Abstract
Background:
The association between oral nutritional supplement use and nutritional parameters among patients with nondialysis chronic kidney disease (CKD-ND) with or at high risk of undernutrition/protein-energy wasting has not been previously studied. The definition of patient subgroups most likely to benefit from oral nutritional supplementation (ONS) is also an area where more research is needed.
Objective:
To assess nutritional parameter trajectories among patients with CKD-ND prescribed oral nutritional supplements in British Columbia, and to compare trajectories by nutritional phenotype.
Design:
Longitudinal cohort study, pre-post design.
Setting:
Multidisciplinary CKD clinics across British Columbia.
Patients:
A total of 3957 adult patients with CKD-ND, who entered multidisciplinary CKD clinics during 2010 to 2019, met criteria for oral nutritional supplement prescription based on dietitian assessment, and received ≥1 oral nutritional supplement prescription.
Measurements:
Longitudinal nutritional parameters, including body mass index (BMI), serum albumin, serum bicarbonate, serum phosphate, and neutrophil-to-lymphocyte ratio (NLR).
Methods:
Using linear mixed models, slopes for nutritional and inflammation parameters were assessed in the 2-year periods before and after the first oral nutritional supplement prescription. Hierarchical cluster analysis was applied to identify nutritional phenotypes using baseline data, and slope analysis was repeated by cluster.
Results:
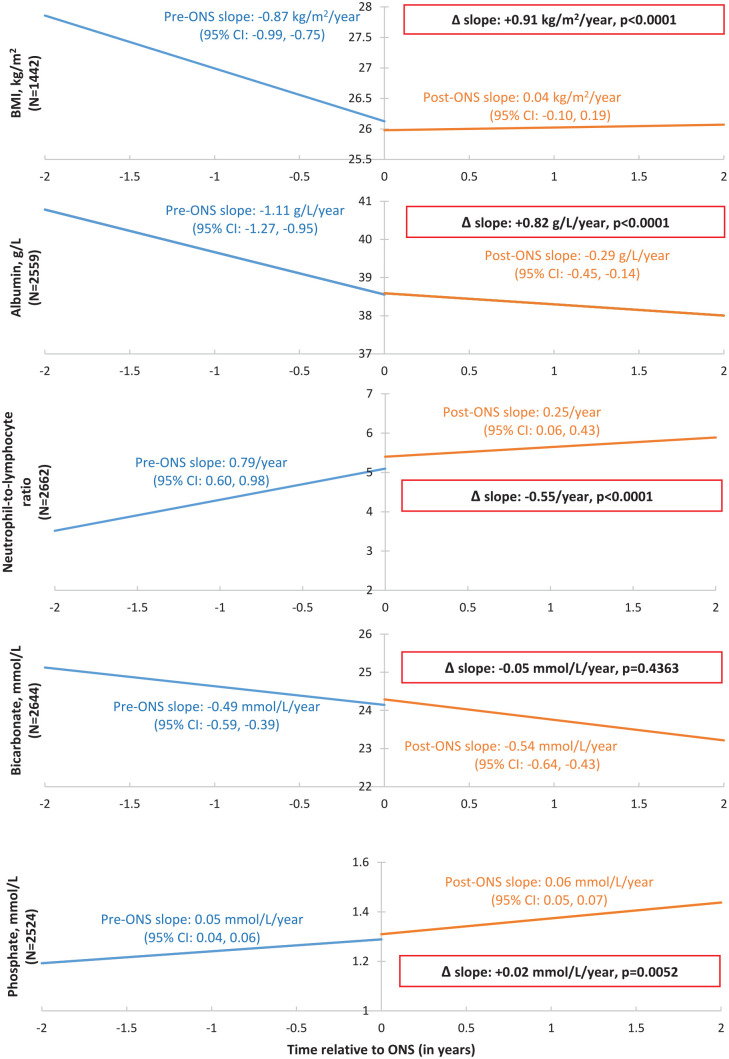
In the pre-oral-nutritional-supplement period, declines in BMI (−0.87 kg/m2/year, 95% confidence interval [CI]: −0.99 to −0.75), albumin (−1.11 g/L/year, 95% CI: −1.27 to −0.95), and bicarbonate (−0.49 mmol/L/year; 95% CI: −0.59 to −0.39), and increases in NLR (+0.79/year; 95% CI: 0.60 to 0.98) and phosphate (+0.05 mmol/L/year; 95% CI: 0.04 to 0.06) were observed. Following oral nutritional supplement prescription, there were statistically significant increases in BMI slope (+0.91 kg/m2/year, P < .0001), albumin slope (+0.82 g/L/year, P < .0001), and phosphate slope (+0.02 mmol/L/year, P = .005), as well as a decline in NLR slope of −0.55/year (P < .0001). There was no significant change in bicarbonate slope. Cluster analysis identified 5 distinct phenotypes. The cluster with the highest mean baseline NLR and lowest mean BMI demonstrated the greatest number of improvements in nutritional parameter slopes in the post-oral-nutritional-supplement period.
Limitations:
Possibility of residual confounding. Data on dietary intake, muscle mass, and nutritional scoring systems were not available in the registry.
Conclusions:
Among patients with CKD-ND prescribed oral nutritional supplements, there were improvements in nutrition/inflammation parameters over time following the first ONS prescription. The heterogeneity in response to ONS by cluster subgroup suggests an individualized approach to nutritional management may be beneficial.
Keywords: malnutrition, protein-energy wasting, oral nutritional supplement, nutritional trajectories, chronic kidney disease
Abrégé
Contexte:
L’association entre la prise de suppléments nutritionnels par voie orale (SNO) et les paramètres nutritionnels n’a jamais été étudiée chez les patients atteints d’insuffisance rénale chronique non dialysés (IRC-ND) présentant un risque élevé de sous-alimentation/dénutrition protéino-énergétique. Des recherches sont également nécessaires pour définir les sous-groupes de patients les plus susceptibles de bénéficier d’une supplémentation par voie orale.
Objectifs:
Évaluer les trajectoires des paramètres nutritionnels des patients atteints d’IRC-ND sous ordonnance de SNO en Colombie-Britannique, puis comparer ces trajectoires selon le phénotype nutritionnel.
Conception:
Étude de cohorte longitudinale avec devis pré-post.
Cadre:
Cliniques multidisciplinaires d’IRC en Colombie-Britannique
Sujets:
3 957 patients adultes IRC-ND ayant fréquenté les cliniques multidisciplinaires d’IRC entre 2010 et 2019, ayant satisfait au critère de prescription de SNO après évaluation par une diététicienne et ayant reçu au moins une ordonnance de SNO.
Mesures:
Paramètres nutritionnels longitudinaux : indice de masse corporelle (IMC), albumine sérique, bicarbonate sérique, phosphate sérique et rapport neutrophiles/lymphocytes (RNL)
Méthodologie:
Des modèles linéaires mixtes ont servi à évaluer les courbes des paramètres nutritionnels et inflammatoires pour des périodes de deux ans précédant et suivant la première prescription de SNO. Une analyse de classification hiérarchique a servi à établir les phénotypes nutritionnels grâce aux données initiales, puis l’analyse de la courbe a été répétée par classe.
Résultats:
Au cours de la période précédant la prescription de SNO, on a observé une réduction de l’IMC (-0,87 kg/m2/année; IC 95 % : -0,99 à -0,75), du taux d’albumine (-1,11 g/L/année; IC 95 % : -1,27 à -0,95) et du taux de bicarbonate (-0,49 mmol/L/année; IC 95 % : -0,59 à -0,39), et une hausse du RNL (+0,79/année; IC 95 % : 0,60 à 0,98) et du taux de phosphate (+0,05 mmol/L/année; IC 95 % : 0,04 à 0,06). Après la prescription d’un SNO, on a noté une hausse statistiquement significative de la courbe de l’IMC (+0,91 kg/m2/année; p<0,0001), de la courbe de l’albumine (+0,82 g/L/année; p<0,0001) et de la courbe du phosphate (+0,02 mmol/L/an; p=0,005), ainsi qu’une réduction de la courbe du RNL de -0,55/année (p<0,0001). Aucun changement significatif n’a été observé pour la courbe du bicarbonate. L’analyse de classification hiérarchique a permis de dégager cinq phénotypes distincts. Dans la période suivant la prescription de SNO, les classes ayant montré le plus grand nombre d’améliorations sont celles qui présentaient le RNL moyen le plus élevé et l’IMC moyen le plus bas au début de l’étude.
Limites:
Possibilité de confusion résiduelle. Les données sur l’apport alimentaire, la masse musculaire et les systèmes de notation de la qualité nutritionnelle n’étaient pas disponibles dans le registre.
Conclusion:
Des améliorations ont été observées au fil du temps dans les paramètres nutritionnels et inflammatoires des patients atteints d’IRC-ND après une première ordonnance de SNO. L’hétérogénéité de la réponse aux SNO dans les sous-groupes de patients suggère qu’une approche individualisée de la gestion nutritionnelle des patients serait bénéfique.
Introduction
Malnutrition and protein-energy wasting (PEW) are complications of advanced kidney disease associated with increased risk of frailty, cardiovascular disease, infectious complications, and mortality.1-6 The prevalence of PEW among patients with nondialysis chronic kidney disease (CKD-ND) ranges from 11% to 54%. 3 ,7-11 Longitudinal cohort studies have demonstrated declines in weight and body mass index (BMI) after estimated glomerular filtration rate (eGFR) declined below 35 ml/min/1.73 m2, and those with >5% weight loss per year prior to starting dialysis were at higher risk of mortality following dialysis initiation. 12 Thus, malnutrition, if amenable to intervention, may be a potentially modifiable determinant of the high mortality rate observed in the early period following dialysis initiation. 13 Moreover, nutritional status tends to be at its lowest at the time of dialysis initiation, and this baseline nutritional status is the most important predictor of nutritional parameter levels in the year following dialysis initiation.14,15 These previous findings highlight the potential importance of managing malnutrition and PEW prior to end-stage kidney disease. International guidelines recommend oral nutritional supplementation (ONS) for treatment of malnutrition and PEW in patients with CKD if intake remains inadequate despite dietary counseling.16-18 However, previous studies have focused on efficacy of ONS among patients on dialysis, 19 while ONS use among patients with CKD-ND with or at high risk of malnutrition and PEW has not been previously studied.
In British Columbia (BC), a government-funded Nutritional Supplement Policy under the direction of renal dietitians guides ONS prescription for patients with CKD, regardless of eGFR, who meet criteria based on degree of weight loss or inadequate nutrient intake. 20 To date, this is the only universal coverage program for ONS that is standardized across a provincial program, and therefore, it is of interest to study its implementation from a stewardship perspective. Based on the premise of “precision nutrition,” 21 responses to nutritional supplementation may also differ substantially among individuals. Therefore, we aimed to assess longitudinal nutritional status parameters in response to ONS treatment in a cohort of patients with CKD-ND using BC registry data. We hypothesized that there would be identifiable change over time in nutritional status parameters with ONS prescription, and that we could identify subgroups of patients using cluster analysis, to explore possible variation in response.
Materials and Methods
This analysis included longitudinal data from adult patients >18 years of age with CKD-ND who entered multidisciplinary CKD clinics in BC between January 1, 2010, and December 31, 2019, and who initiated any ONS during CKD clinic follow-up. According to the current ONS policy for the adult population with CKD, ONS prescription by registered renal dietitians is indicated when any of the following conditions occur and cannot be addressed through nutritional counseling/diet changes alone: unintentional weight loss >10% of the usual body weight in the past 6 months, current weight <90% of desirable body weight, nutrient intake <80% recommended, and/or current hypercatabolic state (surgery, infections, burns, wounds). 20 A list of ONS available under this policy is in Supplemental Table 1. Several types of ONS are available in the BC Renal formulary, including general ONS, renal-specific ONS, diabetes-specific ONS, and protein-only ONS. Renal dietitians determine the type of ONS and frequency of administration needed to meet daily intake requirements for a given patient. Duration of ONS treatment is variable and based on the patient’s medical condition and regular evaluation by a renal dietitian of the patient’s dietary intake and response to supplement(s). 20 Demographic and laboratory data from routine follow-up were extracted from the Patient Records and Outcome Management Information System, an electronic registry of kidney patients in BC. Patients with previous renal transplant were excluded from the analysis. Ethics approval was obtained from the University of British Columbia-Providence Health Care Institute (study number: H19-01154).
Statistical Analysis
Using a before-after study design, linear mixed effects models with segmented regression were used to determine the slopes of the following 5 nutritional parameters: BMI, serum albumin, serum bicarbonate, serum phosphate, and neutrophil-to-lymphocyte ratio (NLR) in the 2 years prior to (pre-ONS) and 2 years after (post-ONS) the first ONS prescription. Included patients were required to have at least 2 measurements pre-ONS and post-ONS for slope measurements. Each model was adjusted for sex, age, eGFR, urine albumin-creatinine ratio, hypertension, cardiovascular disease, diabetes, health region, and calendar year of the first ONS prescription.
Cluster analysis using hierarchical clustering method with consensus clustering was performed. Baseline values of BMI, serum albumin, serum bicarbonate, serum phosphate, and NLR that were the closest measurement within 12 months prior to the first ONS prescription were utilized as clustering variables. Parameters consisted of 80% subsampling with 500 repetitions, and cluster size ranging from 2 to 20. The optimal number of clusters was selected by applying the elbow test to the change in the area under the cumulative distribution function curve. The slope analyses were repeated for each of the resulting cluster subgroups.
Regression analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina). Cluster analysis was performed with the ConsensusClusterPlus package in R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Pre-Post Analysis for Overall Cohort
Of 26 265 patients in BC who entered CKD clinics between January 1, 2010 and December 31, 2019, 3957 patients were prescribed ONS according to policy criteria and were included in the analysis (Supplemental Figure 1). Among the 3957 patients in the cohort, 847 patients initiated dialysis and 17 patients received transplant within 2 years after ONS prescription. Baseline demographics are shown in Table 1. The median age was 76.5 years (IQR: 66.5-83.6), with 46.5% women, and median eGFR of 23 ml/min/1.73 m2 (IQR: 16-31). Median time from clinic entry to the first ONS prescription was 8.6 months (IQR: 2.0-24.4). In the pre-ONS period, patients demonstrated declines in BMI (−0.87 kg/m2/year, 95% confidence interval [CI]: −0.99 to −0.75), serum albumin (−1.11 g/L/year, 95% CI: −1.27 to −0.95), and serum bicarbonate (−0.49 mmol/L/year; 95% CI: −0.59 to −0.39), as well as increases in NLR (+0.79/year; 95% CI: 0.60 to 0.98) and serum phosphate (+0.05 mmol/L/year; 95% CI: 0.04 to 0.06) (Figure 1). Following the first ONS prescription, there were statistically significant increases in BMI slope (+0.91 kg/m2/year, P < .0001) and albumin slope (+0.82 g/L/year, P < .0001), as well as a decline in NLR slope of −0.55/year (P < .0001) (Figure 1). The change in serum phosphate slope was of small magnitude (+0.02 mmol/L/year, P = .005), while there was no significant change in bicarbonate slope associated with ONS use (−0.05 mmol/L/year, P = .4363).
Table 1.
Baseline Characteristics a of Nondialysis Chronic Kidney Disease Patients Prescribed Oral Nutritional Supplements (N = 3957).
| Age (years) | 76.5 (66.5, 83.6) |
| Female (%) | 1838 (46.5%) |
| Comorbidities (%) | |
| Diabetes | 2074 (52.4%) |
| Hypertension | 3141 (79.4%) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 23 (16, 31) |
| Cause of CKD | |
| Hypertension | 683 (17.3%) |
| Diabetes | 577 (14.6%) |
| Polycystic | 59 (1.5%) |
| Congenital | 18 (0.5%) |
| Glomerulonephritis/Autoimmune | 304 (7.7%) |
| Other | 1782 (45.0%) |
| Unknown | 815 (20.6%) |
| Urine albumin-to-creatinine ratio (mg/mmol) | 28.4 (5.1, 141.8) |
| Body mass index (kg/m2) | 24.6 (21.8, 28.3) |
| Serum albumin (g/L) | 39 (35, 42) |
| Serum phosphate (mmol/L) | 1.3 (1.1, 1.5) |
| Serum bicarbonate (mmol/L) | 24 (22, 27) |
| Serum ferritin (µg/L) | 157 (77, 325) |
| Iron saturation (%) | 23 (17, 31) |
| Hemoglobin (g/L) | 108 (97, 120) |
| Parathyroid hormone (pmol/L) | 12.2 (7.3, 19.9) |
| Neutrophil-to-lymphocyte ratio | 3.3 (2.3, 5.2) |
Note. Results expressed as median (interquartile range) or number (percentage). CKD = chronic kidney disease.
Within 12 months prior to the first prescription of oral nutritional supplements.
Figure 1.
Nutritional trajectories for body mass index, serum albumin, neutrophil-to-lymphocyte ratio, serum bicarbonate, and serum phosphate before and after the first oral nutritional supplement prescription among patients with nondialysis chronic kidney disease.
Note. Time 0 refers to time of the first ONS prescription. Changes in slope (post-ONS slope vs pre-ONS slope) are shown in boxes. Linear mixed effect models adjusted for sex, age, eGFR, urine albumin-creatinine ratio, hypertension, cardiovascular disease, diabetes, health region, and calendar year of the first ONS prescription. Graph shown for reference patient with the following characteristics: 60 years old, male sex; no history of cardiovascular disease, diabetes, or hypertension; urine albumin-to-creatinine ratio: 3.0 mg/mmol; eGFR: 60 ml/min/1.73m2; Island Health Authority; initiated ONS prescription in 2015. BMI = body mass index; ONS = oral nutritional supplement; eGFR = estimated glomerular filtration rate; CI = confidence interval.
Cluster Analysis
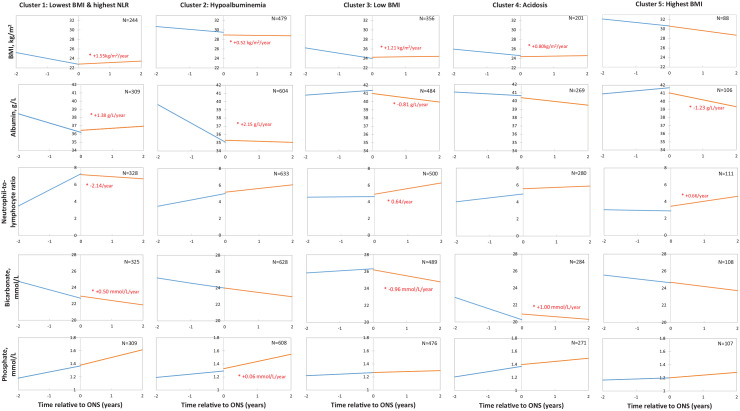
Cluster analysis based on nutritional parameters identified 5 main clusters (Supplemental Figure 1), which were labeled by their most salient features (Table 2). Complete baseline data for the 5 clusters are shown in Supplemental Table 2. Pre- and post-ONS slopes of BMI, serum albumin, phosphate, bicarbonate, and NLR for each cluster are shown in Figure 2 and Supplemental Table 3.
Table 2.
Cluster Analysis of Patients Prescribed Oral Nutritional Supplements and Nutritional Parameter Characteristics a by Cluster.
| Cluster | Main feature of cluster | No. of patients | BMI, kg/m2 | Albumin, g/L | Phosphate, mmol/L | Bicarbonate, mmol/L | NLR |
|---|---|---|---|---|---|---|---|
| 1 | Lowest BMI and highest NLR | 471 | 22.1 | 36.1 | 1.4 | 23.3 | 8.5 |
| 2 | Hypoalbuminemia | 898 | 29.8 | 34.0 | 1.3 | 24.4 | 4.2 |
| 3 | Low BMI | 672 | 22.3 | 42.4 | 1.3 | 27.1 | 3.0 |
| 4 | Acidosis | 367 | 24.1 | 41.8 | 1.4 | 19.9 | 3.6 |
| 5 | Highest BMI | 135 | 30.0 | 42.9 | 1.3 | 24.5 | 2.3 |
| Total | 2543 | 25.6 | 38.2 | 1.3 | 24.3 | 4.5 |
Note. BMI = body mass index; NLR = neutrophil-to-lymphocyte ratio.
Mean nutritional parameters at baseline (closest measurement within 12 months prior to the first ONS prescription).
Figure 2.
Nutritional trajectories, by cluster, for body mass index, serum albumin, neutrophil-to-lymphocyte ratio, serum bicarbonate, serum phosphate before and after the first oral nutritional supplement prescription among patients with nondialysis chronic kidney disease.
Note. Statistically significant changes in post-ONS versus pre-ONS slopes (P < .05) are marked by an asterisk (*). Time 0 refers to time of the first ONS prescription. Linear mixed effect models adjusted for sex, age, eGFR, urine albumin-creatinine ratio, hypertension, cardiovascular disease, diabetes, health region, and calendar year of the first ONS prescription. Graph shown for reference patient with the following characteristics: 60 years old; male sex; no history of cardiovascular disease, diabetes, or hypertension; urine albumin-to-creatinine ratio: 3.0 mg/mmol; eGFR: 60 ml/min/1.73m2; Island Health Authority; initiated ONS prescription in 2015. BMI = body mass index; NLR = neutrophil-to-lymphocyte ratio; ONS = oral nutritional supplement; eGFR = estimated glomerular filtration rate.
Cluster 1 (N = 471) was characterized by the highest mean NLR and the lowest mean BMI. Among patients in this group, 13.8% had glomerulonephritis/autoimmune cause of CKD, compared with 4.4% to 8.2% in the other clusters. Following the first ONS prescription, this cluster demonstrated increases in BMI slope (+1.55 kg/m2/year, P < .0001), serum albumin slope (+1.38 g/L, P < .0001), and serum bicarbonate slope (+0.50 mmol/L/year, P < .005), as well as a decrease in NLR slope (−2.14/year, P < .0001). Cluster 2 (N = 898), the largest cluster, was characterized by hypoalbuminemia. This cluster demonstrated increases in serum albumin slope (+2.15 g/L/year, P < .0001), BMI slope (+0.52 kg/m2/year, P = .0059), and serum phosphate slope (+0.06 mmol/L/year, P < .0001). There were no statistically significant changes in slopes of the other parameters. Among cluster 3 (N = 672), characterized by a low mean BMI, there was a significant increase in BMI slope (+1.21 kg/m2/year, P < .0001) with ONS treatment, accompanied by decreases in serum albumin slope (−0.81 g/L/year, P < .0001) and bicarbonate slope (−0.96 mmol/L/year, P < .0001), and an increase in NLR slope (+0.64/year, P = .004). Cluster 4 (N = 367), characterized by acidosis, demonstrated increases in BMI slope (+0.80 kg/m2/year, P < .0001) and serum bicarbonate slope (+1.00 mmol/L/year, P < .0001) following the first ONS prescription, but there were no statistically significant changes in slopes of other parameters. In cluster 5 (N = 135), characterized by the highest BMI (mean: 30 kg/m2) among clusters, there was a decrease in serum albumin slope (−1.23 g/L/year, P < .0001), an increase in NLR slope (+0.66/year, P = .02), and no statistically significant changes in BMI, bicarbonate, or phosphate slopes.
Discussion
In a large cohort of 3957 patients, we found ONS prescription was associated with increases in BMI and albumin slopes, and a decrease in NLR slope. There was minimal effect on phosphate slope and no significant change in bicarbonate slope in the overall cohort. This analysis is the first, to our knowledge, to assess longitudinal trajectories of nutritional parameters before and after ONS prescription in a large outpatient population of patients with CKD-ND at risk of malnutrition/PEW. Uniquely this population is under the care of multidisciplinary teams, and ONS is fully supported financially in BC.
Our novel approach used cluster analysis, a form of unsupervised machine learning, to create distinct groupings based on similarity across multiple variables. In doing so, we identified 5 specific “phenotypes,” which allowed us to explore heterogeneity of treatment response to ONS. While no previous studies have used cluster analysis to assess nutritional status in CKD-ND, this method was applied in 1 previous study in hemodialysis patients to group variables and to assess relationships between inflammation, malnutrition, and cardiovascular disease markers. 22 However, this study did not report groupings of patient observations. Our data highlighted several examples of between-cluster heterogeneity in the change in nutrition and inflammation parameter slopes following ONS prescription. Cluster 1, with the highest mean NLR and the lowest mean BMI, demonstrated improvements in BMI, albumin, bicarbonate, and NLR slopes. Conversely, cluster 3, characterized by low BMI in the absence of inflammation or abnormalities in other nutritional parameters, demonstrated an increase in BMI slope after ONS prescription, but adverse changes in serum albumin, bicarbonate, and NLR slopes. This result suggests that patients with inflammation may respond better to ONS. This is consistent with a previous study in hemodialysis patients, which assessed the association between ONS prescription protocol and mortality, and suggested a greater benefit in patients with higher WBC, a proxy for inflammation. 23 Cluster 4, characterized by lowest mean serum bicarbonate among clusters, demonstrated increases in BMI and bicarbonate slopes following ONS prescription. This contrasts with the results in the whole cohort, in which serum bicarbonate slopes did not change following ONS prescription. We did not analyze bicarbonate supplementation and therefore cannot exclude an effect of this possible cointervention on nutritional parameter slopes. Bicarbonate supplementation has been associated with improved nutritional status and decreased risk of progression of CKD. 24 Our analysis suggests that the phenotype with the highest mean BMI (cluster 5), representing a small subgroup in the cohort, may be least responsive to ONS in terms of nutritional parameter changes. These patients may have sarcopenic obesity, characterized by low muscle mass and function in combination with obesity. 25 Such patients may respond better to other lifestyle interventions, as suggested by Androga et al. 25
For clinical interpretation, the magnitude of changes in nutritional parameter slopes can be considered in relative terms (change in parameter/parameter mean). In a previous study from the Dialysis Outcomes and Practice Patterns Study, 26 a decline by >3.5% in BMI and a decline by >5.3% in serum albumin were each associated with significantly increased mortality risk in hemodialysis patients. Across cluster phenotypes, we observed relative changes in BMI ranging from −0.72% to +7.0% and relative changes in albumin of −2.9% to +6.3% associated with ONS use in our cohort. The clinical significance of changes in nutritional parameter slopes needs to be evaluated by assessing clinical outcomes including progression to kidney failure, mortality, hospitalization, and quality of life in future randomized controlled trials (RCTs) in the population with CKD-ND. Validation of phenotypes in other cohorts with CKD-ND is warranted, as are prospective studies evaluating the responses among the different patient phenotypes to tailored nutritional interventions.
Previous studies have assessed the clinical significance of longitudinal trajectories of nutritional parameters among patients with kidney disease. Among patients with CKD-ND in the Chronic Renal Insufficiency Cohort and the African American Study of Kidney Disease and Hypertension, patients with >5% annual weight loss prior to starting dialysis had greater risk of death following dialysis initiation compared with patients with stable weight. 12 In the hemodialysis population, longitudinal changes in nutritional parameters and composite nutritional scores have been associated with hospitalization and mortality.27-29 Specifically, NLR slope and serum bicarbonate slope were identified as important predictors of these outcomes. 29 Hospitalizations have also been associated with prolonged declines in nutritional parameters among hemodialysis patients. 14
While most evidence supporting ONS use originates from the population on dialysis, there is a paucity of studies assessing ONS in the population with CKD-ND. One randomized trial evaluated a nonprotein calorie supplement as an adjunct to a low-protein diet in patients with CKD-ND, and found improvements in urine protein excretion and eGFR, but no differences in serum albumin, phosphorus, or C-reactive protein compared with the control group. 30 However, this study did not specifically enroll patients with or at risk of malnutrition or PEW.
There are several clinical implications for our study. First, ONS appears to improve nutritional parameters among CKD-ND, in keeping with the goal of ONS. Second, cluster analysis is the method by which we can empirically identify nutritional phenotypes based on multiple nutritional parameters. In many large dialysis organizations in the United States, ONS supplement programs utilize serum albumin criteria alone. 23 ,31-33 However, because serum albumin is also affected by intercurrent illness, inflammation, and volume status, serum albumin alone is not a reliable marker of nutritional status in kidney patients. 34 Our study found that while nutritional parameters among the hypoalbuminemic cluster were responsive to ONS, other patient phenotypes (eg, with high NLR and low BMI despite normal serum albumin levels) also appear to have favorable responses to ONS treatment. This highlights the heterogeneity of malnutrition and the PEW syndrome and the importance of dietitian assessment of multiple nutritional parameters in the decision to start ONS. Third, we identified variability in ONS response across phenotypes, which suggests that management of malnutrition and PEW with ONS and nutritional status assessment may need to be customized by phenotype.
One of the limitations of the study is its observational design, and therefore, a causal link between ONS prescription and nutritional parameters cannot be inferred. There is also a possibility of residual confounding. We used a pre-post design, rather than a separate control group, due to confounding by indication. However, future studies could perform comparisons between matched patients prescribed ONS versus not prescribed ONS. Data on dietary intake, muscle mass, and nutritional scoring systems were not available in the registry, although dietitians do perform intake assessments at CKD clinics. Therefore, we focused our analysis on readily available weight and lab parameters that are measured as part of routine follow-up. Because specific inflammation markers are not routinely measured among patients with CKD in BC, we selected NLR as an inflammation parameter, an outcome that has been validated in patients with kidney disease. 35 The models assume a linear trajectory of longitudinal nutritional parameters in each segment (pre-ONS and post-ONS). Secular effects over time may affect nutritional parameter outcomes; therefore, we adjusted for year of the first ONS prescription in the regression models. We adjusted for baseline demographic and comorbidity variables but not time-varying variables, such as medications.
One of the strengths of our study is that we included a large, province-wide registry of patients with CKD-ND with longitudinal data and managed with standardized care protocols. Through a government-funded policy, ONS is provided without cost to kidney patients and without eGFR restriction, thereby allowing access to all patients with CKD who meet criteria based on low nutrient intake, and/or weight. This scope is unique, as many other ONS policies in North America have focused on ONS use in the dialysis population only. As the risk of mortality is greatest in the first 120 days after dialysis initiation, nutritional status is a potential contributor to this mortality risk that is amenable to treatment prior to dialysis initiation. 13 Therefore, a future research direction will entail assessment of outcomes associated with ONS treatment and the transition to dialysis.
Conclusion
Our study provides evidence that among patients with CKD-ND at risk of malnutrition/PEW, a dietitian-led ONS policy can improve nutritional status trajectories, as measured by objective weight and laboratory parameters. One of the additional findings of our study is that it may be important to establish nutritional phenotypes, and that cluster analysis appears to be a useful method of integrating multiple variables to establish these phenotypes. From a clinical perspective, our demonstration of the heterogeneity of response to ONS underscores the potential of developing personalized nutritional management strategies to optimize patient outcomes.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581211069008 for Trajectories of Nutritional Parameters Before and After Prescribed Oral Nutritional Supplements: A Longitudinal Cohort Study of Patients With Chronic Kidney Disease Not Requiring Dialysis by Michelle M. Y. Wong, Yuyan Zheng, Dani Renouf, Zainab Sheriff and Adeera Levin in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors wish to acknowledge Ognjenka Djurdjev, BC Renal, the Patient Records and Outcome Management Information System team, the BC Renal Dietitians Group for their assistance and input with this project.
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the University of British Columbia-Providence Health Care Institute (H19-01154).
Consent for Publication: All authors provided their consent for publication of the manuscript.
Availability of Data and Materials: Data and materials are available by contacting the corresponding author.
Author Contributions: Conception and study design: M.M.Y.W. and Y.Z.; data acquisition/statistical analysis/visualization: Y.Z.; data interpretation: M.M.Y.W., Y.Z., D.R., and A.L. Project administration: Z.S.; writing original draft: M.M.Y.W.; writing—review and editing: M.M.Y.W., Y.Z., D.R., Z.S., and A.L. Supervision and mentorship: A.L. Each author contributed important intellectual content during article drafting or revision and approved the final version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Michael Smith Foundation for Health Research [M.M.Y.W., grant number: 18465].
ORCID iD: Michelle M. Y. Wong  https://orcid.org/0000-0002-1334-0763
https://orcid.org/0000-0002-1334-0763
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391-398. [DOI] [PubMed] [Google Scholar]
- 2. Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77-90. [DOI] [PubMed] [Google Scholar]
- 3. Amparo Kamimura MA, Molnar MZ, Cuppari L, et al. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol Dial Transplant. 2015;30(5):821-828. [DOI] [PubMed] [Google Scholar]
- 4. Lopes AA, Bragg-Gresham JL, Elder SJ, et al. Independent and joint associations of nutritional status indicators with mortality risk among chronic hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Ren Nutr. 2010;20(4):224-234. [DOI] [PubMed] [Google Scholar]
- 5. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251-1263. [DOI] [PubMed] [Google Scholar]
- 6. Rambod M, Bross R, Zitterkoph J, et al. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2008;53(2):298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28(6):380-392. [DOI] [PubMed] [Google Scholar]
- 8. Cuppari L, Meireles MS, Ramos CI, Kamimura MA. Subjective global assessment for the diagnosis of protein-energy wasting in nondialysis-dependent chronic kidney disease patients. J Ren Nutr. 2014;24(6):385-389. [DOI] [PubMed] [Google Scholar]
- 9. Campbell KL, Ash S, Davies PS, Bauer JD. Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am J Kidney Dis. 2008;51(5):748-758. [DOI] [PubMed] [Google Scholar]
- 10. Sanches FM, Avesani CM, Kamimura MA, et al. Waist circumference and visceral fat in CKD: a cross-sectional study. Am J Kidney Dis. 2008;52(1):66-73. [DOI] [PubMed] [Google Scholar]
- 11. Westland GJ, Grootendorst DC, Halbesma N, Dekker FW, Verburgh CA. The nutritional status of patients starting specialized predialysis care. J Ren Nutr. 2015;25(3):265-270. [DOI] [PubMed] [Google Scholar]
- 12. Ku E, Kopple JD, Johansen KL, et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis. 2017;71(5):657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thijssen S, Wong MM, Usvyat LA, Xiao Q, Kotanko P, Maddux FW. Nutritional competence and resilience among hemodialysis patients in the setting of dialysis initiation and hospitalization. Clin J Am Soc Nephrol. 2015;10(9):1593-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pupim LB, Kent P, Caglar K, Shyr Y, Hakim RM, Ikizler TA. Improvement in nutritional parameters after initiation of chronic hemodialysis. Am J Kidney Dis. 2002;40(1):143-151. [DOI] [PubMed] [Google Scholar]
- 16. Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096-1107. [DOI] [PubMed] [Google Scholar]
- 17. Fouque D, Vennegoor M, ter Wee P, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007;22(suppl 2):ii45-ii87. [DOI] [PubMed] [Google Scholar]
- 18. Alp Ikizler T, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3)(suppl 1):S1-S107. [DOI] [PubMed] [Google Scholar]
- 19. Liu PJ, Ma F, Wang QY, He SL. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2018;13(9):e0203706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. BC Renal Pharmacy and Formulary Committee and the BC Renal Dietitians Group. Nutritional supplement policy; July 2018. http://www.bcrenalagency.ca/resource-gallery/Documents/Nutritional%20Supplement%20Program%20Guideline.pdf. Accessed January 28, 2021.
- 21. Wang AY, Kalantar-Zadeh K, Fouque D, et al. Precision medicine for nutritional management in end-stage kidney disease and transition to dialysis. Semin Nephrol. 2018;38(4):383-396. [DOI] [PubMed] [Google Scholar]
- 22. Perunicic-Pekovic G, Pljesa S, Rasic-Milutinovic Z, Stankovic S, Ilic M, Maletic R. Inflammatory cytokines and malnutrition as related to risk for cardiovascular disease in hemodialysis patients. Can J Physiol Pharmacol. 2008;86(4):205-209. [DOI] [PubMed] [Google Scholar]
- 23. Weiner DE, Tighiouart H, Ladik V, Meyer KB, Zager PG, Johnson DS. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis. 2014;63(2):276-285. [DOI] [PubMed] [Google Scholar]
- 24. Kraut JA, Madias NE. Metabolic acidosis of CKD: an update. Am J Kidney Dis. 2016;67(2):307-317. [DOI] [PubMed] [Google Scholar]
- 25. Androga L, Sharma D, Amodu A, Abramowitz MK. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep. 2017;2(2):201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62(6):2238-2245. [DOI] [PubMed] [Google Scholar]
- 27. Beberashvili I, Azar A, Sinuani I, et al. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(3):443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blumberg Benyamini S, Katzir Z, Biro A, et al. Nutrition assessment and risk prediction in dialysis patients-a new integrative score. J Ren Nutr. 2014;24(6):401-410. [DOI] [PubMed] [Google Scholar]
- 29. Wong MMY, Thijssen S, Wang Y, et al. Prediction of mortality and hospitalization risk using nutritional indicators and their changes over time in a large prevalent hemodialysis cohort. J Ren Nutr. 2020;30(1):69-78. [DOI] [PubMed] [Google Scholar]
- 30. Wu HL, Sung JM, Kao MD, Wang MC, Tseng CC, Chen ST. Nonprotein calorie supplement improves adherence to low-protein diet and exerts beneficial responses on renal function in chronic kidney disease. J Ren Nutr. 2013;23(4):271-276. [DOI] [PubMed] [Google Scholar]
- 31. Lacson E, Jr, Wang W, Zebrowski B, Wingard R, Hakim RM. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis. 2012;60(4):591-600. [DOI] [PubMed] [Google Scholar]
- 32. Benner D, Brunelli SM, Brosch B, Wheeler J, Nissenson AR. Effects of oral nutritional supplements on mortality, missed dialysis treatments, and nutritional markers in hemodialysis patients. J Ren Nutr. 2018;28(3):191-196. [DOI] [PubMed] [Google Scholar]
- 33. Cheu C, Pearson J, Dahlerus C, et al. Association between oral nutritional supplementation and clinical outcomes among patients with ESRD. Clin J Am Soc Nephrol. 2013;8(1):100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gama-Axelsson T, Heimbürger O, Stenvinkel P, Bárány P, Lindholm B, Qureshi AR. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol. 2012;7(9):1446-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34(2):155-159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581211069008 for Trajectories of Nutritional Parameters Before and After Prescribed Oral Nutritional Supplements: A Longitudinal Cohort Study of Patients With Chronic Kidney Disease Not Requiring Dialysis by Michelle M. Y. Wong, Yuyan Zheng, Dani Renouf, Zainab Sheriff and Adeera Levin in Canadian Journal of Kidney Health and Disease