Abstract
Background:
For chronic hepatitis C (CHC) patients completing pegylated interferon (PegIFN)-α/ribavirin therapy, long-term liver histological changes remain largely unexplored.
Methods:
This observational cohort study included 85 CHC patients completing PegIFN-α/ribavirin therapy with liver biopsies performed at baseline and the end of surveillance (EOS). Median years between paired biopsies were 6.75 (interquartile range: 5.63–7.54).
Results:
In patients with baseline METAVIR fibrosis stages (F) <4 (able to undergo fibrosis progression; n = 77), cases achieving sustained virological response (SVR) (n = 52) had a significantly lower rate of fibrosis progression than non-SVR cases (n = 25) (3.8% versus 24.0%, p = 0.012). Among the entire cohort (n = 85), the rate of activity response [METAVIR activity grades (A) decreasing or maintaining at A0] in SVR cases (n = 59) was significantly higher than that in non-SVR cases (n = 26) (94.9% versus 65.4%, p = 0.001). For SVR cases among the entire cohort, independent predictors of fibrosis clearance included baseline F <2 [odds ratio (OR) = 7.877, p = 0.042] and aspartate transaminase (AST) levels declining by >70% at EOS compared with baseline (OR = 9.013, p = 0.038). For non-SVR cases among the entire cohort, baseline AST levels >80 U/l and glucose levels ⩽ 105 mg/dl independently predicted significant fibrosis (F2/F3/F4) at EOS (OR = 12.558, p = 0.049) and activity response (OR = 17.741, p = 0.047), respectively.
Conclusions:
Among CHC patients completing PegIFN-α/ribavirin therapy, SVR lowers the risk of liver histological progression but does not guarantee fibrosis clearance. For SVR cases, those with baseline F ⩾ 2 or without significantly declined follow-up AST levels should be specifically monitored. As for non-SVR cases, those with a higher baseline AST or glucose level should preferentially receive retreatment.
Keywords: chronic hepatitis C, fibrosis, liver biopsy, necroinflammatory activity, pegylated interferon-α, ribavirin, sustained virological response
Introduction
Hepatitis C is a global health burden with above 175 million people infected with the hepatitis C virus (HCV). 1 For chronic hepatitis C (CHC), therapeutic modalities including interferon-based therapy and direct-acting antivirals (DAAs) have been applied, and achieving sustained virological response (SVR) is the primary treatment goal.2,3 Among interferon-based therapeutics, the combination therapy of pegylated interferon (PegIFN)-α and ribavirin used to be the standard treatment for CHC, 2 while it is no longer recommended and has been replaced by emerging DAAs 3 to reduce side effects and improve SVR rates. 4 However, a great number of CHC patients were treated with PegIFN-α/ribavirin therapy before the DAA era, and it remains unknown whether these patients are still at the risk of advanced liver histological events after treatment completion.
Annually about 400 thousand people worldwide expire from HCV-related cirrhosis or hepatocellular carcinoma (HCC). 5 The progression from HCV infection to liver fibrosis, cirrhosis, and HCC is an extended process, 6 making the surveillance of fibrosis changes an important issue for CHC management. At present, various noninvasive tests have been utilized in the assessment of liver fibrosis. 7 Nonetheless, a liver biopsy remains the reference standard for assessing liver fibrosis 7 and the only method for directly estimating liver injuries. 8 Previous studies have reported factors associated with fibrosis regression in CHC patients receiving interferon-based therapy, such as SVR,8–16 specific baseline features (advanced fibrosis,8,14 no or mild necroinflammatory activity, 14 the absence of steatosis, 17 younger age,8,14 and lower body mass index11,14 or serum viral load 14 ) or clinical changes [inflammation improvement 9 and the normalization of11,15 or a decline in 17 alanine transaminase (ALT) levels], and longer follow-up. 16 Besides, predictors of necroinflammation improvement under interferon-based therapy were proposed as well, including SVR,9–11,14 higher ALT levels at baseline, and the normalization of ALT levels. 11 However, for CHC patients completing PegIFN-α/ribavirin therapy, long-term surveillance studies on liver histological changes are lacking.
Although SVR defines the success of anti-HCV treatment, it requires further confirmation as to whether SVR, an indicator of viral clearance in peripheral serum instead of the hepatic parenchyma, remains predictive for liver histological improvement over a long time frame in CHC patients completing PegIFN-α/ribavirin therapy. Besides, to develop a more comprehensive criterion for monitoring these treatment-experienced patients, it is necessary to explore the predictors of fibrosis changes in SVR and non-SVR cases among these patients. Furthermore, given that necroinflammatory activity directly reflects the severity of the underlying disease process, 18 its changes and related predictors among these patients should also be investigated.
Methods
Study cohort
From July 2008 to June 2017 at China Medical University Hospital, Taichung, Taiwan, 97 treatment-naïve CHC patients (defined as those with the presence of HCV antibody in serum for at least 6 months and detectable serum HCV RNA but not yet receiving anti-HCV treatment) starting PegIFN-α/ribavirin therapy and accepting a baseline liver biopsy for evaluating disease severity were enrolled at baseline to participate in this observational cohort study. PegIFN-α was administered subcutaneously with PegIFN-α 2a prescribed at a dosage of 180 µg per week or PegIFN-α 2b at a weight-based dosage of 1.5 µg/kg per week, and ribavirin was given 1000 (body weight <75 kg) or 1200 mg (body weight ⩾75 kg) orally per day. 2 Dosage reduction was considered if needed. All enrolled patients were followed up extendedly for as long as possible. At each visit during treatment and post-treatment follow-up, patients received detailed physical examination and biochemical evaluation. Besides, serum HCV RNA tests were performed at baseline, 4 weeks after baseline, 12 weeks after baseline, the end of treatment (EOT), and 24 weeks after EOT. Rapid virological response (RVR), early virological response (EVR), virological response at EOT, and SVR were defined as undetectable serum HCV RNA at 4 weeks after baseline, 12 weeks after baseline, EOT, and 24 weeks after EOT, respectively. To evaluate liver histological changes, all enrolled patients were invited to receive the second liver biopsy at the end of surveillance [EOS; spanning from May 2014 to December 2019 among those accepting the second liver biopsy (n = 85)], and those declining the second liver biopsy (n = 12) were excluded from the present study. From baseline to EOS, none of the included patients received retreatment with interferon-based therapy or DAAs.
Liver biopsy
Biopsy specimens obtained percutaneously from the right-lobe liver were assessed by experienced pathologists blinded to patients’ data. Biopsy results were evaluated with the METAVIR scoring system; fibrosis was staged on a 5-point scale (F0: no fibrosis; F1: portal fibrosis without septa; F2: portal fibrosis with rare septa; F3: numerous septa without cirrhosis; F4: cirrhosis), and necroinflammatory activity was graded according to the intensity of necroinflammatory lesions (A0: no activity; A1: mild activity; A2: moderate activity; A3: severe activity).18,19 Besides, steatosis was scored based on parenchymal involvement by steatosis (S0: <5%; S1: 5%–33%; S2: >33%–66%; S3: >66%). 20
Evaluation of liver histological changes
Liver histological changes were assessed by comparing the results of the first (baseline) and the second (EOS) liver biopsy. Fibrosis changes were defined as follows: clearance as fibrosis stages decreasing from F ⩾1 to F0, non-clearance regression as decreased fibrosis stages except those ending up at F0, stabilization as unchanged, and progression as increased fibrosis stages. Changes in necroinflammatory activity were categorized into activity response (defined as activity grades decreasing or maintaining at A0) and nonresponse [defined as unchanged (except maintaining at A0) or increased activity grades].
Statistical analysis
Statistical analysis was performed with SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Macintosh, Version 20.0. Armonk, NY: IBM Corp.). No statistical methods were used to predetermine the sample size. Nominal and ordinal data were presented as absolute frequencies with relative proportions and compared by using the Fisher’s exact test. Continuous data were shown as medians with interquartile ranges and compared by using the Mann–Whitney U test for two independent samples or the Wilcoxon signed-rank test for two related samples. The odds ratio (OR) with its 95% confidence interval (CI) was calculated with binary logistic regression. Variables showing a p-value <0.05 in univariate analysis were entered into multivariate analysis. All statistical tests were two-tailed, and a p-value <0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of China Medical University Hospital (No. CMUH109-REC1-033). All procedures were performed following the ethical standards of the institutional review board and the 1964 Helsinki Declaration with its later amendments. Written informed consent was obtained from all participants.
Results
Patient characteristics
Table 1 provides the demographics of the entire cohort (85 CHC patients completing PegIFN-α/ribavirin therapy with paired liver biopsies) and its separate groups divided by whether achieving SVR or not. Among the entire cohort, 59 (69.4%) cases achieved SVR after completing PegIFN-α/ribavirin therapy, while the other 26 (30.6%) cases did not. The median duration between paired biopsies was 6.75 [interquartile range (IQR): 5.63–7.54], 6.83 (IQR: 5.58–7.67), and 6.38 (IQR: 5.71–7.02) years in the entire cohort, its SVR cases, and its non-SVR cases, respectively (SVR versus non-SVR, p = 0.282) (Table 1). Compared with non-SVR cases among the entire cohort, SVR cases had a significantly higher rate of HCV genotype 2a infection (28.8% versus 7.7%, p = 0.046), mild activity (METAVIR score A1) (72.9% versus 42.3%, p = 0.013), RVR (64.4% versus 26.9%, p = 0.002), or virological response at EOT (96.6% versus 76.9%, p = 0.009) and a significantly lower rate of activity absence (METAVIR score A0) (8.5% versus 34.6%, p = 0.008) or median level of HCV RNA [1.81 (IQR: 0.13–10.60) versus 6.51 (IQR: 2.74–14.12) 106 copies/ml, p = 0.004] at baseline (Table 1).
Table 1.
Patient characteristics of the entire cohort (85 CHC patients completing PegIFN-α/ribavirin therapy with paired liver biopsies) and its separate groups divided by whether achieving SVR or not.
| Variable | Entire cohort (n = 85) | Separate groups | ||
|---|---|---|---|---|
| SVR cases (n = 59) | Non-SVR cases (n = 26) | p-value | ||
| Baseline characteristics | ||||
| PegIFN-α, 2a/2b | 59 (69.4%)/26 (30.6%) | 43 (72.9%)/16 (27.1%) | 16 (61.5%)/10 (38.5%) | 0.317 |
| Sex, male/female | 46 (54.1%)/39 (45.9%) | 29 (49.2%)/30 (50.8%) | 17 (65.4%)/9 (34.6%) | 0.238 |
| Age (years) | 54 (49–61) | 54 (50–61) | 52 (43–61) | 0.366 |
| HBV coinfection | 7 (8.2%) | 3 (5.1%) | 4 (15.4%) | 0.193 |
| HCV genotype | ||||
| 1b | 42 (49.4%) | 26 (44.1%) | 16 (61.5%) | 0.163 |
| 2a | 19 (22.4%) | 17 (28.8%) | 2 (7.7%) | 0.046* |
| Others | 24 (28.2%) | 16 (27.1%) | 8 (30.8%) | 0.796 |
| METAVIR scores | ||||
| Fibrosis stages | ||||
| F0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| F1 | 32 (37.6%) | 21 (35.6%) | 11 (42.3%) | 0.630 |
| F2 | 37 (43.5%) | 25 (42.4%) | 12 (46.2%) | 0.814 |
| F3 | 8 (9.4%) | 6 (10.2%) | 2 (7.7%) | 1.000 |
| F4 | 8 (9.4%) | 7 (11.9%) | 1 (3.8%) | 0.425 |
| Activity grades | ||||
| A0 | 14 (16.5%) | 5 (8.5%) | 9 (34.6%) | 0.008* |
| A1 | 54 (63.5%) | 43 (72.9%) | 11 (42.3%) | 0.013* |
| A2 | 16 (18.8%) | 10 (16.9%) | 6 (23.1%) | 0.553 |
| A3 | 1 (1.2%) | 1 (1.7%) | 0 (0.0%) | 1.000 |
| Steatosis scores | ||||
| S0 | 24 (28.2%) | 17 (28.8%) | 7 (26.9%) | 1.000 |
| S1 | 57 (67.1%) | 39 (66.1%) | 18 (69.2%) | 1.000 |
| S2 | 4 (4.7%) | 3 (5.1%) | 1 (3.8%) | 1.000 |
| S3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Alcohol abuse | 10 (11.8%) | 5 (8.5%) | 5 (19.2%) | 0.271 |
| Diabetes mellitus | 24 (28.2%) | 19 (32.2%) | 5 (19.2%) | 0.298 |
| Anti-HCV (S/CO) | 14.18 (12.76–14.96) | 14.29 (12.84–15.36) | 13.98 (12.34–14.77) | 0.496 |
| HCV RNA (106 copies/ml) | 3.24 (0.28–11.07) | 1.81 (0.13–10.60) | 6.51 (2.74–14.12) | 0.004* |
| AST (U/l) | 62 (40–96) | 62 (41–94) | 63 (39–105) | 0.964 |
| ALT (U/l) | 85 (53–148) | 89 (54–147) | 77 (51–154) | 0.713 |
| Total bilirubin (mg/dl) | 0.90 (0.71–1.12) | 0.86 (0.70–1.09) | 1.00 (0.75–1.26) | 0.159 |
| Albumin (g/dl) | 4.3 (4.1–4.5) | 4.3 (4.1–4.5) | 4.3 (4.1–4.5) | 0.792 |
| INR | 1.03 (0.98–1.08) | 1.03 (0.99–1.09) | 1.02 (0.98–1.08) | 0.775 |
| Hemoglobin (g/dl) | 14.3 (13.4–15.4) | 14.3 (13.4–15.3) | 14.5 (13.4–15.8) | 0.478 |
| Platelet counts (103/µl) | 166 (133–203) | 159 (133–202) | 176 (132–210) | 0.369 |
| AFP (ng/ml) | 5.17 (3.37–10.33) | 5.54 (3.51–10.11) | 4.58 (2.52–12.39) | 0.455 |
| Glucose (mg/dl) | 102 (95–124) | 103 (96–136) | 100 (93–108) | 0.122 |
| HbA1c (%) | 5.8 (5.4–6.2) | 5.8 (5.5–6.4) | 5.8 (5.4–6.0) | 0.312 |
| Total cholesterol (mg/dl) | 178 (151–197) | 179 (158–201) | 176 (129–194) | 0.315 |
| HDL (mg/dl) | 41.1 (36.2–51.0) | 41.3 (38.0–51.6) | 39.5 (29.6–51.0) | 0.148 |
| LDL (mg/dl) | 102.8 (88.3–125.4) | 101.6 (92.9–128.7) | 103.9 (68.5–122.8) | 0.217 |
| Triglyceride (mg/dl) | 95 (68–128) | 96 (66–120) | 93 (70–156) | 0.521 |
| Creatinine (mg/dl) | 0.82 (0.66–0.96) | 0.81 (0.65–0.96) | 0.84 (0.71–0.96) | 0.695 |
| TSH (µIU/ml) | 1.434 (0.988–2.246) | 1.466 (0.935–2.350) | 1.398 (1.034–2.175) | 0.911 |
| Free thyroxine (ng/dl) | 0.85 (0.76–0.98) | 0.84 (0.72–0.99) | 0.88 (0.81–0.95) | 0.776 |
| Virological response | ||||
| RVR | 45 (52.9%) | 38 (64.4%) | 7 (26.9%) | 0.002* |
| EVR | 75 (88.2%) | 54 (91.5%) | 21 (80.8%) | 0.271 |
| At EOT | 77 (90.6%) | 57 (96.6%) | 20 (76.9%) | 0.009* |
| Mean RBV dosage (mg/day) | 875 (800–1000) | 858 (800–1000) | 884 (800–1000) | 0.765 |
| Years between biopsies | 6.75 (5.63–7.54) | 6.83 (5.58–7.67) | 6.38 (5.71–7.02) | 0.282 |
(1) When each variable was assessed, cases with missing data were excluded from analysis. (2) Nominal and ordinal data were presented as absolute frequencies with relative proportions and compared by using the Fisher’s exact test. Continuous data were shown as medians with interquartile ranges and compared by using the Mann–Whitney U test. AFP, alpha-fetoprotein; ALT, alanine transaminase; Anti-HCV, HCV antibody; AST, aspartate transaminase; EOT, end of treatment; EVR, early virological response; HbA1c, hemoglobin A1c; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein; PegIFN, pegylated interferon; RBV, ribavirin; RNA, ribonucleic acid; RVR, rapid virological response; SVR, sustained virological response; TSH, thyroid-stimulating hormone.
A p-value <0.05 was considered statistically significant.
Liver histological and biochemical changes in SVR and non-SVR cases
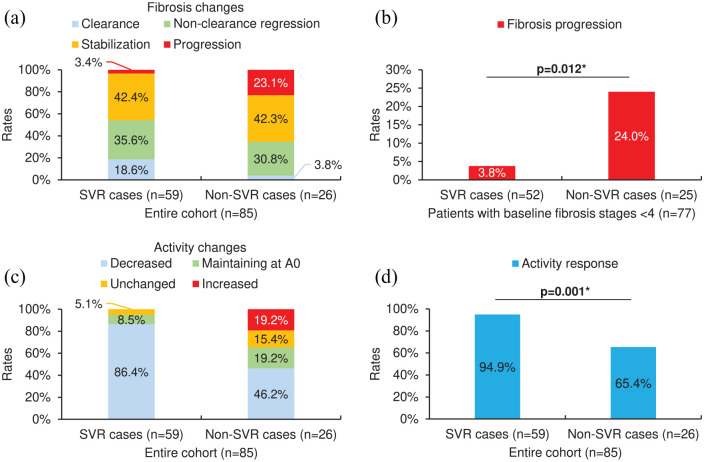
Figures 1(a) and (c) show the distributions of fibrosis and activity changes in SVR and non-SVR cases among the entire cohort. In patients with baseline fibrosis stages <4 who were able to undergo fibrosis progression (n = 77), SVR cases (n = 52) presented a significantly lower rate of fibrosis progression than non-SVR cases (n = 25) [3.8% versus 24.0%, p = 0.012; Figure 1(b)]. Among the entire cohort, the rate of activity response in SVR cases was significantly higher than that in non-SVR cases [94.9% versus 65.4%, p = 0.001; Figure 1(d)].
Figure 1.
Liver histological changes in SVR and non-SVR cases among the entire cohort (n = 85) or patients with baseline fibrosis stages <4 (able to undergo fibrosis progression; n = 77). (a) The distribution of fibrosis changes (clearance, non-clearance regression, stabilization, or progression). (b) The rate of fibrosis progression. (c) The distribution of activity changes [decreased, maintaining at A0, unchanged (except maintaining at A0), or increased]. (d) The rate of activity response (activity grades decreasing or maintaining at A0). For (b), patients with cirrhosis (METAVIR score F4) at baseline (n = 8) were excluded as they were unable to undergo fibrosis progression. For (b, d), asterisks (*) denote a p-value <0.05 (statistically significant; Fisher’s exact test). Additional data can be found in Supplementary Tables S1 and S2 online.
SVR, sustained virological response.
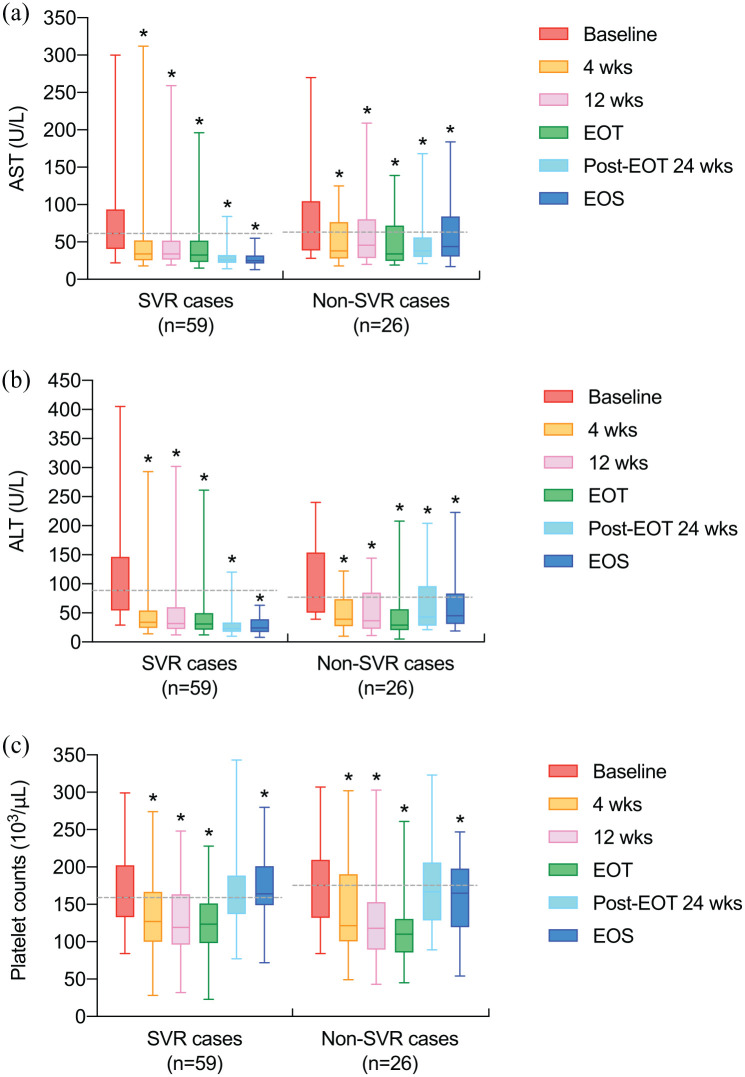
For biochemical changes compared with baseline among the entire cohort, median levels of aspartate transaminase (AST) and ALT significantly declined at 4 weeks after baseline, 12 weeks after baseline, EOT, 24 weeks after EOT, and EOS in both SVR and non-SVR cases [Figures 2(a) and (b)]. Besides, in both SVR and non-SVR cases among the entire cohort, median platelet counts significantly reduced at 4 weeks after baseline, 12 weeks after baseline, and EOT [Figure 2(c)]. From baseline to EOS, median platelet counts significantly increased in SVR cases but decreased in non-SVR cases among the entire cohort [Figure 2(c)].
Figure 2.
Levels of AST, ALT, and platelet counts at different time points in SVR (n = 59) and non-SVR cases (n = 26) among the entire cohort (n = 85). (a) AST (U/l). (b) ALT (U/l). (c) Platelet counts (103/μl). Data are depicted with box and whisker plots; the middle line represents the median, the upper and lower hinges indicate the first and third quartiles, and the upper and lower whiskers display the range (minimum to maximum). Dashed lines indicate the medians of baseline values. Asterisks ( * ) denote that the median significantly decreased or increased compared with baseline (p <0.05; Wilcoxon signed-rank test).
(1) When each variable was assessed, cases with missing data were excluded from analysis. (2) Detailed data can be found in Supplementary Table S3 online. AST, aspartate transaminase; ALT, alanine transaminase; SVR, sustained virological response; 4 wks, 4 weeks after baseline; 12 wks, 12 weeks after baseline; EOT, end of treatment; Post-EOT 24 wks, 24 weeks after EOT; EOS, end of surveillance.
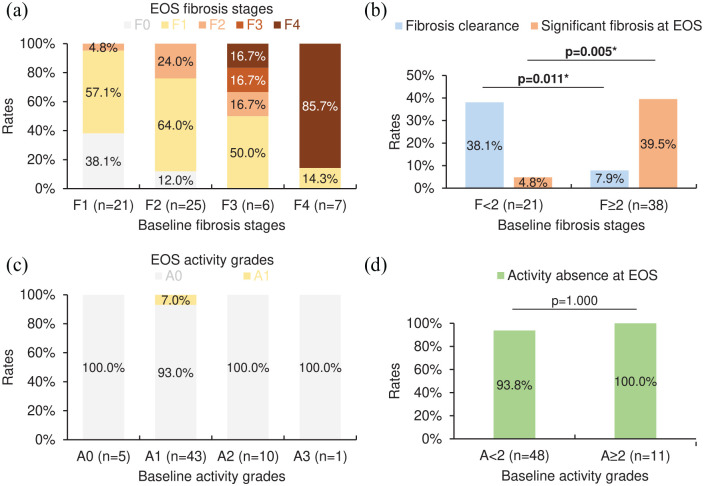
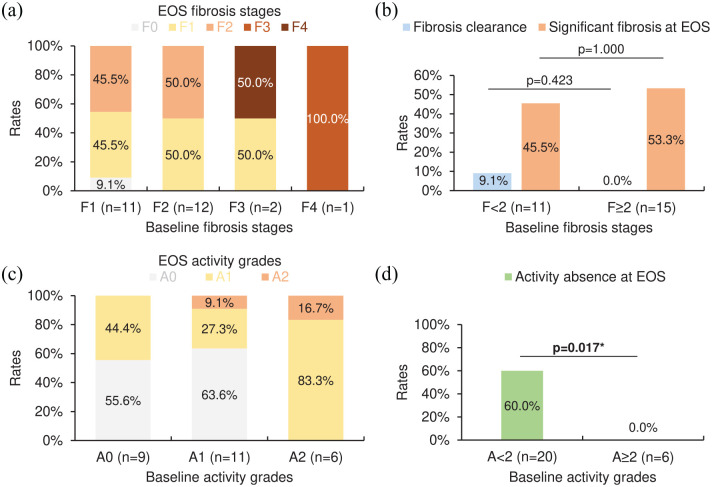
Figures 3(a), (c), 4(a), and (c) provide the distributions of corresponding EOS fibrosis stages or activity grades to each baseline fibrosis stage or activity grade in SVR or non-SVR cases among the entire cohort. For SVR cases among the entire cohort, patients with baseline fibrosis stages <2 (n = 21) were more likely to achieve fibrosis clearance and avoid significant fibrosis (METAVIR score F2/F3/F4) at EOS compared with those with baseline fibrosis stages ⩾2 (n = 38) [rates: fibrosis clearance, 38.1% versus 7.9%, p = 0.011; significant fibrosis at EOS, 4.8% versus 39.5%, p = 0.005; Figure 3(b)], while the rate of activity absence at EOS in patients with baseline activity grades <2 (n = 48) was insignificantly different from that in those with baseline activity grades ⩾2 (n = 11) [93.8% versus 100.0%, p = 1.000; Figure 3(d)]. As for non-SVR cases among the entire cohort, patients with baseline fibrosis stages <2 (n = 11) had no significant advantage in attaining fibrosis clearance and avoiding significant fibrosis at EOS compared with those with baseline fibrosis stages ⩾2 (n = 15) [rates: fibrosis clearance, 9.1% versus 0.0%, p = 0.423; significant fibrosis at EOS, 45.5% versus 53.3%, p = 1.000; Figure 4(b)], whereas the rate of activity absence at EOS in patients with baseline activity grades <2 (n = 20) was significantly higher than that in those with baseline activity grades ⩾2 (n = 6) [60.0% versus 0.0%, p = 0.017; Figure 4(d)].
Figure 3.
EOS METAVIR scores in SVR cases (n = 59) among the entire cohort (n = 85) with varying baseline METAVIR scores. (a) The distribution of EOS fibrosis stages. (b) The rates of fibrosis clearance and significant fibrosis (METAVIR score F2/F3/F4) at EOS. (c) The distribution of EOS activity grades. (d) The rate of activity absence (METAVIR score A0) at EOS. For (b, d), asterisks (*) denote a p-value <0.05 (statistically significant; Fisher’s exact test).
EOS, end of surveillance; SVR, sustained virological response.
Figure 4.
EOS METAVIR scores in non-SVR cases (n = 26) among the entire cohort (n = 85) with varying baseline METAVIR scores. (a) The distribution of EOS fibrosis stages. (b) The rates of fibrosis clearance and significant fibrosis (METAVIR score F2/F3/F4) at EOS. (c) The distribution of EOS activity grades. (d) The rate of activity absence (METAVIR score A0) at EOS. For (b, d), asterisks (*) denote a p-value <0.05 (statistically significant; Fisher’s exact test).
EOS, end of surveillance; SVR, sustained virological response.
Predictors of fibrosis clearance in SVR cases among the entire cohort
For SVR cases among the entire cohort, six factors significantly predicted fibrosis clearance in univariate analysis [baseline characteristics: age <48 (OR = 6.286, p = 0.024), fibrosis stages <2 (OR = 7.179, p = 0.009), platelet counts ⩾170 103/µl (OR = 6.286, p = 0.014), and low-density lipoprotein (LDL) levels ⩾135 mg/dl (OR = 5.429, p = 0.039); biochemical changes: alpha-fetoprotein (AFP) levels declining from ⩾4 ng/ml at baseline to <4 ng/ml at 12 weeks after baseline (OR = 6.167, p = 0.029) and AST levels declining by >70% at EOS compared with baseline (OR = 5.104, p = 0.022); Table 2]. Among these factors, two of them remained statistically significant in multivariate analysis, including baseline fibrosis stages <2 (OR = 7.877, p = 0.042) and AST levels declining by >70% at EOS compared with baseline (OR = 9.013, p = 0.038) (Table 2).
Table 2.
Predictors of fibrosis clearance in SVR cases (n = 59) among the entire cohort (n = 85).
| Variable | n | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Baseline characteristics | |||||
| PegIFN-α, 2a versus 2b | 43 versus 16 | 0.990 (0.227–4.315) | 0.990 | ||
| Sex, male versus female | 29 versus 30 | 0.833 (0.224–3.103) | 0.786 | ||
| Age, <48 versus ⩾48 | 8 versus 51 | 6.286 (1.270–31.102) | 0.024* | 4.592 (0.306–68.994) | 0.270 |
| METAVIR scores | |||||
| Fibrosis stages, <2 versus ⩾2 | 21 versus 38 | 7.179 (1.648–31.279) | 0.009* | 7.877 (1.076–57.637) | 0.042* |
| Activity grades, <2 versus ⩾2 | 48 versus 11 | 2.632 (0.300–23.058) | 0.382 | ||
| Steatosis scores, 0 versus ⩾1 | 17 versus 42 | 1.538 (0.386–6.137) | 0.542 | ||
| Diabetes mellitus, (–) versus (+) | 40 versus 19 | 0.795 (0.202–3.136) | 0.744 | ||
| HCV RNA (106 copies/ml) | |||||
| ⩽2 versus >2 | 31 versus 28 | 0.705 (0.189–2.628) | 0.603 | ||
| ⩽3 versus >3 | 33 versus 26 | 0.933 (0.250–3.482) | 0.918 | ||
| AST (U/l) | |||||
| ⩽40 versus >40 | 14 versus 44 | 1.227 (0.277–5.439) | 0.787 | ||
| ⩽80 versus >80 | 36 versus 22 | 0.680 (0.180–2.565) | 0.569 | ||
| ALT (U/l) | |||||
| ⩽55 versus >55 | 15 versus 43 | 0.581 (0.110–3.057) | 0.522 | ||
| ⩽110 versus >110 | 37 versus 21 | 0.992 (0.253–3.883) | 0.990 | ||
| Total bilirubin (mg/dl), ⩽1.2 versus >1.2 | 46 versus 12 | 0.632 (0.139–2.867) | 0.552 | ||
| Albumin (g/dl), ⩾4.4 versus <4.4 | 26 versus 31 | 0.623 (0.160–2.424) | 0.495 | ||
| Platelet counts (103/µl), ⩾170 versus <170 | 22 versus 36 | 6.286 (1.450–27.250) | 0.014* | 3.131 (0.436–22.478) | 0.256 |
| AFP (ng/ml), <4 versus ⩾4 | 18 versus 40 | 1.347 (0.339–5.345) | 0.672 | ||
| Glucose (mg/dl), ⩽105 versus >105 | 32 versus 24 | 0.556 (0.147–2.097) | 0.386 | ||
| HbA1c (%), <5.7 versus ⩾5.7 | 22 versus 31 | 0.541 (0.123–2.379) | 0.417 | ||
| HDL (mg/dl), ⩾40 versus <40 | 34 versus 19 | 0.972 (0.244–3.869) | 0.968 | ||
| LDL (mg/dl), ⩾135 versus <135 | 8 versus 45 | 5.429 (1.092–26.977) | 0.039* | 1.668 (0.106–26.177) | 0.716 |
| Biochemical changes | |||||
| Baseline versus 12 weeks after baseline | |||||
| AFP levels declining from ⩾4 to <4 ng/ml, (+) versus (–) | 8 versus 43 | 6.167 (1.205–31.550) | 0.029* | 3.562 (0.348–36.474) | 0.285 |
| Baseline versus EOS | |||||
| AST levels declining by >70%, (+) versus (–) | 19 versus 39 | 5.104 (1.268–20.544) | 0.022* | 9.013 (1.130–71.896) | 0.038* |
(1) To calculate the OR, each variable was categorized into a binary form with the latter designated as the reference factor. (2) For each variable, cases with missing data were excluded from both univariate and multivariate analysis. (3) Variables entered into multivariate analysis were those showing a p-value <0.05 in univariate analysis. (4) Additional data can be found in Supplementary Tables S4 and S5 online. AFP, alpha-fetoprotein; ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; EOS, end of surveillance; HbA1c, hemoglobin A1c; HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio; PegIFN, pegylated interferon; RNA, ribonucleic acid.
Binary logistic regression: A p-value <0.05 was considered statistically significant.
Bold values represent statistical significance in univariate or multivariate analysis.
Predictors of significant fibrosis at EOS in non-SVR cases among the entire cohort
For non-SVR cases among the entire cohort, three baseline factors significantly predicted EOS significant fibrosis in univariate analysis, including AST levels >80 U/l (OR = 12.375, p = 0.010), albumin levels <4.4 g/dl (OR = 8.000, p = 0.031), and AFP levels ⩾4 ng/ml (OR = 24.750, p = 0.008) (Table 3). Of these factors, only baseline AST levels >80 U/l maintained statistical significance in multivariate analysis (OR = 12.558, p = 0.049; Table 3).
Table 3.
Predictors of significant fibrosis (METAVIR score F2/F3/F4) at EOS in non-SVR cases (n = 26) among the entire cohort (n = 85).
| Variable | n | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Baseline characteristics | |||||
| PegIFN-α, 2a versus 2b | 16 versus 10 | 1.000 (0.206–4.856) | 1.000 | ||
| Sex, male versus female | 17 versus 9 | 0.350 (0.065–1.895) | 0.223 | ||
| Age, ⩾48 versus <48 | 17 versus 9 | 6.417 (0.999–41.212) | 0.050 | ||
| HBV coinfection, (+) versus (–) | 4 versus 22 | 0.278 (0.025–3.104) | 0.298 | ||
| METAVIR scores | |||||
| Fibrosis stages, ⩾2 versus <2 | 15 versus 11 | 1.371 (0.288–6.535) | 0.692 | ||
| Activity grades, ⩾2 versus <2 | 6 versus 20 | 7.500 (0.733–76.773) | 0.090 | ||
| Steatosis scores, ⩾1 versus 0 | 19 versus 7 | 1.481 (0.258–8.499) | 0.659 | ||
| Alcohol abuse, (+) versus (–) | 5 versus 21 | 5.333 (0.506–56.236) | 0.164 | ||
| Diabetes mellitus, (+) versus (–) | 5 versus 21 | 5.333 (0.506–56.236) | 0.164 | ||
| HCV RNA (106 copies/ml) | |||||
| >2 versus ⩽2 | 21 versus 5 | 1.650 (0.227–11.993) | 0.621 | ||
| >3 versus ⩽3 | 17 versus 9 | 1.406 (0.277–7.131) | 0.681 | ||
| AST (U/l) | |||||
| >40 versus ⩽40 | 18 versus 8 | 4.714 (0.734–30.278) | 0.102 | ||
| >80 versus ⩽80 | 11 versus 15 | 12.375 (1.828–83.767) | 0.010* | 12.558 (1.015–155.295) | 0.049* |
| ALT (U/l) | |||||
| >55 versus ⩽55 | 18 versus 8 | 4.714 (0.734–30.278) | 0.102 | ||
| >110 versus ⩽110 | 7 versus 19 | 3.437 (0.527–22.432) | 0.197 | ||
| Total bilirubin (mg/dl), >1.2 versus ⩽1.2 | 7 versus 19 | 0.291 (0.045–1.898) | 0.197 | ||
| Albumin (g/dl), <4.4 versus ⩾4.4 | 15 versus 10 | 8.000 (1.215–52.693) | 0.031* | 1.995 (0.153–26.063) | 0.598 |
| Platelet counts (103/µl), <170 versus ⩾170 | 13 versus 13 | 2.560 (0.527–12.431) | 0.244 | ||
| AFP (ng/ml), ⩾4 versus <4 | 15 versus 10 | 24.750 (2.333–262.586) | 0.008* | 16.961 (0.960–299.677) | 0.053 |
| Glucose (mg/dl), >105 versus ⩽105 | 10 versus 16 | 3.889 (0.718–21.061) | 0.115 | ||
| HbA1c (%), ⩾5.7 versus <5.7 | 15 versus 10 | 3.500 (0.638–19.195) | 0.149 | ||
| HDL (mg/dl), <40 versus ⩾40 | 12 versus 11 | 1.750 (0.329–9.298) | 0.511 | ||
| LDL (mg/dl), <135 versus ⩾135 | 20 versus 4 | 3.000 (0.265–33.974) | 0.375 | ||
| Biochemical changes | |||||
| Baseline versus 12 weeks after baseline | |||||
| AFP levels declining from ⩾4 to <4 ng/ml, (–) versus (+) | 18 versus 4 | 1.000 (0.115–8.730) | 1.000 | ||
| Baseline versus EOS | |||||
| AST levels declining by >70%, (–) versus (+) | 24 versus 2 | 1.000 (0.056–17.903) | 1.000 | ||
(1) To calculate the OR, each variable was categorized into a binary form with the latter designated as the reference factor. (2) For each variable, cases with missing data were excluded from both univariate and multivariate analysis. (3) Variables entered into multivariate analysis were those showing a p-value <0.05 in univariate analysis. (4) Additional data can be found in Supplementary Tables S6 and S7 online. AFP, alpha-fetoprotein; ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; EOS, end of surveillance; HBV, hepatitis B virus; HbA1c, hemoglobin A1c; HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio; PegIFN, pegylated interferon; RNA, ribonucleic acid.
Binary logistic regression: A p-value <0.05 was considered statistically significant.
Bold values represent statistical significance in univariate or multivariate analysis.
Predictors of activity response in non-SVR cases among the entire cohort
For non-SVR cases among the entire cohort, four factors were significantly associated with activity response in univariate analysis [baseline characteristics: glucose levels ⩽105 mg/dl (OR = 16.333, p = 0.006); biochemical changes: ALT levels remaining ⩽42 or declining from >42 to ⩽42 U/l from baseline to 24 weeks after EOT (OR = 0.114, p = 0.029), platelet counts remaining ⩾200 103/µl at both baseline and 24 weeks after EOT (OR = 0.077, p = 0.041), and AST levels declining by >30% at EOS compared with baseline (OR = 11.429, p = 0.037); Table 4]. Among these factors, only baseline glucose levels ⩽105 mg/dl significantly correlated with activity response in multivariate analysis (OR = 17.741, p = 0.047; Table 4).
Table 4.
Predictors of activity response (activity grades decreasing or maintaining at A0) in non-SVR cases (n = 26) among the entire cohort (n = 85).
| Variable | n | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Baseline characteristics | |||||
| PegIFN-α, 2a versus 2b | 16 versus 10 | 1.467 (0.282–7.627) | 0.649 | ||
| Sex, male versus female | 17 versus 9 | 1.920 (0.358–10.286) | 0.446 | ||
| Age, <48 versus ⩾48 | 9 versus 17 | 7.111 (0.723–69.985) | 0.093 | ||
| METAVIR scores | |||||
| Fibrosis stages, <2 versus ⩾2 | 11 versus 15 | 0.208 (0.037–1.181) | 0.076 | ||
| Activity grades, <2 versus ⩾2 | 20 versus 6 | 0.300 (0.029–3.071) | 0.310 | ||
| Steatosis scores, 0 versus ⩾1 | 7 versus 19 | 0.615 (0.104–3.658) | 0.593 | ||
| Alcohol abuse, (–) versus (+) | 21 versus 5 | 1.333 (0.179–9.912) | 0.779 | ||
| Diabetes mellitus, (–) versus (+) | 21 versus 5 | 1.333 (0.179–9.912) | 0.779 | ||
| HCV RNA (106 copies/ml) | |||||
| ⩽2 versus >2 | 5 versus 21 | 0.267 (0.035–2.019) | 0.201 | ||
| ⩽3 versus >3 | 9 versus 17 | 0.521 (0.097–2.790) | 0.446 | ||
| AST (U/l) | |||||
| ⩽40 versus >40 | 8 versus 18 | 0.833 (0.147–4.723) | 0.837 | ||
| ⩽80 versus >80 | 15 versus 11 | 0.563 (0.105–3.023) | 0.502 | ||
| ALT (U/l) | |||||
| ⩽55 versus >55 | 8 versus 18 | 0.385 (0.068–2.164) | 0.278 | ||
| ⩽110 versus >110 | 19 versus 7 | 0.686 (0.104–4.522) | 0.695 | ||
| Total bilirubin (mg/dl), ⩽1.2 versus >1.2 | 19 versus 7 | 0.686 (0.104–4.522) | 0.695 | ||
| Albumin (g/dl), ⩾4.4 versus <4.4 | 10 versus 15 | 1.167 (0.208–6.559) | 0.861 | ||
| Platelet counts (103/µl), ⩾200 versus <200 | 7 versus 19 | 0.268 (0.044–1.640) | 0.154 | ||
| AFP (ng/ml), <4 versus ⩾4 | 10 versus 15 | 1.167 (0.208–6.559) | 0.861 | ||
| Glucose (mg/dl), ⩽105 versus >105 | 16 versus 10 | 16.333 (2.197–121.425) | 0.006* | 17.741 (1.033–304.675) | 0.047* |
| HbA1c (%), <5.7 versus ⩾5.7 | 10 versus 15 | 2.667 (0.414–17.169) | 0.302 | ||
| HDL (mg/dl), ⩾40 versus <40 | 11 versus 12 | 0.583 (0.097–3.506) | 0.556 | ||
| LDL (mg/dl), ⩾135 versus <135 | 4 versus 20 | 1.615 (0.140–18.581) | 0.700 | ||
| Biochemical changes | |||||
| Baseline versus 24 weeks after EOT | |||||
| ALT levels remaining ⩽42 or declining from >42 to ⩽42 U/l, (+) versus (–) | 11 versus 12 | 0.114 (0.016–0.806) | 0.029* | 0.290 (0.014–6.182) | 0.428 |
| Platelet counts remaining ⩾200 103/µl, (+) versus (–) | 5 versus 17 | 0.077 (0.007–0.901) | 0.041* | 0.178 (0.003–10.261) | 0.404 |
| Baseline versus EOS | |||||
| AST levels declining by >30%, (+) versus (–) | 11 versus 15 | 11.429 (1.155–113.115) | 0.037* | 1.516 (0.063–36.591) | 0.798 |
(1) To calculate the OR, each variable was categorized into a binary form with the latter designated as the reference factor. (2) For each variable, cases with missing data were excluded from both univariate and multivariate analysis. (3) Variables entered into multivariate analysis were those showing a p-value <0.05 in univariate analysis. (4) Additional data can be found in Supplementary Tables S8 and S9 online. AFP, alpha-fetoprotein; ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; EOS, end of surveillance; EOT, end of treatment; HbA1c, hemoglobin A1c; HCV, hepatitis C virus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio; PegIFN, pegylated interferon; RNA, ribonucleic acid.
Binary logistic regression: A p-value <0.05 was considered statistically significant.
Bold values represent statistical significance in univariate or multivariate analysis.
Discussion
For CHC patients completing PegIFN-α/ribavirin therapy, post-treatment changes in liver fibrosis and necroinflammatory activity over a long time scale remain poorly understood. The present study evaluated long-term liver histological changes and their predictors in CHC patients completing PegIFN-α/ribavirin therapy, aiming to identify those at a higher risk of unreversed disease processes. To attain this purpose, detailed clinical factors were included in the study with liver histological status assessed by liver biopsies instead of noninvasive methodologies.
For SVR-achieving CHC patients with pretreatment advanced fibrosis (METAVIR score F3) or cirrhosis, post-SVR surveillance of HCC every 6 months with ultrasound is recommended regardless of received treatment types (interferon-based or interferon-free).2,3 This scenario reflects the concern that SVR may not definitely correspond to the clearance of hepatic injuries. To elucidate the role of SVR in long-term liver histological changes after completing PegIFN-α/ribavirin therapy, our study first evaluated whether SVR was associated with a lower risk of liver histological progression. The results showed that SVR indicated long-term advantages in preventing fibrosis progression (for patients able to undergo fibrosis progression) and attaining activity response (for the entire cohort). However, SVR did not guarantee fibrosis clearance, non-clearance regression, or activity response in the study patients. Besides, some of the non-SVR cases in our study still presented benign liver histological changes. In sum, for CHC patients completing PegIFN-α/ribavirin therapy, long-term changes in fibrosis and necroinflammatory activity are diverse in both SVR and non-SVR cases. Therefore, we further investigated the predictors of varying liver histological changes under the separation between SVR and non-SVR cases among the entire cohort. As activity response was predominantly seen in SVR cases, only non-SVR cases were screened for the predictors of activity changes.
Among the entire cohort, younger age at baseline significantly predicted fibrosis clearance in SVR cases (univariate analysis), implying that deferred anti-HCV treatment attenuates fibrosis clearance despite treatment success. Besides, a baseline fibrosis stage <2 was an independent predictor of fibrosis clearance and meanwhile indicated a significantly lower risk of EOS significant fibrosis for SVR cases among the entire cohort. Similarly, a higher level of platelet counts (⩾170 103/µl) was significantly predictive for fibrosis clearance in SVR cases among the entire cohort (univariate analysis). As for non-SVR cases among the entire cohort, a higher AST level of >80 U/l (multivariate analysis) and a lower albumin level of <4.4 g/dl (univariate analysis) significantly predicted significant fibrosis at EOS. These results jointly suggest that for both SVR- and non-SVR-achieving CHC patients, pretreatment severity of the disease plays a decisive role in long-term fibrosis changes following treatment completion. Future studies are required to investigate whether these results remain consistent in CHC patients treated with DAAs.
In our study, univariate analysis also showed that a baseline LDL level ⩾135 mg/dl was a significant predictor of fibrosis clearance for SVR cases among the entire cohort. Previous studies have proposed that lipids play a vital role in the HCV life cycle in which viral replication and assembly require lipid raft-like domains and lipid droplets, respectively. 21 Besides, the LDL receptor (LDLR) is a surface membrane glycoprotein responsible for the uptake of LDL from serum to hepatocytes. 22 To facilitate the intracellular need of lipids for viral proliferation, HCV enhances LDLR expression via upregulating sterol-regulatory element (SRE) binding proteins, the proteins that activate LDLR transcription by binding to SRE-1 in the LDLR promoter, and downregulating proprotein convertase subtilisin/kexin type 9, a protein that induces LDLR degradation. 23 Furthermore, CHC patients were found to present a lower level of serum LDL compared with healthy blood donors. 24 These findings and our study collectively suggest that a higher level of LDL in serum indicates a less activated LDLR expression which impairs HCV propagation and therefore leads to a higher chance of fibrosis clearance for SVR-achieving CHC patients treated with PegIFN-α/ribavirin therapy.
In terms of factors predicting activity changes in our study, baseline glucose levels ⩽ 105 mg/dl were independently predictive for activity response of non-SVR cases among the entire cohort. Previous studies have reported that HCV infection is linked with altered glucose metabolism. By decreasing insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1, HCV infection induces insulin resistance which leads to the suppression of phosphoinositide 3-kinase and protein kinase B, eventually inhibiting glucose uptake and promoting gluconeogenesis in hepatocytes. 25 Besides, HCV replication downregulates cell surface expression of glucose transporters, which also suppresses glucose uptake in hepatocytes. 26 Furthermore, through reactive oxygen species-dependent JNK activation, HCV infection promotes nuclear accumulations of forkhead box O1, a transcription factor that upregulates gene expression of the enzymes for hepatic gluconeogenesis including phosphoenolpyruvate carboxykinase and glucose 6-phosphatase, thus enhancing glucose production by gluconeogenesis in hepatocytes. 27 Taken together, HCV infection suppresses glucose uptake and induces gluconeogenesis in hepatocytes,25–27 causing hyperglycemia and high glucose levels in HCV-infected hepatocytes. 27 To explain how HCV infection benefits from glucose metabolic disorders, previous studies have proposed that abundant glucose promotes HCV replication or assembly mainly through two mechanisms. First, fatty acid synthesis (FAS) and its downstream production of lipid droplets are required for HCV replication and assembly, and glucose meets the need for FAS through glycolysis and the tricarboxylic acid cycle. 21 Second, glucose shortage leads to the activation of adenosine monophosphate-activated protein kinase (AMPK) which inhibits FAS and hepatic lipid accumulations, and high glucose levels prevent AMPK from being activated, thereby enhancing HCV replication.28,29
In addition to baseline factors mentioned above, several variables of biochemical changes also served as the predictors of liver histological changes in the present study. For SVR cases among the entire cohort, an early decline in AFP levels (from ⩾4 ng/ml at baseline to <4 ng/ml at 12 weeks after baseline) significantly predicted fibrosis clearance in univariate analysis. Previous studies have found that elevated serum AFP levels indicate more advanced fibrosis in treatment-naïve CHC patients.30–32 To explain this phenomenon, Kuhlmann et al. 33 proposed that when the liver is injured, serum AFP levels rise due to AFP synthesis by regenerating adult hepatocytes with AFP gene activation or differentiating biliary epithelial cells with fetal gene reactivation. Therefore, the level of AFP in serum may be considered as an indicator for the severity of liver injuries. Given so, we inferred that diminished liver injuries at an early stage benefit post-SVR clearance of fibrosis. Besides, considering that a higher baseline AFP level (⩾4 ng/ml) significantly predicted EOS significant fibrosis of non-SVR cases in our study cohort (univariate analysis), a more severe pretreatment liver injury may lead to a more advanced final fibrotic outcome if anti-HCV treatment fails. Future studies are needed to validate these inferences in DAA-treated CHC patients. As for other predictive biochemical changes in our study, fibrosis clearance of SVR cases among the entire cohort was featured by a much more significant decline in AST levels (by >70%; multivariate analysis) in the same time frame (from baseline to EOS). A significant decline in AST levels (by >30%) from baseline to EOS also indicated concurrent changes in necroinflammatory activity (activity response) for non-SVR cases among the entire cohort (univariate analysis). However, the normalization of ALT levels (remaining ⩽42 or declining from >42 to ⩽42 U/l) or the maintenance of higher platelet counts (remaining ⩾200 103/µl) from baseline to 24 weeks after EOT was linked with activity nonresponse in non-SVR cases among the entire cohort (univariate analysis), implying that the liver with improved or maintained functions allows for a more progressive disease following the failure of anti-HCV treatment.
The major limitation of this study is that we were unable to enroll and include a large number of participants as most of the CHC patients are unwilling to receive a liver biopsy despite its accuracy. Besides, sample size calculation was not performed. However, the distribution of HCV genotypes in our study cohort (Supplementary Table S10 online) was similar to that in the general CHC population in Taiwan (genotypes 1b and 2a being the dominant types with genotypes 1 and 2 accounting for around 53% and 40% of the CHC population, respectively), 34 suggesting that patients included in our study were somewhat representative and generalizable. Another limitation of this study is that liver biopsies were not performed at time points other than baseline and EOS (such as 4 or 12 weeks after baseline, EOT, and 24 weeks after EOT), making it unfeasible to compare liver histological changes over short and long time frames in CHC patients completing PegIFN-α/ribavirin therapy. Nonetheless, biochemical values measured at different time points reflecting the variation of liver functions helped elucidate the pattern of liver histological changes in our study. However, different biochemical parameters may be inconsistent in indicating liver histological changes. To determine which of them shares the most similar changing pattern with liver histological status over short and long time scales in CHC patients receiving anti-HCV treatment, future studies combining liver biopsies and biochemical tests performed at multiple time points are needed. At last, although our study was conducted in CHC patients completing PegIFN-α/ribavirin therapy, the results providing a preliminary insight could also benefit the DAA era as it remains unknown whether HCV eradication with interferon-based therapy is the same as with DAAs. 35
Conclusion
For CHC patients completing PegIFN-α/ribavirin therapy, SVR indicates a lower risk of liver histological progression but does not guarantee benign fibrosis or activity changes under long-term follow-up. Based on the independent predictors of liver histological changes found in our study, we suggested the following surveillance criterion for CHC patients completing PegIFN-α/ribavirin therapy: (1) for SVR cases, those with baseline fibrosis stages ⩾2 or the absence of significantly declined follow-up AST levels (by >70% compared with baseline) should be specifically monitored for fibrosis changes as fibrosis is less likely to be cleared in these patients; (2) for non-SVR cases, retreatment with DAAs should be preferentially performed in those with baseline AST levels >80 U/l or glucose levels >105 mg/dl as they are more likely to present advanced liver histological outcomes after the failure of PegIFN-α/ribavirin therapy.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-10-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211067631_(1) for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211067631_(2) for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-4-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-5-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-6-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-7-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-8-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-9-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-xlsx-11-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors extend their sincere gratitude to all participants in this study. The authors thank Dr. Shu-Mei Tsai for her assistance in applying for the ethics approval of the institutional review board. The first author Ming-Han Hsieh is sincerely grateful to Dr. Chun-Hsiung Hsieh and Ms. Tsui-Huang Tsai for their encouragement in conducting this study.
Footnotes
Author contributions: Ming-Han Hsieh: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualiza-tion; Writing – original draft; Writing – review & editing.
Tzu-Yu Kao: Data curation; Formal analysis; Investigation; Software; Writing – review & editing.
Ting-Hui Hsieh: Data curation; Formal analysis; Investigation; Software; Writing – review & editing.
Chun-Chi Kao: Data curation; Formal analysis; Investigation; Software; Writing – review & editing.
Cheng-Yuan Peng: Investigation; Resources; Writing – review & editing.
Hsueh-Chou Lai: Investigation; Resources; Writing – review & editing.
Po-Heng Chuang: Investigation; Resources; Writing – review & editing.
Jung-Ta Kao: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Ming-Han Hsieh  https://orcid.org/0000-0001-9751-6692
https://orcid.org/0000-0001-9751-6692
Jung-Ta Kao  https://orcid.org/0000-0002-3801-5342
https://orcid.org/0000-0002-3801-5342
Data accessibility statement: All data generated or analyzed during this study are included in this published article and its supplementary material files.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ming-Han Hsieh, Department of Medicine, School of Medicine, China Medical University, Taichung, Taiwan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Tzu-Yu Kao, Faculty of Medicine, Wroclaw Medical University, Wroclaw, Dolnoslaskie, Poland.
Ting-Hui Hsieh, Interdisciplinary Program for Undergraduates, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Chun-Chi Kao, Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Cheng-Yuan Peng, Department of Medicine, School of Medicine, China Medical University, Taichung, Taiwan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Hsueh-Chou Lai, Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Po-Heng Chuang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
Jung-Ta Kao, Department of Medicine, School of Medicine, China Medical University, No. 91, Hsueh-Shih Road, Taichung 40402, Taiwan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan.
References
- 1. Petruzziello A, Marigliano S, Loquercio G, et al. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 2016; 22: 7824–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015; 63: 199–236. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol 2020; 73: 1170–1218. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO). Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection, https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/ (2018, accessed 1 June 2021). [PubMed]
- 5. World Health Organization (WHO). Global hepatitis report, https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (2017, accessed 1 June 2021).
- 6. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010; 15(Suppl. 4): 14–22. [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol 2021; 75: 659–689. [DOI] [PubMed] [Google Scholar]
- 8. Poynard T, Moussalli J, Munteanu M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol 2013; 59: 675–683. [DOI] [PubMed] [Google Scholar]
- 9. Shiffman ML, Sterling RK, Contos M, et al. Long term changes in liver histology following treatment of chronic hepatitis C virus. Ann Hepatol 2014; 13: 340–349. [PubMed] [Google Scholar]
- 10. George SL, Bacon BR, Brunt EM, et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009; 49: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cammà C, Di Bona D, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology 2004; 39: 333–342. [DOI] [PubMed] [Google Scholar]
- 12. Abergel A, Darcha C, Chevallier M, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol 2004; 16: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 13. Toccaceli F, Laghi V, Capurso L, et al. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J Viral Hepat 2003; 10: 126–133. [DOI] [PubMed] [Google Scholar]
- 14. Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002; 122: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 15. Kojima H, Hongo Y, Harada H, et al. Long-term histological prognosis and serum fibrosis markers in chronic hepatitis C patients treated with interferon. J Gastroenterol Hepatol 2001; 16: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 16. Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000; 132: 517–524. [DOI] [PubMed] [Google Scholar]
- 17. Kurosaki M, Matsunaga K, Hirayama I, et al. The presence of steatosis and elevation of alanine aminotransferase levels are associated with fibrosis progression in chronic hepatitis C with non-response to interferon therapy. J Hepatol 2008; 48: 736–742. [DOI] [PubMed] [Google Scholar]
- 18. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007; 47: 598–607. [DOI] [PubMed] [Google Scholar]
- 19. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24: 289–293. [DOI] [PubMed] [Google Scholar]
- 20. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez EL, Lagunoff M. Viral activation of cellular metabolism. Virology 2015; 479–480: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneider WJ, Beisiegel U, Goldstein JL, et al. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem 1982; 257: 2664–2673. [PubMed] [Google Scholar]
- 23. Syed GH, Tang H, Khan M, et al. Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation. J Virol 2014; 88: 2519–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siagris D, Christofidou M, Theocharis GJ, et al. Serum lipid pattern in chronic hepatitis C: histological and virological correlations. J Viral Hepat 2006; 13: 56–61. [DOI] [PubMed] [Google Scholar]
- 25. Hammerstad SS, Grock SF, Lee HJ, et al. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne) 2015; 6: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasai D, Adachi T, Deng L, et al. HCV replication suppresses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J Hepatol 2009; 50: 883–894. [DOI] [PubMed] [Google Scholar]
- 27. Shoji I, Deng L, Hotta H. Molecular mechanism of hepatitis C virus-induced glucose metabolic disorders. Front Microbiol 2012; 2: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakashima K, Takeuchi K, Chihara K, et al. Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways. Microbiol Immunol 2011; 55: 774–782. [DOI] [PubMed] [Google Scholar]
- 29. Mankouri J, Tedbury PR, Gretton S, et al. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci U S A 2010; 107: 11549–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tai W-C, Hu T-H, Wang J-H, et al. Clinical implications of alpha-fetoprotein in chronic hepatitis C. J Formos Med Assoc 2009; 108: 210–218. [DOI] [PubMed] [Google Scholar]
- 31. Bruce MG, Bruden D, McMahon BJ, et al. Clinical significance of elevated alpha-fetoprotein in Alaskan Native patients with chronic hepatitis C. J Viral Hepat 2008; 15: 179–187. [DOI] [PubMed] [Google Scholar]
- 32. Hu K-Q, Kyulo NL, Lim N, et al. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol 2004; 99: 860–865. [DOI] [PubMed] [Google Scholar]
- 33. Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol 2006; 87: 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol 2009; 24: 336–345. [DOI] [PubMed] [Google Scholar]
- 35. Rockey DC, Friedman SL. Fibrosis regression after eradication of hepatitis C virus: from bench to bedside. Gastroenterology 2021; 160: 1502–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-10-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211067631_(1) for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211067631_(2) for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-4-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-5-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-6-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-7-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-8-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-9-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-xlsx-11-taj-10.1177_20406223211067631 for Long-term surveillance of liver histological changes in chronic hepatitis C patients completing pegylated interferon-α plus ribavirin therapy: an observational cohort study by Ming-Han Hsieh, Tzu-Yu Kao, Ting-Hui Hsieh, Chun-Chi Kao, Cheng-Yuan Peng, Hsueh-Chou Lai, Po-Heng Chuang and Jung-Ta Kao in Therapeutic Advances in Chronic Disease