Introduction
Multiple sclerosis (MS) is a leading cause of disability affecting people typically between the ages of 20 and 50 years, with negative impacts on their quality of life. 1 Although medications may reduce the risk of relapses for those with relapsing remitting MS (RRMS), there are limited treatments to slow disability accrued from the progressive subtypes of MS. 2 Measuring disability accrual relies on self-report or the use of clinical measures. Clinical measures for MS suffer from limitations in their ability to detect changes in function that relate to disease progression or intervention responsiveness. For example, the Expanded Disability Status Scale is a widely accepted rater-based categorical measure that provides an overview of disability in people with MS; however, it has limited reliability and sensitivity for detecting small meaningful changes in motor function. 3,4 Walk tests such as the Timed 25-foot Walk and Timed-Up and Go are objective and reliable but only provide quantitative information about a moment in time, limiting the capture of daily (or even hourly) performance fluctuations that may provide an early indication of progression. Self-reported outcomes offer valuable personal perspectives but rely on memory recall, which could be confounded with cognitive changes or depression and anxiety. The heterogeneity of impairments in MS makes it challenging to find an objective outcome measure that reflects a person’s overall disability including daily fluctuations, that can be implemented in a standard way and demonstrates ecological validity. New biophysical markers that can be tailored to a person’s disability, applied in a person’s natural environment, and are simple to apply are greatly needed. Recent studies have turned to the use of motion sensors, such as accelerometers, aiming to develop a new gold standard for quantifying walking mobility.5,6,7
The wearable accelerometer is a non-invasive, objective, and inexpensive technology that records human movement in real-time in a real-world context. Accelerometry data are simple to acquire, making it possible to objectively study physical activity in a wide range of individuals at an unprecedented temporal level (i.e., at minute level) in a person’s free‐living environment.8,9 However, the methods used to analyze accelerometry data often fall well short of the richness of the accelerometry data. Current analysis methods largely rely on aggregated data summaries of either activity intensity or duration of active times defined for activity counts above a certain threshold; the data are often summarized in daily totals10–13 which leads to a loss of detail about diurnal distribution of physical activity over 24-h. Use of aggregated data removes the ability to tailor the accelerometry data to a person’s diurnal profile or to use it as a guide for interventions, ultimately expanding its clinical usefulness.
To better understand how disease progression affects physical activity, it is possible to evaluate data variations through a 24-h period at refined resolutions rather than summing activity counts over a whole day. Use of functional data analysis tools, 14 allows for study of the entire activity profile, capturing unique information from each accelerometry dataset. Over the past decade, with an increasing interest in studying the more complex data structures, new functional data analysis methods have been developed for fast and accurate parameter estimations, expanding the study of imaging data,15,16 accelerometry data, 17–19 and other methods that capture continuous time series observations.
Although using accelerometry to study physical activity in persons with MS has drawn increasing attention, to our knowledge, no studies have yet applied functional data analysis to better understand how diurnal patterns of activity are affected by the levels of disability. Thus, in this study, our primary goal is to use wrist-worn accelerometer data to characterize diurnal patterns of physical activity over 24-h. A comparison of accelerometry with both clinical measures of disability (i.e., walking) and the EDSS, is critical for establishing a reliable biomarker of disability. Our second objective is to compare the traditional linear models that study the single daily summary (24-h aggregated data) to a functional data analysis technique, Function-On-Scalar Regression (FOSR), 19 which can model a diurnal pattern as a functional outcome. Quantification of mobility-specific indices, and detailed minute-by-minute analyses of the accelerometry data will allow us to detect differences in activity among individuals with MS. This work presents an application of FOSR to accelerometery data emphasizing the interpretation of the models for uncovering associations with global disability, walking, and device use.
Methods
This study is a cross-sectional analysis of baseline accelerometry data from two cohorts of individuals with MS, collected between 2016 and 2018 at Johns Hopkins University and the Kennedy Krieger Institute. The goal was to include as many participants as possible, as such participants were included in all analyses if they were diagnosed with relapsing-remitting MS with an EDSS score between 1.0–6.5 (recruitment from the larger of the two cohorts only included individuals with EDSS of 1–2 and 4–6.5), had been stable on immunomodulatory therapy for >6 months (if applicable), had no relapse within 3 months of the study, could follow complex directions, and had no activity restrictions. Participants were excluded from the study if they were not medically stable, had other neurological deficits, cancer, or other diagnoses that prevented them from participating in the study tests, or had plans that would significantly alter their activity during the study ( time off work, vacation, etc.). All participants gave written informed consent prior to participation, and the Institutional Review Boards at Johns Hopkins Medical Institutes and Kennedy Krieger Institute approved all procedures.
The following participant demographics were recorded: sex, age, height, weight, and device use for walking.
Clinical measures
Expanded disability status scale (EDSS).20,21
Timed 25 Foot Walk (T25FW): The average of two trials, reported in seconds, is used in the analysis.22,23
Timed Up and Go (TUG): The second trial, reported in seconds, is used in the analysis. 24,25,26
Accelerometry
Immediately following in-person informed consent the clinical measures were completed and the accelerometer was placed on the participant. Participants wore the Actigraph GTX-9 physical activity monitor for 2 weeks in a free-living environment. The accelerometry devices were registered to measure activity counts in 1-min epochs; only the time of day was visible to the participant. Participants were asked to wear the monitor at all times, including when bathing. The device was worn on the non-dominant wrist.
For processing the collected accelerometery data, we followed previously validated processing pipelines to exclude invalid data and to summarize the activity counts into total physical activity volume. The ActiGraph accelerometery software generated tri-axial activity data, which we then summarized via minute-level activity counts. 27 We further exclude non-valid wearing days following the algorithms described in literature, 28 (1) we excluded time intervals with minute-level zero counts longer than 90 min; two non-zero counts are allowed within each 90 min non-wear window, (2) a day with more than 12 non-wear hours were defined as non-valid and excluded from our analysis. Subjects with less than 3 days of valid wearing data were excluded.
Since the activity count data exhibit high skewedness, taking a log-transformation (i.e., natural log) makes the minute level data follow a more symmetric distribution. Therefore, we applied log (1 + activity counts) transformation to the minute-level activity counts and summed over all 1440 min to obtain the daily total log-transformed activity count (TLAC).17,29,30,31–33,34 We further averaged the daily TLAC across all valid days for each subject. The daily TLAC was used as our measurement to quantify the total volume of activity. The diurnal patterns of physical activity were calculated as follows. First, 30-min binning was performed by averaging log-transformed activity counts within 48 non-overlapping 30-min intervals. Finally, the mean subject-specific diurnal patterns of physical activity were obtained by averaging daily curves over all valid days. Thus, in the main analysis, each mean subject-specific diurnal patterns of physical activity has been represented via 48 30-min log-transformed activity counts.
Statistical analyses
The primary predictor variables in this study include EDSS, T25FW, and TUG, adjusted for scalar covariates of age, body mass index (BMI) and sex. In models studying the walking measurements, the use of an assistive device for walking (Device) was also included as a covariate. We did not add assistive walking device to the EDSS model to avoid bias, as it is incorporated into how the EDSS is scored.
To explore associations between the total daily volume of physical activity and TUG, T25FW, and EDSS, three simple linear regressions were adjusted for age, sex, and BMI and fitted using Ordinary Least Squares. To build some intuition behind functional regression models and bridge a gap between linear and functional regressions, we considered 12 separate linear regressions between physical activity in twelve non-overlapping two-hour intervals representing the 24-h day and TUG, T25FW, and EDSS. For these regressions, we obtained the responses by summing-up minute-level log-transformed activity count within each of the 12 consecutive non-overlapping 2-h time windows. Specifically, we split the 24-h period into 12 intervals, such that the first interval started at 0:01 a.m. and ends at 2:00 a.m. and the second interval started at 2:01 a.m. and ended at 4:00 a.m., etc., we model activity at each time interval with the interested predictors and covariates. Finally, to explore time-of-day specific associations between physical activity and TUG, T25FW and EDSS, three function-on-scalar regression (FOSR) were fitted. FOSR model diurnal activity profiles are treated as functional responses. Thus, FOSR flexibly assesses the time-varying associations between subject-specific diurnal physical activity profile and subject-specific clinical characteristics and demographics. Specifically, after adjusting for age, sex, and BMI, FOSR models the association between the diurnal pattern of physical activity, denoted as , and as follows
Thus, FOSR model (1) estimates the adjusted relationship between the functional outcome and at each 30-min epoch t Assuming smoothness of functional outcomes, FOSR enforces similar smoothness on functional regression parameters for the functional intercept, the functional effect of age, , the functional effect of sex, , the functional effect of BMI, , and the functional effect of EDSS, . Thus, FOSR could be viewed as an extension of the standard scalar linear regression with a flexibility of having a time-varying association between the outcome and the predictor of interest, described by functional or time-varying regression parameter The penalized generalized least square (GLS) method was chosen for estimation and selection of the tuning parameters in FOSR model. 34 To test statistical significance, we used pointwise confidence intervals and assumed 0.05 significance level. All statistical analyses were conducted using R (version 3.6.1). Functional regression models are performed using the refund package (version 0.1.17).
Results
For this study we enrolled 66 individuals with MS. Participants with less than 3 days of valid wearing data were excluded. Based on this criterion, two subjects were excluded due to zero wear time. After processing the accelerometry data following the above algorithms (using R accelerometry package, version 3.1.2), we got 899 valid days for the remaining 64 subjects (14 days per subject). Additionally, four participants with non-functional walking speeds (T25FW ranging from 35-53 s) were removed from all analyses. Table 1 shows the participant summary information for 60 participants (43, 72%, female), ordered by disability, based on EDSS. This study included people with a wide range of disability, from EDSS 1.0–6.5, T25FW (2.89–20 s) and TUG (3.85–41.12 s) with 13 participants requiring a device for the walking tests. The three individuals with an EDSS of 6.5 each used a walker. The average age is 52.84 years and the average BMI score is 26.63 kg/m2. Total log-transformed activity counts (TLAC), are described as a measure of the total volume of physical activity and trends down with the increase in EDSS.
Table 1.
Characteristics of population by EDSS score.
| EDSS | Participants (N) | Assistive device (N) | Age Mean (SD) | BMI Mean (SD) | T25FW Mean (SD) | TUG Mean (SD) | TLAC Mean (SD) |
|---|---|---|---|---|---|---|---|
| 1 | 7 | 0 | 38.86 (13.01) | 26 (5.03) | 3.72 (0.66) | 5.65 (0.64) | 4.71 (0.44) |
| 1.5 | 12 | 0 | 47 (10.22) | 28.02 (6.04) | 3.9 (0.34) | 6.09 (0.8) | 4.39 (0.61) |
| 2 | 7 | 0 | 42.29 (11.31) | 28.35 (5.75) | 4.04 (0.86) | 5.92 (1.07) | 4.73 (0.42) |
| 3 | 2 | 0 | 56 (8.49) | 30.68 (4.18) | 5.15 (0.48) | 7.38 (0.45) | 4.85 (0.32) |
| 3.5 | 3 | 0 | 56 (10.58) | 27.85 (5.61) | 4.59 (0.78) | 7.66 (1.65) | 4.09 (0.4) |
| 4 | 11 | 1 | 49 (13.75) | 27.37 (6.22) | 5.14 (1.24) | 7.65 (1.31) | 4.21 (0.69) |
| 4.5 | 4 | 2 | 48 (5.48) | 26.33 (7.9) | 5.51 (1.54) | 8.52 (3.92) | 4.23 (0.67) |
| 5 | 2 | 0 | 37.5 (9.19) | 28 (8.77) | 7.8 (0.48) | 10.4 (0.88) | 3.78 (0.86) |
| 5.5 | 1 | 0 | 46 (−) | 27.64 (−) | 6.68 (−) | 8.45 (−) | 4.25 (−) |
| 6 | 8 | 7 | 51.62 (11.78) | 32.25 (7.26) | 9.33 (3.6) | 12.77 (4.09) | 4.17 (0.21) |
| 6.5 | 3 | 3 | 58.33 (5.13) | 22.64 (4.46) | 9.88 (2.31) | 15.34 (6.06) | 4.24 (0.48) |
| Total | 60 | 13 | 47.53 (11.73) | 27.96 (6.08) | 5.50 (2.58) | 8.13 (3.56) | 4.36 (0.56) |
Abbreviations: N=sample size for that variable; SD=standard deviation; BMI=body mass index; T25FW= Time (seconds) for The Timed 25-Foot Walk Test; TUG=Time (seconds) for the Timed Up and Go Test; TLAC=Total Log Activity Count.
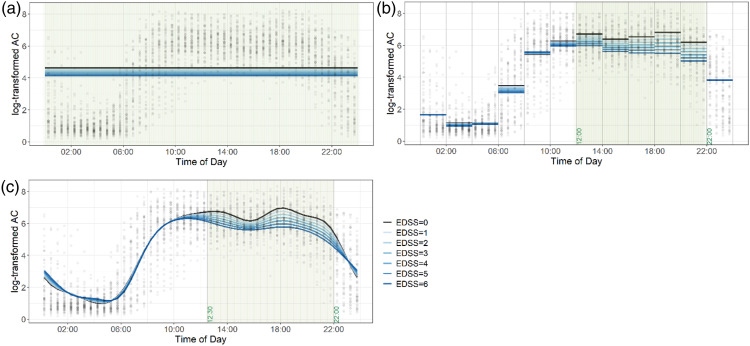
Figures 1(a)–(c) show the diurnal variability of activity of all participants via the “cloud” of black dots and how well this variability is explained via a simple linear regression for the total daily physical activity outcome (Figure 1(a)), 12 linear regressions for two-hour physical activity outcomes (Figure 1(b)), and function-on-scalar regression for the diurnal physical activity outcome (Figure 1(c)). The gray background highlights time periods that are (pointwise for FOSR) statistically significant at 0.05 level of significance. In each Figure, black dots represent participant’s time-of-day specific 30-min TLACs. In Figure 1(a), horizontal lines correspond to the fitted values from simple linear regression presented in the first column of Table 2 with TLAC as the main outcome and EDSS as the main predictor variable. Results from this analysis (i.e., representing different EDSS levels) are overlaid on the individual data (i.e., the “cloud” of dots). Dark blue lines correspond to higher disability (i.e., higher EDSS), and light blue represents lower disability (i.e., dark blue). The shaded region indicates that the EDSS significantly explains variability in physical activity across the 24-h period, with lower EDSS (less disability, light blue) indicative of more activity and higher EDSS (more disability, dark blue) indicative of less activity. This figure clearly shows the limitations of simple linear regression that assumes constant level of activity across the day and results in an inability to capture significant diurnal variability of physical activity.
Figure 1.
Scatter plot showing diurnal physical activity of all participants, overlaid with regression results for the EDSS as the main predictor variable. Each black dot represents the average log-transformed activity count over every 30-min, across 24-h days for an individual participant. 1(a). Results from a simple linear regression for the total daily physical activity outcome. 1(b). Results from twelve linear regressions for the two-hour physical activity outcome. 1(c). Results from the function-on-scalar regression for the diurnal physical activity outcome. The gray background highlights time periods that are statistically significant at 0.05 level of significance.
Table 2.
Results of 24-h TLAC linear models.
| EDSS model R^2 = 0.167 | T25FW model R^2 = 0.146 | TUG model R^2 = 0.130 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| — | Coefficient (95% CI | p-value | var.R^2 | — | Coefficient (95% CI | p-value | var.R^2 | — | Coefficient (95% CI | p-value | var.R^2 |
| EDSS | −0.08 (–0.16, 0) | 0.044 | 0.072 | T25FW | −0.04 (−0.12, 0.03) | 0.249 | 0.024 | TUG | −0.01 (−0.06, 0.03) | 0.557 | 0.006 |
| Age | −0.01 (−0.02, 0) | 0.236 | 0.025 | Age | −0.01 (−0.02, 0) | 0.137 | 0.040 | Age | −0.01 (−0.02, 0) | 0.162 | 0.036 |
| Female | 0.01 (−0.29, 0.32) | 0.932 | 0.000 | Female | −0.01 (−0.33, 0.3) | 0.93 | 0.000 | Female | −0.02 (−0.34, 0.3) | 0.907 | 0.000 |
| BMI | −0.02 (−0.04, 0.01) | 0.137 | 0.040 | BMI | −0.02 (−0.04, 0.01) | 0.166 | 0.035 | BMI | −0.02 (−0.04, 0.01) | 0.142 | 0.040 |
| — | — | — | — | Device | −0.03 (−0.49, 0.43) | 0.896 | 0.000 | Device | −0.13 (−0.56, 0.29) | 0.531 | 0.007 |
Abbreviations: EDSS= Expanded Disability Status Scale; BMI= Body Mass Index; T25FW= Time (seconds) for The Timed 25-Foot Walk Test; TUG=Time (seconds) for the Timed Up and Go Test.
Tables 3–5 report the R-squares and p-values for the significant time intervals identified by the 2-h linear models which when compared to the time windows identified by the FOSR modelling show consistency but also illustrate arbitrarily selecting time intervals limits the ability to fully identify significant periods of time such as nighttime hours, as seen in this sample.
Table 3.
Linear model results of EDSS over 2-h intervals.
| time.interval | EDSS Coefficient (95% CI) | p-value | var.R^2 a | model.R^2 |
|---|---|---|---|---|
| 00:00–02:00 | 0.002 (−0.18, 0.18) | 0.983 | 0.038 | 0.118 |
| 02:00–04:00 | −0.031 (−0.13, 0.07) | 0.533 | 0.022 | 0.104 |
| 04:00–06:00 | −0.008 (−0.1, 0.09) | 0.871 | −0.073 | 0.017 |
| 06:00–08:00 | −0.074 (−0.32, 0.17) | 0.544 | −0.058 | 0.031 |
| 08:00–10:00 | 0.021 (−0.19, 0.23) | 0.837 | −0.018 | 0.067 |
| 10:00–12:00 | −0.048 (−0.19, 0.09) | 0.496 | −0.003 | 0.081 |
| 12:00–14:00 | −0.121 (-0.23, –0.01) | 0.028 | 0.078 | 0.155 |
| 14:00–16:00 | −0.127 (-0.24, –0.02) | 0.024 | 0.074 | 0.152 |
| 16:00–18:00 | −0.167 (-0.27, –0.07) | 0.00138 | 0.29 | 0.35 |
| 18:00–20:00 | −0.219 (-0.34, –0.1) | 0.0005 | 0.267 | 0.329 |
| 20:00–22:00 | −0.196 (-0.36, –0.03) | 0.021 | 0.131 | 0.204 |
| 22:00–24:00 | −0.004 (-0.23, 0.23) | 0.975 | 0 | 0.084 |
avar.R^2 indicates R^2 value for EDSS 2-h model; Abbreviations: EDSS= Expanded Disability Status Scale.
Table 5.
Linear model results of TUG over 2-h intervals.
| time.interval | TUG Coefficient (95% CI) | p-value | var.R^2 a | model.R^2 |
|---|---|---|---|---|
| 00:00–02:00 | 0.092 (−0.01, 0.2) | 0.089 | 0.089 | 0.166 |
| 02:00–04:00 | 0.007 (−0.05, 0.07) | 0.817 | 0.016 | 0.099 |
| 04:00–06:00 | −0.017 (−0.07, 0.04) | 0.56 | −0.068 | 0.023 |
| 06:00–08:00 | −0.152 (-0.29, -0.01) | 0.036 | 0.02 | 0.103 |
| 08:00–10:00 | −0.049 (−0.18, 0.08) | 0.435 | −0.008 | 0.077 |
| 10:00–12:00 | −0.018 (−0.1, 0.07) | 0.678 | −0.004 | 0.081 |
| 12:00–14:00 | −0.013 (−0.08, 0.05) | 0.706 | 0.034 | 0.116 |
| 14:00–16:00 | −0.074 (-0.14, -0.01) | 0.031 | 0.071 | 0.149 |
| 16:00–18:00 | −0.013 (−0.08, 0.05) | 0.675 | 0.217 | 0.283 |
| 18:00–20:00 | −0.059 (−0.14, 0.02) | 0.129 | 0.188 | 0.257 |
| 20:00–22:00 | −0.007 (−0.11, 0.1) | 0.89 | 0.077 | 0.155 |
| 22:00–24:00 | 0.130 (−0.01, 0.27) | 0.061 | 0.064 | 0.143 |
avar.R^2 indicates R^2 value for TUG 2-h model. TUG=Time (seconds) for the Timed Up and Go Test.
Figure 1(b) and Table 3 show the relationship between EDSS and physical activity for each of the twelve 2-h increments. As can be seen from the figure, EDSS explains very different amounts of the physical activity variability (i.e., the “cloud”) across different times of the day. From 12:00–22:00, participants with greater disability (dark blue) have significantly lower activity then participants with less disability (light blue). By contrast, overnight and earlier in the day, the EDSS does not significantly distinguish among participants with higher or lower disability (dark blue and light blue lines overlap). The shaded regions, 12:00–22:00 indicate times when the EDSS significantly explains the variability in physical activity.
Finally, Figure 1(c) shows the results of the FOSR model with EDSS as the main predictor. The main advantage of this model is that it does not require any pre-specification of time intervals and adapt to the data. Thus, as it can be seen at the figure, it results in more flexible and less restrictive determination of the times of the day when EDSS best explains the diurnal variability of physical activity. Using the FOSR model, the EDSS significantly explains variability in activity from 12:00–22:00; our model shows that participants with greater disability (dark blue) are less physically active during these times.
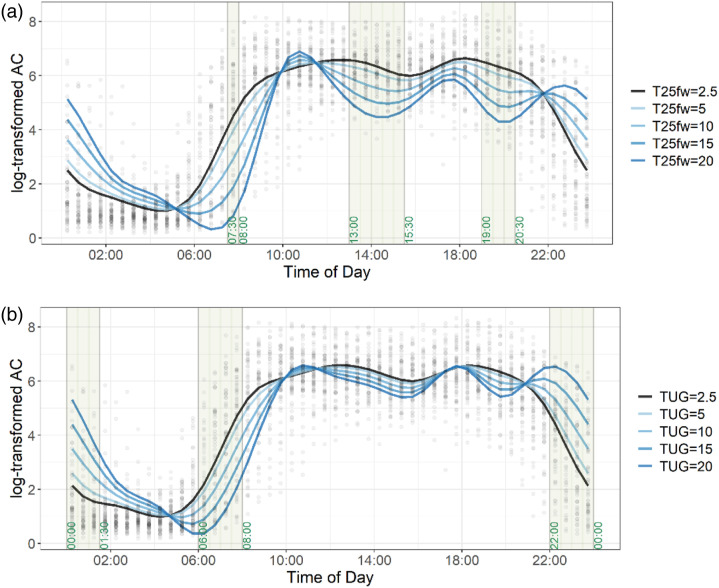
Tables 3 and 4 show the results for the 12 regression models with 2-h physical activity outcomes and T25FW and TUG as the predictors, respectively. In Figure 2, we plot results for the FOSR model for T25FW (Figure 2(a)) and for TUG (Figure 2(b)), respectively. The FOSR fits display diurnal variability of physical activity corresponding to the different levels of performance on these walking tests (dark blue lines indicate longer times). The model for T25FW (Figure 2(a)) indicates the importance of three specific times (highlighted), early morning 7:30–08:00, mid-afternoon 13:00–15:30, and early evening 19:00–20:30 when the T25FW significantly explains variability in physical activity (p < 0.01). During these times, slower walking is significantly associated with lower physical activity. In the last FOSR model (Figure 2(b)), TUG is significantly associated with activity during the night 22:00–01:30 and early morning 06:00–08:00 h and is not significantly associated with physical activity during other times. Specifically, between 22:00 and 01:30 participants with slower walking times were more active than participants with faster walking time (lighter blue). By contrast, there are times that do not distinguish among participants with slower walking. For example, in Figure 2(a) and (b)10:00–12:00 h show dark blue (slower walkers) and light blue (faster walkers) largely overlap.
Table 4.
Linear model results of T25FW over 2-h intervals.
| time.interval | T25FW Coefficient (95% CI) | p-value | var.R^2 a | model.R^2 |
|---|---|---|---|---|
| 00:00–02:00 | 0.065 (−0.1, 0.23) | 0.432 | 0.05 | 0.13 |
| 02:00–04:00 | −0.002 (−0.09, 0.09) | 0.973 | 0.015 | 0.098 |
| 04:00–06:00 | −0.001 (−0.09, 0.09) | 0.981 | −0.074 | 0.016 |
| 06:00–08:00 | −0.203 (−0.42, 0.01) | 0.065 | 0.002 | 0.087 |
| 08:00–10:00 | −0.053 (−0.24, 0.14) | 0.575 | −0.014 | 0.072 |
| 10:00–12:00 | −0.010 (−0.14, 0.12) | 0.882 | −0.007 | 0.078 |
| 12:00–14:00 | −0.038 (−0.14, 0.06) | 0.45 | 0.042 | 0.123 |
| 14:00–16:00 | −0.126 (-0.23, –0.03) | 0.014 | 0.094 | 0.17 |
| 16:00–18:00 | −0.025 (−0.12, 0.07) | 0.602 | 0.218 | 0.284 |
| 18:00–20:00 | −0.105 (−0.22, 0.01) | 0.071 | 0.203 | 0.27 |
| 20:00–22:00 | −0.086 (−0.24, 0.07) | 0.267 | 0.098 | 0.174 |
| 22:00–24:00 | 0.075 (−0.14, 0.28) | 0.479 | 0.009 | 0.093 |
avar.R^2 indicates R^2 value for T25FW 2-h model.; Abbreviations, T25FW= Time (seconds) for The Timed 25-Foot Walk Test.
Figure 2.
Scatter plot showing diurnal physical activity of all participants, overlaid with regression results for each walking measures as the main predictor variable. Each black dot represents the average log transformed activity count over every 30-min, across 24-h days for an individual participant. 2(a). Results from the function-on-scalar regression for the diurnal physical activity outcome, with T25FW as the main predictor variable. 2(b). Results from the function-on-scalar regression for the diurnal physical activity outcome, with TUG as the main predictor variable. The gray background highlights time periods that are statistically significant at 0.05 level of significance for the predictor variable.
Discussion
Everyday physical activity measured with accelerometry may provide a sensitive near real-time modality for tracking MS disease severity. In this study, we examined the objective mobility measurements of accelerometry in persons with MS by looking at the 24-h activity patterns in comparison to the total daily physical activity. The accelerometry-measured physical activity showed significant diurnal variability across 24-h, a phenomenon that has not been previously characterized in MS. By using time-of-day specific linear models as well as modeling with FOSR, we identified the unique relationships between physical activity at specific times of day and global disability (i.e., EDSS) and measures of walking (i.e., T25FW and TUG). The EDSS is highly correlated with physical activity between the hours of 12:00 and 22:00. The timed walking tests correlate better with morning and evening activity. By comparing the models at different temporal resolution, expanding the time span of the data has revealed the complexity of physical activity throughout the day. For example, activity is not simply decreased across the entire 24-h period in individuals who have more severe disease. Analyzing the activity profiles in relation to the disability in MS provides novel insights to time periods that may be targeted to better track the progression of MS or more precisely estimate the treatment effect in clinical trials and can also be used for more accurate clinical guidance about physical activity to individuals with MS.
Daytime activity in this MS sample shows a high correlation with the EDSS, our measure of disease severity. The most significant period is from 12:00 to 22:00-h, not in the morning hours or overnight. This is an important and novel observation; since the EDSS is considered the gold standard for measuring global disability and progression in MS, we might assume that this would consistently relate to activity throughout the day. However, our data show that people across the disability spectrum have similar levels of morning activity, presumably taking care of essential tasks of daily living (e.g., getting up, getting out of bed, and eating breakfast). Also, it is not uncommon for people with MS to report that fatigue limits them later in the day, or that tasks are completed more slowly later in the day. The time period between 12:00 and 22:00-h is likely reflective of a sum of worsening neurological symptoms, weakness, sensory loss, walking endurance, ataxia, cognitive changes, etc. that is often described as global disability
It is interesting that clinical tests of walking and balance give us information beyond the afternoon hours. Different from the EDSS, which focuses on global measures of disability, walking, and balance tests relate more specifically to functional capacity. Capacity reflects a person’s ability to complete a task. 35 Timed walking measures, such as the T25FW and TUG give us a measure of capacity, as they focus on speed, not endurance. Earlier and greater participation (i.e., physical activity) in activities of daily living in the morning hours (06:00–08:00) correlates with faster timed walking. These measures, in contrast, correlate with less rest at night (i.e., more physical activity). Additionally, each walking measure gives us information about the relationship of 24-h activity patterns in MS and functional capacity. The TUG correlates with less rest at night (i.e., more physical activity). The T25FW shows that while all disability levels reach a similar activity level at 10:00, there are two times (13:00 and 19:00) in the afternoon hours when physical activity more greatly diverges. We know that a person with greater disability (slower walking) has lower overall activity at these times. However, the data from this study show that at night, people are more active if they are slower walkers. This could reflect that the daytime activity of slower walkers is quite low and thus it does not take much activity at night to be notable. Another possibility for this is that individuals who are slower walkers have additional symptoms that are either not captured in the EDSS and/or they may not sleep as well ( restless legs, spasticity, nocturia, etc.), perhaps reflecting spinal cord pathology. Another explanation has been described in animal models, which show that greater use of leg muscles during the day can affect sleep at night. 36 Although the mechanism is unclear it is important to consider that sleep impairment may contribute. We know that individuals with MS are less physically active than other healthy individuals their age. 37 Both circadian rhythm disturbance and sleep impairment occur in MS and both may be associated with disease worsening.38–40 Circadian rhythm abnormalities are commonly linked with sleep disturbance, we know that sleep disturbance affects nearly 50% of people with MS.41–43 Furthermore, while fatigue in MS is multifactorial, sleep impairment is a primary contributor to its severity and previous studies have suggested a link between higher fatigue levels and subsequent brain atrophy in people with MS. 44 The mechanism for the finding that slower walking speed is related to less rest at night (i.e., more activity at a time when rest is important) requires further study. The use of participant reported outcomes in future work may offer needed insights into strategies people may use to control fatigue and other symptoms.
Accelerometry
Body-worn accelerometers offer an opportunity to study physical activity in individuals in their free-living environment. However, assessing the immense data that is captured continuously using accelerometry is challenging. A variety of methods have been used. Some studies apply total activity counts which sums the magnitude of the one-minute activity counts, or the TLAC 29 while others adopt summary metrics to measure specific information of interest. For instance, Di et al., 11 used various accelerometry-derived measures such as the total volume of sedentary behavior, intra-daily variability, and relative amplitude, to study joint and individual associations between physical activity, sleep and circadian rhythm, and gait speed. For the past few years, studies using accelerometry in MS have primarily focused on aggregated data summaries using primary outcome measures such as counts per minute summarized over 24 hours45–47 or maximal walking distance. 48 However, as shown in our results, use of only aggregate data summaries misses much of the rich information from activity fluctuations that can be quantified when we directly evaluate physical activity patterns.
Accelerometry also offers the potential for improving clinical recommendations and offers information that could be used for designing tailored rehabilitation interventions. We know that increased physical activity relates to better quality of life in healthy and MS populations. Current conventional practice is to simply provide guidelines for “ideal” activity levels and motivate patients with MS to do more. However, this study shows that there are particular times of the day when activity is lower for people with MS at differing levels of disability. And the data show, EDSS nor timed walking, explain broad variability in physical activity from 08:30 to 12:30, making this time period interesting for further study. This analysis therefore offers key information useful for designing more specific targeted interventions offering insight into times of day when activity is particularly lacking, for identifying poor sleep patterns and a quantitative way to measure change.
Lack of effective statistical tools to study accelerometry data could be one of the reasons that activity profiles have not been previously analyzed. In this study, we have introduced two possible methods which could utilize the data at a refined resolution to evaluate physical activity patterns with other covariates. One approach is to perform multiple linear regression models on activity count summaries over a specified time span across the whole day. In this study, we expanded the data resolution to 2-h intervals and have identified significant periods through the day that related specifically to each of the clinic measures of interest. However, the issue with performing multiple linear regression models over disjoint time intervals is that we lose information at the boundary of the neighboring intervals. To account for the time-to-time covariances, we further applied FOSR modeling that simultaneously evaluates the associations between physical activity and the clinic measurements using the whole dataset. By expanding the resolution of the physical activity data, we are able to show the extent to which MS disease severity is reflected in daytime activity and sleep. On average, individuals with MS show a pattern of lower activity during the night with activity ramping up through the morning and sustained higher activity in the afternoon and declining activity in the evening. This is perhaps only the first step for better understanding the rich data collected using accelerometry. Alternative approaches could be used to better distinguish between types of activity. For example, one could identify walking, and other common daily activities, summarizing each using key metrics such as time spent in each activity and quality of walking (quantified using accelerometry-derived gait parameters), then relate those summaries to the disease severity and functional status of individuals with MS. This type of work is needed for future research.
Strengths and limitations
There are strengths and limitations from this study that are important to consider. Strengths of this study include the use of accelerometry data and novel statistical methods for data analysis. Accelerometry allows for the quantification and tracking of subtle symptomatic changes that may fluctuate over time and otherwise may be missed or ignored by participant memory. Accelerometry provides a potential tool available to the person with MS with a broad range of disability, their caregivers or physician to objectively monitor their activity patterns, which may be useful for early detection of disease changes. The use of FOSR modeling allows for the identification of particular times to focus on physical activity this may be useful for clinical trials or devising times to participate in activities. A limitation of this study is that it is only a cross-sectional analysis on a relatively small sample, thus limiting our knowledge of the directionality and magnitude of the relationships discovered. Consequently, we are unable to make conclusions about changes in accelerometry measures with longitudinal disease progression. We are currently collecting longitudinal data and will apply similar analyses. We limited our analysis to individuals with moderate disability; however, the analysis used here could provide critical new information about less active behavior during specific times of the day in individuals with more severe disability. Given the small sample size we did not look at other individual factors that are known to contribute to physical activity such as medication effects, disease duration, other comorbidities, employment status, seasonal activity changes, or living environment; all of which would be important considerations for future investigations. The inclusion of a non-impaired control group would also be important. As with all accelerometry studies, without a current standard method to evaluate activity it is difficult to compare the data from this study with other studies. An important step would be to establish universal consensus on data analyses for accelerometry where evaluating activity patterns may contribute greatly.
Conclusion
Using novel data analytical methods, we used the readily available temporal resolution and richness of 24-h accelerometry data to associate MS disability and diurnal 24-h patterns of physical activity. Our analysis using function-on-scalar modelling technique provides a deeper understanding of disability manifestation through a discovery of functionally meaningful insights extracted from 24-h accelerometry data.
Acknowledgments
We thank the participants and their families for their generous offering of time and commitment to this project.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sanofi Genzyme, Investigator initiated pilot grant, Johns Hopkins Institute for Data Intensive Engineering and Science Seed Funding Program, and Seed Funding Award.
ORCID iDs
Fan Tian https://orcid.org/0000-0003-2117-3659
Kathryn C. Fitzgerald https://orcid.org/0000-0003-3137-0322
Kathleen M. Zackowski https://orcid.org/0000-0002-2399-5704
References
- 1.Compston A, Coles A. Multiple sclerosis. The Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol 2015; 14: 194–207. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444. [DOI] [PubMed] [Google Scholar]
- 4.Baldassari LE, Salter AR, Longbrake EE, et al. Streamlined EDSS for use in multiple sclerosis clinical practice: development and cross-sectional comparison to EDSS. Mult Scler J 2018; 24: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 5.Pearson OR, Busse ME, van Deursen RWM, et al. Quantification of walking mobility in neurological disorders. QJM 2004; 97: 463–475. [DOI] [PubMed] [Google Scholar]
- 6.Saint-Maurice PF, Troiano RP, Bassett DR, et al. Association of daily step count and step intensity with mortality among US adults. JAMA 2020; 323: 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block VJ, Bove R, Zhao C, et al. Association of continuous assessment of step count by remote monitoring with disability progression among adults with multiple sclerosis. JAMA Netw Open 2019; 2: e190570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey B, Coote S, Donnelly A. Objective physical activity measurement in people with multiple sclerosis: a review of the literature. Disabil Rehabil Assistive Tech 2018; 13: 124–131. [DOI] [PubMed] [Google Scholar]
- 9.Sparaco M, Lavorgna L, Conforti R, et al. The role of wearable devices in multiple sclerosis. Mult Sclerosis International 2018; 2018: 7627643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karas M, Bai J, Strączkiewicz M, et al. Accelerometry data in health research: challenges and opportunities. Stat Biosciences 2019; 11: 210–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di J, Spira A, Bai J, et al. Joint and individual representation of domains of physical activity, sleep, and circadian rhythmicity. Stat Biosciences 1–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair 2009; 23: 108–116. [DOI] [PubMed] [Google Scholar]
- 13.Sandroff BM, Motl RW. Comparison of actigraph activity monitors in persons with multiple sclerosis and controls. Disabil Rehabil 2013; 35: 725–731. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay JO, Silverman BW. Functional Data Analysis, 2005, New York, NY: Springer-Verlag. [Google Scholar]
- 15.Zipunnikov V, Caffo B, Yousem DM, et al. Multilevel functional principal component analysis for high-dimensional data. J Comput Graphical Stat 2011; 20: 852–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith J, Greven S, Crainiceanu C. Corrected confidence bands for functional data using principal components. Biometrics 2013; 69: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Huang L, Schrack JA, et al. Quantifying the lifetime circadian rhythm of physical activity: a covariate-dependent functional approach. Biostatistics 2015; 16: 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shou H, Zipunnikov V, Crainiceanu CM, et al. Structured functional principal component analysis. Biometrics 2015; 71: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith J, Zipunnikov V, Schrack J. Generalized multilevel function-on-scalar regression and principal component analysis. Biometrics 2015; 71: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014; 14: 58–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 2000; 123: 1027–1040. [DOI] [PubMed] [Google Scholar]
- 22.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler J 2012; 18: 914–924. [DOI] [PubMed] [Google Scholar]
- 23.Rosti-Otajärvi E, Hämäläinen P, Koivisto K, et al. The reliability of the MSFC and its components. Acta Neurol Scand 2008; 117: 421–427. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil 2006; 28: 789–795. [DOI] [PubMed] [Google Scholar]
- 26.Nilsagard Y, Lundholm C, Gunnarsson L-G, et al. Clinical relevance using timed walk tests and ‘timed up and go’ testing in persons with multiple sclerosis. Physiother Res Int 2007; 12: 105–114. [DOI] [PubMed] [Google Scholar]
- 27.Wolff-Hughes DL, Bassett DR, Churilla JR, et al. Waist-worn actigraphy: population-referenced percentiles for total activity counts in U.S. adults. J Phys Activity Health 2015; 12: 447–453. [DOI] [PubMed] [Google Scholar]
- 28.Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011; 43: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varma VR, Watts A. Daily physical activity patterns during the early stage of alzheimer’s disease. J Alzheimer’s Disease : JAD 2017; 55: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. Journals Gerontol Ser A: Biol Sci Med Sci 2014; 69: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varma VR, Dey D, Leroux A, et al. Total volume of physical activity: TAC, TLAC or TAC( λ ). Prev Med 2018; 106: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma VR, Dey D, Leroux A, et al. Re-evaluating the effect of age on physical activity over the lifespan. Prev Med 2017; 101: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JS, Carroll RJ. Wavelet-based functional mixed models. J R Stat Soc Ser B (Statistical Methodology) 2006; 68: 179–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JS. Comparison and contrast of two general functional regression modelling frameworks. Stat Model 2017; 17: 59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cesari M, Araujo de Carvalho I, Thiyagarajan JA, et al. Evidence for the domains supporting the construct of intrinsic capacity. The Journals Gerontol Ser A 2018; 73: 1653–1660. [DOI] [PubMed] [Google Scholar]
- 36.Ehlen JC, Brager AJ, Baggs J, et al. Bmal1 function in skeletal muscle regulates sleep. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler J 2005; 11: 459–463. [DOI] [PubMed] [Google Scholar]
- 38.Hedström A, Åkerstedt T, Olsson T, et al. Shift work influences multiple sclerosis risk. Mult Scler J 2015; 21: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 39.Hedström AK, Åkerstedt T, Hillert J, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol 2011; 70: 733–741. [DOI] [PubMed] [Google Scholar]
- 40.Farez MF, Mascanfroni ID, Méndez-Huergo SP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015; 162: 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veauthier C, Gaede G, Radbruch H, et al. Sleep disorders reduce health-related quality of life in multiple sclerosis (nottingham health profile data in patients with multiple sclerosis). Int J Mol Sci 2015; 16: 16514–16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veauthier C. Sleep disorders in multiple sclerosis. review. Curr Neurol Neurosci Rep 2015; 15: 21. [DOI] [PubMed] [Google Scholar]
- 43.Brass SD, Li CS, Auerbach S. The underdiagnosis of sleep disorders in patients with multiple sclerosis. J Clinical Sleep Medicine : JCSM : Official Publication Am Acad Sleep Med 2014; 10: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler J 2006; 12: 481–486. [DOI] [PubMed] [Google Scholar]
- 45.Motl RW, McAuley E, Sandroff BM. Longitudinal change in physical activity and its correlates in relapsing-remitting multiple sclerosis. Phys Therapy 2013; 93: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 46.Sandroff BM, Motl RW, Pilutti LA, et al. Accuracy of stepwatch and actigraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS ONE 2014; 9: e93511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebastião E, Learmonth YC, Motl RW. Lower physical activity in persons with multiple sclerosis at increased fall risk. Am J Phys Med Rehabil 2017; 96: 357–361. [DOI] [PubMed] [Google Scholar]
- 48.Dalla-Costa G, Radaelli M, Maida S, et al. Smart watch, smarter EDSS: improving disability assessment in multiple sclerosis clinical practice. J Neurol Sci 2017; 383: 166–168. [DOI] [PubMed] [Google Scholar]