Abstract
Background
Enteral feeding for very preterm or very low birth weight (VLBW) infants is often delayed for several days after birth due to concern that early introduction of feeding may not be tolerated and may increase the risk of necrotising enterocolitis. Concerns exist, however, that delaying enteral feeding may diminish the functional adaptation of the gastrointestinal tract and prolong the need for parenteral nutrition with its attendant infectious and metabolic risks.
Objectives
To determine the effects of delayed introduction of progressive enteral feeds on the risk of necrotising enterocolitis, mortality and other morbidities in very preterm or VLBW infants.
Search methods
Search strategies were developed by an information specialist in consultation with the review authors. The following databases were searched in October 2021 without date or language restrictions: CENTRAL (2021, Issue 10), MEDLINE via OVID (1946 to October 2021), Embase via OVID (1974 to October 2021), Maternity and Infant Care via OVID (1971 to October 2021), CINAHL (1982 to October 2021). We also searched for eligible trials in clinical trials databases, conference proceedings, previous reviews, and reference lists of retrieved articles.
Selection criteria
Randomised controlled trials that assessed the effects of delayed (four or more days after birth) versus earlier introduction of progressive enteral feeds on necrotising enterocolitis, mortality and other morbidities in very preterm or VLBW infants.
Data collection and analysis
Two review authors separately evaluated trial risk of bias, extracted data, and synthesised effect estimates using risk ratio (RR), risk difference (RD), and mean difference. We used the GRADE approach to assess the certainty of evidence for effects on necrotising enterocolitis, mortality, feed intolerance, and invasive infection.
Main results
We included 14 trials in which a total of 1551 infants participated. Potential sources of bias were lack of clarity on methods to generate random sequences and conceal allocation in half of the trials, and lack of masking of caregivers or investigators in all of the trials. Trials typically defined delayed introduction of progressive enteral feeds as later than four to seven days after birth and early introduction as four days or fewer after birth. Infants in six trials (accounting for about half of all of the participants) had intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery.
Meta‐analyses showed that delayed introduction of progressive enteral feeds may not reduce the risk of necrotising enterocolitis (RR 0.81, 95% confidence interval (CI) 0.58 to 1.14; RD ‐0.02, 95% CI ‐0.04 to 0.01; 13 trials, 1507 infants; low‐certainty evidence due risk of bias and imprecision) nor all‐cause mortality before hospital discharge (RR 0.97, 95% CI 0.70 to 1.36; RD ‐0.00, 95% CI ‐0.03 to 0.03; 12 trials, 1399 infants; low‐certainty evidence due risk of bias and imprecision). Delayed introduction of progressive enteral feeds may slightly reduce the risk of feed intolerance (RR 0.81, 95% CI 0.68 to 0.97; RD ‐0.09, 95% CI ‐0.17 to ‐0.02; number needed to treat for an additional beneficial outcome = 11, 95% CI 6 to 50; 6 trials, 581 infants; low‐certainty evidence due to risk of bias and imprecision) and probably increases the risk of invasive infection (RR 1.44, 95% CI 1.15 to 1.80; RD 0.10, 95% CI 0.04 to 0.15; number needed to treat for a harmful outcome = 10, 95% CI 7 to 25; 7 trials, 872 infants; moderate‐certainty evidence due to risk of bias).
Authors' conclusions
Delaying the introduction of progressive enteral feeds beyond four days after birth (compared with earlier introduction) may not reduce the risk of necrotising enterocolitis or death in very preterm or VLBW infants. Delayed introduction may slightly reduce feed intolerance, and probably increases the risk of invasive infection.
Plain language summary
Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants
Background
Very preterm (born more than eight weeks early) or very low birth weight (VLBW; less than 1500 grams) newborn babies are at risk of developing a severe bowel disorder called necrotising enterocolitis (where the bowel becomes inflamed and dies). Infants whose growth in the womb is compromised are thought to have a high risk of developing necrotising enterocolitis. Very preterm or VLBW infants are initially fed low amounts of milk, with amounts gradually increased over several days. Delaying the introduction and increase in volume of milk feeds for several days (or longer) after birth may be one possible way to reduce the risk of this condition.
Study characteristics
We searched for clinical trials assessing the effect of delayed (more than four days after birth) versus earlier introduction of milk feeds (where human milk or formula is fed directly by a tube into the stomach) on the risk of necrotising enterocolitis, death and general health in very preterm or VLBW infants. The search is up‐to‐date as of October 2021.
Key results
We found 14 trials with 1551 infants participating. About half of these infants had evidence of compromised growth while in the womb. Combined analysis of these trials showed that delayed introduction of progressive enteral feeds may not reduce the risk of necrotising enterocolitis or death. Delayed feeding may slightly reduce the risk of feed intolerance, but probably increases the risk of serious infection occurring.
Conclusions and certainty of evidence
This review provides low‐certainty evidence that delaying the introduction of enteral feeds may not reduce the risk of necrotising enterocolitis or death for very preterm or VLBW infants, including infants whose growth in the womb was compromised.
Summary of findings
Summary of findings 1. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very preterm or very low birth weight infants.
| Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very preterm or very low birth weight infants | |||||
| Patient or population: very preterm (< 32 weeks' gestation) or very low birth weight (< 1500 g) infants Setting: neonatal care facilities in Argentina, India, Iran, Colombia, Qatar, Turkey, North America, Ireland, and the UK. Intervention: delayed (≥ 4 days after birth) introduction of progressive enteral feeds Comparison: early (< 4 days after birth) introduction of progressive enteral feeds | |||||
| Outcomes | Anticipated absolute effects* | Relative effect (95% CI) | No. of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with early introduction | Risk with delayed introduction | ||||
| Necrotising enterocolitis prior to hospital discharge | 85 per 1000 | 69 per 1000 (95% CI 49 to 97) | RR 0.81 (95% CI 0.58 to 1.14] | 1507 (13) | ⊕⊕⊝⊝ LOWa,b |
| Mortality prior to hospital discharge | 84 per 1000 | 81 per 1000 (95% CI 59 to 114) | RR 0.97 (95% CI 0.70 to 1.36) | 1399 (12) | ⊕⊕⊝⊝ LOWa,b |
| Feed intolerance prior to hospital discharge | 461 per 1000 | 374 per 1000 (95% CI 314 to 447) | RR 0.81 (95% CI 0.68 to 0.97) | 581 (6) | ⊕⊕⊝⊝ LOWa,c |
| Invasive infection prior to hospital discharge | 266 per 1000 | 383 per 1000 (95% CI 306 to 479) | RR 1.44 (95% CI 1.15 to 1.80) | 872 (7) | ⊕⊕⊕⊝ MODERATEa |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio. | |||||
GRADE Working Group certainty of evidence
| |||||
aDowngraded one level for serious study limitations (risk of bias due to lack of masking of clinicians, caregivers, and investigators in trials)
bDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or harm)
cDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial benefit or slight/no benefit)
Background
Description of the condition
Necrotising enterocolitis (NEC), a syndrome of acute intestinal necrosis of unknown aetiology, affects about 5% of very preterm (< 32 weeks' gestation) or very low birth weight (VLBW) (< 1500 g) infants (Horbar 2012; Samuels 2017). Intrauterine growth restriction may be an additional risk factor, especially if associated with circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in Doppler studies of the fetal aorta or umbilical artery (Bernstein 2000; Garite 2004; Kamoji 2008). Infants who develop NEC experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not develop NEC (Battersby 2018; Berrington 2012). The associated mortality rate is more than 20%, and infants who develop NEC, especially if associated with bloodstream infections, have a higher risk of developmental delay and neurodisability compared with their unaffected peers (Hickey 2018; Shah 2012).
Most very preterm or VLBW infants who develop NEC have received enteral milk feeds. Feeding with human milk rather than cow milk formula reduces the risk of NEC (Quigley 2019). Other differences in enteral feeding regimens, such as the timing of introduction of feeds and the size of daily feeds volume increments, may also contribute to inter‐unit variation in the incidence of NEC (Walsh 2019). Observational studies have suggested that delaying the introduction of enteral feeds beyond the first few days after birth, or increasing the volume of feeds by less than about 24 mL/kg body weight each day, is associated with a lower risk of developing NEC in very preterm or VLBW infants (Henderson 2009; Patole 2005).
Description of the intervention
Oral feeding for very preterm or VLBW infants is not usually possible because of neurological immaturity or respiratory distress challenging breathing, sucking, and swallowing coordination (Viswanathan 2019). Consequently, most very preterm or VLBW infants receive milk via a gastric feeding tube. The timing of introduction of milk feeds, and the rate of advancement of feed volumes, is determined and monitored by clinicians and care‐givers. Substantial variation in early enteral feeding practices for very preterm or VLBW infants exists (Hay 2018). In high‐income countries, clinical policy and practice has tended to favour a conservative approach to introducing enteral feeds because of concerns about adverse effects including gastro‐oesophageal reflux and aspiration of stomach contents, and whether early feeding might increase the risk of NEC (de Waard 2018). One commonly recommended and widely used approach is to limit any enteral feeding to 'trophic' levels (minimal enteral nutrition) during the first few days after birth, and to delay introducing progressive enteral feeding (beyond trophic levels) until clinicians are reassured that the trophic feeding volumes are well‐tolerated and absorbed (Klingenberg 2012). In many low‐ and middle‐income countries with fewer resources for neonatal care, practice has tended to be more pragmatic and to favour early introduction and advancement of enteral feeds (often facilitated by 'kangaroo' mother care) for clinically stable very preterm or VLBW infants (Conde‐Agudelo 2016).
How the intervention might work
Delaying the introduction of milk feeds aims to reduce the risk of feed intolerance (inability to absorb and digest milk) and NEC by limiting the physiological and metabolic stresses on the immature gastrointestinal tract during the first few days after birth. Potential disadvantages, however, are associated with this conservative approach to early enteral feeding (Flidel‐Rimon 2004; Leaf 2013). Because gastrointestinal hormone secretion and motility are stimulated by milk feeds, delayed introduction of progressive enteral feeds may delay the functional adaptation of the gastrointestinal tract and disrupt the patterns of microbial colonisation (Burrin 2002; Embleton 2017). Intestinal dysmotility and dysbiosis might exacerbate feed intolerance and delay the establishment of enteral feeding independently of parenteral nutrition (Pammi 2017). Prolonging the duration of parenteral nutrition is associated with infectious and metabolic complications that increase mortality and morbidity, prolong hospital stay, and adversely affect growth and development (Embleton 2013; el Manouni el Hassani 2019). It has been argued that the risk of NEC should not be considered in isolation from these other potential clinical outcomes when evaluating enteral feeding practices for very preterm or VLBW infants (Flidel‐Rimon 2006; Hartel 2009).
Why it is important to do this review
Given the potential for the timing of the introduction of progressive enteral feeding to affect important outcomes for very preterm or VLBW infants, it is important to identify, appraise, and synthesise available evidence from randomised controlled trials to inform practice and research. This review focuses on the comparison of delayed versus earlier introduction of progressive enteral feeding; that is, advancing the volume of milk feeds beyond minimal enteral nutrition levels. Other Cochrane Reviews have assessed the evidence for the effect of prolonged minimal enteral nutrition (restricting feed volumes to trophic levels) versus a period of enteral fasting, and different rates advancement of enteral feed volumes (including full enteral feeding from birth) on the risk of NEC and mortality in very preterm or VLBW infants (Morgan 2013; Oddie 2017; Walsh 2020).
Objectives
To determine the effectiveness of delayed introduction of progressive enteral feeds on reducing the risk of NEC, mortality and other morbidities in very preterm or VLBW infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs or cluster‐RCTs.
Types of participants
VLBW (< 1500 g) or very preterm (< 32 weeks' gestation) newborn infants.
If studies included some infants of > 32 weeks' gestation and > 1500 g birth weight (and subgroup data were not provided), we included data if they had enrolled a majority (> 50%) of very preterm or VLBW infants.
Types of interventions
Delayed introduction (four or more days after birth) of progressive enteral feeds versus earlier introduction of enteral feeds. We defined progressive enteral feeding as the intention to advance feed volumes in excess of minimal enteral nutrition levels (24 mL/kg/day) within five days of commencement or by one week after birth.
Infants in each group should have received the same type of milk (breast milk or formula), the same route and mode of feeding (intragastric or transpyloric, bolus gavage or continuous) and the same rate of feed volume advancement in both groups.
Types of outcome measures
We focused on assessing effects on infant‐ and family‐important outcomes, principally neonatal morbidities that plausibly affect rates of mortality or neurodevelopmental impairment or disability.
Primary outcomes
-
NEC confirmed at surgery or autopsy or using standardised clinical and radiological criteria (VON 2020):
at least one of: bilious gastric aspirate or emesis; or abdominal distention; or blood in stool; and
at least one of: abdominal radiograph showing pneumatosis intestinalis; or gas in the portal venous system; or free air in the abdomen.
All‐cause mortality before discharge from hospital
Secondary outcomes
-
Growth
Time to regain birth weight and rates of weight gain, linear growth, head growth, or skinfold thickness growth up to six months (corrected for preterm birth)
Long‐term growth: weight, height, or head circumference (or proportion of infants who remained below the 10th percentile for the index population's distribution) assessed at intervals from six months of age
Neurodevelopmental disability defined as one or more of: moderate or severe developmental delay (> two standard deviations (SD) below the mean of standardised infant developmental assessment aged > 18 months), and classifications of disability, including non‐ambulant cerebral palsy and auditory or visual impairment
Time to establish full enteral feeding independently of parenteral nutrition
Time to establish oral feeding (independently of parenteral nutrition or enteral tube feeding, or both)
Feed intolerance (requirement to cease enteral feeds > 4 hours)
Invasive infection confirmed by culture of bacteria or fungus from blood, cerebrospinal fluid, or another normally sterile body space
Duration of hospital stay (days)
Search methods for identification of studies
An information specialist developed search strategies in consultation with the authors. Search strategies used three conceptual approaches:
enteral nutrition terms and neonate;
necrotising enterocolitis and prevention and neonate;
parenteral nutrition and adverse effects and neonate.
The neonatal terms are a standardised set developed by Cochrane Neonatal.
A methodological filter was used to limit retrieval to RCTs.
Electronic searches
The following databases were searched in October 2021 without language, publication year, publication status, or publication type restrictions:
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 10) (Appendix 1)
MEDLINE via OVID (1946 to October 2021) (Appendix 2)
Embase via OVID (1974 to October 2021) (Appendix 3)
Maternity and Infant Care via OVID (1971 to October 2021) (Appendix 4)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to October 2021) (Appendix 5)
We searched the US National Library of Medicine trial registry (ClinicalTrials.gov) for ongoing or recently completed trials (Appendix 6).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review.
Data collection and analysis
We used the standard methods of Cochrane Neonatal (neonatal.cochrane.org/).
Selection of studies
WM screened titles and abstracts of all records identified by the search and coded records as “order” or “exclude". A second review author (LY or SO) assessed all records coded as “order” and made the final decision about which records should be ordered as full‐text articles. Two review authors read the full texts and used a checklist to assess each article's eligibility for inclusion on the basis of prespecified inclusion and exclusion criteria.
Data extraction and management
WM and LY extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from trial reports were insufficient, we contacted trialists to ask for further information.
Assessment of risk of bias in included studies
Two review authors (WM and SO) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 7 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When we deemed it appropriate to combine two or more study arms, we obtained treatment effects from combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We determined the number needed to treat for an additional beneficial outcome (NNTB) or harmful outcome (NNTH) for a statistically significant difference in RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. For cluster‐randomised trials (had we identified any for inclusion), we planned to undertake analyses at the level of the individual while accounting for clustering in the data by using methods recommended in the Cochrane Handbook (Higgins 2020).
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or were reported unclearly. When data remained missing, we examined the impact on effect size estimates by performing sensitivity analyses.
Assessment of heterogeneity
We examined treatment effects in individual trials and heterogeneity between trial results by inspecting forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high (I² > 50%) levels of heterogeneity, we explored possible causes by performing subgroup analyses.
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. Studies using the interventions in a potentially eligible infant population but not reporting on any of the primary and secondary outcomes were documented in the Characteristics of included studies tables. We used the funnel plots to screen for publication bias where there were a sufficient number of studies (at least 10) reporting the same outcome. If publication bias was suggested by substantial asymmetry of the funnel plot on visual assessment, we planned to assess this statistically use Harbord's modification of Egger's test (Harbord 2006).
Data synthesis
We used a fixed‐effect model inverse variance meta‐analysis for combining data where trials examined the same intervention and the populations and methods of the trials were judged to be similar.
Subgroup analysis and investigation of heterogeneity
We pre‐specified subgroup analyses for primary outcomes to compare effects in trials:
in which most infants were exclusively formula‐fed versus trials in which most infants were exclusively or partially fed with human milk (maternal or donor);
in which most participants were extremely low birth weight (ELBW; < 1000 g) or extremely preterm (< 28 weeks' gestation at birth) versus trials in which most infants were ≥ 28 weeks' gestation at birth or of birth weight ≥ 1000 g; and
which restricted participation to infants with intrauterine growth restriction or absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials which did not do so.
Sensitivity analysis
We planned to perform sensitivity analyses if:
there was unexplained high heterogeneity (I² > 75%) (explored by removing the outlying trial or trials);
a trial with high risk of bias (including high level of missing outcome data) was included in the meta‐analysis of an outcome where the other studies had low risk of bias (removed the study with high risk of bias).
Summary of findings and assessment of the certainty of the evidence
Two review authors (WM and LY) used the GRADE approach, as outlined in the GRADE handbook (Schünemann 2013; Walsh 2021), to assess the certainty of the evidence for effects on infant‐ and family‐important outcomes, principally NEC, all‐cause mortality, feed intolerance, and invasive infection. We included these four outcomes in Table 1.
Two review authors (WM and LY) independently assessed the certainty of the evidence for each of the outcomes above. We initially considered evidence from RCTs to be of high certainty. We downgraded this certainty by one level for serious limitations or by two levels for very serious limitations based on five GRADE criteria: design weakness (risk of bias), inconsistency across studies, indirectness, imprecision of estimates, and presence of publication bias. We used the GRADEpro GDT software to create Table 1 for reporting the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence for a given outcome as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
After the removal of duplicates from the search results, we screened 6450 titles and abstracts, which included forward and backward citation searches, clinical trials registers and grey literature. We evaluated 21 new articles sourced as full‐text reports. We included five of these new trials alongside the nine previously included trials (Figure 1).
1.
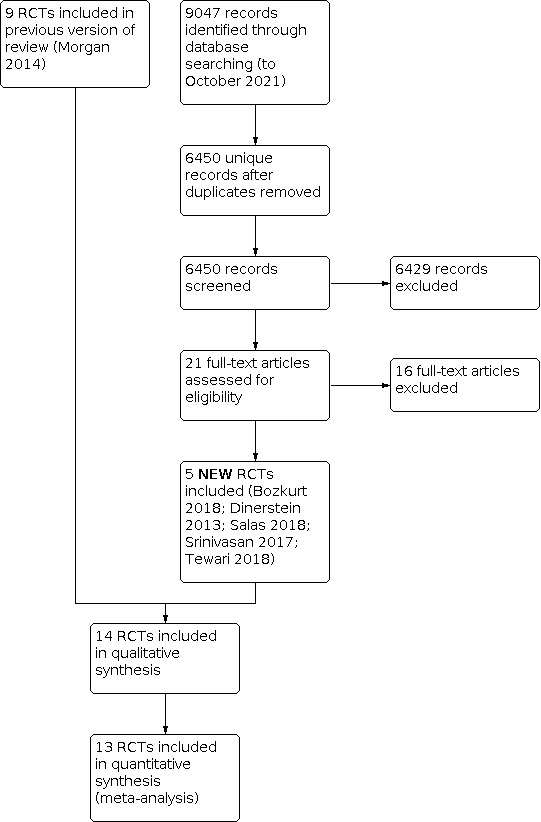
Study flow diagram: review update.
Included studies
Fourteen trials fulfilled the review eligibility criteria (Abdelmaaboud 2012; Armanian 2013; Arnon 2013; Bozkurt 2020; Davey 1994; Dinerstein 2013; Karagianni 2010; Khayata 1987; Leaf 2012; Ostertag 1986; Pérez 2011; Salas 2018; Srinivasan 2017; Tewari 2018). See Characteristics of included studies.
Population
A total of 1551 infants participated in the included trials.
Three small trials were undertaken in neonatal care centres in North America during the 1980s and early 1990s.
Davey 1994: 62 clinically stable preterm infants of birth weight less than 2000 grams who had a low umbilical artery catheter in place (most participants were of birth weight less than 1500 grams or gestational age less than 32 weeks).
Khayata 1987: 12 VLBW infants.
Ostertag 1986: 38 VLBW infants assessed to be at high risk of developing NEC.
The more recent trials were performed in neonatal care centres in various countries during the 2000s to 2010s.
Abdelmaaboud 2012: single‐centre study in Qatar, 125 preterm infants with intrauterine growth restriction and abnormal Doppler flow patterns on ultrasound of the umbilical artery (most participants were of birth weight less than 1500 grams).
Armanian 2013: single‐centre study in Iran, 82 VLBW infants.
Arnon 2013: single‐centre study in Israel, 60 small for gestational age preterm infants (most participants were of birth weight less than 1500 grams).
Bozkurt 2020: single‐centre study in Turkey, 229 preterm infants with birth weight less than 1251 grams.
Dinerstein 2013: single‐centre study in Argentina, 62 appropriate for gestation preterm infants (< 31 weeks' gestation).
Karagianni 2010: single‐centre study in Greece, 84 infants less than 35 weeks' gestation with a birth weight less than 10th percentile and evidence of abnormal fetal blood flow patterns on Doppler ultrasound of the umbilical artery.
Leaf 2012: 54‐centre trial in the UK and Ireland, 404 infants: (a) less than 35 weeks' gestation, (b) birth weight less than 10th percentile and (c) evidence of abnormal fetal blood flow patterns on Doppler ultrasound studies. Since most participants were of birth weight less than 1500 grams, we made a consensus decision to include the trial.
Pérez 2011: single‐centre study in Colombia, 239 very preterm or VLBW infants.
Salas 2018: single‐centre study in the USA, 60 preterm infants (< 29 weeks' gestation), appropriate weight for gestation.
Srinivasan 2017: single centre study in India, 32 preterm infants with evidence of intrauterine growth restriction associated with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery.
Tewari 2018: single centre study in India, 62 preterm (27 to 32 weeks' gestation) infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery.
In six trials (accounting for about half of the total participants), all participating infants had evidence of intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery (Abdelmaaboud 2012; Arnon 2013; Davey 1994; Karagianni 2010; Leaf 2012; Srinivasan 2017). We included these trials since most (> 50%) of the infant were of < 32 weeks' gestational age at birth or of birth weight < 1500 g.
Interventions/comparisons
Trials typically defined delayed introduction of progressive enteral feeds as later than day four to day seven after birth. Early feeding varied from day one to day four after birth.
In nine trials, infants received expressed human milk or artificial formula or both (Abdelmaaboud 2012; Armanian 2013; Arnon 2013; Bozkurt 2020; Davey 1994; Karagianni 2010; Leaf 2012; Pérez 2011; Salas 2018). Infants in three of the trials received only expressed maternal milk or donor human milk (Dinerstein 2013; Srinivasan 2017; Tewari 2018). In two trials, infants received only formula (Ostertag 1986; Khayata 1987).
Infants received enteral feeds by gavage at one‐ or two‐hourly intervals in all the trials except Ostertag 1986, where infants received feeds by continuous intragastric infusion. Most trials specified criteria and indications for advancing (daily increments of 15 to 30 mL/kg) or interrupting enteral feed (e.g. residual gastric contents not greater than 3 to 5 mL or one‐third to one‐half of the previous feed volume, frequent vomiting, abdominal distention or detection of blood in the stools).
Outcomes
All the trials except Khayata 1987 reported NEC (stage II/III modified Bell criteria; confirmed radiologically or at surgery or autopsy). Other reported outcomes included mortality, time to establish full enteral feeding, invasive infection and duration of hospital stay. None of the trials assessed long term growth or neurodevelopment.
Excluded studies
We excluded 16 reports after full‐text screening (see Characteristics of excluded studies table). Several studies were excluded for design reasons (not randomised), and most trials were excluded either because both groups received early introduction of progressive enteral feeds, or the primary comparison was rate of feed volume advancement rather than timing of introduction.
Characteristic of studies awaiting classification
There is one study awaiting classification (Li 2016).
Risk of bias in included studies
'Risk of bias' assessments are described in the Characteristics of included studies table. The methodological quality of the included trials was generally high, but the nature of the intervention meant that parents, caregivers, or clinical investigators were aware of each infant's allocated feeding group (Figure 2).
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In half of the trial reports, methods to ensure adequate allocation concealment were not described. The other trials employed adequate methods to generate random sequences (typically computer‐generated) and to ensure adequate allocation concealment (typically using sealed opaque envelopes).
Blinding
None of the included trials was able to mask feeding strategies from parents, caregivers, or clinical investigators (though some may have masked assessment of abdominal radiographs for diagnosis of NEC). All the trials were assessed as being at high risk of performance or detection bias.
Incomplete outcome data
All trials reported complete or near‐complete assessments of primary outcomes (low risk of attrition bias).
Selective reporting
Although trial protocols were not available for most trials, selective reporting bias was not considered a major threat given that all relevant clinical outcomes were reported.
Other potential sources of bias
We did not find evidence of important between‐group baseline differences in participant characteristics or demographics in any other trials.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Necrotising enterocolitis
Meta‐analysis of data from 13 trials (1507 infants) showed that delayed introduction of progressive enteral feeds may not reduce the risk of NEC: RR 0.81, 95% CI 0.58 to 1.14 (I² = 0%); RD ‐0.02, 95% CI ‐0.04 to 0.01 (Analysis 1.1). The funnel plot was not markedly asymmetrical (Figure 3). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations (risk of bias) and imprecision.
1.1. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 1: Necrotising enterocolitis (NEC)
3.

Funnel plot‐ Necrotising enterocolitis
Subgroup analyses
We found no evidence of subgroup differences by type of milk: test for subgroup differences: Chi² = 2.45, degrees of freedom (df) = 2 (P = 0.29), I² = 18.3% (Figure 4)
None of the trials recruited predominantly ELBW or extremely preterm infants
We found no evidence of subgroup differences by trials that restricted participation to growth‐restricted infants or infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials that did not: test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.90), I² = 0% (Analysis 1.2 )
4.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.
1.2. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 2: NEC (subgroup analysis of infants growth‐restricted or with AREDFV)
Mortality
Meta‐analysis of data from 12 trials (1399 infants) showed that delayed introduction of progressive enteral feeds may not affect the risk of death before hospital discharge: RR 0.97, 95% CI 0.70 to 1.36 (I² = 0%); RD ‐0.00, 95% CI ‐0.03 to 0.03 (Analysis 1.3).
1.3. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 3: Mortality prior to discharge
The funnel plot was not markedly asymmetrical (Figure 5). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations and imprecision.
5.

Funnel plot‐ mortality prior to discharge
Subgroup analyses
We found no evidence of subgroup differences by type of milk: test for subgroup differences: Chi² = 2.05, df = 2 (P = 0.36), I² = 2.2% (Figure 6).
None of the trials recruited predominantly ELBW or extremely preterm infants.
We found no evidence of subgroup differences by trials that restricted participation to growth‐restricted infants or infants with absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery versus trials that did not: test for subgroup differences: Chi² = 0.84, df = 1 (P = 0.36), I² = 0% (Analysis 1.4).
6.

Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding (all trials), outcome: 1.3 Mortality prior to discharge.
1.4. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 4: Mortality (subgroup analysis of infants growth‐restricted or with AREDFV)
Secondary outcomes
Growth
Four trials reported the median time to regain birth weight, and none of these showed a between‐group difference (Abdelmaaboud 2012; Bozkurt 2020; Davey 1994; Tewari 2018). The data available were insufficient for meta‐analysis. Three trials reported rate of weight gain during the trial period. Two did not show a between‐group difference (Khayata 1987; Pérez 2011). Bozkurt 2020 reported that infants in the delayed introduction group had a slower rate of weight gain (15 g/day versus 19 g/day). The data available were insufficient for meta‐analysis.
None of the other trials reported growth parameters.
Neurodevelopment
None of the trials assessed neurodevelopmental outcomes.
Time to establish full enteral feeding
The median time to establish full enteral feeding was longer in the delayed introduction group in the included trials:
Abdelmaaboud 2012: two days
Armanian 2013: five days
Arnon 2013: three days
Bozkurt 2020: two days
Davey 1994: three days
Dinerstein 2013: two days
Karagianni 2010: three days
Khayata 1987: data not reported
Leaf 2012: three days
Ostertag 1986: data not reported
Pérez 2011: four days
Salas 2018: two days
Srinivasan 2017: four days
Tewari 2018: 5.5 days (extremely preterm), four days (very preterm)
The reports did not provide data (mean and SD) in a form to allow meta‐analysis.
Time to establish full oral feeding
None of the trials assessed time to establish full oral feeding.
Feed intolerance
Meta‐analysis of data from six trials (581 infants) showed that delayed introduction of progressive enteral feeds slightly reduces the risk of feed intolerance: RR 0.81, 95% CI 0.68 to 0.97 (I² = 18%); RD ‐0.09, 95% CI ‐0.17 to ‐0.02; NNTB 11, 95% CI 6 to 50 (Analysis 1.5). We assessed the certainty of evidence as low using GRADE methods, downgraded for serious study design limitations and imprecision.
1.5. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 5: Feed intolerance
One trial did not detect a difference, but the report did not provide data to allow quantitative synthesis (Davey 1994).
Invasive infection
Meta‐analysis of data from seven trials (872 infants) showed that delayed introduction of progressive enteral feeds probably increases the risk of invasive infection: RR 1.44, 95% CI 1.15 to 1.80 (I² = 0%); RD 0.10, 95% CI 0.04 to 0.15; NNTH 10, 95% CI 7 to 25 (Analysis 1.6). We assessed the certainty of evidence as moderate using GRADE methods, downgraded for serious study design limitations.
1.6. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 6: Invasive infection
Duration of hospital stay
Meta‐analysis from four trials (368 infants) showed a longer duration of hospitalisation in the delayed feeding group: MD 4.57 days, 95% CI 1.53 to 7.61; I² = 24% (Analysis 1.7).
1.7. Analysis.

Comparison 1: Delayed versus early introduction of progressive enteral feeding (all trials), Outcome 7: Duration of hospital admission (days)
Another three trials did not show an effect, but the reports did not provide data to allow quantitative synthesis (Abdelmaaboud 2012; Leaf 2012; Tewari 2018).
Sensitivity analyses for heterogeneity or risk of bias
We had planned sensitivity analyses for high heterogeneity (I² > 75%) and for risk of bias. However, none of the pre‐specified meta‐analyses contained high levels of heterogeneity, nor did any include data from a trial with high risk of bias where the other studies had low risk of bias.
Discussion
Summary of main results
The trial data included in this review provide low‐certainty evidence that delaying the introduction of progressive enteral feeds beyond about four days after birth may not reduce the risk of NEC in very preterm or VLBW infants. The boundaries of the 95% CI for the estimate of effect are consistent with either two fewer cases or three more cases of NEC in every 100 infants who have delayed introduction of progressive enteral feeds. Meta‐analysis of data from these trials did not show evidence of an effect on all‐cause mortality, with the 95% CI boundaries being consistent with either three fewer or three more deaths in every 100 infants who had delayed introduction of progressive enteral feeds. Prespecified analyses did not show subgroup effects on risk of NEC or death among infants with growth restriction or evidence of absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery. Meta‐analysis showed that delayed introduction of progressive enteral feeds may result in a slight reduction in feed intolerance. Data from seven trials showed a higher risk of late‐onset infection among infants who had delayed introduction of progressive enteral feeds. The point estimate suggests that an extra episode of late‐onset infection occurs for every 10 infants who have delayed rather than early introduction of progressive enteral feeds. None of the included trials has reported effects on long term growth or neurodevelopmental outcomes.
Overall completeness and applicability of evidence
These data are relevant to current practice since most of the included trials were conducted since the year 2000. Six of these trials (767 infants) specifically recruited infants thought to be at high risk of developing NEC due to intrauterine growth restriction and abnormal fetal circulatory distribution or flow. This widens the applicability of the findings since this is the population for which most clinical uncertainty and variation in practice with regard to early feeding strategies exists (Klingenberg 2012). Previously, this population of infants has been specifically excluded from participating in many trials of early enteral feeding practices (Tyson 2007).
Artificial formula feeding increases the risk NEC (Quigley 2019). The risk‐benefit balance of enteral feeding strategies may differ between human milk‐fed and formula‐fed very preterm or VLBW infants (Young 2020). Subgroup analyses at trial‐level by type of milk did not show evidence of differences in effect. Most trials, however, included infants who received either human milk or cow milk formula or both, and subgroup data were not reported. If such subgroup data were available, an individual participant‐level meta‐analysis could be conducted to explore this issue further. It is also unclear whether the findings can be applied to infants who receive continuous infusion of intragastric feeds, as most of the infants in the included trials received enteral feeds as interval gastric boluses. Randomised controlled trials have reported conflicting findings about the effect on continuous enteral infusion on feed tolerance in VLBW (and especially ELBW) infants (Premji 2021).
The included trials were mainly undertaken in neonatal care centres in middle‐ or high‐income countries. It is less clear how applicable this evidence is to neonatal care practices in low‐income countries. Conservative strategies, such as delayed introduction of enteral feeds, may confer substantial nutritional disadvantage in settings with less technologically developed healthcare provision where adjunctive parenteral nutrition is not readily and safely available (Akindolire 2020). In some low‐ or middle‐income countries where severe infection is a much more important cause of mortality and morbidity, the nutritional and immunological advantages of early feeding, particularly with breast milk, may outweigh any risks associated with enteral feeding for very preterm or VLBW infants (de Silva 2004). A recent Cochrane Review has assessed the data from six trials undertaken in India since the late 2000s that compared exclusive enteral feeding (no parenteral fluid) from birth with gradual introduction of enteral feeds over several days in VLBW infants with birth weight greater than 1000 grams (Walsh 2020). While these trials were not eligible for inclusion in this review, none found evidence of an effect on NEC or other adverse outcomes.
Quality of the evidence
The GRADE‐assessed certainty (quality) of evidence for primary outcomes was downgraded because of lack of masking in the included trials and imprecision of estimates of effect (Table 1). Although these trials were otherwise of good methodological quality, in common with other trials of feeding interventions in this population, it was not possible to mask parents, caregivers and clinical assessors to the nature of the intervention. Lack of masking may have resulted in surveillance and ascertainment biases. It is more likely, however, to have caused an overestimation of feed intolerance and NEC among infants whose feed volumes were advanced faster. Assessment of abdominal radiographs for signs of NEC was masked in some trials to try to ensure that the diagnosis of severe NEC (confirmed by radiological detection of gas in the bowel wall or portal tract) was not prone to bias. As microbial generation of gas in the bowel wall is substrate dependent, however, infants who received more enteral milk (substrate) may have been more likely to demonstrate this radiological sign than infants with equally severe bowel disease who had less intraluminal substrate. This 'substrate effect' is also more likely to cause over‐ascertainment of NEC among infants who had faster rates of feed volume advancement (Tyson 2007).
The other reason for downgrading the certainty of evidence was the existence of substantial imprecision in estimates of effect, with meta‐analyses generating 95% CI that included benefit as well as no benefit or harm. Although the total number of participants in the 14 included trials was more than 1500, not all trials contributed data to all outcome estimates, and estimates of effect were consequently imprecise, especially for less common outcomes including NEC and mortality.
The definition of delayed introduction of progressive feeds varies between subpopulations of very preterm or VLBW infants who have different empiric risks for developing feed intolerance and NEC. The effects of enteral feeding are likely to be very different, for example, for an inotrope‐supported infant of birth weight less than 750 grams compared with a clinically stable infant of birth weight greater than 1000 grams. For this Cochrane Review, we defined delayed introduction as later than four days after birth since some observational studies have found the risk of NEC to be lower when feeds are introduced five to seven days after birth (Patole 2005). For ELBW or extremely preterm infants, it may be more appropriate to define delayed introduction as more than seven days after birth (or even later) since small‐intestinal motility is poorly organised before about 28 weeks' gestation resulting in a high risk of feed intolerance. In addition, enteral feeds are often delayed in this population because of respiratory or metabolic instability or because of other putative risk factors for NEC, such as the existence of a patent ductus arteriosus, the use of non‐steroidal anti‐inflammatory drugs or the presence of an umbilical arterial catheter (McGuire 2004).
Potential biases in the review process
The main concern with the review process is the possibility that findings are subject to publication and other reporting biases (Hopewell 2009). Data from trials which show statistically significant or potentially important effects tend to be more readily available for inclusion in meta‐analyses (Gale 2020). Publication bias, as well as other sources of small‐study bias, can inflate effect size estimates in meta‐analyses of interventions to improve outcomes in very preterm or VLBW infants (Young 2021). The Cochrane Review of probiotics to prevent NEC in very preterm or VLBW infants, for example, shows a large reduction in the risk of NEC, but the funnel plot and regression analysis indicate that publication bias is likely to have inflated the pooled effect size estimate (Sharif 2020). We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of major international perinatal conferences to identify trial reports that are not published in full form in academic journals. Inspection of funnel plots of meta‐analyses that included at least 10 data points did not show sufficient asymmetry to raise concerns about possible publication or small study bias.
In six trials (accounting for about half of all the participants), all participating infants needed to have evidence of intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery (Abdelmaaboud 2012; Arnon 2013; Davey 1994; Karagianni 2010; Leaf 2012; Srinivasan 2017). Most infants who participated in these trials were very preterm or VLBW infants, but subgroup data were not available. We included data from these trials since intrauterine growth restriction or circulatory redistribution demonstrated by absent or reversed end‐diastolic flow velocities in the fetal aorta or umbilical artery have been associated with a high risk of developing NEC and associated complications, and the findings are applicable to enteral feeding policies and practices (Embleton 2017).
Agreements and disagreements with other studies or reviews
This review focused specifically on the comparison of delayed versus early introduction of progressive enteral feeds. Other Cochrane Reviews have assessed how (i) enteral fasting versus trophic feeding (minimal enteral nutrition), (ii) slow versus faster rates of feed volume advancement, and (iii) early full enteral feeding versus gradual introduction of feeds affects important outcomes in very preterm or VLBW infants (Morgan 2013; Oddie 2017; Walsh 2020). These reviews, consistent with the findings of this review, have found evidence that conservative feeding strategies probably do not reduce the risk of NEC, mortality, or associated morbidity.
Authors' conclusions
Implications for practice.
Delaying the introduction of progressive enteral feeds beyond four days after birth may not reduce the risk of necrotising enterocolitis (NEC) or death in very preterm or very low birth weight (VLBW) infants (low‐certainty evidence), including infants who are growth‐restricted or compromised in utero, but may slightly reduce the risk of feed intolerance (low‐certainty evidence). Introducing progressive feed volumes earlier (typically before four days after birth) probably reduces the risk of late‐onset invasive infection slightly (moderate‐certainty evidence). Clinicians, policy‐makers, and guideline‐producers can consider how to apply this evidence to practice in their local context (Soll 2019).
Implications for research.
Further trial data may alter these effect estimates for NEC or death, particularly for extremely preterm or extremely low birth weight (ELBW) infants. Effects on long term growth and neurodevelopment have not been studied. With regard to very preterm or VLBW infants who are clinically‐stable after birth (typically infants with birth weight > 1000 g or gestational age > 27 weeks), the key research question is now whether exclusive enteral feeding from birth is better than delayed introduction and slow advancement (Walsh 2020).
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2021 | New citation required but conclusions have not changed | The updated search identified five new trials (Bozkurt 2020; Dinerstein 2013; Salas 2018; Srinivasan 2017; Tewari 2018). |
| 1 October 2021 | New search has been performed | This updates the review "Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants" (Morgan 2014). |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 13 January 2011 | New search has been performed | This updates the review "Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants" published in the Cochrane Database of Systematic Reviews, Issue 2, 2008 (Bombell 2008). Updated search includes three new trials (Ostertag 1986; Karagianni 2010; Leaf 2012). New authorship for this review update. |
| 13 January 2011 | New citation required and conclusions have changed | The addition of new trial data has increased the total number of participating infants to 600 and modified the implications for practice and research. |
| 2 February 2008 | New search has been performed | This updates the review "Early versus delayed initiation of progressive enteral feedings for parenterally fed low birth weight or preterm infants" published in the Cochrane Database of Systematic Reviews, Issue 1, 2000 (Kennedy 2000). The title has been changed to "Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants" and has a new authorship of Sarah Bombell and William McGuire. Changes made to the original protocol are outlined below: 1. Introduction of progressive enteral feeds is defined as feed volumes more than 24 ml/kg/day (1 ml/kg/hour). 2. The population has been restricted to very low birth weight and very preterm infants 3. Subgroup analyses of extremely low birth weight and extremely preterm infants, and infants with evidence of intrauterine growth restriction or absent or reversed end‐diastolic flow velocities in Doppler studies of the fetal aorta or umbilical artery were prespecified. Search updated December 2007. No new trials were included, but one on‐going trial was identified. The findings and implications for practice and research of the review have not changed overall. |
| 11 January 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the corresponding authors who provided further information about their trials.
We thank Cochrane Neonatal for methods and technical support: Colleen Ovelman and Jane Cracknell, former Managing Editors, Roger Soll, Coordinating Editor, and Michelle Fiander, Information Specialist.
We thank Melissa Harden, University of York, UK for designing and managing the electronic literature searches.
Editorial and peer‐reviewer contributions:
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, Senior Editor of the Children and Families Network, Cochrane
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Helen Wakeford, Executive Editor, Cochrane Editorial and Methods Department
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Editorial and Methods Department
Copy Editor (copy‐editing and production): Hacsi Horvath, Cochrane Copy Edit Support
Peer‐reviewers (provided comments and recommended an editorial decision): Sivam Thanigainathan, Assistant Professor, Department of Neonatology, AIIMS, Jodhpur, Rajasthan, India (clinical/content review), Brenda B Poindexter, MD, MS Emory University and Children's Healthcare of Atlanta, USA (clinical/content review), Jacqueline J Ho, RCSI & UCD Malaysia Campus, Co‐Director Cochrane Malaysia (clinical/content review)*, Senior Editor Cochrane Neonatal Nuala Livingstone, Cochrane Editorial and Methods Department (methods review), Robin Paynter (MLIS) Cochrane Fertility Regulation Information Specialist (search review)
*Jacqueline J Ho is a member of Cochrane Neonatal and provided peer‐review comments on this article, but was not otherwise involved in the editorial process or decision‐making for this article.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL (via Wiley)
Search date 21st October 2021
2308 records identified
#1 MeSH descriptor: [Infant, Newborn] explode all trees 16781
#2 MeSH descriptor: [Premature Birth] this term only 1617
#3 (neonat* or neo next nat*):ti,ab,kw 23965
#4 (newborn* or new next born* or newly next born*):ti,ab,kw 29310
#5 (preterm or preterms or pre next term or pre next terms):ti,ab,kw 14633
#6 (preemie* or premie or premies):ti,ab,kw 53
#7 (prematur* near/3 (birth* or born or deliver*)):ti,ab,kw 3122
#8 (low near/3 (birthweight* or birth next weight*)):ti,ab,kw 5718
#9 (lbw or vlbw or elbw):ti,ab,kw 1756
#10 infan*:ti,ab,kw 66526
#11 (baby or babies):ti,ab,kw 9291
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 84601
#13 MeSH descriptor: [Enteral Nutrition] this term only 1938
#14 ((enteral or enteric) NEAR/2 (nutrition or feed*)):ti,ab,kw 6024
#15 ((oral or sip or tube) NEAR/2 feeding*):ti,ab,kw 2264
#16 ((nasogastric or gastrostomy or jejunostomy) NEAR/2 tube*):ti,ab,kw 1972
#17 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral feed*):ti,ab,kw 1147
#18 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric feed*):ti,ab,kw 37
#19 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral intake*):ti,ab,kw 292
#20 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric intake*):ti,ab,kw 9
#21 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral nutrition):ti,ab,kw 1092
#22 ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric nutrition):ti,ab,kw 34
#23 ((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 feed*):ti,ab,kw 332
#24 ((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 volume*):ti,ab,kw 323
#25 ((gut or gastrointestinal) NEAR/2 priming):ti,ab,kw 15
#26 {OR #13‐#25} 9348
#27 #12 and #26 2120
#28 MeSH descriptor: [Parenteral Nutrition] explode all trees and with qualifier(s): [adverse effects ‐ AE] 235
#29 MeSH descriptor: [Enterocolitis, Necrotizing] explode all trees and with qualifier(s): [etiology ‐ ET, epidemiology ‐ EP, prevention & control ‐ PC] 166
#30 MeSH descriptor: [Infections] this term only and with qualifier(s): [epidemiology ‐ EP] 208
#31 ((prevent* or risk*) NEAR/3 necrotising enterocolitis):ti,ab,kw 281
#32 ((prevent* or risk*) NEAR/3 necrotizing enterocolitis):ti,ab,kw 281
#33 {OR #28‐#32} 770
#34 #12 and #33 440
#35 #27 or #34 in Trials 2308
Appendix 2. MEDLINE search strategy
MEDLINE via OVID
Date range: 1946 to October 18, 2021
Search date 21st October 2021
3067 records identified
1 exp Infant, Newborn/ (637173)
2 Premature Birth/ (16542)
3 (neonat$ or neo nat$).ti,ab. (282741)
4 (newborn$ or new born$ or newly born$).ti,ab. (175484)
5 (preterm or preterms or pre term or pre terms).ti,ab. (83058)
6 (preemie$ or premie or premies).ti,ab. (190)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (16799)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (37075)
9 (lbw or vlbw or elbw).ti,ab. (9226)
10 infan$.ti,ab. (463721)
11 (baby or babies).ti,ab. (74639)
12 or/1‐11 (1115571)
13 Enteral Nutrition/ (20760)
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (15234)
15 ((oral or sip or tube) adj2 feeding$).ti,ab. (10976)
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (9396)
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (1762)
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (14)
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab. (43)
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab. (0)
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (1555)
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (5)
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (3166)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (4202)
25 trophic feeding$.ti,ab. (91)
26 ((gut or gastrointestinal) adj2 priming).ti,ab. (35)
27 or/13‐26 (46363)
28 12 and 27 (7617)
29 Parenteral Nutrition/ae [Adverse Effects] (2855)
30 Enterocolitis, Necrotizing/ep, et, pc [Epidemiology, Etiology, Prevention & Control] (1860)
31 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (151)
32 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (648)
33 Infections/ep [Epidemiology] (4307)
34 or/29‐33 (9366)
35 12 and 34 (3558)
36 28 or 35 (10531)
37 randomized controlled trial.pt. (546615)
38 controlled clinical trial.pt. (94462)
39 randomized.ab. (537193)
40 placebo.ab. (222257)
41 drug therapy.fs. (2387403)
42 randomly.ab. (367871)
43 trial.ab. (571925)
44 groups.ab. (2259383)
45 or/37‐44 (5146346)
46 exp animals/ not humans.sh. (4899490)
47 45 not 46 (4476845)
48 36 and 47 (3067)
Appendix 3. EMBASE search strategy
Embase via OVID
Date range: 1974 to 2021 October 20
Search date 21st October 2021
3287 records identified
1 newborn/ (555540)
2 prematurity/ (111368)
3 exp low birth weight/ (66811)
4 (neonat$ or neo nat$).ti,ab. (367728)
5 (newborn$ or new born$ or newly born$).ti,ab. (205522)
6 (preterm or preterms or pre term or pre terms).ti,ab. (116337)
7 (preemie$ or premie or premies).ti,ab. (298)
8 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (23263)
9 (low adj3 (birthweight$ or birth weight$)).ti,ab. (46930)
10 (lbw or vlbw or elbw).ti,ab. (12593)
11 infan$.ti,ab. (531512)
12 (baby or babies).ti,ab. (104266)
13 or/1‐12 (1214010)
14 enteric feeding/ (35123)
15 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (24631)
16 ((oral or sip or tube) adj2 feeding$).ti,ab. (16620)
17 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (14616)
18 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (1797)
19 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (11)
20 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (2259)
21 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (7)
22 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake).ti,ab. (42)
23 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake).ti,ab. (0)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (3641)
25 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (5622)
26 trophic feeding$.ti,ab. (115)
27 ((gut or gastrointestinal) adj2 priming).ti,ab. (43)
28 or/14‐27 (71660)
29 13 and 28 (11075)
30 randomized controlled trial/ (680435)
31 controlled clinical trial/ (464191)
32 random$.ti,ab. (1716294)
33 randomization/ (92016)
34 intermethod comparison/ (276126)
35 placebo.ti,ab. (330951)
36 (compare or compared or comparison).ti. (548705)
37 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2384026)
38 (open adj label).ti,ab. (91699)
39 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (249445)
40 double blind procedure/ (188791)
41 parallel group$1.ti,ab. (28260)
42 (crossover or cross over).ti,ab. (113098)
43 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (365027)
44 (assigned or allocated).ti,ab. (430146)
45 (controlled adj7 (study or design or trial)).ti,ab. (390486)
46 (volunteer or volunteers).ti,ab. (261511)
47 human experiment/ (557170)
48 trial.ti. (341316)
49 or/30‐48 (5548092)
50 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) (8737)
51 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) (285551)
52 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. (19040)
53 (Systematic review not (trial or study)).ti. (189119)
54 (nonrandom$ not random$).ti,ab. (17321)
55 "Random field$".ti,ab. (2599)
56 (random cluster adj3 sampl$).ti,ab. (1387)
57 (review.ab. and review.pt.) not trial.ti. (932582)
58 "we searched".ab. and (review.ti. or review.pt.) (38703)
59 "update review".ab. (118)
60 (databases adj4 searched).ab. (45955)
61 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1125374)
62 Animal experiment/ not (human experiment/ or human/) (2361522)
63 or/50‐62 (3807709)
64 49 not 63 (4922568)
65 29 and 64 (2430)
66 parenteral nutrition/ (31248)
67 complication/ (271464)
68 safety/ or patient safety/ (384313)
69 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab. (719190)
70 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab. (6322560)
71 67 or 68 or 69 or 70 (6700517)
72 66 and 71 (11542)
73 necrotizing enterocolitis/co, ep, et, pc [Complication, Epidemiology, Etiology, Prevention] (2784)
74 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (216)
75 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (839)
76 Infection/ep [Epidemiology] (4742)
77 72 or 73 or 74 or 75 or 76 (19622)
78 13 and 64 and 77 (1223)
79 65 or 78 (3287)
Appendix 4. Maternity & Infant Care search strategy
Maternity & Infant Care via OVID
Date range: 1971 to October 19, 2021
Search date 28th October 2021
126 records identified
1 (neonat$ or neo nat$).ti,ab. (51554)
2 (preterm or preterms or pre term or pre terms).ti,ab. (30680)
3 (preemie$ or premie or premies).ti,ab. (61)
4 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (4455)
5 (low adj3 (birthweight$ or birth weight$)).ti,ab. (11921)
6 (lbw or vlbw or elbw).ti,ab. (3469)
7 infan$.ti,ab. (72038)
8 (baby or babies).ti,ab. (31834)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (129545)
10 (Infant ‐ premature or Infant ‐ very low birth weight or Infant ‐ newborn).de. (31397)
11 9 or 10 (134036)
12 10 or 11 (134036)
13 Enteral nutrition.de. (292)
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (831)
15 ((oral or sip or tube) adj2 feeding$).ti,ab. (522)
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (179)
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (343)
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (1)
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab. (9)
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab. (0)
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (75)
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (0)
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (79)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (31)
25 trophic feeding$.ti,ab. (23)
26 ((gut or gastrointestinal) adj2 priming).ti,ab. (5)
27 or/13‐26 (1574)
28 12 and 27 (1463)
29 limit 28 to randomised controlled trial (119)
30 Parenteral nutrition.de. (209)
31 Enterocolitis ‐ necrotizing.de. (1)
32 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab. (14940)
33 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab. (100517)
34 Complications.de. (201)
35 safety.de. (2191)
36 (30 or 31) and (32 or 33 or 34 or 35) (97)
37 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (77)
38 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (236)
39 36 or 37 or 38 (404)
40 limit 39 to randomised controlled trial (18)
41 29 or 40 (126)
Appendix 5. CINAHL search strategy
CINAHL via EBSCO
Search date 22nd October 2021
1199 records identified
| S1 | MH "Infant, Newborn+" |
| S2 | MH "Childbirth, Premature" |
| S3 | TI ( neonat* or neo‐nat* ) OR AB ( neonat* or neo‐nat* ) |
| S4 | TI ( newborn* or new‐born* or (newly N1 born*) ) OR AB ( newborn* or new‐born* or (newly N1 born*) ) |
| S5 | TI ( preterm or preterms or pre‐term or pre‐terms ) OR AB ( preterm or preterms or pre‐term or pre‐terms) |
| S6 | TI ( preemie* or premie or premies ) OR AB ( preemie* or premie or premies ) |
| S7 | TI ( prematur* N3 (birth* or born or deliver*) ) OR AB ( prematur* N3 (birth* or born or deliver*) ) |
| S8 | TI ( low N3 (birthweight* or birth‐weight*) ) OR AB ( low N3 (birthweight* or birth‐weight*) ) |
| S9 | TI ( lbw or vlbw or elbw ) OR AB ( lbw or vlbw or elbw ) |
| S10 | TI infan* OR AB infan* |
| S11 | TI ( baby or babies ) OR AB ( baby or babies ) |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 |
| S13 | (MH "Enteral Nutrition") |
| S14 | TI ( (enteral* or enteric*) N2 (nutrition or feed*) ) OR AB ( (enteral* or enteric*) N2 (nutrition or feed*) ) |
| S15 | TI ( (advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed* ) OR AB ( (advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed* ) |
| S16 | TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) |
| S17 | TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) ) |
| S18 | TI ( (oral or sip or tube) N2 feeding*) OR AB ( (oral or sip or tube) N2 feeding*) |
| S19 | TI ( (nasogastric or gastrostomy or jejunostomy) N2 tube* ) OR AB ( (nasogastric or gastrostomy or jejunostomy) N2 tube* ) |
| S20 | TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) |
| S21 | TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR TI trophic feeding OR AB trophic feeding* OR TI ( ((gut or gastrointestinal) N2 priming) ) OR AB ( ((gut or gastrointestinal) N2 priming) ) |
| S22 | TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) ) |
| S23 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 |
| S24 | S12 AND S23 |
| S25 | MH randomized controlled trials |
| S26 | MH double‐blind studies |
| S27 | MH single‐blind studies |
| S28 | MH random assignment |
| S29 | MH cluster sample |
| S30 | TI (randomised OR randomized) |
| S31 | AB (random*) |
| S32 | TI (trial) |
| S33 | MH (sample size) AND AB (assigned OR allocated OR control) |
| S34 | MH (placebos) |
| S35 | PT (randomized controlled trial) |
| S36 | AB (control W5 group) |
| S37 | MH (crossover design) OR MH (comparative studies) |
| S38 | AB (cluster W3 RCT) |
| S39 | S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 |
| S40 | S24 AND S39 |
| S41 | MH "Parenteral Nutrition/AE" |
| S42 | MH "Enterocolitis, Necrotizing/CO/ET/EP/PC" |
| S43 | MH "Infection/EP" |
| S44 | TI ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR TI ( ((prevent* or risk*) N3 necrotizing enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotizing enterocolitis) ) |
| S45 | S41 OR S42 OR S43 OR S44 |
| S46 | S12 AND S39 AND S45 |
| S47 | S40 OR S46 |
Appendix 6. ClinicalTrials.gov search strategy
ClinicalTrials.gov
Search date 28th October 2021
74 records identified
2 searches carried out:
1. 21 Studies found for: enteral nutrition | Enteral Feeding Intolerance | Child
Also searched for Enteral Feeding, Enteral Nutrition, and Enteral feeds. See Search Details
Applied Filters: Child (birth–17)
2. 53 Studies found for: enteral nutrition | Necrotizing Enterocolitis | Child
Also searched for Enterocolitis, necrotizing, Enteral Feeding, and Enteral feeds. See Search Details
Applied Filters: Child (birth–17)
Appendix 7. Risk of bias tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Delayed versus early introduction of progressive enteral feeding (all trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Necrotising enterocolitis (NEC) | 13 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.14] |
| 1.1.1 Human milk or formula‐fed (or mixed) infants | 9 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.24] |
| 1.1.2 Only human milk‐fed infants | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.28] |
| 1.1.3 Only formula‐fed infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.40, 2.94] |
| 1.2 NEC (subgroup analysis of infants growth‐restricted or with AREDFV) | 13 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.14] |
| 1.2.1 Trials including only growth‐restricted/AREDFV infants | 6 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.32] |
| 1.2.2 Trials including all infants | 7 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.30] |
| 1.3 Mortality prior to discharge | 12 | 1399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3.1 Human milk or formula‐fed (or mixed) infants | 9 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.27] |
| 1.3.2 Only human milk‐fed infants | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.55, 5.57] |
| 1.3.3 Only formula‐fed infants | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.57, 3.61] |
| 1.4 Mortality (subgroup analysis of infants growth‐restricted or with AREDFV) | 12 | 1399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 1.4.1 Trials including only growth‐restricted/AREDFV infants | 5 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.68, 2.12] |
| 1.4.2 Trials including all infants | 7 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.31] |
| 1.5 Feed intolerance | 6 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.97] |
| 1.5.1 Human milk or formula‐fed (or mixed) infants | 4 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.02] |
| 1.5.2 Only human milk‐fed infants | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 1.6 Invasive infection | 7 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.15, 1.80] |
| 1.6.1 Human milk or formula‐fed (or mixed) infants | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.68] |
| 1.6.2 Only human milk‐fed infants | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.21, 5.46] |
| 1.7 Duration of hospital admission (days) | 4 | 378 | Mean Difference (IV, Fixed, 95% CI) | 4.57 [1.53, 7.61] |
| 1.7.1 Human milk or formula‐fed (or mixed) infants | 3 | 346 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [1.62, 8.17] |
| 1.7.2 Only human milk‐fed infants | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐5.76, 10.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmaaboud 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants, 28 to 36 weeks' gestation with birth weight < 10th centile, and antenatal ultrasound showing intrauterine growth restriction, absent or reversed end diastolic flow on Doppler waveforms of the umbilical artery with evidence of cerebral redistribution Setting: Women's Hospital, Hamad Medical Corporation, Qatar (2010 to 2011) |
|
| Interventions | Delayed introduction of progressive enteral feeds on day 6 (63 infants) versus earlier introduction on day 2 (62 infants) | |
| Outcomes |
|
|
| Notes | > 90% of participants were VLBW | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on masking of radiological assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Armanian 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | VLBW infants without a congenital anomaly Setting: Isfahan Faculty of Medicine, Iran (2010 to 2012) |
|
| Interventions | Delayed introduction of progressive enteral feeds until day 7 (47 infants) versus earlier introduction on day 3 (35 infants) Infants received either maternal milk or formula (no subgroup data available) Volumes and rates of advancement or progressive feeds were the same in both groups (20 mL/kg/day) |
|
| Outcomes |
|
|
| Notes | Mean gestation at birth: 30 weeks' Mean birth weight: 1200 g |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Arnon 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants, birth weight < 10th centile, and antenatal evidence of absent or reversed end diastolic flow on Doppler waveforms of the umbilical artery Setting: Meir Medical Centre, Kfar Saba, Tel Aviv, Israel (2011 to 2012) |
|
| Interventions | Delayed progressive enteral feeding (≥ day 4 after birth, 30 infants) versus earlier enteral feeding (day 2 after birth, 30 infants) Infants received expressed breast or formula or both |
|
| Outcomes |
|
|
| Notes | Most participants were VLBW (mean 1480 g‐ range 963 to 1683 g) Mean gestation at birth: 31 weeks' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Bozkurt 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight ≤ 1250 g Zekai Tahir Burak Maternity Teaching Hospital, Istanbul, Turkey (2016 to 17) |
|
| Interventions | Delayed progressive enteral feeding (minimal enteral nutrition for 5 days after birth, 110 infants) versus earlier progressive enteral feeding (within 48 hours, 109 infants), with feed volumes advanced by 20 to 25 mL/kg/d until 150 mL/kg/d feed volume achieved Infants received expressed maternal milk (first choice) or formula or both |
|
| Outcomes |
|
|
| Notes | Mean gestation at birth: 27weeks' Mean birth weight: 963 g Further information courtesy of Dr Ozlem Bozturk (March 2020) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Davey 1994.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants with birth weight < 2000 g who were clinically stable and who had an umbilical artery catheter in place Infants were excluded if they had a lethal condition or had received a double‐volume exchange transfusion Setting: Division of Neonatology, Department of Pediatrics, University of Rochester Medical Center, USA (study dates not stated) |
|
| Interventions | Delayed introduction of enteral feeds (median 5 days; 31 infants) versus earlier introduction (median 2 days; 31 infants) Infants received either breast milk or diluted formula (no subgroup data available) |
|
| Outcomes |
|
|
| Notes | A trial inclusion criterion for birth weight was < 2000 g (mean birth weight 1100 g; > 80% of infants were VLBW or very preterm) Infants in the delayed introduction group commenced enteral feeds when the umbilical artery catheter had been removed for 24 hours and the infant was clinically stable and infants in the earlier introduction group commenced feeds with the umbilical artery catheter in situ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Dinerstein 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | VLBW infants (appropriate weight for gestation) Neonatal Unit, Hospital Sarda, Buenos Aires, Argentina (2011 to 2012) |
|
| Interventions | Delayed progressive enteral feeding (> 96 hours after birth, 32 infants) versus earlier enteral feeding (within 48 hours, 30 infants), with feed volumes advanced by 15 to 20 mL/kg/d. Infants received expressed maternal milk (first choice) or pasteurised donor human milk |
|
| Outcomes |
|
|
| Notes | Median gestation at birth 28 weeks' (range 25‐ 30 weeks') Further information courtesy of Dr Alejandro Dinerstein (April 2021) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Karagianni 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of gestational age 27 to 34 weeks' and birth weight < 10th percentile who also had antenatal Doppler ultrasound evidence within 7 days before birth of 'pathological fetal perfusion', defined as uterine or umbilical arterial pulsatility index > 90th percentile and middle cerebral arterial pulsatility index < 10th percentile for gestational age Setting: Neonatology Department, Aristotle University, Thessaloniki, Greece (2007 to 2009) |
|
| Interventions | Delayed (> 5 days after birth; 42 infants) versus early (≤ 5 days; 42 infants) introduction of progressive enteral feeds (expressed maternal milk or preterm formula) Feed volumes were advanced at daily targeted increments of 15 mL/kg |
|
| Outcomes |
|
|
| Notes | Median birth weight 1100 g (range 440‐1440 g) *Unpublished data courtesy of Dr Karagianni |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 84 infants enrolled, 81 completed the study 3 infants died before 5 days after birth ‐ we have included these infants in the intention‐to‐treat analysis of mortality |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Khayata 1987.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | VLBW infants | |
| Interventions | Delayed introduction of enteral feeds (day 10 after birth; 7 infants) versus earlier introduction (< 4 days; 5 infants) All infants received artificial formula Setting: Neonatal Unit, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, USA (study dates not stated, likely early 1980s) |
|
| Outcomes |
|
|
| Notes | This trial was reported as an abstract only ‐ unpublished methodological or outcome data were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described but unlikely to be masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described but unlikely to be masked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine |
| Other bias | Unclear risk | Not described |
Leaf 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants < 35 weeks' gestation and birth weight < 10th percentile and antenatal Doppler ultrasound evidence of:
Infants were excluded with a major congenital anomaly, receipt of in‐utero transfusion, multi‐organ failure or need for inotrope support Setting: 54 neonatal care centres in UK and Ireland (2006 to 2009) |
|
| Interventions | Delayed (day 5 after birth; 202 infants) versus early (day 2 after birth; 202 infants) introduction of milk feeds | |
| Outcomes |
|
|
| Notes | Median gestation at birth: 31 weeks' (> 90% of infants were VLBW) Further analysis of infants < 29 weeks' gestation reported separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort assessed |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Ostertag 1986.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | VLBW infants at 'high risk' of developing NEC based on a risk assessment score Setting: Perinatology Center, New York Hospital‐Cornell Medical Center, New York, USA (early 1980s) |
|
| Interventions | Delayed introduction of enteral feeds (day 7 after birth; 20 infants) versus earlier introduction (day 1; 18 infants) Infants received feeds by continuous intragastric infusion starting initially with sterile water, then progressing to 2.5% dextrose, diluted formula, then full‐strength formula. |
|
| Outcomes |
|
|
| Notes | Mean gestation at birth: 28 weeks' Mean birth weight: 1020 g (range 700‐ 1450 g) Further details about exclusions after randomisation kindly provided by Dr La Gamma (March 2009) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants died before 7 days after birth Investigators excluded 1 infant before day 14 because of a feeding protocol violation ‐ we have included all of these infants in the relevant intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Pérez 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Very preterm (27 to 32 weeks') or VLBW (750 to 1500 g) infants without congenital anomalies or evidence of intrauterine growth restriction Setting: Ramón González Valencia de Bucaramanga University Hospital, Columbia (1997 to 2005) |
|
| Interventions | Delayed enteral feeding (day 5 after birth, 104 infants) versus earlier enteral feeding (day 1 to 2 after birth, 135 infants) All infants received a combination of human milk and formula |
|
| Outcomes |
|
|
| Notes | Mean birth weight 1230 g Mean gestation at birth 30 weeks' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described (states "randomly assigned", and "controlled clinical trial") |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment for primary outcomes |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Salas 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (< 29 weeks', appropriate weight for gestation) Neonatal Unit, Beth Israel Deaconess Medical Center, Boston, and University of Alabama at Birmingham Hospital, USA (2016 to 2017) |
|
| Interventions | Delayed progressive enteral feeding (> 96 hours after birth, 30 infants) versus earlier enteral feeding (< 48 hours, 30 infants), with feed volumes advanced by 24 mL/kg/d Infants received expressed maternal milk (first choice), pasteurised donor human milk (second choice), or artificial formula (third choice). |
|
| Outcomes |
|
|
| Notes | Mean gestation at birth: 26 weeks' Mean birth weight: 833 g |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete cohort |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Srinivasan 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 32 preterm (< 37 weeks' gestation) infants with evidence of intrauterine growth restriction associated with absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery Setting: Neonatal Unit, KEM Hospital, Mumbai, India (2016 to 2017) |
|
| Interventions | Delayed enteral feeding (120 to 143 hours after birth, 16 infants) versus earlier enteral feeding (24 to 48 hours after birth, 16 infants) All infants received a combination of maternal or donor human milk. |
|
| Outcomes |
|
|
| Notes | Mean birth weight: 1139 g (SD 263 g) Mean gestation at birth: 32 weeks' *one episode of NEC occurred‐ associated with intestinal perforation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
Tewari 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 62 preterm (27 to 32 weeks' gestation) infants with evidence of intrauterine growth restriction associated with absent or reversed end‐diastolic flow velocities in antenatal Doppler studies of the umbilical artery Setting: Army Hospital, Hospital, New Dehli, India (2014 to 2015) |
|
| Interventions | Delayed enteral feeding (120 to 144 hours after birth, 31 infants) versus earlier enteral feeding (12 to 48 hours after birth, 31 infants) All infants received a combination of maternal or donor human milk. |
|
| Outcomes |
|
|
| Notes | Mean gestation at birth: 30 weeks' Mean birth weight: 1195 g Additional information and data courtesy of Dr Tewari (March 2020) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete cohort |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence baseline imbalance |
NEC: necrotising enterocolitis; VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2020 | RCT of progressive feeds introduction < 48 hours vs later initiation of feeds ‐ both groups are "early" according to the criteria for this review |
| Chetry 2014 | Both groups received early enteral feeds |
| Glass 1984 | Infants were allocated alternately to either early (first day after birth) or delayed transpyloric enteral feeding. The delayed feeding group commenced enteral nutrition when assessed to be "clinically stable" but this included initiation within 4 days after birth |
| Higgs 1974 | Infants in the delayed progressive enteral feeds group received total parenteral nutrition as a co‐intervention |
| Jajoo 2021 | Both groups received early introduction of progressive enteral feeds |
| Jayaraman 2017 | RCT examining the effect on breast milk feeding of early vs delayed kangaroo mother care in low birth weight infants (no intention to delay introduction) |
| LaGamma 1985 | This was not a randomised controlled trial |
| Modi 2015 | RCT of different rates of advancement of enteral feed volumes (not delayed versus early introduction of progressive feeding) |
| Montealegre‐Pomar 2021 | RCT of different rates of advancement of enteral feed volumes (not delayed versus early introduction of progressive feeding) |
| Nangia 2019 | RCT of early full enteral feeding (not delayed versus early introduction of progressive feeding) |
| Said 2008 | Infants in the delayed progressive enteral feeding group received minimal enteral nutrition prior to feed advancement as a co‐intervention |
| Sanghvi 2013 | Both groups received early enteral feeds |
| Tottman 2020 | Retrospective cohort study (epoch comparison) |
| Viswanathan 2017 | Retrospective cohort study |
| Weiler 2006 | Infants in both groups received some enteral feeds before 4 days after birth |
| Wilson 1997 | Infants in the delayed progressive enteral feeds group also received delayed advancement of parenteral nutrition as a co‐intervention |
RCT: randomised controlled trial; vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
Li 2016.
| Methods | Randomised controlled trial (unclear) |
| Participants | 128 VLBW infants |
| Interventions | Delayed progressive enteral feeding (10 days after birth, 63 infants) versus earlier enteral feeding (2 days after birth, 64 infants) All infants received a combination of maternal or donor human milk. |
| Outcomes |
|
| Notes | Uncertainty about methods and outcomes (further information sought from investigators in May 2020, and October 2021) |
NEC: necrotising enterocolitis; VLBW: very low birth weight.
Differences between protocol and review
We made the following changes to the previous publication of the review (Morgan 2014):
We updated the certainty of evidence and the 'Risk of bias' tool in Appendix 2;
-
We revised text in these sections:
How the intervention might work
Assessment of reporting biases
Sensitivity analysis
Allocation (selection bias)
Masking (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential sources of bias
We added new external sources of support
We included trials in which some infants were > 32 weeks' gestation and > 1500 g birth weight (and subgroup data were not provided), provided most (> 50%) infants were very preterm or VLBW.
Contributions of authors
Lauren Young and William McGuire updated the search, independently determined the eligibility of identified studies, assessed the methodological quality of the included trials, and extracted the relevant information and data.
All authors completed the final review.
Sources of support
Internal sources
-
Centre for Reviews and Dissemination, UK
Host institution
External sources
-
National Institute for Health Research, UK
Funder (NIHR133131)
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, Other
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent of the Gerber Products Company. The grantor has no input on the content of the review or the editorial process
Declarations of interest
SO works as a healthcare professional at Bradford Teaching Hospitals NHS Foundation Trust; no conflict of interest to declare.
LY works as a healthcare professional at Guy's and St Thomas' NHS Foundation Trust; no conflict of interest to declare.
WM is co‐ordinating editor for Cochrane Neonatal. He was not involved in the editorial process for this review, which was conducted by Cochrane's Central Editorial Service.
*Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent of the Gerber Products Company. The grantor has no input on the content of the review or the editorial process. Please see Sources of support.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdelmaaboud 2012 {published data only}
- Abdelmaaboud M, Mohammed A.A randomized controlled trial on early versus late minimal enteral feeding in preterm growth-restricted neonates with abnormal antenatal Doppler studies. Journal of Neonatal-Perinatal Medicine 2012;5(2):1-8. [DOI: 10.3233/NPM-2012-57311] [DOI] [PubMed] [Google Scholar]
Armanian 2013 {published and unpublished data}
- Armanian AM, Mirbod SM, Kazemipour S, Hassanzade A.Comparison of prolonged low volume milk and routine volume milk on incidence of necrotizing enterocolitis in very low birth weight neonates. Pakistan Journal of Medical Sciences 2013;29(1 Suppl):312-6. [DOI: 10.12669/pjms.291(Suppl).3523] [DOI] [Google Scholar]
Arnon 2013 {published data only}
- Arnon S, Sulam D, Konikoff F, Regev RH, Litmanovitz I, Naftali T.Very early feeding in stable small for gestational age preterm infants: a randomized clinical trial. Jornal de Pediatria 2013;89(4):388-93. [DOI: 10.1016/j.jped.2012.12.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bozkurt 2020 {published and unpublished data}
- Bozkurt O, Alyamac Dizdar E, Bidev D, Sari FN, Uras N, Suna Oguz S.Prolonged minimal enteral nutrition versus early feeding advancements in preterm infants with birth weight ≤1250 g: a prospective randomized trial. Journal of Maternal-Fetal & Neonatal Medicine 2020;25:1-7. [DOI: 10.1080/14767058.2020.1716723] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davey 1994 {published data only (unpublished sought but not used)}
- Davey AM, Wagner CL, Cox C, Kendig JW.Feeding premature infants while low umbilical artery catheters are in place: a prospective, randomized trial. Journal of Pediatrics 1994;124(5 Pt 1):795-9. [DOI: 10.1016/s0022-3476(05)81376-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dinerstein 2013 {published data only}
- Dinerstein A, Nieto RM, Solana CL, Ventura D, General F.Early minimal enteral feeding with human milk in very low birth weight infants (VLBW): when to start? Pediatric Academic Societies Annual Meeting 2013;1514:579.
Karagianni 2010 {published data only}
- Karagianni P, Briana DD, Mitsiakos G, Elias A, Theodoridis T, Chatziioannidis E, et al.Early versus delayed minimal enteral feeding and risk for necrotizing enterocolitis in preterm growth-restricted infants with abnormal antenatal Doppler results. American Journal of Perinatology 2010;27(5):367-73. [DOI: 10.1055/s-0029-1243310] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khayata 1987 {published data only (unpublished sought but not used)}
- Khayata S, Gutcher G, Bamberger J, Heimler R.Early versus late feeding of low birth weight (LBW) infants: effect on growth and hyperbilirubinemia. Pediatric Research 1987;21:431A. [Google Scholar]
Leaf 2012 {published data only}
- Kempley S, Gupta N, Linsell L, Dorling J, McCormick K, Mannix P, et al, ADEPT Trial Collaborative Group.Feeding infants below 29 weeks' gestation with abnormal antenatal Doppler: analysis from a randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(1):F6-11. [DOI: 10.1136/archdischild-2013-304393] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Brocklehurst P.Abnormal Doppler enteral prescription trial study: the results of a trial of feeding in a high risk group of premature babies. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95:Fa9. [DOI: 10.1136/adc.2010.192310.4.1] [DOI] [Google Scholar]
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Brocklehurst P.ADEPT - Abnormal Doppler Enteral Prescription Trial. BMC Pediatrics 2009;9:63. [DOI: 10.1186/1471-2431-9-63] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Brocklehurst P.When should feeds be started in the high risk preterm infant? The Abnormal Doppler Enteral Prescription Trial (ADEPT). In: E-PAS20101670.7. 2010.
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al, Abnormal Doppler Enteral Prescription Trial Collaborative Group.Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics 2012;129(5):e1260-8. [DOI: 10.1542/peds.2011-2379] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ostertag 1986 {published and unpublished data}
- Ostertag SG, LaGamma EF, Reisen CE, Ferrentino FL.Early enteral feeding does not affect the incidence of necrotizing enterocolitis. Pediatrics 1986;77(3):275-80. [PMID: ] [PubMed] [Google Scholar]
Pérez 2011 {published data only}
- Pérez LA, Pradilla GL, Díaz G, Bayter SM.Necrotising enterocolitis among preterm newborns with early feeding [Incidencia de enterocolitis necrosante en niños prematuros alimentados precozmente]. Biomédica 2011;31(4):485-91. [DOI: 10.1590/S0120-41572011000400003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Salas 2018 {published data only}
- Salas AA, Li P, Parks K, Lal CV, Martin CR, Carlo WA.Early progressive feeding in extremely preterm infants: a randomized trial. American Journal of Clinical Nutrition 2018;107(3):365-70. [DOI: 10.1093/ajcn/nqy012] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 2017 {published data only}
- Srinivasan A, Nanavati RN, Kabra NS.Comparison of feeding regimens in preterm neonates with abnormal antenatal Doppler: a randomised controlled trial. Global Journal for Research Analysis 2017;6(4):44-7. [DOI: 10.36106/gjra] [DOI] [Google Scholar]
Tewari 2018 {published data only}
- Tewari VV, Dubey SK, Kumar R, Vardhan S, Sreedhar CM, Gupta G.Early versus late enteral feeding in preterm intrauterine growth restricted neonates with antenatal doppler abnormalities: an open-label randomized trial. Journal of Tropical Pediatrics 2018;64(1):4-14. [DOI: 10.1093/tropej/fmx018] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2020 {published data only}
- Ahmed F, Dey SK, Shahidullah M, Mannan MA, Raj AY, Sharmin S.Early versus delayed enteral feeding for achieving full feeding in preterm growth-restricted infants: a randomized clinical trial. Mymensingh Medical Journal 2020;29(3):638-45. [PMID: ] [PubMed] [Google Scholar]
Chetry 2014 {published data only}
- Chetry S, Kler N, Saluja S, Garg P, Soni A, Thakur A, et al.A randomised control trial comparing initiation of total enteral feeds on 1st day of life with standard feeding regimen in stable very low birth weight infants born between > 30-34 weeks gestation and 1000-1500 gms. In: Pediatric Academic Societies Annual Meeting; 2014 May 3–6; Vancouver (BC). 2014:2390;442.
Glass 1984 {published data only}
- Glass EJ, Hume R, Lang MA, Forfar JO.Parenteral nutrition compared with transpyloric feeding. Archives of Disease in Childhood 1984;59(2):131-5. [DOI: 10.1136/adc.59.2.131] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgs 1974 {published data only}
- Higgs SC, Malan AF, De Heese HV.A comparison of oral feeding and total parenteral nutrition in infants of very low birthweight. South African Medical Journal 1974;48(52):2169-73. [PMID: ] [PubMed] [Google Scholar]
Jajoo 2021 {published data only}
- Jajoo M, Singh A, Arora N, Bhaskar V, Mandal A.Early total versus gradually advanced enteral nutrition in stable very-low-birth-weight preterm neonates: a randomized, controlled trial. Indian Journal of Pediatrics 2021;89:Online ahead of print. [DOI: 10.1007/s12098-021-03778-6] [DOI] [PubMed] [Google Scholar]
Jayaraman 2017 {published data only}
- Jayaraman D, Mukhopadhyay K, Bhalla AK, Dhaliwal LK.Randomized controlled trial on effect of intermittent early versus late kangaroo mother care on human milk feeding in low-birth-weight neonates. Journal of Human Lactation 2017;33(3):533-9. [DOI: 10.1177/0890334416685072] [PMID: ] [DOI] [PubMed] [Google Scholar]
LaGamma 1985 {published data only}
- LaGamma EF, Ostertag S, Birenbaum H.Failure of delayed oral feedings to prevent necrotizing enterocolitis. Results of study in very-low-birth-weight neonates. American Journal of Diseases of Children 1985;139(4):385-9. [DOI: 10.1001/archpedi.1985.02140060067031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Modi 2015 {published data only}
- Modi M, Ramji S, Jain A, Kumar P, Gupta N.Early aggressive enteral feeding in neonates weighing 750–1250 grams: a randomized controlled trial. Indian Pediatrics 2019;56(4):294-8. [PMID: ] [PubMed] [Google Scholar]
- Modi M, Ranji S, Jain A, Sharma P, Gupta N.A randomised trial of aggressive feeding regimen in infants with birth weight ≤ 1250 grams. In: Proceedings of the Pediatric Academic Societies Annual Conference; 2015 Apr 25-28; San Diego, CA. 2015. [CENTRAL: CN-01344271]
Montealegre‐Pomar 2021 {published data only}
- Montealegre‐Pomar AP, Bertolotto-Cepeda AM, Romero-Marquez Y, Munoz-Ramirez KJ.Effectiveness and safety of fast enteral advancement In preterm infants between 1000 and 2000 g of birth weight. Journal of Parenteral and Enteral Nutrition 2021;45(3):578-86. [DOI: 10.1002/jpen.1925] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nangia 2019 {published data only}
- Nangia S, Vadivel V, Thukral A, Saili A.Early total enteral feeding versus conventional enteral feeding in stable very-low-birth-weight infants: a randomised controlled trial. Neonatology 2019;115(3):256-62. [DOI: 10.1159/000496015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Said 2008 {published data only}
- Said H, Elmetwally D, Said N.Randomized controlled trial of early versus late enteral feeding in prematurely born infants with birth weight ≤ 1200 grams. Kasr Al Aini Medical Journal 2008;14:1-10. [Google Scholar]
Sanghvi 2013 {published data only}
- Sanghvi KP, Joshi P, Nabi F, Kabra N.Feasibility of exclusive enteral feeds from birth in VLBW infants >1200 g - an RCT. Acta Paediatrica 2013;102(7):e299-304. [DOI: 10.1111/apa.12254] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tottman 2020 {published data only}
- Tottman AC, Alsweiler JM, Bloomfield FH, Gamble GD, Jiang Y, Leung M, et al.Relationships between early neonatal nutrition and neurodevelopment at school age in children born very preterm. Journal of Pediatric Gastroenterology and Nutrition 2020;70(1):72-8. [DOI: 10.1097/MPG.0000000000002471] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viswanathan 2017 {published data only}
- Viswanathan S, Merheb R, Wen X, Collin M, Groh-Wargo S.Standardized slow enteral feeding protocol reduces necrotizing enterocolitis in micropremies. Journal of Neonatal-Perinatal Medicine 2017;10(2):171-80. [DOI: 10.3233/NPM-171680] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weiler 2006 {published data only}
- Weiler HA, Fitzpatrick-Wong SC, Chellenberg JM, Fair DE, McCloy UR, Veitch RR, et al.Minimal enteral feeding within 3 d of birth in prematurely born infants with birth weight < or = 1200g improves bone mass by term age. American Journal of Clinical Nutrition 2006;83(1):155-62. [DOI: 10.1093/ajcn/83.1.155] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilson 1997 {published data only}
- Wilson DC, Cairns P, Halliday HL, Reid M, McClure G, Dodge JA.Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;77(1):F4-11. [DOI: 10.1136/fn.77.1.f4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Li 2016 {published data only}
- Li QF, Wang H, Liu D, Tang Y, Xu XF.Application of prolonging small feeding volumes early in life to prevent of necrotizing enterocolitis in very low birth weight preterm infants. International Journal of Nursing Sciences 2016;3:45-9. [DOI: 10.1016/j.ijnss.2016.02.015] [DOI] [Google Scholar]
Additional references
Akindolire 2020
- Akindolire A, Talbert A, Sinha I, Embleton N, Allen S, Neonatal Nutrition Network (NeoNuNet).Evidence that informs feeding practices in very low birthweight and very preterm infants in sub-Saharan Africa: an overview of systematic reviews. BMJ Paediatrics Open 2020;4(1):e000724. [DOI: 10.1136/bmjpo-2020-000724] [PMID: 32821859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Battersby 2018
- Battersby C, Santhalingam T, Costeloe K, Modi N.Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Archives of Disease in Childhood Fetal and Neonatal Edition 2018;103(2):F182-9. [DOI: 10.1136/archdischild-2017-313880] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernstein 2000
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A.Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 1):198-206. [DOI: 10.1016/s0002-9378(00)70513-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND.Deaths in preterm infants: changing pathology over 2 decades. Journal of Pediatrics 2012;160(1):49-53. [DOI: 10.1016/j.jpeds.2011.06.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Burrin 2002
- Burrin DG, Stoll B.Key nutrients and growth factors for the neonatal gastrointestinal tract. Clinics in Perinatology 2002;29(1):65-96. [DOI: 10.1016/s0095-5108(03)00065-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2016
- Conde-Agudelo A, Diaz-Rossello JL.Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD002771. [DOI: 10.1002/14651858.CD002771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Silva 2004
- Silva A, Jones PW, Spencer SA.Does human milk reduce infection rates in preterm infants? A systematic review. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(6):F509-13. [DOI: 10.1136/adc.2003.045682] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Waard 2018
- Waard M, Li Y, Zhu Y, Ayede AI, Berrington J, Bloomfield FH, et al.Time to full enteral feeding for very low-birth-weight infants varies markedly among hospitals worldwide but may not be associated with incidence of necrotizing enterocolitis: the NEOMUNE-NeoNutriNet cohort study. Journal of Parenteral and Enteral Nutrition 2018;43:658-67. [DOI: 10.1002/jpen.1466] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
el Manouni el Hassani 2019
- el Manouni el Hassani S, Berkhout DJ, Niemarkt HJ, Mann S, de Boode WP, Cossey V, et al .Risk factors for late-onset sepsis in preterm infants: a multicenter case-control study. Neonatology 2019;116:42-51. [DOI: 10.1159/000497781] [DOI] [PMC free article] [PubMed] [Google Scholar]
Embleton 2013
- Embleton ND.Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2017
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al.Mechanisms affecting the gut of preterm infants in enteral feeding trials. Frontiers in Nutrition 2017;4:14. [DOI: 10.3389/fnut.2017.00014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flidel‐Rimon 2004
- Flidel-Rimon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell ES.Early enteral feeding and nosocomial sepsis in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(4):F289-92. [DOI: 10.1136/adc.2002.021923] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flidel‐Rimon 2006
- Flidel-Rimon O, Branski D, Shinwell ES.The fear of necrotizing enterocolitis versus achieving optimal growth in preterm infants - an opinion. Acta Paediatrica 2006;95(11):1341-4. [DOI: 10.1080/08035250600719713] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gale 2020
- Gale C, McGuire W, Juszczak E.Randomised controlled trials for informing perinatal care. Neonatology 2020;117(1):8-14. [DOI: 10.1159/000499881] [DOI] [PubMed] [Google Scholar]
Garite 2004
- Garite TJ, Clark R, Thorp JA.Intrauterine growth restriction increases morbidity and mortality among premature neonates. American Journal of Obstetrics and Gynecology 2004;191(2):481-7. [DOI: 10.1016/j.ajog.2004.01.036] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Version accessed 12 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2017. Available at gradepro.org.
Harbord 2006
- Harbord RM, Egger M, Sterne JA.A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hartel 2009
- Hartel C, Haase B, Browning-Carmo K, Gebauer C, Kattner E, Kribs A, et al.Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? Journal of Pediatric Gastroenterology and Nutrition 2009;48(4):464-70. [DOI: 10.1097/MPG.0b013e31818c5fc3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hay 2018
- Hay WW Jr.Nutritional support strategies for the preterm infant in the neonatal intensive care unit. Pediatric Gastroenterology, Hepatology & Nutrition 2018;21(4):234-47. [DOI: 10.5223/pghn.2018.21.4.234] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2009
- Henderson G, Craig S, Brocklehurst P, McGuire W.Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(2):F120-3. [DOI: 10.1136/adc.2007.119560] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hershkovitz 2000
- Hershkovitz R, Kingdom JC, Geary M, Rodeck CH.Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound in Obstetrics & Gynecology 2000;15(3):209-12. [DOI: 10.1046/j.1469-0705.2000.00079.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hickey 2018
- Hickey M, Georgieff M, Ramel S.Neurodevelopmental outcomes following necrotizing enterocolitis. Seminars in Fetal and Neonatal Medicine 2018;23(6):426-32. [DOI: 10.1016/j.siny.2018.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K.Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horbar 2012
- Horbar JH, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al.Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kamoji 2008
- Kamoji VM, Dorling JS, Manktelow B, Draper ES, Field DJ.Antenatal umbilical Doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatrica 2008;97(3):327-31. [10.1111/j.1651-2227.2008.00671.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2012
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA.Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(1):F56-61. [DOI: 10.1136/adc.2010.204123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leaf 2013
- Leaf A.Introducing enteral feeds in the high-risk preterm infant. Seminars in Fetal & Neonatal Medicine 2013;18(3):150-4. [DOI: 10.1016/j.siny.2013.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGuire 2004
- McGuire W, Henderson G, Fowlie PW.Feeding the preterm infant. BMJ 2004;329(7476):1227-30. [DOI: 10.1136/bmj.329.7476.1227] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2013
- Morgan J, Bombell S, McGuire W.Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD000504. [DOI: 10.1002/14651858.CD000504.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oddie 2017
- Oddie SJ, Young L, McGuire W.Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pammi 2017
- Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al.Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017;5(1):31. [DOI: 10.1186/s40168-017-0248-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patole 2005
- Patole SK, Klerk N.Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(2):F147-51. [DOI: 10.1136/adc.2004.059741] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Premji 2021
- Premji SS, Chessell L, Stewart F.Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2019
- Quigley M, Embleton ND, McGuire W.Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD002971. [DOI: 10.1002/14651858.CD002971.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuels 2017
- Samuels N, de Graaf RA, Jonge RC, Reiss IK, Vermeulen MJ.Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics 2017;17(1):105. [DOI: 10.1186/s12887-017-0847-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shah 2012
- Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, et al.Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. Journal of Perinatology 2012;32(7):552-8. [DOI: 10.1038/jp.2011.176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharif 2020
- Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W.Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD005496. [DOI: 10.1002/14651858.CD005496.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2019
- Soll RF, McGuire W.Evidence-based practice: improving the quality of perinatal care. Neonatology 2019;116(3):193-8. [DOI: 10.1159/000496214] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tyson 2007
- Tyson JE, Kennedy KA, Lucke JF, Pedroza C.Dilemmas initiating enteral feedings in high risk infants: how can they be resolved? Seminars in Perinatology 2007;31(2):61-73. [PMID: 10.1053/j.semperi.2007.02.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viswanathan 2019
- Viswanathan S, Jadcherla SR.Development of gastrointestinal motility reflexes. In: Gastroenterology and nutrition: neonatology questions and controversies. Philadelphia: Elsevier, 2019:15-28. [Google Scholar]
VON 2020
- Vermont Oxford Network.Manual of Operations. Data Definitions & Infant Data Booklets 2020;Part 2(Release 25.0).
Walsh 2019
- Walsh V, McGuire W.Immunonutrition for preterm infants. Neonatology 2019;115(4):398-405. [DOI: 10.1159/000497332] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2020
- Walsh V, Brown JVE, Copperthwaite BR, Oddie SJ, McGuire W.Early full enteral feeding for preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD013542. [DOI: 10.1002/14651858.CD013542-pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2021
- Walsh V, McGuire W, Halliday HL.Evaluation of the quality of perinatal trials: making the GRADE. Neonatology 2021;118(3):378-383. [DOI: 10.1159/000516239] [PMID: ] [DOI] [PubMed] [Google Scholar]
Young 2020
- Young L, McGuire W.Immunologic properties of human milk and clinical implications in the neonatal population. Neoreviews 2020;21(12):e809-e816. [DOI: 10.1542/neo.21-12-e809] [DOI] [PubMed] [Google Scholar]
Young 2021
- Young L, McGuire W, Fowlie PW.Commentary on "enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants". Neonatology 2021;118(2):139-42. [DOI: 10.1159/000512988] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bombell 2008
- Bombell S, McGuire W.Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub2] [DOI] [PubMed] [Google Scholar]
Morgan 2011
- Morgan J, Young L, McGuire W.Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub3] [DOI] [PubMed] [Google Scholar]
Morgan 2014
- Morgan J, Young L, McGuire W.Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


