Abstract
Objective:
We examined parental diabetes monitoring behaviors in a cohort of children at increased genetic risk for type 1 diabetes. We hypothesized that being informed of a positive islet autoantibody (IA) would increase monitoring behaviors.
Research Design and Methods:
The Environmental Determinants of Diabetes in the Young (TEDDY) study follows 8676 children with high-risk human leucocyte antigen-DQ genotypes from birth to age 15, including general population (GP) children and those with a first-degree relative (FDR) with diabetes. Data on parental monitoring behaviors were solicited yearly. Serum samples were tested for IA and parents were informed of child results. We examined parental monitoring behaviors during the first 7 years of TEDDY.
Results:
In IA− children, the most common monitoring behavior was participating in TEDDY study tasks; up to 49.8% and 44.2% of mothers and fathers, respectively, reported this. Among FDRs, 7%–10% reported watching for diabetes symptoms and 7%–9% reported monitoring the child’s glucose, for mothers and fathers, respectively. After IA+ notification, all monitoring behaviors significantly increased in GP parents; only glucose monitoring increased in FDR parents and these behaviors continued for up to 4 years. FDR status, accurate diabetes risk perception, and anxiety were associated with glucose monitoring in IA+ and IA− cohorts.
Conclusions:
Many parents view TEDDY participation as a way to monitor for type 1 diabetes, a benefit of enrollment in a longitudinal study with no prevention offered. IA+ notification increases short- and long-term monitoring behaviors. For IA− and IA+ children, FDR parents engage in glucose monitoring, even when not instructed to do so.
Keywords: blood glucose self-monitoring, genetic screening, observational study, pediatrics, type 1 diabetes mellitus
1 |. INTRODUCTION
Research-based genetic testing for chronic childhood conditions is becoming increasingly common to help elucidate links between genetic and environmental factors in the development of diseases, including type 1 diabetes.1–3 Parents often desire information about their child’s genetic risk for type 1 diabetes even though there are currently no efficacious strategies for prevention.4,5 However, there are important ethical considerations when conducting pediatric research that provides genetic risk information but no preventive measures.6,7,8
Given this, it is important to understand not only the emotional impact of genetic testing9,10 but its behavioral impact. While a number of studies have documented changes that individuals or their parents may undertake to prevent diabetes, fewer studies have examined behaviors to monitor for the occurrence of diabetes in those at genetic risk.11,12 When a sibling has type 1 diabetes, many parents report monitoring the glucose levels of unaffected siblings, even in the absence of clinical symptoms.13 Given this, it is not surprising that parents of children genetically at-risk for diabetes may engage in behaviors to monitor for the onset of the condition. Although adults rarely report intentions to monitor for diabetes if they are found to be at genetic risk,11,14 parents often report engaging in health surveillance behaviors, such as watching for symptoms of diabetes or checking the child’s blood glucose levels.15
However, the literature on parent monitoring behaviors has focused almost exclusively on mothers in families with a first-degree relative (FDR) affected by type 1 diabetes. Although these mothers often report monitoring an unaffected child for the disease, a more detailed classification of the types of monitoring behaviors and the natural course of these behaviors is needed. Further, very little is known about the characteristics of parents who engage in these diabetes monitoring behaviors or whether these behaviors increase or change in response to information about increased diabetes risk.
The Environmental Determinants of Diabetes in the Young (TEDDY) study seeks to elucidate environmental factors that contribute to the development of type 1 diabetes in genetically at-risk children.16 As a large, prospective, international study, TEDDY represents a valuable source of data on parental diabetes monitoring behaviors in genetically at-risk children recruited from the general population (GP), with no history of type 1 diabetes, as well as from FDR families. Using TEDDY data, we addressed the following questions: (a) Do parents monitor their child for type 1 diabetes when the child is genetically at-risk for type 1 diabetes but islet autoantibody negative (IA−)?; (b) If monitoring does occur, what are the characteristics of parents who monitor their IA− children?, (c) Is an islet autoantibody positive (IA+) test result associated with a change in the type or frequency of monitoring behaviors?, and (d) What is the long term impact of IA+ test results on parent monitoring behaviors? We hypothesized that diabetes monitoring behaviors would be more often reported by mothers than fathers, would be more common among FDR parents than GP parents, would increase following notification of an IA+ result, and would be highest among parents whose children have multiple IA+ results compared to IA− single IA+ results. The examination of these hypotheses has relevance to the scientific community better understand how parents may change their behavior due to genetic results. Study findings may also provide guidance for clinicians working with families of children perceived to be at higher risk (e.g., those with FDRs with diabetes). Finally, results will elucidate ways participants may change behaviors even in naturalistic studies.
2 |. METHODS
2.1 |. The TEDDY study
TEDDY is a natural history study designed to identify environmental triggers of autoimmunity and type 1 diabetes onset in genetically at-risk children identified at six centers (Colorado, Georgia/Florida, Washington in the United States and Finland, Germany, and Sweden in Europe). At birth, 424,788 infants were screened using human leucocyte antigen (HLA) genotyping, 21,589 were eligible for TEDDY participation, and 8676 HLA-eligible infants joined the TEDDY study before 4.5 months of age. Families were recruited from the GP and from families with a FDR affected by type 1 diabetes. Following enrollment, families are asked to participate in clinic visits every 3 months during the first 4 years of the child’s life and for every 6 months thereafter up to the age of 15 years or the diagnosis of type 1 diabetes. Children with persistent, confirmed IA+ results continue to participate in quarterly study visits throughout their time in TEDDY. A variety of data are collected at study visits including biological samples (e.g., blood, saliva, and stool), records of the child’s diet, illnesses, life stressors, caregiver and child psychosocial functioning, and impact of study participation. The TEDDY study design has been previously published.16
2.2 |. TEDDY IA testing and risk notification process
Parents were fully informed of the infant’s increased genetic risk for type 1 diabetes at study enrollment. IA testing was conducted at each study visit. Based on IA results, risk information was provided to parents following each visit, either by letter, phone, or at the next study visit. For IA− children, parents were informed via letter that their child’s risk for type 1 diabetes remained increased and had not changed. Parents of children with a first IA+ result were told that their child’s risk for diabetes may have increased slightly but that positive results sometimes return to normal levels. If at subsequent study visits, the child’s IA+ test results reverted to negative (i.e., single, nonpersistent IA+), parents were told that IA test results often change over time and that their child’s negative result does not indicate a reduction in the child’s risk for type 1 diabetes unless future test findings are negative. For children testing positive for one IA for the second time (i.e., single, persistent IA+), parents were informed that their child’s risk of diabetes had increased (e.g., “your child’s risk of diabetes is 15 out of 100”). In cases where children had multiple persistent IAs, parents were informed that their child’s risk for diabetes had increased significantly (i.e., “out of 100 children with your child’s test results, 50 will go on to develop type 1 diabetes*”) and were given information about the signs and symptoms of type 1 diabetes and encouraged to discuss the increased risk with the child’s pediatrician. These children were also asked to complete a periodic oral glucose tolerance test (OGTT) as part of the TEDDY study.
Children with IA− results, nonpersistent IA+ results, or a single persistent IA+ results were not instructed to engage in glucose monitoring. The situation was different for children testing positive for multiple persistent IAs. TEDDY study sites varied in terms of whether they asked parents to engage in glucose monitoring for children positive for multiple IAs. At the United States and German TEDDY sites, parents were given glucose meters and were instructed to engage in glucose monitoring periodically (e.g., once a week or if they noticed behavioral signs of diabetes). In Finland, one clinical center (Tampere) instructed every parent with a child positive for multiple IAs to engage in blood glucose monitoring, while two other clinical centers only provided this instruction to families with further clinical signs of diabetes (e.g., impaired OGTT) or to families who expressed a desire to monitor glucose levels. In Sweden, parents were not instructed to monitor glucose levels unless there were further clinical signs of diabetes.
2.3 |. Participants
The current study focused on the first 7 years of TEDDY families’ participation as of August 31, 2016. From a total of 8676 participants who joined the TEDDY study, 7319 were still enrolled after the first year. Using these participants, three cohorts were created. The first cohort included 5944 children consistently IA− during the 7-year study window and with parental monitoring data available at least once during this time (mother n = 5929; father n = 5628) The second cohort, created to assess the short-term impact of IA+ notification on monitoring, included 867 parents who were notified of their child’s IA+ result and for whom parent monitoring behavior data was available before and after the first IA+ notification (mother n = 839; father n = 704). The third cohort, created to assess the long-term impact of IA+ test notification on parent monitoring, included 777 IA+ children with parent monitoring data available at least once and up to 4 years following the child’s first IA+ notification (mother n = 771; father n = 712).
2.4 |. Measures
2.4.1 |. Sociodemographic variables
Child sociodemographic characteristics included child age, child gender (male/female), ethnic minority status (United States: the TEDDY child’s mother’s first language is not English or the mother was not born in the United States or the child is a member of an ethnic minority group – yes/no; Europe: the child’s mother’s first language or country of birth is other than that of the TEDDY country in which the child resides – yes/no), whether the child is a first born child (yes/no), and whether the child has a FDR with type 1 diabetes (yes/no). Parent sociodemographic characteristics included parent gender (male/female), parent’s age at the TEDDY child’s birth (years), parent’s education (primary education or high school, trade school or some college, graduated from college), and marital status (married/living together versus single parent). Data on ethnic minority status, first born child status, marital/living together status, and parental education were collected when the child was 9 months of age.
2.4.2 |. Parent post-partum depression
Post-partum depression was measured at the 6-month study visit using the Edinburgh Postnatal Depression Scale17,18 (coefficient α = 0.844).
2.4.3 |. Parent anxiety about the child’s diabetes risk
An abbreviated 6-item version of the state component of the Spielberg State–Trait Anxiety Inventory (SAI)19 was used to assess parent anxiety about the child’s risk for developing type 1 diabetes. This abbreviated form showed excellent internal consistency (coefficient α = 0.901 at 6-month study visit; coefficient α = 0.904 at 15-month study visit).
2.4.4 |. Parent diabetes risk perception
Parents were asked about their perception of their child’s risk for developing type 1 diabetes at the 6-month visit, the 15-month visit, and annually thereafter. Their responses were coded as accurate (the child’s diabetes risk was higher or much higher than other children’s risk) or an underestimate (the child’s diabetes risk was the same, somewhat lower, or much lower than other children’s risk).
2.4.5 |. Parent belief that T1D risk can be reduced
At the 6-month visit, the 15-month visit, and annually thereafter, parents were asked if they believed something can be done to reduce their child’s risk for developing type 1 diabetes using three items. Responses were given using a 5-point Likert scale (1 = strongly agree to 5 = strongly disagree); high internal consistency was demonstrated (Cronbach’s α mothers = 0.821; fathers = 0.793).
2.4.6 |. Parental actions to monitor for type 1 diabetes
At the 6-month visit, the 15-month visit, and annually thereafter, parents were asked the following in questionnaire format: “In the past year have you done anything to monitor or keep an eye on your child’s risk of developing diabetes” If the parent responded “yes,” they were asked to list the actions taken. Response(s) were then coded into one of 17 possible actions. Codes that represented similar themes were collapsed into categories, such as study-related tasks (e.g., study venipuncture, “being in TEDDY”), watching for specific diabetes symptoms (e.g., increased thirst, weight loss), and glucose monitoring (at home or at a medical clinic).
2.5 |. Statistical analysis
Possible differences in the proportion of parents reporting monitoring behaviors between mother and fathers were examined using McNemar’s test. Factors were next examined for association with parent monitoring behaviors in children with consistently IA− test results over time for mothers and fathers separately, using marginal logistic regression models as estimated from generalized estimating equations (GEE). All sociodemographic variables and the postpartum depression measure were considered fixed effects. Child age and the remaining psychosocial variables were considered time-varying. All GEE models assumed an exchangeable correlation structure. The empirically based estimates were compared to the model based estimates to ensure the working correlation was reasonable. Results are provided as marginal odds ratios (OR) with 95% confidence intervals (95%CI), and differences in the marginal odds across groups were tested for statistical significance using Wald tests.
Next, the change in the proportion of parents reporting a monitoring behavior after a first IA+ test result was examined using McNemar’s test. Multiple logistic regression models were used to evaluate if factors were associated with monitoring behaviors after a first IA+ test result when adjusting for whether or not the parent indicated monitoring behavior before the first IA+ test.
Finally, GEE was used to examine long term trends in the monitoring behavior up to 4 years in cases with an initial IA+ test result, adjusting for the parents monitoring behavior immediately after first IA+ test. Comparisons were made between those with single nonpersistent IA+ results, single persistent IA+ results, and multiple persistent IA+ results; IA status was examined as time-varying. Statistical analysis was performed using SAS 9.4. p-values less than 0.05 were considered statistically significant.
3 |. RESULTS
3.1 |. Parental monitoring in IA− children
Figure 1 depicts the proportion of parents reporting three types of monitoring behaviors in children with consistently IA− test results: study-related task, glucose monitoring, and watching for signs and symptoms of diabetes. Proportions are presented for GP mothers and fathers as well FDR mothers and fathers. By the 15 month study visit, close to half of parents of IA−s children reported at least one monitoring behavior (mothers: 56.7%, fathers: 48.7%, p < 0.0001); with more mothers reporting a monitoring behavior than fathers. Parents of children with a FDR with type 1 diabetes reported monitoring more often than parents of GP children (mothers: GP = 56.2%, FDR = 61.6%, p = 0.03; fathers: GP = 47.9%, FDR = 57.8%, p = 0.0001). By far, the monitoring behavior most often reported was participating a TEDDY study-related task; up to 49.8% of mothers and 44.2% of fathers reported this at a given time point; there was no difference in reports of this behavior by FDR status. However, there were differences between FDR and GP parents’ reports of glucose monitoring (mothers: GP = 0.6%, FDR = 8.7%, p-value < 0.0001; fathers: GP = 0.3%, FDR = 7.4%, p < 0.0001) and watching for diabetes symptoms (mothers: GP = 5.0%, FDR = 9.6%, p < 0.0001; fathers: GP = 2.1%, FDR = 6.9%, p < 0.0001), with FDR mothers and fathers reporting these behaviors more often than GP families. Trends remained relatively consistent after the 15 month study visit.
FIGURE 1.
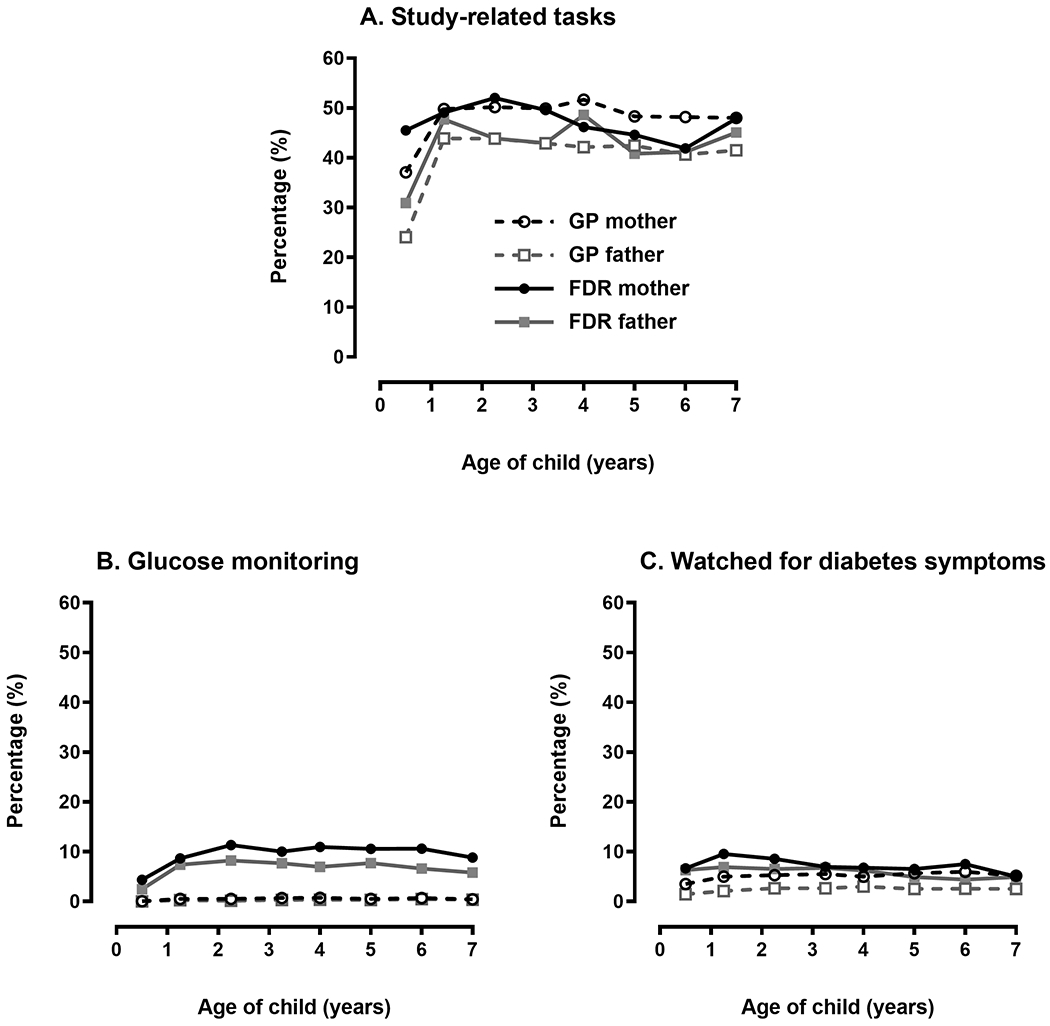
Monitoring behaviors in parents of islet autoantibody (IA) negative children in the first 7 years of TEDDY. FDR, first-degree relative; GP, general population
Factors associated with any parental monitoring behavior (yes/no) in this sample of consistently IA− children were examined separately for mothers and fathers using multiple marginal logistic regression GEE models (see Table 1). The strongest predictors of monitoring behaviors for both mothers and fathers were having an FDR with type 1 diabetes (mother’s OR = 1.31, 95% CI = 1.12–1.53, p < 0.001), being from United States (mother’s OR = 2.26, 95% CI = 2.01–2.54), Germany (OR = 2.99, 95%CI = 2.47–3.63), or Sweden (mother’s OR = 4.18, 95% CI 3.70–4.72) as compared to mothers in Finland (p < 0.001), and having an accurate versus underestimated diabetes risk perception (mother’s OR = 1.29, 95% CI = 1.22–1.36, p < 0.001) (see Table 1 for fathers data, which is similar). Additionally, a monitoring behavior was more likely to be reported by parents of nonethnic minority children (mother and father p < 0.001), parents of first born children (mother, p = 0.04; father, p < 0.001), parents who believe that something can be done to reduce the child’s diabetes risk (mother and father p < 0.001), and those who were anxious about the child’s diabetes risk (mother and father p < 0.001). Mothers (p < 0.001) but not fathers (p = 0.29) were more likely to report a monitoring behavior in older versus younger children. Postpartum depression was weakly associated with increased monitoring (statistically significant only for mothers.) Obtaining a higher educational degree (compared to trade school/college degree and basic education) was associated with monitoring only for fathers.
TABLE 1.
Factors associated with any monitoring behavior reported by mothers and fathers of IA− childrena
| Mothers |
Fathers |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Age of child | ||||||
| Years | 1.04 | 1.03-1.05 | <0.001 | 1.01 | 1.00-1.02 | 0.29 |
|
| ||||||
| FDR | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.31 | 1.12-1.53 | <0.001 | 1.42 | 1.21-1.66 | <0.001 |
|
| ||||||
| Country of residence | ||||||
| Finland | 1.00 | Reference | 1.00 | Reference | ||
| United States | 2.26 | 2.01-2.54 | 2.43 | 2.14-2.76 | ||
| Germany | 2.99 | 2.47-3.63 | 3.46 | 2.83-4.23 | ||
| Sweden | 4.18 | 3.70-4.72 | <0.001 | 3.97 | 3.48-4.52 | <0.001 |
|
| ||||||
| Child ethnic minority status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.78 | 0.69-0.89 | <0.001 | 0.77 | 0.67-0.88 | <0.001 |
|
| ||||||
| First born child statusb | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.09 | 1.01-1.18 | 0.04 | 1.19 | 1.09-1.30 | <0.001 |
|
| ||||||
| Parent’s education | ||||||
| Higher education | 1.00 | Reference | 1.00 | Reference | ||
| Graduated trade school/college | 0.92 | 0.83-1.02 | 0.83 | 0.74-0.92 | ||
| Basic education | 0.95 | 0.84-1.06 | 0.24 | 0.98 | 0.87-1.09 | 0.003 |
| Parental postnatal depressionc | 1.05 | 1.00-1.11 | 0.04 | 1.06 | 1.00-1.14 | 0.06 |
| Anxiety about diabetes (SAI)d | 1.09 | 1.05-1.12 | <0.001 | 1.09 | 1.06-1.13 | <0.001 |
|
| ||||||
| Parental risk perception | ||||||
| Underestimated | 1.00 | Reference | 1.00 | Reference | ||
| Accurate | 1.29 | 1.22-1.36 | <0.001 | 1.28 | 1.21-1.35 | <0.001 |
| Belief that TID risk can be reduced (LOC)e | 1.04 | 1.02-1.05 | <0.001 | 1.06 | 1.05-1.08 | <0.001 |
Parent marital/living together status and crowding was not associated with monitoring behaviors and was excluded from the model.
First born child status collected at 9 months of age.
Higher score (per 5 unit increase) suggests more depressive symptomology.
Higher SAI score (per 10 unit increase) suggests more anxiety about diabetes.
Higher locus of control score (per unit score) suggests stronger belief in the ability to prevent diabetes.
Table 2 provides the results of a similar analysis focusing specifically on the behavior of glucose monitoring and some of these associations were different from associations found with monitoring behaviors overall. For example, the rates of glucose monitoring were more disparate between FDR and GP families (FDR mother’s OR = 15.5, 95% CI = 11.4–21.1, p < 0.001). Mothers (p < 0.001), but not fathers (p = 0.72), also reported glucose monitoring more often if they were Finnish compared to other countries and if they had higher postnatal depressive symptoms (mothers p = 0.03, fathers p = 0.45). Contrary to findings with all monitoring behaviors, parents belief that something can be done to reduce the child’s diabetes risk was not associated with glucose monitoring.
TABLE 2.
Factors associated with glucose monitoring reported by mothers and fathers of IA− children
| Factorsa | Parent |
|||||
|---|---|---|---|---|---|---|
| Mothers |
Fathers |
|||||
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Age of child | ||||||
| Year | 1.12 | 1.07-1.17 | <0.001 | 1.13 | 1.06-1.20 | <0.001 |
|
| ||||||
| FDR | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 15.5 | 11.4-21.1 | <0.001 | 22.0 | 14.5-33.4 | <0.001 |
|
| ||||||
| Country of residence | ||||||
| Finland | 1.00 | Reference | 1.00 | Reference | ||
| United States | 0.34 | 0.34-0.80 | 1.02 | 0.61-1.73 | ||
| Germany | 0.44 | 0.44-1.24 | 0.87 | 0.46-1.64 | ||
| Sweden | 0.30 | 0.30-0.71 | <0.001 | 0.76 | 0.41-1.41 | 0.72 |
| First born child statusb | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.63 | 0.46-0.86 | 0.003 | 0.62 | 0.41-0.94 | 0.02 |
|
| ||||||
| Parental postnatal depressionc | ||||||
| Higher postnatal depression | 1.21 | 1.02-1.43 | 0.03 | 1.11 | 0.85-1.44 | 0.45 |
|
| ||||||
| Anxiety about diabetes (SAI)d | ||||||
| Higher anxiety | 1.64 | 1.43-1.89 | <0.001 | 1.22 | 1.03-1.46 | 0.02 |
|
| ||||||
| Parental risk perception | ||||||
| Underestimated | 1.00 | Reference | 1.00 | Reference | ||
| Accurate | 1.41 | 1.02-1.96 | 0.03 | 1.53 | 1.09-2.13 | 0.01 |
Child’s ethnic minority status, parental education, marital/living together status of parents, and parental locus of control were not associated with monitoring behaviors and were excluded from model.
First born child status collected at 9 months of age.
Higher score (per 5 unit increase) suggests more depressive symptomology.
Higher SAI score (per 10 unit increase) suggests parents’ higher anxiety about diabetes.
3.2 |. Impact of first IA+ test result on parental monitoring
We compared reports of monitoring behaviors before and after a child’s first IA+ result notification by parent (mother/father) and FDR status (FDR/GP) (see Figure 2). The percentage of GP parents reporting monitoring behaviors significantly increased after the child’s first IA+ test result: study related task (mothers: 49% pre-IA+ notification, 53% post, p < 0.04; fathers: 39% pre-IA+ notification, 48% post, p < 0.0001), glucose monitoring (mothers: 1% pre-IA+ notification, 4% post, p < 0.0001; fathers: 1% pre-IA+ notification, 3% post, p < 0.007), and watching for diabetes symptoms (mothers: 4% pre-IA+ notification, 9% post; p < 0.0001, fathers: 3% pre-IA+ notification, 5% post; p = 0.008). In contrast, following the first IA+ notification, FDR mothers and fathers only reported increased glucose monitoring (mothers: 9% pre-IA+ notification, 31% post, p < 0.0001; fathers: 3% pre-IA+ notification, 23% post, p < 0.0001), and the increase was much larger than that in the GP parents. Overall, 31% of FDR mothers and 23% of FDR fathers reported glucose monitoring after their first IA+ notification. A small percentage of parents were notified that their child had multiple IA+ results at the time of their first IA+ notification and were enrolled at a TEDDY site that instructed them to monitor their child’s blood glucose (approximately 11% of parents, data not shown). We conducted an analysis excluding these parents who were instructed to glucose monitor. Findings continued to show a significant increase in glucose monitoring in FDR mothers and fathers and in GP mothers, but did not support a significant increase in GP fathers (p = 0.11). We also examined the time between completion of the pre-notification survey and the post-notification survey, but no significant changes in results were found when adjusting for this variable.
FIGURE 2.
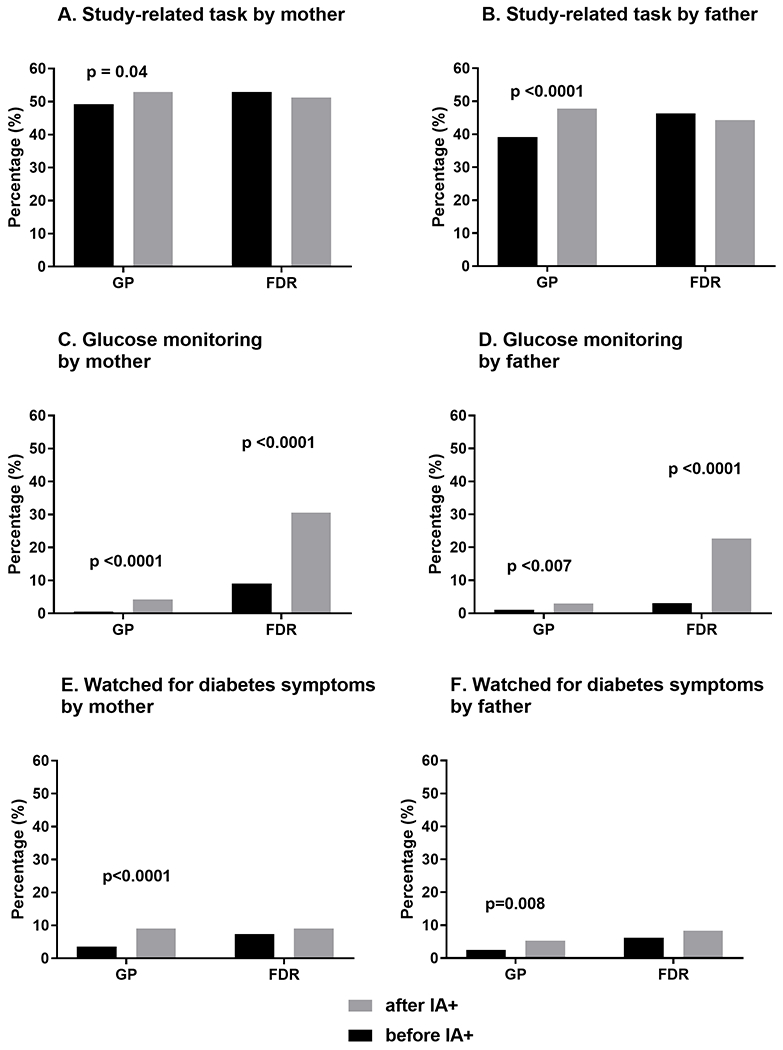
Impact of initial IA+ notification on monitoring behaviors in mothers and fathers by type of behavior and first-degree relative (FDR) status. GP, general population
3.3 |. Long-term impact of IA+ test results on parental monitoring
Figure 3 depicts parent monitoring behavior up to 4 years after child’s first IA+ for three groups: (a) Parents of children with a single nonpersistent IA+ test result (i.e., an IA+ test result occurred once and then disappeared), (b) parents of children with a single persistent IA+ result (i.e., the IA+ test result re-occurred at a subsequent visit), and (c) parents of children with multiple persistent IA+ test results (i.e., the child was positive for two or more different antibodies on two or more occasions). IA status was treated as time-varying, therefore participants in each group changed at each time point based on their current IA status. Tables S1 and S2 show n and CI for each time point. IA status had the strongest impact on glucose monitoring behaviors for both mothers (Figure 3(C)) and fathers (Figure 3(D)). After adjusting for parents’ report of glucose monitoring behaviors immediately after IA+ notification, as well as for other factors associated with glucose monitoring (see Table 2), mothers (OR = 2.30, 95% CI = 1.25–8.20, p < 0.008) but not fathers (OR = 1.55, 95% CI = 0.55–4.42, p = 0.30) of children with single persistent IA+ results were more likely to engage in glucose monitoring as compared to mothers of children with a single nonpersistent IA+ results (Table 3). However, if the child was multiple persistent IA+, fathers were also more likely to monitor glucose as compared to those in the other IA+ groups. If the site recommended glucose monitoring was recommended for children with multiple IAs, the odds of monitoring was much higher than in the other groups (mothers OR = 18.6, 95% CI = 10.5–32.7; fathers OR = 30.9, 95% CI = 13.1–72.8, p < 0.001); but even if glucose monitoring was not recommended, both mothers (OR = 4.47, 95% CI = 2.44–8.20, p < 0.001) and fathers (OR = 6.10, 95% CI = 2.49–14.9, p < 0.001) were still significantly more likely to engage in glucose monitoring if their children were multiple IA+ compared to other IA+ classifications. Data for multiple IA+ participants instructed to monitor glucose levels compared to those not instructed to monitor are shown in Table S1(A,B). FDR status, diabetes risk perception, and anxiety about child’s diabetes risk were also still associated with glucose monitoring after IA+ notification (Table 3).
FIGURE 3.
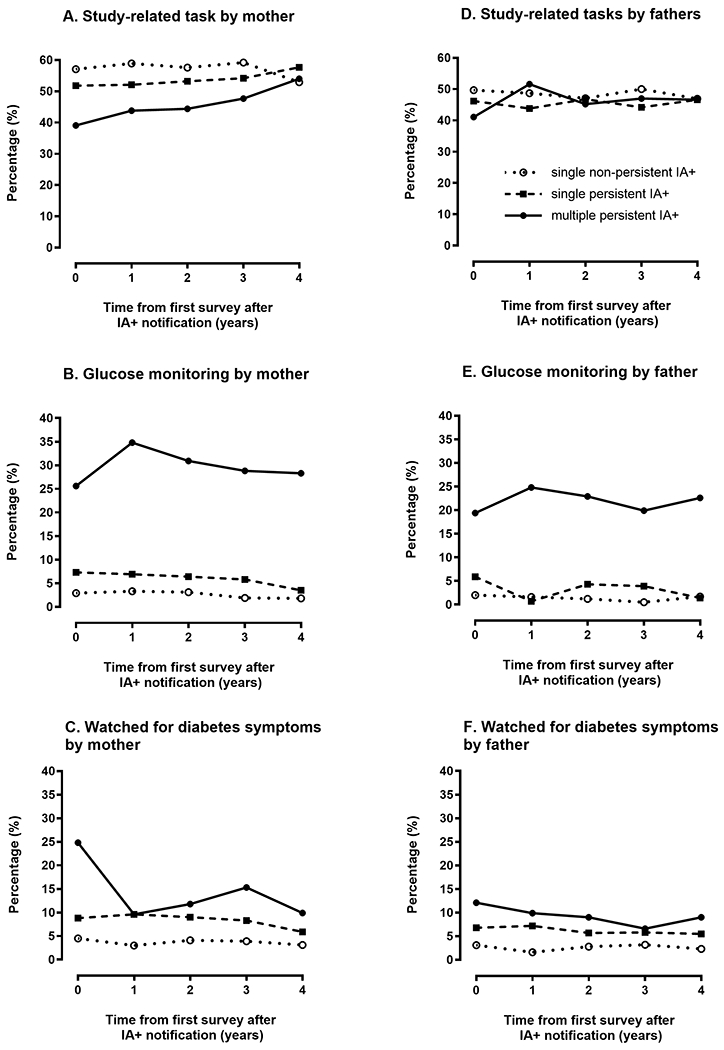
Long term impact of IA+ test results on parental monitoring behaviors
TABLE 3.
Factors associated with glucose monitoring reported by mothers and fathers up to 4 years after a child’s first positive IA+ test result
| Factorsa | Parent |
|||||
|---|---|---|---|---|---|---|
| Mothers |
Fathers |
|||||
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Monitoring immediately after impact | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 5.21 | 2.81-9.65 | <0.001 | 7.93 | 3.60-17.5 | <0.001 |
|
| ||||||
| FDR | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 3.09 | 1.86-5.11 | <0.001 | 3.66 | 1.98-6.74 | <0.001 |
|
| ||||||
| IA status of child | ||||||
| Single, nonpersistent IA | 1.00 | Reference | 1.00 | Reference | ||
| Single persistent IA | 2.30 | 1.25-8.20 | 0.008 | 1.55 | 0.55-4.42 | 0.30 |
| Multiple persistent IA | ||||||
| Glucose monitoring recommended | ||||||
| No | 4.47 | 2.44-8.20 | <0.001 | 6.10 | 2.49-14.9 | <0.001 |
| Yes | 18.6 | 10.5-32.7 | <0.001 | 30.9 | 13.1-72.8 | <0.001 |
|
| ||||||
| Risk perception | ||||||
| Underestimated | 1.00 | Reference | 1.00 | Reference | ||
| Accurate | 2.23 | 1.13-4.41 | 0.02 | 2.78 | 1.71-4.53 | <0.001 |
|
| ||||||
| Anxiety about diabetes (SAI)b | 1.22 | 1.02-1.45 | 0.03 | 1.31 | 1.05-1.64 | 0.02 |
Time from impact, child’s age, ethnic minority status, being a first born child, postnatal depression score and locus of control score were not associated with parents reporting monitoring behaviors.
Higher SAI score (per 10 unit increase) suggests higher anxiety about diabetes.
4 |. DISCUSSION
To our knowledge, this is the first study to longitudinally examine parental behaviors to monitor for type 1 diabetes in children at genetic risk for the condition. Our findings suggest that parents frequently report behaviors to monitor for the onset of type 1 diabetes and that the most common monitoring behaviors reported were TEDDY study-related tasks. This finding suggests that one potential benefit of enrollment in an observational study like TEDDY—that offers no diabetes preventive intervention—is that parents perceive study participation as a way to monitor for the onset of the condition. This perception is accurate as all TEDDY children are more intensively monitored depending on IA+ status and clinical signs of diabetes. In fact, TEDDY children diagnosed with type 1 diabetes have lower rates of diabetic ketoacidosis, lower HbA1cs, and less insulin requirements at diagnosis than children diagnosed in the community in part due to these monitoring strategies.20–22
We examined parental monitoring behaviors across three different cohorts. For consistently IA− children, up to 57% of TEDDY parents reported a monitoring behavior during the first 6 years of the study. TEDDY study-related tasks were by far the most commonly reported, but watching for diabetes-specific symptoms and glucose monitoring were also endorsed at lower rates. Longitudinally, in IA− children, monitoring behaviors appear to remain relatively stable over time after an initial increase in very young children, between 6 and 15 months of age. This initial increase may be attributable to the fact that monitoring behaviors would be more difficult to initiate in infants (e.g., glucose monitoring, watching for signs of diabetes). Overall, our results show that mothers consistently report more monitoring behaviors than fathers, which mirrors previous TEDDY findings suggesting that fathers generally participate less in TEDDY, have less accurate risk perception, and have less anxiety about their child developing diabetes.23 Monitoring was also more common in certain demographic groups including older children, first born children, nonethnic minority children, and FDR families compared to GP families. Within this cohort, psychological and cognitive factors such as having an accurate diabetes risk perception, stronger belief that diabetes can be prevented, postnatal depression, and higher anxiety about diabetes also predicted parental engagement in monitoring behaviors. Parents who are more anxious/depressed and who recognize that their child is at increased risk of developing type 1 diabetes may view monitoring their child for the condition as a way to mitigate their concerns. Parents who believe they can do something to prevent type 1 diabetes, likely view monitoring as a way to detect the condition earlier to prevent it, despite the fact that no empirically supported preventive interventions exist at this time. We have previously shown that despite the lack of preventive intervention(s), many TEDDY parents do engage in behaviors intended to prevent type 1 diabetes.9
Due to the invasive nature of glucose monitoring and given that this was not recommended to TEDDY families of IA− children, we examined this behavior separately. We found that up to 11% of FDR mothers and 8% of FDR fathers endorsed this behavior and this increased report in FDR compared to GP families was even more pronounced than what was observed for all monitoring behaviors. This may be due to FDR parents having more access to and familiarity with glucose monitoring due to their family history of the condition. Further, the association between increased glucose monitoring in parents with higher anxiety about diabetes was also stronger than what was seen in associations with all monitoring behaviors. It is not surprising that anxiety is a strong predictor of parents’ willingness to check their child’s glucose levels, even in the absence of expert recommendation to do so. In addition, although a belief that diabetes could be prevented was associated with any monitoring behavior, it was not associated with glucose monitoring specifically. Perhaps parents who engage in glucose monitoring are more familiar with type 1 diabetes and recognize that no preventive intervention currently exists. Finally, we found stronger relationships between glucose monitoring and being from Finland, being an older child within this young cohort, and not being a first born child, suggesting that glucose monitoring is more salient in these groups, perhaps due to cultural beliefs, parental ideas about the child’s willingness to accept glucose monitoring (e.g., older children may be more accepting) or parental beliefs about the child’s vulnerability (e.g., parents may be more protective of first born children and less willing to engage in an invasive behavior such as glucose monitoring).
As expected, many parents initiated monitoring after their child’s IA+ result. Interestingly, there were meaningful differences in monitoring behavior changes following notification between FDR and GP parents. In GP parents, following IA+ notification, rates of watching for diabetes symptoms, reporting study-related tasks as a monitoring behavior, and glucose monitoring all significantly increased. In FDR mothers and fathers, only glucose monitoring significantly increased following IA+ notification. Given their close personal experience with type 1 diabetes, FDR parents may believe that the most valuable method of monitoring for the condition is glucose monitoring – rather than watching for symptoms or performing study related tasks - after learning of their child’s increased risk despite the fact that there was no encouragement to do so on the part of TEDDY study staff for those with single IA+ status. However, it is important to acknowledge that while glucose monitoring increased significantly in the FDR parents after IA+ notification, this group also had much higher levels of glucose monitoring compared to GP parents even prior to IA+ notification.
Finally, we found that in the 4 years following IA+ notification, monitoring behaviors remain relatively stable for parents of children with single nonpersistent and single persistent IA+ results, with TEDDY-related tasks being the most commonly reported monitoring behavior. However, there were some interesting trends observed in children with multiple IAs. In this subset, up to 35% report glucose monitoring. Given that within TEDDY most study sites recommend that parents initiate glucose monitoring following notification that their child has multiple IAs, this finding is, at least in part, a reflection of compliance with study protocol. Interestingly, parents of children with multiple IAs endorse TEDDY-related tasks as a monitoring behavior less commonly than parents of single persistent and single nonpersistent IAs although these reports do increase over the 4 years following IA notification (e.g., from 39% to 54% of mothers report this over time). Further, while parents of multiple IA+ children watch for diabetes symptoms at higher rates that other IA+ groups, this monitoring behavior declines quickly in the year following IA+ notification, particularly in mothers. Taken together, it appears that as parents of children positive for multiple IAs engage in more glucose monitoring, they watch for diabetes symptoms less often, perhaps perceiving that glucose monitoring is a more meaningful method of monitoring for early signs of the condition. Also, over time, TEDDY-related tasks (e.g., OGTT) may be viewed as an increasingly important way to monitor for type 1 diabetes in parents of children positive for multiple IAs.
The current study has several limitations worth noting. Monitoring data was self-reported by parents and thus may be subject to reporting biases, although this was minimized by the use of a recall item in which we asked parents to report behaviors rather than providing them with a list of behaviors (i.e., recognition item). Further, while we have data showing monitoring behaviors over time, we do not have data about how often certain behaviors are occurring. For example, a better understanding of how often parents are checking their children’s blood glucose levels would be helpful to elucidate whether this is a regular or merely intermittent behavior in parents who undertake it. TEDDY data is based on a unique, well-characterized, high-risk population in which children are closely tracked and parents are regularly informed of their child’s increased risk of type 1 diabetes. Therefore, families that chose to enroll in TEDDY are self-selected and may be predisposed to view the study participation as a benefit for ongoing monitoring of their child. Our results, therefore, may not be generalizable to the GP or even FDR families not enrolled in TEDDY.
In conclusion, we have documented that parents enrolled in a longitudinal, observational study for children at genetic risk for type 1 diabetes frequently monitor their children for the condition. Findings suggest that parental monitoring is a complex behavior, differentially impacted by a variety of factors such as demographic and psychological constructs, as well as the level of type 1 diabetes risk communicated to parents (i.e., IA status). A small but relevant group of parents engage in unnecessary glucose monitoring, despite not being instructed to do so and despite there being no extant literature suggesting that intermittent glucose monitoring may be helpful in predicting the development of type 1 diabetes. Our data suggest that additional education regarding more watchful waiting strategies (e.g., monitoring for symptoms) is needed given that even within a high risk population such as TEDDY, this behavior was reported by relatively few parents, regardless of FDR or IA status. Watching for diabetes symptoms is an easy, noninvasive monitoring behavior that can be recommended to both high risk populations, like TEDDY families, and the GP within clinical settings. We found that many TEDDY families view study participation as a way to monitor for diabetes and this can provide guidance for researchers conducting other longitudinal studies. Participants may be more likely to remain in a lengthy study if they perceive monitoring for the condition as a benefit of participation. Future work within TEDDY will examine monitoring behaviors vis-à-vis study participation to better understand how they may influence study satisfaction, protocol adherence, and study retention.
Supplementary Material
ACKNOWLEDGMENTS
Funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: HHSN267200700014C; University of Colorado, Grant/Award Number: UL1 TR001082; University of Florida, Grant/Award Number: UL1 TR000064; Centers for Disease Control and Prevention; Juvenile Diabetes Research Foundation - United States of America; National Institute of Environmental Health Sciences; National Institute of Child Health and Human Development; National Institute of Allergy and Infectious Diseases
Abbreviations:
- EDPS
Edinburgh Postnatal Depression Scale
- FDR
first degree relative
- GP
general population
- HLA
human leucocyte antigen
- PANDA
Prospective Assessment of Newborn for Diabetes Autoimmunity
- SAI
Spielberg State Anxiety Inventory
- TEDDY
The Environmental Determinants of Diabetes in the Young
- WBQ
Well-Being Questionnaire
Footnotes
The multiple IA+ risk estimate of 50 out of 100 was communicated to TEDDY families up to 2017 and is accurate for the cohort and data examined in this paper. Since 2017, the TEDDY Study has changed their risk communication slightly. Families of children with multiple IA+ results are currently told that their child’s risk of developing diabetes is 70 out of 100.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Kimpimaki T, Kulmala P, Savola K, et al. Disease-associated autoantibodies as surrogate markers of type 1 diabetes in young children at increased genetic risk. Childhood diabetes in Finland study group. J Clin Endocrinol Metab. 2000;85(3):1126–1132. 10.1210/jcem.85.3.6466. [DOI] [PubMed] [Google Scholar]
- 2.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia. 1996;39(7):807–812. [DOI] [PubMed] [Google Scholar]
- 3.Wahlberg J, Fredriksson J, Nikolic E, Vaarala O, Ludvigsson J. Environmental factors related to the induction of beta-cell autoantibodies in 1-yr-old healthy children. Pediatr Diabetes. 2005;6(4):199–205. 10.1111/j.1399-543X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen L, Schatz D. Current and future efforts toward the prevention of type 1 diabetes. Pediatr Diabetes. 2016;17(22):78–86. 10.1111/pedi.12333. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson Stolt U, Liss PE, Ludvigsson J. Parents want to know if their child is at high risk of getting diabetes. Ann N Y Acad Sci. 2003; 1005:395–399. 10.1196/annals.1288.066. [DOI] [PubMed] [Google Scholar]
- 6.Ross LF. Minimizing risk: the ethics of predictive diabetes mellitus screening research in newborns. Arch Pediatr Adolesc Med. 2003;157:89–95. [DOI] [PubMed] [Google Scholar]
- 7.Roth LR. Psychological and ethical aspects of prevention trials. J Pediatr Endocrinol Metab. 2001;14(1):669–674. [DOI] [PubMed] [Google Scholar]
- 8.Schatz D, Krischer J, She J. To screen or not to screen for pre-type 1 diabetes? Horm Res. 2002;57(1):12–17. [DOI] [PubMed] [Google Scholar]
- 9.Hood KK, Johnson SB, Baughcum AE, She JX, Schatz DA. Maternal understanding of infant diabetes risk: differential effects of maternal anxiety and depression. Genet Med. 2006;8(10):665–670. 10.1097/01.gim.0000237794.24543.4d. [DOI] [PubMed] [Google Scholar]
- 10.Bennett Johnson S, Baughcum AE, Carmichael SK, She JX, Schatz DA. Maternal anxiety associated with newborn genetic screening for type 1 diabetes. Diabetes Care. 2004;27(2):392–397. 10.2337/diacare.27.2.392. [DOI] [PubMed] [Google Scholar]
- 11.Bennett Johnson S, Tercyak KP Jr. Psychological impact of islet cell antibody screening for IDDM on children, adults, and their family members. Diabetes Care. 1995;18(10):1370–1372. 10.2337/diacare.18.10.1370. [DOI] [PubMed] [Google Scholar]
- 12.Smith LB, Lynch KF, Baxter J, et al. Factors associated with maternal-reported actions to prevent type 1 diabetes in the first year of the TEDDY study. Diabetes Care. 2014;37(2):325–331. 10.2337/dc13-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucidarme N, Domingues-Muriel E, Castro D, Czernichow P, Levy-Marchal C. Appraisal and implications of predictive testing for insulin-dependent diabetes mellitus. Diabetes Metab. 1998;24(6):550–553. [PubMed] [Google Scholar]
- 14.Hendrieckx C, De Smet F, Kristoffersen I, Bradley C. Risk assessment for developing type 1 diabetes: intentions of behavioural changes prior to risk notification. Diabetes Metab Res Rev. 2002;18(1):36–42. 10.1002/dmrr.234. [DOI] [PubMed] [Google Scholar]
- 15.Baughcum AE, Johnson SB, Carmichael SK, Lewin AB, She JX, Schatz DA. Maternal efforts to prevent type 1 diabetes in at-risk children. Diabetes Care. 2005;28(4):916–921. 10.2337/diacare.28.4.916. [DOI] [PubMed] [Google Scholar]
- 16.Hagopian WA, Erlich H, Lernmark A, et al. The environmental determinants of diabetes in the young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):733–743. 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psych. 1987;150:782–786. 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 18.Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. N Engl J Med. 2002;347(3):194–199. 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- 19.Spielberger CDGR, Lushene R. Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 20.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–126. 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steck AK, Larsson HE, Liu X, et al. Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes. 2017;18:794–802. 10.1111/pedi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes. 2018;19(2):314–319. 10.1111/pedi.12541. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SB, Lynch KF, Roth R, Schatz D. My child is islet autoantibody positive: impact on parental anxiety. Diabetes Care. 2017;40(9): 1167–1172. 10.2337/dc17-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


