Abstract
To characterize changes in serum cytokine levels in human immunodeficiency virus type 1 (HIV-1)-infected persons with Mycobacterium avium complex (MAC) bacteremia, the levels of IL-1α (interleukin-1α), IL-6, IL-10, tumor necrosis factor alpha (TNF-α), soluble type II TNF receptor (sTNF-RII), and transforming growth factor β (TGF-β) in serum were measured in two cohorts of HIV-1-infected persons with MAC bacteremia. The first cohort was part of a MAC prophylaxis study. Patients with bacteremia were matched with controls without bacteremia. Elevated IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β levels were noted at baseline for all subjects, a result consistent with advanced HIV-1 disease. IL-1α was not detected. No differences in cytokine levels in serum were noted at baseline and at the time of bacteremia between patients with MAC and controls. In the second cohort, subjects had serum samples collected at the time of MAC bacteremia and thereafter while on macrolide therapy. Serum samples at time of bacteremia were collected from HIV-1-infected persons at a time when neither highly active antiretroviral therapy (HAART) nor MAC prophylaxis was used routinely. MAC treatment resulted in decreased levels of IL-6 and TNF-α in serum, which were evident for IL-6 by 4 to 6 weeks and for TNF-α by 8 to 16 weeks. Thus, antibiotic treatment for MAC results in decreased levels of IL-6 and TNF-α in serum in HIV-1-infected persons who are not on HAART.
Mycobacterium avium complex (MAC) bacilli and mycobacterial constituents readily stimulate mononuclear phagocytes to secrete cytokines such as interleukin-1α (IL-1α), IL-6, IL-10, tumor recrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), and soluble type II TNF receptor (sTNF-RII) (3–7, 19, 20). MAC also may use IL-6 as a growth factor (17). As immunodeficiency progresses in human immunodeficiency virus type 1 (HIV-1) infection, elevated levels of some of these same molecules are detected in serum (8, 15, 16). Thus, dysregulation of the in vivo cytokine environment in advanced HIV-1 infection not only favors HIV-1 replication but may also contribute to host susceptibility to MAC infection and dissemination.
To determine the relationship between the in vivo cytokine environment and MAC dissemination, changes in the levels of IL-1α, IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β in serum were measured before and at the time of disseminated MAC infection. Furthermore, the effect of antibiotic treatment of disseminated MAC on these same serum cytokine levels was determined. These studies used stored serum samples obtained from patients in a randomized trial of drug regimens for the prevention of disseminated MAC infection, and serial samples from a second set of patients before and during treatment for disseminated MAC were used (11). IL-1α, IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β were chosen for these studies because of their role(s) in immune responses to MAC and HIV-1 and because elevated levels of these molecules in serum can be measured readily. Serum samples were collected from HIV-1-infected persons at a time when neither highly active antiretroviral therapy (HAART) nor MAC prophylaxis was used routinely.
MATERIALS AND METHODS
Study population and study design.
To address the role of cytokine levels prior to disseminated MAC, a case-control design was used. Patients and controls were selected from the California Collaborative Treatment Group 552 trial, which compared the efficacy of azithromycin to that of rifabutin with the combination of both drugs for the prevention of MAC infection (11). At entry, HIV-1-infected persons had CD4 counts of <100 cells/mm3 and no history of MAC bacteremia. Disseminated MAC was determined on the basis of a positive culture of blood or another normally sterile site. Blood was cultured for MAC monthly, and serum was banked bimonthly and stored at −70°C. Patients (n = 15) were defined as individuals who developed disseminated MAC. Patients were selected from among subjects with available serum at baseline and within 1 month of MAC bacteremia. Serum obtained 1 month before disseminated MAC was considered pre-MAC, and serum obtained at or up to 1 month after MAC bacteremia was considered post-MAC. All other subjects were considered controls. Controls (n = 15) were matched for entry CD4 count, prophylaxis regimens to prevent MAC, prior antiretroviral therapy, and duration of follow-up. Serum HIV-1 RNA levels were analyzed with the Amplicor assay (Roche Diagnostic Systems, Branchburg, N.J.).
To determine the effect of MAC treatment on serum cytokine levels, patients with disseminated MAC were identified at the University of California at San Diego (UCSD) and Case Western Reserve University (CWRU) who had serum samples frozen at the time of MAC diagnosis and after MAC treatment was initiated. All patients were diagnosed with MAC between March 1993 and May 1996. Eligible subjects had a frozen sample at baseline and two additional samples up to 16 weeks after initiation of MAC treatment. Treatment of disseminated MAC included clarithromycin and at least one other antimicrobial. At UCSD six patients were part of a randomized treatment trial of combination therapy for MAC bacteremia, which included clarithromycin and clofazamine with or without ethambutol. At CWRU, four patients were identified by using a computerized database which stores information on patients receiving care at the John T. Carey Special Immunology Unit. Serum samples from these patients were collected during clinic visits and stored in a repository. None of the patients at CWRU and UCSD were receiving HAART therapy at the time of disseminated MAC diagnosis and during the first 6 months of MAC treatment.
Sera were collected from whole blood after centrifugation and stored at −70°C. Sera were batch tested in a blinded manner in the cytokine core facility of the CWRU Center for AIDS Research. Sera were tested in duplicate by enzyme-linked immunosorbent assay (ELISA) for IL-1α, IL-6, and IL-10 (Endogen, Woburn, Mass.); TNF-α (Medgenix, Stillwater, Minn.); and sTNF-RII and TGF-β (R&D Systems, Minneapolis, Minn.). The lower limits of detection were as follows: IL-1α, 2 pg/ml; IL-6, 1 pg/ml; IL-10, 3 pg/ml; TNF-α, 3 pg/ml; sTNF-RII, 1 pg/ml; TGF-β, 7 pg/ml. Informed consent was obtained from patients or their guardians for participation in the above-described clinical studies and trials. Studies were approved by institutional review boards at UCSD and CWRU.
Statistical analysis.
For the case-control study, mean cytokine levels for patients and controls were compared at baseline and at event time (i.e., The time of MAC diagnosis) using a paired t test. In a subgroup analysis, the patients were divided on the basis of whether the event cytokine measurement for patients was obtained prior to MAC bacteremia versus at the time of or up to 30 days after MAC bacteremia. Changes in cytokine levels from the baseline level were compared on the basis of paired t tests. A P value of <0.05 was considered significant.
To evaluate changes in cytokine levels in patients with MAC initiating treatment, cytokine levels at baseline and up to 16 weeks after initiation of antimycobacterial treatment were analyzed using “mean test with one-way analysis of variance.”
RESULTS
Serum cytokine levels in HIV-1-infected persons with or without MAC bacteremia.
For the case-control study, 15 patients with MAC and 15 controls had adequate serial serum samples available for study. Among the 15 patients and 15 controls, baseline CD4 cell counts were <100 cells/μl. Baseline HIV-1 RNA levels were 4.83 versus 4.44 and 4.95 versus 4.39 log10 copies/ml at the time of the event. IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β were detectable in serum at baseline and at the time of MAC bacteremia (Table 1). IL-1α levels of >2 pg/ml were not detected in any of the samples tested. No differences in baseline IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β levels were noted between patients and controls. Furthermore, at the time of MAC bacteremia no differences were noted in the levels of cytokine or sTNF-RII in serum between patients and controls. Since sera were collected every 2 months and mycobacterial blood cultures were obtained every month, for some MAC bacteremia patients a serum sample was obtained in the month before MAC bacteremia and for some patients it was obtained at the time of or within 30 days of MAC bacteremia. Thus, MAC bacteremia patient sera could be divided into pre-MAC (n = 7) and post-MAC (n = 8) cases. Examining the cohort in this way also did not reveal significant differences in the levels of cytokine and sTNF-RII in serum between patients and controls (data not shown). Increased sTNF-RII levels did correlate with increased HIV-1 RNA levels for patients with MAC bacteremia. Thus, overall in this population with advanced HIV-1 disease, where disseminated MAC infection was detected early by monthly blood culture, the levels of IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β in serum did not discriminate among patients with MAC bacteremia and controls.
TABLE 1.
Comparison of IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β levels in serum at baseline and at the time of MAC bacteremia in patients with disseminated MAC and in controls
| Cytokine or receptor and time of measurementa | Mean levelb (SD) for:
|
Pc | |
|---|---|---|---|
| Patients (n = 15) | Controls (n = 15) | ||
| IL-6 (pg/ml) | |||
| Baseline | 0.8 (1.0) | 1.6 (3.3) | NS |
| Event | 4.5 (12.3) | 1.7 (4.7) | NS |
| IL-10 (pg/ml) | |||
| Baseline | 8.3 (7.3) | 9.1 (8.1) | NS |
| Event | 22.7 (14.9) | 20.9 (14.7) | NS |
| sTNF-α (pg/ml) | |||
| Baseline | 16.8 (13.1) | 20.9 (15.7) | NS |
| Event | 37.1 (22.8) | 31.4 (12.4) | NS |
| TNF-RII (ng/ml) | |||
| Baseline | 7.7 (2.1) | 6.7 (3.2) | NS |
| Event | 9.1 (2.3) | 6.9 (2.5) | NS |
| TGF-β (ng/ml) | |||
| Baseline | 45.1 (11.0) | 41.5 (11.3) | NS |
| Event | 37.8 (15.9) | 39.4 (13.1) | NS |
Event refers to MAC bacteremia for patients and a similar length of follow-up for controls.
Units are as indicated in column 1.
NS, not significant.
Effect of treatment of disseminated MAC infection on serum cytokine levels.
Next, we determined if changes in the same group of cytokines and cytokine receptor could be detected in the serum of patients diagnosed with disseminated MAC and then treated with clarithromycin-based antibiotic therapy. Ten patients (six at UCSD and four at CWRU) with adequate serum samples at the time of disseminated MAC infection and follow-up sera were identified for this study. Except for IL-1α, the same serum cytokines and sTNF-RII were measured by ELISA as for the case-control study. The mean and standard deviation values of the serum cytokine levels at the time of disseminated MAC infection are shown in Table 2. These values correlated well with the IL-6, IL-10, TNF-α, TGF-β, and sTNF-RII levels at the time of the event for the case-control study in Table 1.
TABLE 2.
Levels of IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β in serum at time of disseminated MAC infection for cohorts 1 and 2 and posttreatment for cohort 2
| Cytokine or receptor | Mean levela (SD)b
|
||||
|---|---|---|---|---|---|
| At time of disseminated MAC infection
|
Postreatment (cohort 2 [n = 10]) at:
|
||||
| Cohort 1 (n = 15) | Cohort 2 (n = 10) | Wk 4 | Wk 8 | ||
| IL-6 (pg/ml) | 4.5 (12.3) | 10.5 (3.1) [0.15] | 6.6 (2.4) | 6.9 (4.6) | |
| IL-10 (pg/ml) | 22.7 (14.9) | 62.3 (69.0) [0.4] | 27.0 (20.5) | 53.1 (70.7) | |
| TNF-α (pg/ml) | 37.1 (22.8) | 24.5 (18.1) [0.7] | 19.7 (18.5) | 16.5 (12.2) | |
| sTNF-RII (ng/ml) | 9.1 (2.3) | 8.1 (5.9) [0.16] | 8.1 (5.9) | 8.7 (5.5) | |
| TGF-β (ng/ml) | 37.8 (15.9) | 35.4 (14.4) [0.56] | 34.2 (11.0) | 24.9 (13.3) | |
Units are as indicated in column 1.
P values are in brackets.
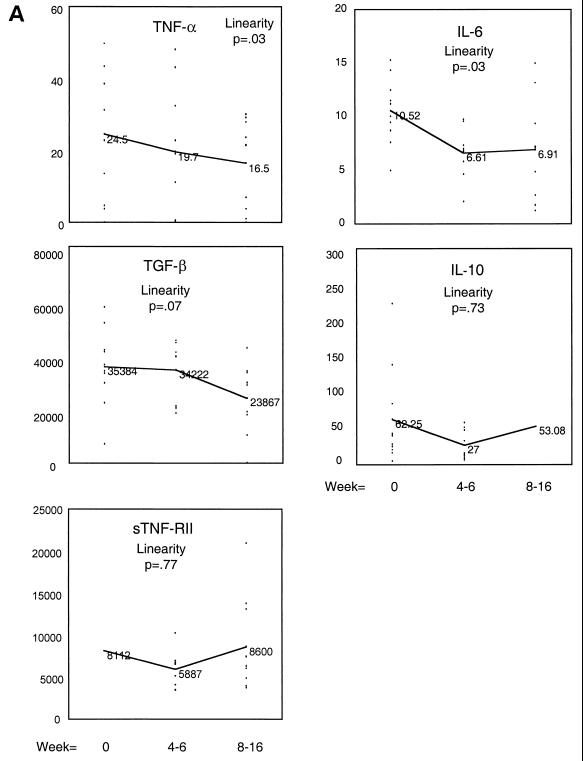
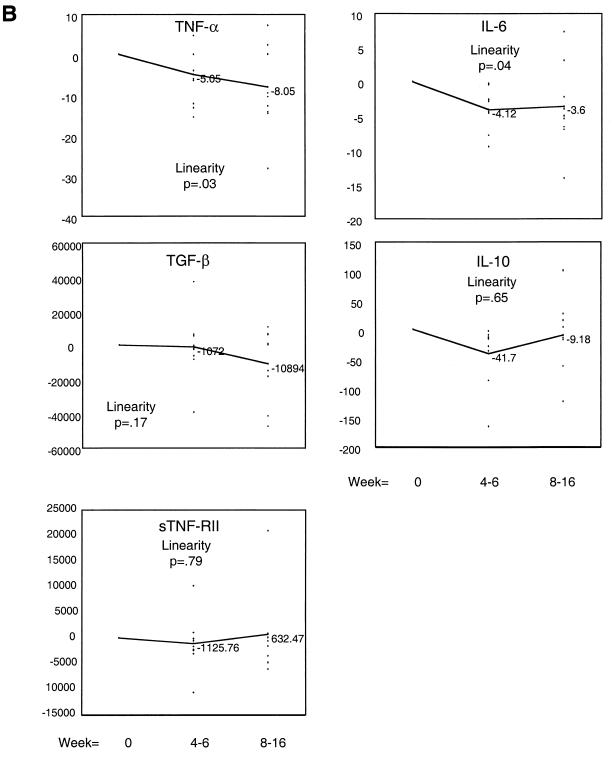
The IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β levels over time with MAC treatment are shown in Fig. 1. Figure 1A shows the mean level for each cytokine for weeks 0, 4 to 6, and 8 to 16. Mean levels of TNF-α in serum decreased gradually and by 8 to 16 weeks a significant change in the mean level had occurred. Mean IL-6 levels decreased more rapidly, reaching significance by weeks 4 to 6 and maintaining this level through weeks 8 to 16. Mean TGF-β levels demonstrated a downward trend which was not significant for this sample size. Levels of TNF-RII or IL-10 in serum did not decrease with MAC treatment. When a change from the baseline was measured, the same trends for IL-6 and TNF-α were observed (Fig. 1B). All patients had documented bacterial and clinical improvement on antibiotic treatment. There was no correlation between the number of MAC CFU in blood and the cytokine levels at the time of disseminated MAC infection.
FIG. 1.
Changes in levels of IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β in serum after intitiation of antibiotic treatment for disseminated MAC infection. Cytokine and cytokine receptor protein levels in serum were measured by ELISA as described in Materials and Methods. Results are expressed either as the mean cytokine level for each time point (weeks 0 to 16) (A) or as the mean change from the baseline level (B). The values (mean cytokine level and mean change from the baseline level) for IL-6, IL-10, and TNF-α are in picograms per milliliter, and those for TGF-β and sTNF-RII are in nanograms per milliliter. The P values listed are for the linearity of change in cytokine over time compared to the baseline level.
DISCUSSION
HIV-1-infected persons with advanced immune deficiency have elevated serum IL-6, IL-10, TNF-α, sTNF-RII, and TGF-β levels. However, the cytokine levels were the same for persons who developed MAC bacteremia and those who did not. Given the relatively large variation in levels in serum among both patients and control individuals, we cannot exclude the possibility that, with a larger sample size, differences between patients and controls could be detected. In healthy non-HIV-1-infected persons, IL-6, IL-10, and TNF-α levels are normally below the level of detection for the ELISAs used (i.e., <2 pg/ml). sTNF-RII and TGF-β are measurable in the serum of healthy individuals but at much lower levels than were observed in these HIV-1-infected persons (2 to 4 versus 6 to 10 ng of sTNF-RII per ml; 2 to 3 versus 36 to 45 ng of TGF-β per ml).
These elevated serum cytokine and sTNF-RII levels suggest an in vivo-dysregulated cytokine environment due to advanced immune deficiency. Elevated levels of IL-6, IL-10, TNF-α, and sTNF-RII in serum have been described previously in cases of advanced HIV-1 disease (2, 8, 10, 15, 16, 18). sTNF-RII is upregulated in vivo by TNF-α, and sTNF-RII levels correlate with the progression of HIV-1 infection. The elevated sTNF-RII levels seen in this study are consistent both with elevated TNF-α and with advanced HIV-1 infection. Although the effects of TGF-β in vitro to modulate responses to HIV-1 or staining for TGF-β in tissues from HIV-1-infected persons have been described, few studies have measured TGF-β levels in HIV-1-infected persons (13). Our results indicate that the immune deficiency of advanced HIV-1 infection results in serum TGF-β levels that are 5 to 10 times higher than normal.
In contrast to a study by Haas et al. (9), our results did not show levels of IL-6 in serum to be elevated at the time of MAC bacteremia. Haas et al. analyzed sera from persons receiving no antibiotic for MAC prophylaxis, whereas our patients received at least one antibiotic for MAC prophylaxis. Antibiotic prophylaxis may have attenuated MAC infection, partly resulting in less-vigorous cytokine responses. However, in this study, cytokine levels at the time of MAC bacteremia were no different between the case-control study and the MAC treatment group (who were not receiving prophylaxis), which would argue against an effect of antibiotic prophylaxis on serum cytokine levels (Table 2).
In vitro, M. avium and M. tuberculosis are potent stimuli for TNF-α production by macrophages (5, 7, 20). Furthermore, TNF-α has a major role in the bidirectional pathogenic interactions between M. tuberculosis and HIV-1 in dually infected persons. Levels of TNF-α in serum, however, did not differentiate here between those with and those without MAC bacteremia, which was also a finding of Haas et al. (9).
TGF-β and IL-10 are produced by macrophages infected with mycobacteria and can inhibit proliferation and gamma interferon production by T cells. Elevated levels of these cytokines in serum in advanced HIV infection may enhance immune suppression, allowing opportunistic pathogens such as MAC to disseminate. However, dissemination of MAC did not result in further increases in already elevated IL-10 and TGF-β levels.
Antibiotic treatment for disseminated MAC infection resulted in decreased levels of cytokine in serum. TNF-α and IL-6 levels decreased after 4 to 16 weeks of MAC treatment. IL-10 and sTNF-RII were not affected. For TGF-β there was a suggestive downward trend. MacArthur et al. recently reported similar results for IL-6 and TNF-α in a study with a smaller number of patients (14). Decreases in TNF-α levels have also been measured in HIV-1-infected persons treated for tuberculosis (21). TNF-α can increase HIV-1 replication. Thus, decreasing TNF-α production in vivo may enhance the control of viral replication.
Mechanisms for decreased TNF-α and IL-6 levels include the clearance of immunostimulatory mycobacterial products. Alternatively, the clearance of MAC from lymphoid organs may have decreased local immune activation and HIV-1 replication, thereby improving HIV-1-mediated dysregulation of the in vivo cytokine environment. A third explanation would be a direct effect of antibiotics. Macrolides have unique immunomodulatory properties in addition to their antibiotic activities and can modulate macrophage cytokine production in vitro (1, 12). Clofazamine and ethambutol are not known to have immunomodulatory properties, and the sample size in cohort 2 was too small to determine specific drug effects. Decreases in TNF-α levels in serum were not associated with decreased sTNF-RII levels, even though these two molecules regulate one another. IL-10, although readily produced by mycobacterium-infected macrophages, also was not affected by treatment. Studies of serum cytokine levels in HIV-1-infected persons with pneumocystis or cytomegalovirus infections will determine whether decreased IL-6 and TNF-α with treatment for MAC are specific for mycobacterial infection or simply the result of a correction in cytokine milieu associated with treatment of any opportunistic infection. The effect of treatment for MAC on the levels of TNF-α and IL-6 in serum suggests that these cytokines may be useful for monitoring the response to treatment. In addition, decreased cytokine levels after MAC treatment suggest that antibiotic-mediated control of this opportunistic infection may result in restoration of immune function, resulting in better control of HIV-1 replication.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-41717, the Case Western Reserve University Center for AIDS Research (AI-36219), the AIDS Clinical Trials Units at Case Western Reserve University (AI-25879), and the University of California at San Diego (AI-27670).
We thank Julie Sherman for performing the cytokine ELISAs (Cytokine Core-CWRU-CFAR).
REFERENCES
- 1.Abe S, Nakamura H, Inoue S, Takeda H, Saito H, Kato S, Mukaida N, Matsushima K, Tomoike H. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;22:51–60. doi: 10.1165/ajrcmb.22.1.3400. [DOI] [PubMed] [Google Scholar]
- 2.Aukrust P, Liabakk N B, Muller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-alpha (TNF alpha) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. . (Erratum, 169:1186–1187.) [DOI] [PubMed] [Google Scholar]
- 3.Balcewicz-Sablinska M K, Gan H, Remold H G. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J Infect Dis. 1999;180:1230–1237. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 4.Balcewicz-Sablinska M K, Keane J, Kornfeld H, Remold H G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 5.Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 6.Blanchard D K, Michelini-Norris M B, Pearson C A, Freitag C S, Djeu J Y. Mycobacterium avium-intracellulare induces interleukin-6 from human monocytes and large granular lymphocytes. Blood. 1991;77:2218–2224. [PubMed] [Google Scholar]
- 7.Fulton S A, Cross J V, Toossi Z T, Boom W H. Regulation of interleukin-12 by interleukin-10, transforming growth factor-beta, tumor necrosis factor-alpha, and interferon-gamma in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–1114. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 8.Godfried M H, van der Poll T, Weverling G J, Mulder J W, Jansen J, van Deventer S J, Sauerwein H P. Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis. 1994;169:739–745. doi: 10.1093/infdis/169.4.739. [DOI] [PubMed] [Google Scholar]
- 9.Haas D W, Lederman M M, Clough L A, Wallis R S, Chernoff D, Crampton S L. Proinflammatory cytokine and human immunodeficiency virus RNA levels during early Mycobacterium avium complex bacteremia in advanced AIDS. J Infect Dis. 1998;177:1746–1749. doi: 10.1086/517437. [DOI] [PubMed] [Google Scholar]
- 10.Haug C J, Aukrust P, Lien E, Muller F, Espevik T, Froland S S. Disseminated Mycobacterium avium complex infection in AIDS: immunopathogenic significance of an activated tumor necrosis factor system and depressed serum levels of 1,25-dihydroxyvitamin D. J Infect Dis. 1996;173:259–262. doi: 10.1093/infdis/173.1.259. [DOI] [PubMed] [Google Scholar]
- 11.Havlir D V, Dube M P, Sattler F R, Forthal D N, Kemper C A, Dunne M W, Parenti D M, Lavelle J P, White A C, Jr, Witt M D, Bozzette S A, McCutchan J A. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 12.Khan A A, Slifer T R, Araujo F G, Remington J S. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–132. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 13.Lazdins J K, Klimkait T, Woods-Cook K, Walker M, Alteri E, Cox D, Cerletti N, Shipman R, Bilbe G, McMaster G. In vitro effect of transforming growth factor-beta on progression of HIV-1 infection in primary mononuclear phagocytes. J Immunol. 1991;147:1201–1207. [PubMed] [Google Scholar]
- 14.MacArthur R D, Lederman M M, Benson C A, Chernoff M C, MacGregor R R, Spritzler J, Mahon L F, Yen-Lieberman B, Purvis S. Effects of Mycobacterium avium complex-infection treatment on cytokine expression in human immunodeficiency virus-infected persons: results of AIDS clinical trials group protocol 853. J Infect Dis. 2000;181:1486–1490. doi: 10.1086/315370. [DOI] [PubMed] [Google Scholar]
- 15.Rautonen J, Rautonen N, Martin N L, Philip R, Wara D W. Serum interleukin-6 concentrations are elevated and associated with elevated tumor necrosis factor-alpha and immunoglobulin G and A concentrations in children with HIV infection. AIDS. 1991;5:1319–1325. doi: 10.1097/00002030-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Scott-Algara D, Vuillier F, Marasescu M, de Saint Martin J, Dighiero G. Serum levels of IL-2, IL-1 alpha, TNF-alpha, and soluble receptor of IL-2 in HIV-1-infected patients. AIDS Res Hum Retrovir. 1991;7:381–386. doi: 10.1089/aid.1991.7.381. [DOI] [PubMed] [Google Scholar]
- 17.Shiratsuchi H, Johnson J, Ellner J J. Bidirectional effects of cytokines on the growth of M. avium within human macrophages. J Immunol. 1991;146:3165–3170. [PubMed] [Google Scholar]
- 18.Thea D M, Porat R, Nagimbi K, Baangi M, St. Louis M E, Kaplan G, Dinarello C A, Keusch G T. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Toossi Z, Young T G, Averill L E, Hamilton B D, Shiratsuchi H, Ellner J J. Induction of transforming growth factor beta 1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–228. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis R S, Amir T M, Ellner J J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallis R S, Vjecha M, Amir-Tahmasseb M, Okwera A, Byekwaso F, Nyole S, Kabengera S, Mugerwa R D, Ellner J J. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated beta 2-microglobulin in HIV-1-associated tuberculosis. J Infect Dis. 1993;167:43–48. doi: 10.1093/infdis/167.1.43. [DOI] [PubMed] [Google Scholar]