia |
| Control | |||
| C1 | Wound, M, 54 yr | 22/02/1989 | VII |
| C2 | Urine, M, 45 yr | 21/08/1990 | VIII |
| C3 | Wound, F, 27 yr | 23/08/1992 | IV |
| C4 | Urine, F, 83 yr | 23/01/1993 | V |
| C5 | Blood, M, 27 yr | 27/08/1993 | IX |
| C6 | Blood, M, 27 yr | 27/08/1993 | IX |
| C7 | Stomach, F, 0 yr | 11/02/1994 | Ib |
| C8 | Sputum, F, 69 yr | 06/08/1994 | X |
| C9 | Urine, F, 1 yr | 15/05/1995 | IX |
| C10 | Blood, F, 47 yr | 08/04/1996 | VI |
F, female; M, male.
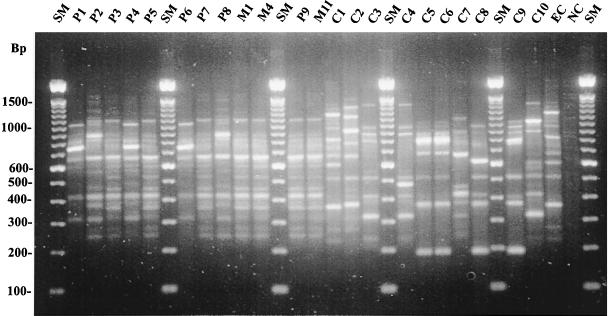
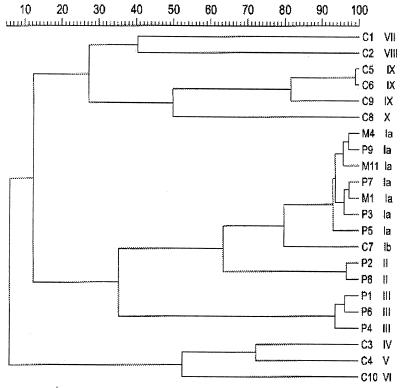
Three different profiles of E. sakazakii (Ia, II, and III) were found among the nine patient isolates (Fig. 1 and 2). The 14 milk isolates and four patient isolates from three patients (patients 8, 9, and 11) shared profile Ia. Profile III isolates were recovered from patients 1, 4 and 9, while profile II was found only for patient 4. The two morphologically different E. sakazakii isolates from the blood culture of patient 4 and the anal swab of patient 9 also had different molecular profiles, II/III and Ia/III, respectively.
FIG. 1.
AP-PCR profiles of the E. sakazakii isolates. P1 to P9, patient isolates; M1, M4, and M11, milk isolates; C1 to C10, control isolates; EC, a laboratory strain of E. cloacae; NC, negative control; SM, molecular size marker (see Table 2 for origins of isolates).
FIG. 2.
Dendrogram of the AP-PCR profiles as analyzed with GelCompar v4.1. P1 to P9, patient isolates; M1, M4, and M11, milk isolates; C1 to C10, control isolates (see Table 2 for origins of isolates).
Eight different profiles (Ib, IV, V, VI, VII, VIII, IX, and X) were found among the 10 control isolates. The two isolates from the same patient (C5 and C6) showed the same profile (IX). One control isolate (C7), collected in 1994 from a gastrostomy tube of a premature infant, showed profile Ib, almost identical to the milk-related profile Ia.
Actions.
The use of Alfaré powdered milk formula was stopped in our NICU on 10 July 1998, immediately after we suspected a possible link between Alfaré milk, E. sakazakii, and development of NEC. However, because our initial cultures demonstrated the presence of E. sakazakii only in prepared milk and not in Alfaré powder, the formula was released again on 20 July 1998 for the feeding of one infant (patient 8). This patient developed symptoms of NEC on 23 July 1998, and E. sakazakii was isolated from her stomach aspirate and anal swab. At the same time further cultures demonstrated the intrinsic contamination of Alfaré powdered milk with E. sakazakii. From then on, feeding with Alfaré was completely stopped.
The manufacturer's microbiological quality control data for the batch SPNAV showed that, of the five samples analyzed, one yielded 20 coliforms/g whereas in the other four samples fewer than 1 coliform/g was found. These results fulfilled the requirements of the Codex Alimentarius (11), i.e., a minimum of four of five control samples with <3 coliforms/g and a maximum of one of five control samples with >3 but ≤20 coliforms/g. However, the manufacturer's microbiological quality control data did not fulfil the requirements of Belgian law (3), i.e., <1 coliform/g in all control samples. This observation led to the recall of the contaminated batch SPNAV from the Belgian market.
After this incident, the production facility in Nunspeet, The Netherlands, was upgraded, appropriate hygienic measures were taken, and more stringent release norms for dietetic specialities (<0.3 coliform/g, 0 E. sakazakii isolates/10 g) were applied by Nestlé. In our NICU, Alfaré powdered milk formula was administered again in April 1999. Until now no further cases of NEC associated with the isolation of E. sakazakii were observed.
DISCUSSION
We described a cluster of 12 neonates with NEC treated at our NICU in June-July 1998. E. sakazakii, a rare pathogen known to cause severe neonatal sepsis and meningitis (1, 2, 4, 12–14, 16, 19, 22, 25, 27–28, 30) and to contaminate powdered milk formula (21), was isolated from 6 of the 12 neonates. After a review of feeding procedures, a significant association was found between the development of NEC, the consumption of a brand of powdered milk formula, and the isolation of E. sakazakii in neonates. E. sakazakii could be isolated from several unopened cans of a single batch of the implicated formula powder. Molecular typing by AP-PCR confirmed strain similarity between all milk powder isolates and three patient isolates. When the use of this formula was discontinued, the outbreak of NEC came to an end. One infant who received the implicated formula after its use was stopped developed NEC and was colonized with E. sakazakii. From all these elements of our cohort study, we conclude that there is a strong, if not causal, relationship between intrinsic contamination of powdered milk formula with E. sakazakii and the development of NEC.
The AP-PCR assay used for the molecular typing of E. sakazakii has high discriminatory power, as shown by the typing results for the control isolates. The results of the AP-PCR, confirmed by a ribotyping assay performed independently at the Nestlé Research Centre, Lausanne, Switzerland (data not shown), were surprising, as we expected an identical profile for all patient and milk isolates. Because Nazarowec-White and Farber already had demonstrated the presence of different genotypes of E. sakazakii in different samples of formula from one company (24) and because another source of a rarely isolated organism such as E. sakazakii coinciding with the formula source seems unlikely, we still suspect the formula to be the source of the three molecularly different patient isolates. The molecular profile Ia found in all milk isolates may possibly suggest that this was the most predominant profile present in the Alfaré milk.
It is interesting that one control isolate (C7) had the profile Ib, almost identical to the milk-related profile Ia. This strain was isolated in 1994 from a gastrostomy tube of a prematurely born girl. The infant suffered from an infection at the gastrostomy tube insertion site after she received Alfaré formula through the tube. The isolation of a closely related E. sakazakii strain 4 years before the outbreak may point to an already long-lasting contamination problem.
Only a few outbreaks linking E. sakazakii with contaminated milk have been reported in the literature. The first report from The Netherlands described eight cases of neonatal meningitis and sepsis due to E. sakazakii (22). Two of the eight cases had NEC and meningitis simultaneously. E. sakazakii was isolated from prepared milk formula, a dish brush, and a stirring spoon, but different plasmid profiles were observed for patient and environmental isolates. In Iceland meningitis caused by E. sakazakii was reported in three cases (4). There is no mention of NEC in any of these cases. E. sakazakii was isolated at low concentrations from the milk powder. Patient and environmental isolates had identical biotypes, antibiograms, and plasmid profiles (8). There was evidence that the formula bottles were occasionally kept at 35 to 37°C for extended periods in bottle heaters. An outbreak of E. sakazakii in Memphis, Tennessee, involved a total of four neonates (27). Three patients had sepsis, and three had bloody diarrhea. All patients had stool colonization. E. sakazakii isolates with the same plasmid profile were cultured from the patients, an open can of powdered milk formula, and the blender, which showed heavy growth of the organism (8).
The role of powdered milk formula in the development of NEC should not be underestimated. Milk formula can serve not only as an ideal substrate for bacterial growth but also as a source of possible pathogens, as most formula products are intrinsically contaminated. Outbreaks of NEC linked to contaminated milk formula might be missed if the isolated microorganisms are frequent nosocomial pathogens that do not arouse immediate suspicion as E. sakazakii does. It has also been shown that confirmed NEC is 10 times as common in babies fed only formula than in those fed only breast milk (18). Until now this observation has been explained by the presence of protective immunoglobulins (immunoglobulin A) in breast milk. Alternatively, we suggest that breast milk is less frequently contaminated with pathogens that can be held responsible for the development of NEC. The frequent isolation of Enterobacteriaceae, especially those belonging to the genus Enterobacter, in NEC and in powdered milk formula may also suggest the involvement of orally administered contaminated formula in the development of NEC. In a study of the preantibiotic bacteriology in 125 neonates with NEC (7), Enterobacter spp. were the most common organisms, isolated in 29% of the patients. On the other hand Muytjens et al. examined a total of 141 different powdered formulas obtained in 35 countries for the presence of members of the Enterobacteriaceae (21). Members of the genus Enterobacter were most frequently isolated: E. agglomerans was cultured from 35 formulas (25%), E. cloacae was cultured from 30 formulas (21%), and E. sakazakii was cultured from 20 formulas (14%) of the 141 formulas examined. The high thermal resistance of Enterobacter spp. in comparison to other members of the Enterobacteriaceae can possibly explain their high prevalence in powdered and prepared formula milk (23).
When a neonate develops NEC, especially when Enterobacter spp. are cultured, a careful examination of feeding procedures is mandatory. Strict hygienic measures must be taken in preparing formula milk (6). Milk bottles should never stay in a bottle heater for more than 15 min to keep the possibility of multiplication of microorganisms to a minimum. The use of a microwave to warm up milk is preferable because of its bactericidal properties (15).
The contaminated lot of Alfaré milk involved in this outbreak fulfilled the requirements of the Food and Agricultural Organization of the United Nations (11). As recommended before (20–21, 25, 27), more stringent release norms regarding microbial contamination of powdered infant milk formula need to be applied, especially in the neonatal setting. The presence of even low-grade pathogens in powdered formula cannot be allowed. The use of commercial, sterilized liquid formula can be a solution to this problem, avoiding the intrinsic powder contaminants and the potential for extrinsic contamination at the time of rehydration. However, liquid formulas are generally more expensive and require larger transport and storage facilities. Furthermore, lower quantities and different concentrations of a formula are used in neonatal care to suit specific nutritional needs. Therefore, this solution is commercially not feasible for most formula milk producers, although it could probably save children's lives.
REFERENCES
- 1.Adamson D H, Rogers J R. Enterobacter sakazakii meningitis with sepsis. Clin Microbiol Newsl. 1981;3:19–20. [Google Scholar]
- 2.Arseni A, Malamou-Ladas E, Koutsia C, Xanthou M, Trikka E. Outbreak of colonization of neonates with Enterobacter sakazakii. J Hosp Infect. 1987;9:143–150. doi: 10.1016/0195-6701(87)90052-1. [DOI] [PubMed] [Google Scholar]
- 3.Belgisch Staatsblad. Koninklijk besluit van 18 februari 1991 betreffende voedingsmiddelen bestemd voor bijzondere voeding. Belgisch Staatsblad publication 30/08/1991. 1991. pp. 18864–18887. . Belgisch Staatsblad, Brussels, Belgium. [Google Scholar]
- 4.Biering G, Karlsson S, Clark N C, Jonsdottir K E, Ludvigsson P, Steingrimsson O. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J Clin Microbiol. 1989;27:2054–2056. doi: 10.1128/jcm.27.9.2054-2056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Book L S, Overall J C, Herbst J J, Britt M R, Epstein B, Jung A L. Clustering of necrotizing enterocolitis: interruption by infection-control measures. N Engl J Med. 1977;297:984–986. doi: 10.1056/NEJM197711032971805. [DOI] [PubMed] [Google Scholar]
- 6.Burnett I A, Wardley B L, Magee J T. The milk kitchen, Sheffield Children's Hospital, before and after a review. J Hosp Infect. 1989;13:179–185. doi: 10.1016/0195-6701(89)90025-x. [DOI] [PubMed] [Google Scholar]
- 7.Chan K L, Saing H, Yung R W, Yeung Y P, Tsoi N S. A study of pre-antibiotic bacteriology in 125 patients with necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:45–48. doi: 10.1111/j.1651-2227.1994.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark N C, Hill B C, O'Hara C M, Steingrimsson O, Cooksey R C. Epidemiological typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn Microbiol Infect Dis. 1990;13:467–472. doi: 10.1016/0732-8893(90)90078-a. [DOI] [PubMed] [Google Scholar]
- 9.Farmer J J, III, Asbury M A, Hickman F W, Brenner D J the Enterobacteriaceae Study Group. Enterobacter sakazakii: a new species of Enterobacteriaceae isolated from clinical materials. Int J Syst Bacteriol. 1980;30:568–584. [Google Scholar]
- 10.Farmer J J., III . Enterobacteriaceae: introduction and identification. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 438–449. [Google Scholar]
- 11.Food and Agriculture Organization. Codex Alimentarius: code of hygienic practice for foods for infants and children. CAC/RCP 21–1979. Rome, Italy: Food and Agriculture Organization of the United Nations; 1994. [Google Scholar]
- 12.Gallagher P G, Ball W S. Cerebral infarctions due to CNS infection with Enterobacter sakazakii. Pediatr Radiol. 1991;21:135–136. doi: 10.1007/BF02015629. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez E B, Gimenez C. Septic shock due to Enterobacter sakazakii. Clin Microbiol Newsl. 1982;4:30. [Google Scholar]
- 14.Jø´ker, R. N., T. Nø´rholm, and K. E. Siboni. 1965. A case of neonatal meningitis caused by a yellow Enterobacter. Dan. Med. Bull. 12:128–130. [PubMed]
- 15.Kindle G, Busse A, Kampa D, Meyer-König U, Daschner F D. Killing activity of microwaves in milk. J Hosp Infect. 1996;33:273–278. doi: 10.1016/s0195-6701(96)90013-4. [DOI] [PubMed] [Google Scholar]
- 16.Kleiman M B, Allen S D, Neal P, Reynolds J. Meningoencephalitis and compartmentalization of the cerebral ventricles caused by Enterobacter sakazakii. J Clin Microbiol. 1981;14:352–354. doi: 10.1128/jcm.14.3.352-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosloske A M. Pathogenesis and prevention of necrotizing enterocolitis: a hypothesis based on personal observation and a review of the literature. Pediatrics. 1984;74:1086–1092. [PubMed] [Google Scholar]
- 18.Lucas A, Cole T J. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 19.Monroe P W, Tift W L. Bacteremia associated with Enterobacter sakazakii (yellow-pigmented Enterobacter cloacae) J Clin Microbiol. 1979;10:850–851. doi: 10.1128/jcm.10.6.850-851.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muytjens H L, Kollée L A. Enterobacter sakazakii meningitis in neonates: causative role of formula? Pediatr Infect Dis J. 1990;9:372–373. doi: 10.1097/00006454-199005000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Muytjens H L, Roelofs-Willemse H, Jaspar G H. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol. 1988;26:743–746. doi: 10.1128/jcm.26.4.743-746.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muytjens H L, Zanen H C, Sonderkamp H J, Kollée L A, Kaye Wachsmuth I, Farmer J J., III Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J Clin Microbiol. 1983;18:115–120. doi: 10.1128/jcm.18.1.115-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarowec-White M, Farber J M. Thermal resistance of Enterobacter sakazakii in reconstituted dried infant formula. Lett Appl Microbiol. 1997;24:9–13. doi: 10.1046/j.1472-765x.1997.00328.x. [DOI] [PubMed] [Google Scholar]
- 24.Nazarowec-White M, Farber J M. Phenotypic and genotypic typing of food and clinical isolates of Enterobacter sakazakii. J Med Microbiol. 1999;48:559–567. doi: 10.1099/00222615-48-6-559. [DOI] [PubMed] [Google Scholar]
- 25.Noriega F R, Kotloff K L, Martin M A, Schwalbe R S. Nosocomial bacteremia caused by Enterobacter sakazakii and Leuconostoc mesenteroides resulting from extrinsic contamination of infant formula. Pediatr Infect Dis J. 1990;9:447–449. [PubMed] [Google Scholar]
- 26.Peter C S, Feuerhahn M, Bohnhorst B, Schlaud M, Ziesing S, von der Hardt H, Poets C F. Necrotising enterocolitis: is there a relationship to specific pathogens? Eur J Pediatr. 1999;158:67–70. doi: 10.1007/s004310051012. [DOI] [PubMed] [Google Scholar]
- 27.Simmons B P, Gelfand M S, Haas M, Metts L, Ferguson J. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol. 1989;10:398–401. doi: 10.1086/646060. [DOI] [PubMed] [Google Scholar]
- 28.Urmenyi A M, White-Franklin A. Neonatal death from pigmented coliform infection. Lancet. 1961;i:313–315. doi: 10.1016/s0140-6736(61)91481-7. [DOI] [PubMed] [Google Scholar]
- 29.Walsh M C, Kliegman R M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis J, Robinson J E. Enterobacter sakazakii meningitis in neonates. Pediatr Infect Dis J. 1988;7:196–199. doi: 10.1097/00006454-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Willoughby R E, Pickering L K. Necrotizing enterocolitis and infection. Clin Perinatol. 1994;21:307–315. doi: 10.1016/S0095-5108(18)30347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]