Abstract
Electrochemical energy storage relies essentially on the development of innovative electrode materials with enhanced kinetics of ion transport. Pseudocapacitors are excellent candidates to bridge the performance gap between supercapacitors and batteries. Highly porous, anhydrous Ni0.5Co0.5C2O4 is envisaged here as a potential electrode for pseudocapacitor applications, mainly because of its open pore framework structure, which poses inherent structural stability due to the presence of planar oxalate anions (C2O42–), and active participation of Ni2+/3+ and Co2+/3+ results in high intercalative charge storage capacity in the aqueous KOH electrolyte. The Ni0.5Co0.5C2O4 electrode shows specific capacitance equivalent to 2396 F/g at 1 A/g in the potential window of 0.6 V in the aqueous 2 M KOH electrolyte by galvanostatic charge/discharge experiments. Predominant pseudocapacitive mechanism seems to operative behind high charge storage due to active participation of Ni2+/3+ and Co2+/3+ redox couple as intercalative (inner) and surface (outer) charges stored by porous anhydrous Co0.5Ni0.5C2O4 were close to high 38 and 62% respectively. Further, in full cell asymmetric supercapacitors (ASCs) in which porous anhydrous Co0.5Ni0.5C2O4 was used as the positive electrode and activated carbon (AC) was utilized as the negative electrode, in the operating potential window 1.6 V, the highest specific energy of 283 W h/kg and specific power of ∼817 W/kg were achieved at 1 A/g current rates. Even at a very high power density of 7981 W/kg, the hybrid supercapacitor still attains an energy density of ∼75 W h/kg with high cyclic stability at a 10 A/g current rate. The detailed electrochemical studies confirm higher cyclic stability and a superior electrochemical energy storage property of porous anhydrous Co0.5Ni0.5C2O4, making it a potential pseudocapacitive electrode for large energy storage applications.
Introduction
Uninterrupted fuel and power supply is a driving force for the innovations and growth of mankind to sustain modern civilization, and continuous depletion of natural sources of fossil fuels and associated environmental concerns has boosted the demand for sustainable, clean, and green energy generation.1 Various classes of cleaner energy sources such as wind energy, solar power, and sea tides were explored, and continuous and controlled supply of energy from these sources requires the development and growth of devices meant for energy conversion and storage.2 Electrochemical energy storage is the most suitable technology for energy conversion and storage due to high theoretical efficiency of converting chemical energy to electrical energy.3 The energy storage process at electrode surfaces involves different phenomena due to the distinctive nature of the electrode and electrolyte interactions. Generally, three types of interaction occur on the electrode surface between the electrode and electrolyte known as (1) EDLC, (2) surface redox, and (3) intercalation of ions.4 Surface redox and intercalation are followed by the faradic law because of charge transformation reaction involved in the energy storage mechanism.5 Surface absorption, surface redox, and intercalation are responsible for pseudocapacitance and involve thermodynamic and kinetic behavior of electrosorption/desorption.6,7 Interaction of species on the electrode surface is via either attraction or repulsion; surface attraction (redox) is followed by Langmuir electrosorption (sharp peak in the cyclic voltammetry curve) and repulsion (peak broadening) is followed by Frumkin electrosorption (broad peak in the cyclic voltammetry curve).8 RuO2 was the first reported material to show pseudocapacitive charge storage behavior.9 MnO2·xH2O performed as a capacitor in a neutral electrolyte.10 According to the charge storage mechanism, pseudocapacitors have access to different oxidation states for redox charge transfer that can enable higher energy density compared to EDLC.11,12 To increase higher energy density, an asymmetry cell shows better performance when the capacitor component stores electrochemical energy by electrostatic force, and the battery component enhances the electron transfer in the hybrid electrode system, which leads to better charge transfer reaction at high current rates.13 Many studies are being carried out on transition metal oxide-based materials such as NiO, V2O5, spinel Co3O4, Fe2O3, and mixed spinel NiCo2O4 to explore electrodes for the pseudocapacitor.14−20 However, structural instability and performance degradation issues related to transition metal oxide lead to investigation of the novel framework structure for higher surface charge storage and better structural stability.21−23 Metal–organic frameworks (MOFs) are used as an interesting open framework structure, where materials are constructed by joining metal-containing units with organic linkers, generating an interesting three-dimensional or two-dimensional network with permanent porosity.24 Highly porous metal–organic framework structures, especially utilizing an oxalate linker with active participation metal ion redox, are known to show faradic pseudocapacitive characteristics.25−27 However, most of the oxalate materials have a high open structural space to accommodate the hydration of water, and that is why, most of the transition metal oxalates contain a structural water molecule. We have envisaged the controlled removal of structural water from the material to develop a novel porous structure that can accommodate a high degree of charge or anion intercalation/deintercalation couple with double layer capacitance to achieve superior capacitance.22b The controlled water removal can maintain the high porosity of the structure that can enable fast charge/ion transfer.
Here, in this article, we present the synthesis, characterizations, and electrochemical performances of hydrated Co0.5Ni0.5C2O4·2H2O and porous anhydrous Co0.5Ni0.5C2O4 electrodes. The porous anhydrous Co0.5Ni0.5C2O4 electrode shows the specific capacitance value of 2396 F/g at 1 A/g, whereas hydrated Co0.5Ni0.5C2O4·2H2O shows the capacitance equivalent to 810 F/g at 1 A/g in an aqueous 2 M KOH electrolyte. Furthermore, we assembled aqueous asymmetric supercapacitors (ASCs), in which porous anhydrous Co0.5Ni0.5CO4 was used as the positive electrode and activated carbon (AC) was utilized as the negative electrode. Highest specific energy equivalent to 283 W h/kg and specific power of ∼817 W/kg were achieved at 1 A/g current rates by the combination of porous anhydrous Co0.5Ni0.5C2O4 and AC with high cyclic stability.
Experimental Section
Synthesis
Synthesis of Co0.5Ni0.5C2O4·2H2O was carried out by the precipitation method. Highly porous anhydrous Co0.5Ni0.5CO4 was prepared in two step synthesis. 1.49 g of cobolt(II) nitrate hexahydrate (Co(NO3)2·6H2O) and 1.46 g of nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O) were dissolved in 200 mL of deionized water with continuous stirring using a hot plate magnetic stirrer, and 1.27 g of oxalic acid dehydrate (H2C2O4·2H2O) was added in solution. The entire mixture was stirred vigorously at 80 °C for 5 h. After 5 h of stirring, a white color precipitate of product Co0.5Ni0.5C2O4·2H2O was obtained. The obtained product is then washed several times with deionized water. Finally, the washed product, Co0.5Ni0.5C2O4·2H2O, was dried in a hot air oven at 90 °C for overnight. Porous anhydrous Co0.5Ni0.5C2O4 was formed after heating the material at 230 °C for 5 h in a N2 atmosphere.
| 1 |
| 2 |
Characterizations
The crystal structure and phase purity of synthesized products were characterized through a Rigaku Miniflex desktop X-ray diffractometer (XRD) with Cu-Ka radiation (λ = 0.154 nm) in the range of 2θ = 10–90° with a step size of 0.02°. Xpert High Score (PANanylytical) software was used to identify the required phase. FE-SEM (FP 5022/22) was used to determine the surface morphology and structure of the sample. Infrared spectra of the samples were recorded using a Nicolet iS5 FTIR spectrometer in the range of 400–4000 cm–1. Pore size distribution and specific surface area of the sample were measured by BET (MicrotracBEL). All electrochemical performances of the sample including cyclic voltammetry (CV), galvanostatic charge discharge (GCD), and electrochemical impedance spectroscopy (EIS) were conducted using a conventional three-electrode arrangement measured by Metrohm Autolab (PGSTAT204) equipped with a FRA32M module. Electrochemical measurements were analyzed using NOVA1.1 software.
Preparation of Electrode
Hydrated Co0.5Ni0.5C2O4·2H2O and anhydrous porous Co0.5Ni0.5C2O4 working electrodes were prepared in a 7:2:1 ratio of the active material, activated carbon, and binder (PVDF) in NMP solvent. Homogenous slurry was prepared using a mortar, and slurry containing ∼1 mg of active materials was cast over a 1 cm2 area of Toray carbon paper. The coated electrode was dried at 80 °C for 12 h. The electrode loading was calculated through taking the weight of the electrode using an electronic balance (error limit: 0.01 mg). For that, the weight of Torrey paper was taken first, and then, the weight of the coated electrode (after drying the coated ink on Torrey carbon paper on 1 × 1 cm2 area) was taken for the study. Then, from the difference in the weight, the exact loading of the electrode material was calculated.
Results and Discussion
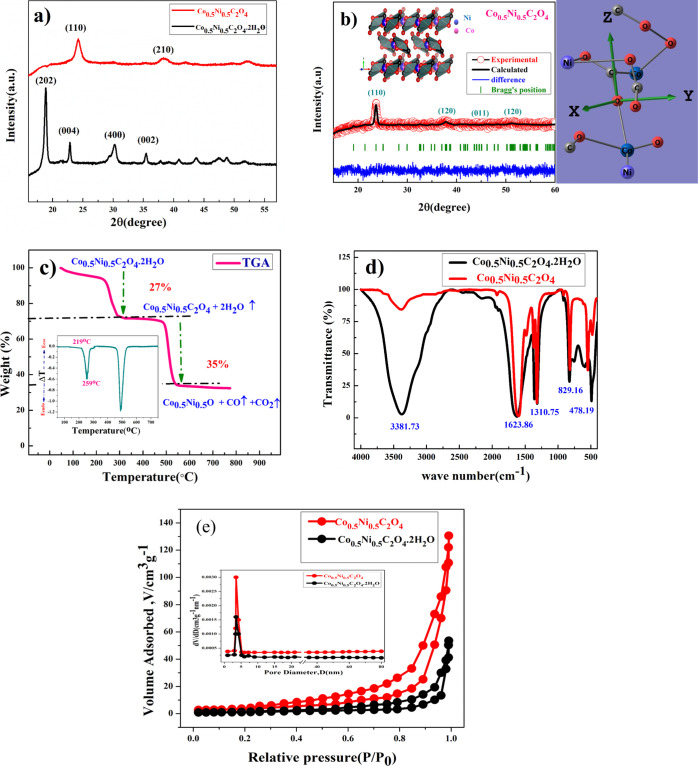
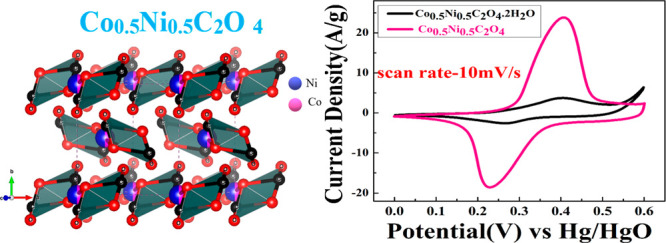
The XRD peak pattern of Co0.5Ni0.5C2O4·2H2O and anhydrous Co0.5Ni0.5C2O4 powder confirms the phase purity and formation of the single phase material. Figure 1a shows the XRD plot of Co0.5Ni0.5C2O4·2H2O and anhydrous Co0.5Ni0.5C2O4 in the 2θ range of 10–60° with a step size of 0.02°. The prominent sharp diffraction peaks at 18.84, 22.78, 30.35, 35.6, and 49.08 represent the (202), (004), (400), (022), and (602) planes of Co0.5Ni0.5C2O4·2H2O in the orthorhombic cell (space group: Cccm) and matches very well with the diffraction pattern of NiC2O4·2H2O (JCPDS no. 25-0582).28,29 After annealing at 230 °C for 5 h, Co0.5Ni0.5C2O4.2H2O transformed into anhydrous Co0.5Ni0.5C2O4 in the α-monoclinic structure (space group P21/n, JCPDS no.: 37-0719). Figure 1b shows the Rietveld Refined XRD profile of Co0.5Ni0.5C2O4 with lattice parameters a = 5.23400 Å, b = 5.653000 Å, c = 7.15900 Å, α-90o, β-118.88o, and γ-90o, and the VESTA image is shown in the inset.30
Figure 1.
(a) XRD pattern of Ni0.5Co0.5C2O4·2H2O and Ni0.5Co0.5C2O4, (b) Rietveld refinement of the XRD profile of anhydrous Ni0.5Co0.5C2O4 (vista image in the inset), (c) TGA of Ni0.5Co0.5C2O4·2H2O in a N2 atmosphere (inset shows the DTA plot), (d) FT-IR spectra of Ni0.5Co0.5C2O4·2H2O and Ni0.5Co0.5C2O4, and € BET surface area measurement plot of Ni0.5Co0.5C2O4·2H2O and Ni0.5Co0.5C2O4.
Thermogravimetric analysis (TGA) as shown in Figure 1c was used to understand the thermal stability of Co0.5Ni0.5C2O4·2H2O. The first weight loss occurred at the temperature from 100 to 300 °C, which corresponds to the removal of structural water from the sample; in this temperature range, phase transformation also occurred from orthorhombic to monoclinic. The TGA curve determines the weight loss of 27%, as 2 mol of water was removed from the sample in the temperature range of 100–300 °C. DTA shown in the inset clearly shows structure water leaving the structure at 219 °C, and the second weight loss step or decomposition of the oxalate group occurs in the temperature range of 350–500 °C, in which the weight loss of 35% was observed for decomposition of Co0.5Ni0.5C2O4. That is why, to perform the control dehydration of materials, we carried out dehydration or annealing at 230 °C to avoid rapid loss of structure water that can damage the porous structure and can result in particle segregation. Thus, to protect the porous structure of the anhydrated materials, annealing was carried out at 230 °C in a N2 atmosphere. Weight loss steps can be represented as follows
| 3 |
| 4 |
FTIR spectra of Co0.5Ni0.5CO4·2H2O and anhydrous Co0.5Ni0.5CO4 powder samples shown in Figure 1d reveal the presence of different functional groups in the material. The broad peak at 3381.71 cm–1 belongs to the stretching vibration of the hydroxyl group (−OH), which signifies the presence of water in Co0.5Ni0.5CO4·2H2O. The observed peak at 1620.75 cm–1 was assigned to the antisymmetric carbonyl stretching band (C=O) specific to the oxalate group.30 Two weak peaks at 1326.86 and 1310.75 cm–1 were attributed to vibrations of C2O42– (C–O) + (C–C) and (C–O) + (O–C=O), respectively. The peak at 829.16 cm–1 was assigned to the vibration mode of C2O42– and O–C=O bending vibrations (O–C=O). The absorption peak at 478.19 cm–1 can be attributed to both Ni–O and Co–O bonding present in the prepared sample of Cobalt oxalate dehydrate (Co0.5Ni0.5C2O4·2H2O). The annealing product after structural water removal represents Co0.5Ni0.5C2O4. The FT-IR study clearly shows the distinctive decrease in peak intensity of stretching vibration of the hydroxyl group (−OH) near 3381.73 cm–1.27Figure 1e shows the BET results of the Co0.5Ni0.5C2O4 sample. The nitrogen adsorption and desorption isotherm shows characteristics, which corresponds to the mesoporous structure of the oxalate; Co0.5Ni0.5C2O4 sample. The calculated BET specific surface area and average pore diameter is 129.82 cm2/g and both micropores and mesopores with diameters of 1.5–3.92 nm, respectively. Mesoporous structures attribute to excellent electrochemical kinetics due to high porosity. The calculated pore diameter of the Co0.5Ni0.5C2O4 sample is much higher than that of the ions present in aqueous electrolytes.28,29
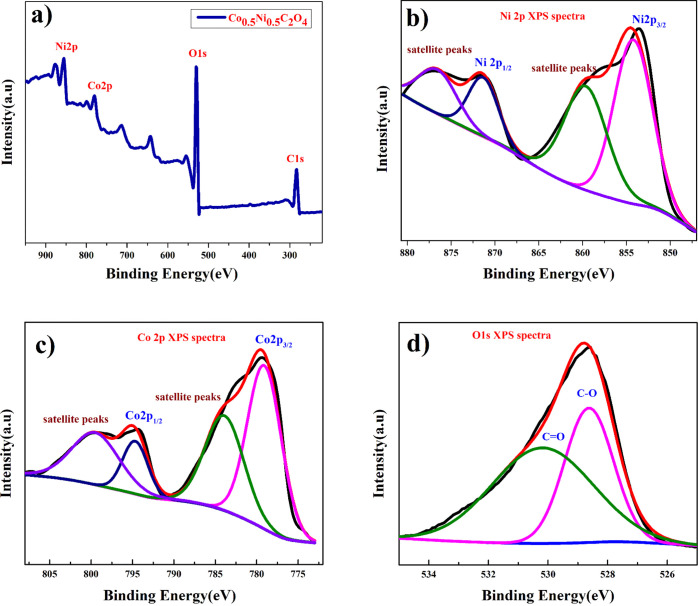
Figure 2a shows the X-ray photoelectron spectroscopy (XPS) survey plot of the Co0.5Ni0.5C2O4 sample, further confirming the presence of Ni and Co in the material. The Ni (2p) spectrum shown in Figure 2b could be assigned to 2p3/2 of Ni2+ (854.12 eV) and 2p1/2 of Ni2+ (871.71 eV) ions, as well as the corresponding satellite peaks at 859.83 and 876.71 eV. The Co(2p) spectrum shown in Figure 2c could be divided into peaks, which can be assigned to 2p3/2 of Co2+ (779.11 eV) and 2p1/2 of Co2+ (794.81 eV) ions, as well as the corresponding satellite peaks at 783.87 and 799.87 eV that arise from Co2+ ions. The O(1s) spectra shown in Figure 2d represent two binding energies at 530.23 and 528.58 eV for different C=O and C–O bond stretchings.30
Figure 2.
XPS plot of (a) full survey Ni0.5Co 0.5C2O4, (b) Ni (2p) spectra, (c) Co 2p spectra, and (d) O (1s) spectra.
The SEM image shown in Figure 3a displays the particle distribution and morphology of the Co0.5Ni0.5C2O4 powder sample. Its shows spongy-like arrangement. The inset (energy-dispersive X-ray analysis) image represents the elemental analysis of anhydrous Co0.5Ni0.5C2O4. To determine the diameter of grains, imageJ software was used. Agglomerated sub-micron size grains are visible in the SEM image. TEM shows atomic arrangements at localized regions within the sample shown in Figure 3b. The inset image represents FFT (fast Furrier transformation) and inverse FFT, and the calculated d spacing was found to be 0.227 nm, which matches the (110) plane of Co0.5Ni0.5C2O4.
Figure 3.
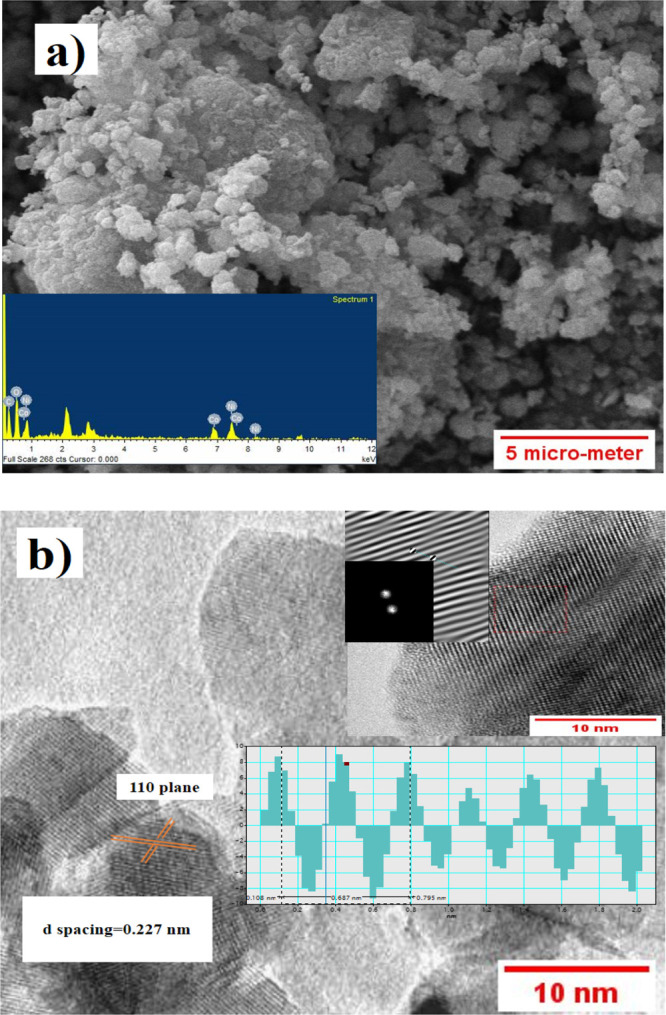
(a) SEM image showing morphology and particle size distribution of anhydrous Ni0.5Co0.5 C2O4 powder; inset shows the EDX image of anhydrous Ni0.5Co 0.5C2O4. (b) TEM image at localized regions; inset shows enlarged lattice fringes (with FFT and inverse FFT) and also (110) plane d spacing of porous anhydrous Ni0.5Co0.5 C2O4.
Electrochemical Studies
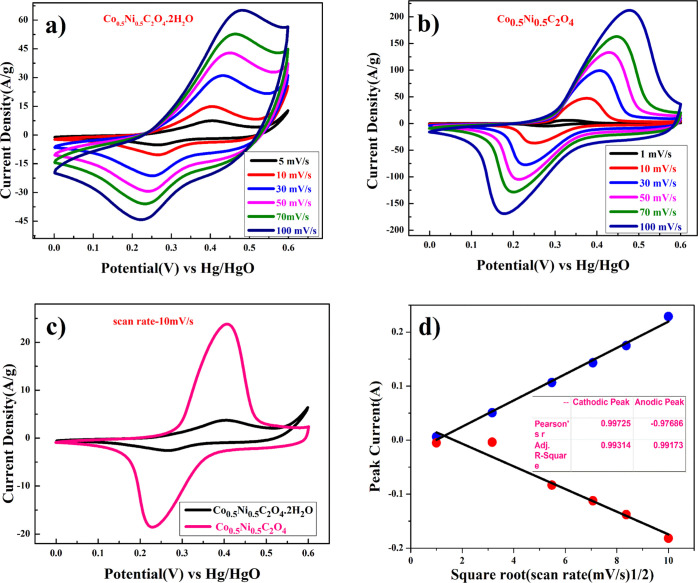
Electrochemical performance of Co0.5Ni0.5C2O4·2H2O and porous anhydrous Co0.5Ni0.5C2O4 as the working electrode was characterized using a three-electrode system, where Co0.5Ni0.5C2O4·2H2O and porous anhydrous Co0.5Ni0.5C2O4 act as working electrodes, saturated Hg/HgO (1 M KOH) as a reference electrode, and a platinum wire as a counter electrode in 2 M KOH as an electrolyte. The charge storage behavior of Co0.5Ni0.5C2O4·2H2O and porous anhydrous Co0.5Ni0.5C2O4 was characterized using cyclic voltammetry (CV) curves in the potential range of 0–0.6 V. Figure 4a represents the CV curve of Co0.5Ni0.5C2O4·2H2O. The nature of the curve explains the pseudocapacitive behavior coupled with surface redox (electrosorption). The CV curve of highly porous anhydrous Co0.5Ni0.5C2O4 shown in Figure 4b shows that pseudocapacitive storage followed intercalative association with surface redox.31 Redox peaks are originated due to the reversible transformation between Co2+ to Co3+ and Ni2+ to Ni3+ through electrosorption (redox) of OH– ions.31
| 5 |
Figure 4.
(a) Cyclic voltammetry of Ni0.5Co0.5C2O4·2H2O, (b) cyclic voltammetry of porous anhydrous Ni0.5Co0.5C2O4, (c) comparative cyclic voltammetry curves for Ni0.5Co0.5C2O4·2H2O and Ni0.5Co0.5C2O4 electrodes at 10 mV/s, and (d) plot of log (peak current vs square root of the scan rate for porous anhydrous Ni0.5Co0.5C2O4).
From the CV curve, specific capacitance C (F/g) can also be calculated as one of the significant parameters to understand the electrochemical performance of the working electrode.32
| 6 |
where “m” is the mass of active material in the electrode (g), “V” is the potential window (V), and “ϑ” is the scan rate (mV/s).
The specific capacitances of Co0.5Ni0.5C2O4·2H2O and anhydrous Co0.5Ni0.5C2O4 were calculated using eq 6, and capacitance was found to be close to 671 and 1993 F/g at 1 mV/s, respectively. The highly porous anhydrous Co0.5Ni0.5C2O4 attains higher charge storage, resulting in much higher capacity compared to hydrated Co0.5Ni0.5C2O4·2H2O. In the voltage window of 0.6 V, the theoretical capacity of Co0.5Ni0.5C2O4·2H2O and anhydrous Co0.5Ni0.5C2O4 will be 879.55 F/g and 1096 F/g, respectively, with 1e–/OH– charge transfer coupled with reversible intercalation/de-intercalation of OH– ions. This suggests that there is at least transfer/exchange of 0/76e–/OH– per Co0.5Ni0.5C2O4·2H2O and 1.82 e–/OH– per Co0.5Ni0.5C2O4 molecule, suggesting participation of both Ni2+/3+ and Co2+/3+ redox couples in charge storage. The redox reaction for high capacitance of Co0.5Ni0.5C2O4·2H2O can be represented as
| 7 |
As given in eq 8, the capacitance of Co0.5Ni0.5C2O4 can be represented as a combination of redox reaction as well as double layer formation as electron transfer is more than 1.
| 8 |
The value of x can vary with scan rates, and the detailed electrochemistry Co0.5Ni0.5C2O4 is described later to understand the charge storage mechanism of the electrode. We believe that as Co0.5Ni0.5C2O4 can easily accommodate two structural water molecule to form Co0.5Ni0.5C2O4·2H2O, the anhydrous Co0.5Ni0.5C2O4 accommodate high charge transfer (1.82e–/OH–) coupled with double layer capacitance formation to result in very high capacity for the anhydrous Co0.5Ni0.5C2O4 electrode. Figure 4c shows a comparative plot of the CV curve of Co0.5Ni0.5C2O4·2H2O and Co0.5Ni0.5C2O4 at a scan rate of 10 mV/s. The plot clearly reveals that there are two different types of redox phenomena occurring in the charge storage process; Co0.5Ni0.5C2O4·2H2O follows surface redox and Co0.5Ni0.5C2O4 surface redox with intercalation and double layer formation.33
As the anhydrous Co0.5Ni0.5CO4 electrode showed much superior pseudocapacitive storage, we focused our study mainly on the anhydrous Co0.5Ni0.5CO4 only. Figure 4d shows the linear relation between anodic and cathodic peak current with respect to square root of scan rate and indicates that anhydrous Co0.5Ni0.5CO4 undergoes the semi-infinite diffusion-controlled process. Furthermore, kinetics of the electrode can be understood by determining the diffusion coefficient. The diffusion coefficient for the electrode was determined using the Randles–Sevick equation.34
| 9 |
where ip is peak current (A), n is the number of electrons transferred in the redox event (usually 1), A is the electrode area in cm2, D is the diffusion coefficient in cm2/s, Co is the OH– ion concentration in mol/cm3, and ν is the scan rate in V/s. According to the equation, the diffusion coefficient of Co0.5Ni0.5C2O4 were calculated to be 1.916 × 10–11 cm2/s for oxidation and 4.8931 × 10–11 cm2/s for reduction.
To further estimate qualitative contribution of the different charge storage kinetics/mechanisms of the electrode, the power law equation given in eq 10 was utilized.
| 10 |
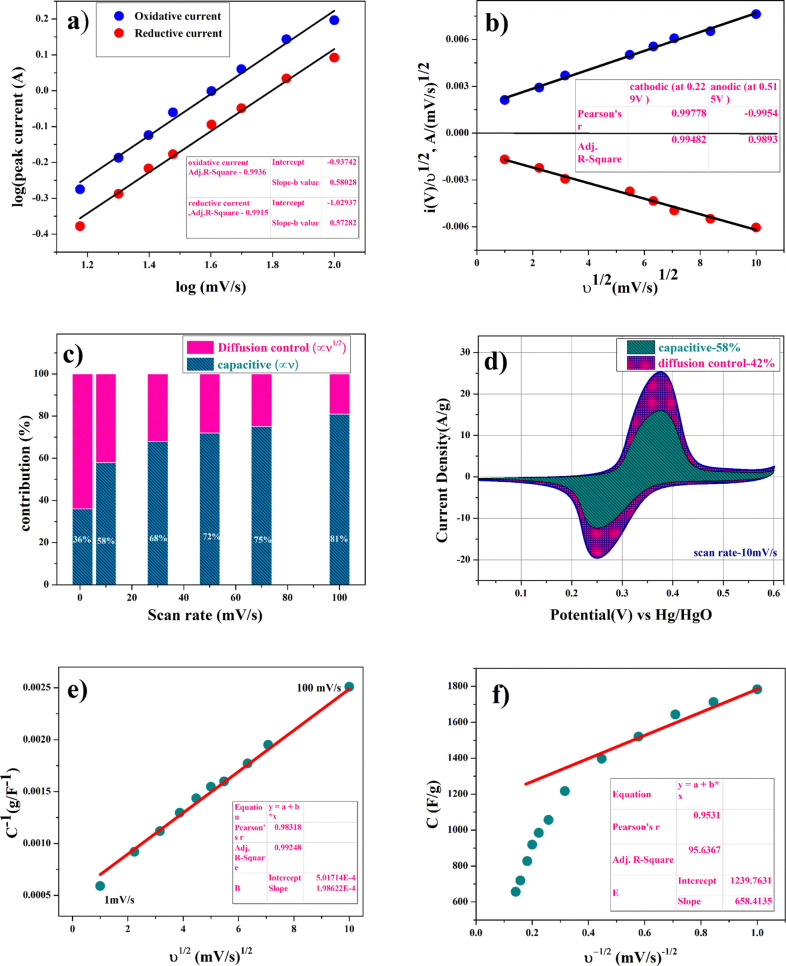
where a and b are adjustable values, i is the current (A), and ν is the scan rate (V/s). The value of b lies between 0.5 and 1, b = 0.5 stands for the semi-infinite diffusion control reaction, that is, battery type intercalative behavior, while b = 1 stands for the surface control reaction or electrosorption. Figure 5a shows the slopes (b value) of the corresponding log [peak current (ip) versus log(v) plots]. The b-values of oxidative and reductive current were found to be 0.58 and 0.57, respectively, indicating the dominance of semi-infinite diffusion-controlled intercalative processes resulting in battery-type supercapacitor behavior during the electrochemical reaction.35
Figure 5.
Electrodynamic characteristics of the Ni0.5Co0.5C2O4 electrode; (a) plot of the linear relationship between log (peak current) and log (scan rate) at two different scan rate regions, (b) plot of power law of the charged state at a potential and discharged state at a potential, (c) contribution of diffusive and capacitive contribution at different scan rates, (d) analysis of kinetic contribution at 10 mV/s, and (e,f) Trasatti plot at different scan rates.
Figure 5b shows the voltammetry sweep rate dependence that can distinguish quantitatively the capacitive contribution to the current response. The current response at a fixed potential is the contribution of two separate mechanisms, surface capacitive effects, and diffusion-controlled insertion or intercalation.
| 11 |
For better understanding, eq 11 was modified as
| 12 |
In eq 11, k1υ and k2υ1/2 represent the current contributions from the surface capacitive process and the diffusion-controlled intercalation process, respectively. Thus, after determination of k1 and k2, we can quantify their contribution in the current density at specific potentials.36k1 and k2 were determined from obtaining the slope and intercept of y axis from linear fit. The representative curve (i(V)/υ1/2 vs υ1/2) shown in Figure 5c represents the contribution of surface capacitance and diffusion-controlled intercalatio at different scan rates. Figure 5d represents specific contribution at a 10 mV/s scan rate, and contribution of surface capacitance or electrosorption was found to be 58% and that of diffusion-controlled intercalation was found to be close to 42%.
According to Trassati, the total specific capacitance is the sum of inner and outer surface capacitance of the electrode. It can be expressed as
| 13 |
The specific capacitance contributed from the inner and outer surface of the electrode is dependent on scan rates.37Figure 5e shows the linear fit C–1 versus υ1/2 at different scan rates, and the y-intercept represents the amount of total charge storage or capacitance of the electrode. Figure 5f shows the linear fit C versus υ–1/2, and the y-intercept represents the outer surface charge storage or capacitance of the electrode. After calculating the y-intercept value applied on the Trassati plot, the total capacitance value (Ctotal) was found to be 1993F/g, Cin was found to be 754 F/g (38% of the total capacitance value), and Cout was found to be 1239 F/g (62% of the total capacitance value).
Galvanostatic experiments were carried out to get more accurate capacity assessment of Co0.5Ni0.5CO4·2H2O and highly porous anhydrous Co0.5Ni0.5CO4 electrodes. From the charge/discharge curve, the specific capacitance of the electrode was calculated using eq 14.32
| 14 |
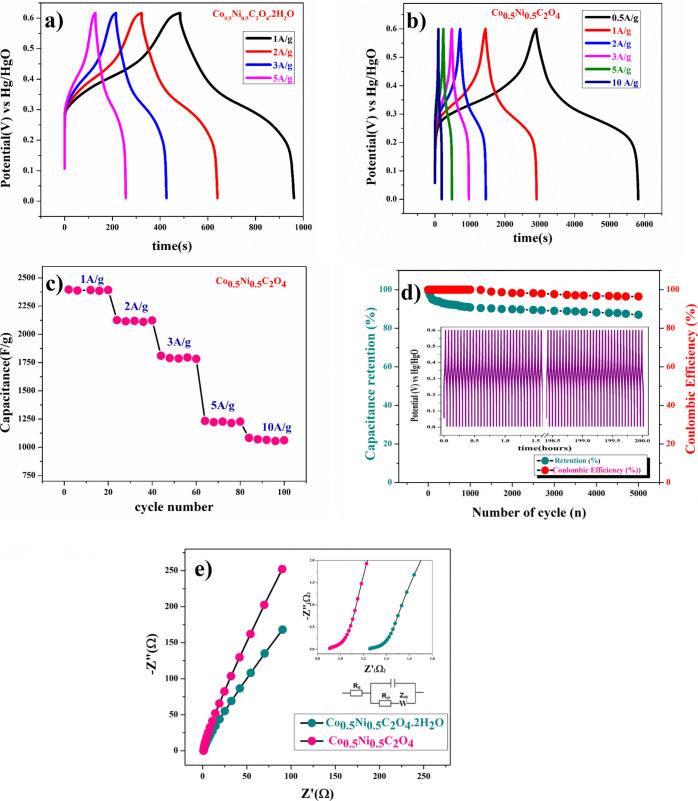
where I is the discharge current (A), Δt is the discharge time (s), m is the mass of the active material in the electrode (g), and ΔV is the potential change during discharge (V). Figure 6a depicts the specific capacitances of Co0.5Ni0.5C2O4·2H2O, and the values were found to be 810, 350, and 216 F/g at current densities of 1, 2, and 5A/g, respectively. Figure 6b shows the specific capacitances of the highly porous anhydrous Co0.5Ni0.5C2O4 electrode, and the values were found to be 2409, 2396, 2126, 1226, and 1083 F/g at current densities of 0.5, 1, 2, 5, and 10 A/g, respectively.
Figure 6.
(a) Charge/discharge curve of Ni0.5Co0.5C2O4·2H2O, (b) charge/charge curve of porous anhydrous Ni0.5Co0.5C2O4, (c) capacitance performance of porous anhydrous Ni0.5Co0.5C2O4 at different constant current rates, (d) capacitance retention and Coulombic efficiency porous anhydrous Ni0.5Co0.5C2O4, and (e) EIS plot and enlarged (zoom) view of the EIS plot of Ni0.5Co0.5C2O4·2H2O and porous anhydrous Ni0.5Co0.5C2O4 electrode at 10 mV (AC).
It has been observed that with increase in current density, there was decrease in specific capacitance of the electrode. In the desired range of current density, the specific capacitance decreases to 55% of its initial value. Figure 6c shows the capacitance value of the cycle number with different currents of the highly porous anhydrous Co0.5Ni0.5C2O4 electrode. Figure 6d exhibits the excellent long-term cyclic stability of highly porous anhydrous Co0.5Ni0.5C2O4 electrodes at 10 A/g for 5000 cycles. 87% capacity retention reflects that the specific capacitance of the electrode did not change much from the initial capacitance after 5000 cycles. The columbic efficiency (η = td/tc) of the electrode was 94.8% after 5000 cycles of charge/discharge, which reveals the high reversibility of the highly porous anhydrous Co0.5Ni0.5C2O4 electrode. In addition to electrochemical stability, we performed AC electrochemical impedance spectroscopy (EIS) at 10 mV, as shown in the Nyquist plot in Figure 6e, in the frequency range of 1 MHz to 0.1 Hz. The specific impedance contribution was attributed to the impedance distributions over electric series resistance (Rs), charge transfer resistance (Rct), and Warburg impedance (Rw). Higher frequency resistance was found for Co0.5Ni0.5C2O4·2H2O than porous anhydrous Co0.5Ni0.5C2O4 electrodes, as the intercept of the EIS spectra on the real axis was found to be at 1.43 and 0.8 Ω, respectively, indicating very small internal resistance for the anhydrous Co0.5Ni0.5C2O4 electrode. The small semicircle in the high frequency region shows the fast charge transport between the electrode and electrolyte. Lower frequency data represent the Warburg diffusion resistance for the samples. The straight line in the low frequency region for the porous anhydrous Co0.5Ni0.5C2O4 electrode is close to a 90° angle [very close to −Z″(Ω) axis], and the horizontal line represents the characteristic of more pseudocapacitance behavior of the electrode. The straight line in the low frequency region also represents fast OH– ion diffusion in the porous structure.38
Two Electrode Test
To understand the real charge storage behavior of the porous anhydrous Co0.5Ni0.5C2O4 sample relative to AC (activated carbon), two electrode measurements have been conducted in 2 M KOH. To determine the maximum specific capacitance during the full test, storage capacity of positive and negative electrodes needs to be balanced as per the following equation
| 15 |
For balancing the charge storage capacity of the cell, the mass ratio (m+/m–) of positive and negative electrode material was measured using the following equation
| 16 |
m+, m–, C+, C–, ΔE+, and ΔE– are mass, specific capacitance, and potential window of positive and negative electrodes estimated by three-electrode measurement, respectively.39,40
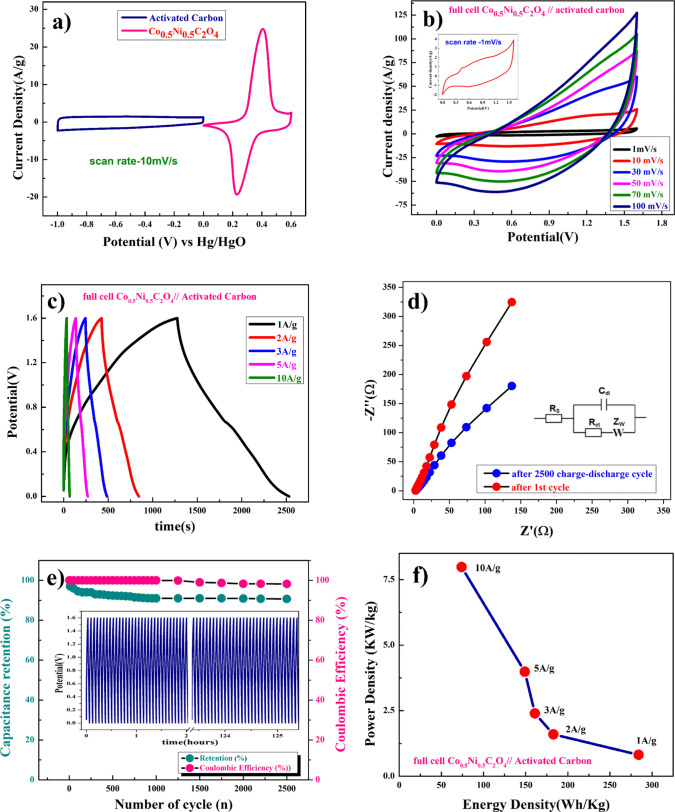
Figure 7a shows CV curves at a 10 mV/s scan rate, where AC (activate carbon) was used as the negative electrode and porous anhydrous Co0.5Ni0.5C2O4 was used as the positive electrode. The calculated mass ratio (m+/m–) was found to be 1: 5.3 for the asymmetric cell, and the weight of the active material was measured to be 4.41 mg (excluding the weight of acetylene black and PVDF). Figure 7b demonstrates the CV curve of porous anhydrous Co0.5Ni0.5C2O4//AC two-electrode ASCs [asymmetry supercapacitor cell at scan rates of 1–100 mV/s in this potential window (1.6 V)]. Figure 7c subsequently shows the galvanostatic charge/discharge curve, and the capacitance values were calculated by eq 13. Capacitance values were found to be 796, 515, 453, 421, and 211F/g at current densities of 1, 2, 3, 5, and 10 A/g, respectively. Figure 7d shows the EIS plot (Nyquist) in the frequency range of 1 MHz to 0.1 Hz at 10 mV/s, confirming the retention of the electronic structure and resistance of the full cell (anhydrous Co0.5Ni0.5C2O4//AC), as impedance of the material decreases after completion of 2500 cycles compared to the first cycle. Figure 7e shows the columbic efficiency of the two-electrode cell, and the cell has lost only 3% efficiency after completion of 2500 cycles with higher capacity retention (90.7%) of its initial value after 2500 cycles. Specific energy and specific power of asymmetric capacitors were calculated using the following equations
| 17 |
| 18 |
where CASCs is specific capacitance, V is operating voltage and tdis is discharge time.40
Figure 7.
(a) Representative CV for activated carbon (AC) and porous anhydrous Ni0.5Co0.5C2O4 at 10 mV/s, (b) plot for activated carbon and the porous anhydrous Ni0.5Co0.5C2O4 cell in ASC mode CV at different scan rates, (c) charge/discharge at different current rates, (d) EIS at 10 mV (AC), (e) capacitance retention and columbic efficiency, and (f) power density and energy density of two electrode cells in ASC mode.
Figure 7f shows the plot of specific energy versus specific power with different constant current rates. Resultant values confirm the highest specific energy equivalent to 283 W h/kg at 1 A/g current density with specific power equivalent to ∼817 W/kg. The maximum specific power of ∼7981 W/kg was obtained when specific energy reduced to ∼75.37 W h/kg at 10 A/g of current density. The capacitances of bulk/pristine transition-metal oxalate-based pseudocapacitors are summarized in Table 1 and are similar to those of anhydrous Co0.5Ni0.5C2O4 electrodes. The charge storage pseudocapacitive behavior of the Co0.5Ni0.5C2O4 electrode and the capacitance value are comparable or superior to that of most of bulk/pristine transition-metal oxalate-based pseudocapacitors reported to date.29,41−44 We believe that the control release of the water molecule from hydrated transition oxalate molecule results in anhydrous porous structured material that can accommodate storage of two molecules/ions (OH–) (intercalation couple with double layer capacitance) over the electrode is the key step in developing superior capacitance or charge storage materials.45
Table 1.
| material | morphology | capacitance (F g–1) | operating potential (V) | electrolyte | reference |
|---|---|---|---|---|---|
| CoC2O4 | thin sheet | 1269 F/g at 6 A/g | 0–0.5 | 6 M KOH | (41) |
| Co0.5Mn0.4Ni0.1C2O4·nH2O | micropolyhedrons | 990 F/g at 0.6 A/g | 0–0.4 | 3 M KOH | (42) |
| CoC2O4·2H2O | 2D porous thin sheets | 1.631 F/cm2 at 1.20 mA/cm | 0–0.4 | 6 M KOH | (43) |
| NiC2O4 | 2D thin sheet | 2835 F/g at 1 A/g | 0–0.4 | 6 M KOH | (44) |
| Ni0.55Co0.45C2O4 | microcuboid | 562 F/g at 1 A/g | 0–0.6 | 6 M KOH | (29) |
| MnC2O4/GO | olive-like | 122 F/g at 0.5 A/g | –0.1–0.55 | 6 M KOH | (35) |
| Co0.5Ni0.5C2O4·2H2O anhydrous | 810 F/g at 1 A/g | 0–0.6 | 2 M KOH | present work | |
| Co0.5Ni0.5C2O4 | nanoflakes | 2409 F/g at 1 A/g and 1993 F/g at 1 mV/s | 0–0.6 | 2 M KOH | present work |
Conclusions
In summary, porous anhydrous Co0.5Ni0.5C2O4 was successfully synthesized using a two-step process; first, Co0.5Ni0.5C2O4·2H2O was synthesized by the co-precipitation method in aqueous medium, and then Co0.5Ni0.5C2O4·2H2O was heated at 230 °C for 5 h, which resulted in porous anhydrous Co0.5Ni0.5C2O4. The anhydrous Co0.5Ni0.5C2O4 electrode showed a highly pseudocapacitive performance with a specific capacitance of 2396 F/g at a current density of 1 A/g and excellent cyclic stability. Predominant intercalative mechanism seems to operative behind high charge storage capacity of the materials as intercalative (inner) and surface (outer) charges stored by porous anhydrous Co0.5Ni0.5C2O4 were close to high 38 and 62%, respectively. The porous anhydrous Co0.5Ni0.5C2O4//AC full cell resulted in 283 W h/kg of maximum specific energy with a specific power equivalent to 817 W/kg in the voltage window of 1.6 V in the 2 M KOH electrolyte at a 1 A/g current rate. These results confirm that porous anhydrous Co0.5Ni0.5C2O4 can act as a potential pseudocapacitive electrode for large-scale energy storage application.
Acknowledgments
Authors thank Department of Ceramic Engineering, IIT (BHU), for its facility and support. P.S. thanks Science and Engineering Research Board (SERB), India, for the financial support (project no.: EMR/2016/006840). The authors also thankfully acknowledge the financial support from the Scheme for Promotion of Academic and Research Collaboration (SPARC) of the Ministry of Human Resource Development (MHRD), Government of India, SPARC grant no. SPARC/2018-2019/P1122/SL.
Author Contributions
P.S. conceptualized and supervised the work. T.M. supervised the electrochemical studies. R.M. and N.K.M. completed the study, and N.K.M. has organized the manuscript.
The authors declare no competing financial interest.
References
- Shafiee S.; Topal E. When will fossil fuel reserves be diminished?. Energy Pol. 2009, 37, 181–189. 10.1016/j.enpol.2008.08.016. [DOI] [Google Scholar]
- Xiang C.; Zhao X.; Tan L.; Ye J.; Wu S.; Zhang S.; Sun L. A solar tube: Efficiently converting sunlight into electricity and heat. Nano Energy 2019, 55, 269–276. 10.1016/j.nanoen.2018.10.077. [DOI] [Google Scholar]
- Augustyn V.; Simon P.; Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597. 10.1039/c3ee44164d. [DOI] [Google Scholar]
- a Brezesinski T.; Wang J.; Tolbert S. H.; Dunn B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. 10.1038/nmat2612. [DOI] [PubMed] [Google Scholar]; b Kamila S.; Mohanty B.; Samantara A. K.; Guha P.; Ghosh A.; Jena B.; Satyam P. V.; Mishra B. K.; Jena B. K. Highly Active 2D Layered MoS 2 -rGO Hybrids for Energy Conversion and Storage Applications. Sci. Rep. 2017, 7, 8378. 10.1038/s41598-017-08677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brad A. J.; Inzelt G.; Scholz F.. Electrochemical Dictionary; Springer Science & Business Media, 2008. (ISBN 978-3-642-29551-5). [Google Scholar]
- Conway B. E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. 10.1149/1.2085829. [DOI] [Google Scholar]
- Conway B. E.; Gileadi E. Kinetic theory of pseudo-capacitance and electrode reactions at appreciable surface coverage. Trans. Faraday Soc. 1962, 58, 2493–2509. 10.1039/tf9625802493. [DOI] [Google Scholar]
- Conway B. E.; Angerstein-Kozlowska H. The electrochemical study of multiple-state adsorption in monolayers. Acc. Chem. Res. 1981, 14, 49–56. 10.1021/ar00062a004. [DOI] [Google Scholar]
- Trasatti S.; Buzzanca G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, A1–A5. 10.1016/s0022-0728(71)80111-0. [DOI] [Google Scholar]
- Lee H. Y.; Goodenough J. B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. 10.1006/jssc.1998.8128. [DOI] [Google Scholar]
- Gogotsi Y.; Penner R. M. Energy Storage in Nanomaterials - Capacitive, Pseudocapacitive, or Battery-like?. ACS Nano 2018, 12, 2081–2083. 10.1021/acsnano.8b01914. [DOI] [PubMed] [Google Scholar]
- Costentin C.; Porter T. R.; Savéant J.-M. How Do Pseudocapacitors Store Energy? Theoretical Analysis and Experimental Illustration. ACS Appl. Mater. Interfaces 2017, 9, 8649–8658. 10.1021/acsami.6b14100. [DOI] [PubMed] [Google Scholar]
- Dubal D. P.; Ayyad O.; Ruiz V.; Gómez-Romero P. Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. 10.1039/c4cs00266k. [DOI] [PubMed] [Google Scholar]
- Devaraj S.; Munichandraiah N. Effect of Crystallographic Structure of MnO2 on Its Electrochemical Capacitance Properties. J. Phys. Chem. C 2008, 112, 4406–4417. 10.1021/jp7108785. [DOI] [Google Scholar]
- Liu K. C.; Anderson M. A. Porous Nickel Oxide/Nickel Films for Electrochemical Capacitors. J. Electrochem. Soc. 1996, 143, 124–130. 10.1149/1.1836396. [DOI] [Google Scholar]
- a Lee H. Y.; Goodenough J. B. Ideal Supercapacitor Behavior of Amorphous V2O5·nH2O in Potassium Chloride (KCl) Aqueous Solution. J. Solid State Chem. 1999, 148, 81–84. 10.1006/jssc.1999.8367. [DOI] [Google Scholar]; b Kamila S.; Mane P.; Mohanty R. I.; Chakraborty B.; Jena B. K. Supercapacitor properties of V10O14(OH)2 and reduced graphene oxide hybrids: Experimental and theoretical insights. Electrochim. Acta 2021, 399, 139357. 10.1016/j.electacta.2021.139357. [DOI] [Google Scholar]; c Kamila S.; Chakraborty B.; Basu S.; Jena B. K. Combined Experimental and Theoretical Insights into Energy Storage Applications of a VO2(D)-Graphene Hybrid. J. Phys. Chem. C 2019, 123, 24280–24288. 10.1021/acs.jpcc.9b03563. [DOI] [Google Scholar]
- Gao Y.; Chen S.; Cao D.; Wang G.; Yin J. Electrochemical capacitance of Co3O4 nanowire arrays supported on nickel foam. J. Power Sources 2010, 195, 1757–1760. 10.1016/j.jpowsour.2009.09.048. [DOI] [Google Scholar]
- Xia X.-H.; Tu J.-P.; Wang X.-L.; Gu C.-D.; Zhao X.-B. Mesoporous Co3O4 monolayer hollow-sphere array as electrochemical pseudocapacitor material. Chem. Commun. 2011, 47, 5786–5788. 10.1039/c1cc11281c. [DOI] [PubMed] [Google Scholar]
- Wang S.-Y.; Ho K.-C.; Kuo S.-L.; Wu N.-L. Investigation on Capacitance Mechanisms of Fe[sub 3]O[sub 4] Electrochemical Capacitors. J. Electrochem. Soc. 2006, 153, A75–A80. 10.1149/1.2131820. [DOI] [Google Scholar]
- a Liu X. Y.; Zhang Y. Q.; Xia X. H.; Shi S. J.; Lu Y.; Wang X. L.; Gu C. D.; Tu J. P. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sources 2013, 239, 157–163. 10.1016/j.jpowsour.2013.03.106. [DOI] [Google Scholar]; b Samantara A. K.; Kamila S.; Ghosh A.; Jena B. K. Highly ordered 1D NiCo2O4 nanorods on graphene: An efficient dual-functional hybrid materials for electrochemical energy conversion and storage applications. Electrochim. Acta 2018, 263, 147–157. 10.1016/j.electacta.2018.01.025. [DOI] [Google Scholar]
- Jabeen N.; Xia Q.; Savilov S. V.; Aldoshin S. M.; Yu Y.; Xia H. Enhanced Pseudocapacitive Performance of α-MnO2 by Cation Preinsertion. ACS Appl. Mater. Interfaces 2016, 8, 33732–33740. 10.1021/acsami.6b12518. [DOI] [PubMed] [Google Scholar]
- a Singh A. K.; Jaiswal P.; Singh P. A Review on Transition-metal Oxalate Based Electrode for Supercapacitors. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1166, 012032. 10.1088/1757-899x/1166/1/012032. [DOI] [Google Scholar]; b Mishra N. K.; Mondal R.; Singh P. Synthesis, characterizations and electrochemical performances of anhydrous CoC2O4 nanorods for pseudocapacitive energy storage applications. RSC Adv. 2021, 11, 33926–33937. 10.1039/d1ra05180f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gao Y.; Wu J.; Zhang W.; Tan Y.; Gao J.; Zhao J.; Tang B. Synthesis of nickel oxalate/zeolitic imidazolate framework-67 (NiC2O4/ZIF-67) as a supercapacitor electrode. New J. Chem. 2015, 39, 94–97. 10.1039/c4nj01719f. [DOI] [Google Scholar]; b Zhang Y.-Z.; Zhao J.; Xia J.; Wang L.; Lai W.-Y.; Pang H.; Huang W. Room temperature synthesis of cobalt-manganese-nickel oxalates micropolyhedrons for high-performance flexible electrochemical energy storage device. Sci. Rep. 2015, 5, 8536. 10.1038/srep08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf M.; Rajak R.; Mobin S. M. A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J. Mater. Chem. A 2016, 4, 16432–16445. 10.1039/c6ta06470a. [DOI] [Google Scholar]
- Han X.; Tao K.; Wang D.; Han L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. 10.1039/c7nr07931a. [DOI] [PubMed] [Google Scholar]
- Yang J.; Xiong P.; Zheng C.; Qiu H.; Wei M. Metal-organic frameworks: a new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. 10.1039/c4ta04140b. [DOI] [Google Scholar]
- Wang J.; Yang L.; Fu Y.; Yin P.; Guan X.; Wang G. Delicate control of crystallographic Cu2O derived Ni-Co amorphous double hydroxide nanocages for high-performance hybrid supercapacitors: an experimental and computational investigation. Nanoscale 2021, 13, 8562–8574. 10.1039/d1nr01016f. [DOI] [PubMed] [Google Scholar]
- Małecka B.; Małecki A.; Drożdż-Cieśla E.; Tortet L.; Llewellyn P.; Rouquerol F. Some aspects of thermal decomposition of NiC2O4·2H2O. Thermochim. Acta 2007, 466, 57–62. 10.1016/j.tca.2007.10.010. [DOI] [Google Scholar]
- Wang L.; Zhang R.; Jiang Y.; Tian H.; Tan Y.; Zhu K.; Yu Z.; Li W. Interfacial synthesis of micro-cuboid Ni0.55Co0.45C2O4 solid solution with enhanced electrochemical performance for hybrid supercapacitors. Nanoscale 2019, 11, 13894–13902. 10.1039/c9nr03790j. [DOI] [PubMed] [Google Scholar]
- Chenakin S.; Kruse N. XPS characterization of transition metal oxalates. Appl. Surf. Sci. 2020, 515, 146041. 10.1016/j.apsusc.2020.146041. [DOI] [Google Scholar]
- Evanko B.; Boettcher S. W.; Yoo S. J.; Stucky G. D. Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation. ACS Energy Lett. 2017, 2, 2581–2590. 10.1021/acsenergylett.7b00828. [DOI] [Google Scholar]
- Shi F.; Li L.; Wang X.-L.; Gu C.-D.; Tu J.-P. Metal oxide/hydroxide-based materials for supercapacitors. RSC Adv. 2014, 4, 41910–41921. 10.1039/c4ra06136e. [DOI] [Google Scholar]
- Jha M. K.; Babu B.; Parker B. J.; Surendran V.; Cameron N. R.; Shaijumon M. M.; Subramaniam C. Hierarchically engineered nanocarbon florets as bifunctional electrode materials for adsorptive and intercalative energy storage. ACS Appl. Mater. Interfaces 2020, 12, 42669–42677. 10.1021/acsami.0c09021. [DOI] [PubMed] [Google Scholar]
- Hanzu I.; Djenizian T.; Knauth P. Electrical and Point Defect Properties of TiO2 Nanotubes Fabricated by Electrochemical Anodization. J. Phys. Chem. C 2011, 115, 5989–5996. 10.1021/jp1111982. [DOI] [Google Scholar]
- Augustyn V.; Come J.; Lowe M. A.; Kim J. W.; Taberna P.-L.; Tolbert S. H.; Abruña H. D.; Simon P.; Dunn B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. 10.1038/nmat3601. [DOI] [PubMed] [Google Scholar]
- Kim H.-S.; Cook J. B.; Lin H.; Ko J. S.; Tolbert S. H.; Ozolins V.; Dunn B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3–x. Nat. Mater. 2017, 16, 454–460. 10.1038/nmat4810. [DOI] [PubMed] [Google Scholar]
- Ren W.; Chen X.; Zhao C. Ultrafast Aqueous Potassium-Ion Batteries Cathode for Stable Intermittent Grid-Scale Energy Storage. Adv. Energy Mater. 2018, 8, 1801413. 10.1002/aenm.201801413. [DOI] [Google Scholar]
- Basiricò L.; Lanzara G. Moving towards high-power, high-frequency and low-resistance CNT supercapacitors by tuning the CNT length, axial deformation and contact resistance. Nanotechnology 2012, 23, 305401. 10.1088/0957-4484/23/30/305401. [DOI] [PubMed] [Google Scholar]
- Subramani K.; Sudhan N.; Divya R.; Sathish M. All-solid-state asymmetric supercapacitors based on cobalt hexacyanoferrate-derived CoS and activated carbon. RSC Adv. 2017, 7, 6648–6659. 10.1039/c6ra27331a. [DOI] [Google Scholar]
- Hu N.; Gong W. H.; Huang L.; Shen P. K. Ultrahigh energy density asymmetric electrochemical capacitors based on flower-like ZnO/Co3O4 nanobundle arrays and stereotaxically constricted graphene. J. Mater. Chem. A 2019, 7, 1273–1280. 10.1039/c8ta10113b. [DOI] [Google Scholar]
- Cheng G.; Si C.; Zhang J.; Wang Y.; Yang W.; Dong C.; Zhang Z. Facile fabrication of cobalt oxalate nanostructures with superior specific capacitance and super-long cycling stability. J. Power Sources 2016, 312, 184–191. 10.1016/j.jpowsour.2016.02.046. [DOI] [Google Scholar]
- Zhang Y.-Z.; Zhao J.; Xia J.; Wang L.; Lai W.-Y.; Pang H.; Huang W. Room temperature synthesis of cobalt-manganese-nickel oxalates micropolyhedrons for high-performance flexible electrochemical energy storage device. Sci. Rep. 2015, 5, 8536. 10.1038/srep08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu T.; Li J.; Jiang Y.; Huang B.; Wang W.; Zhao C.; Xie L.; Chen L. Size and crystallinity control of two-dimensional porous cobalt oxalate thin sheets: tuning surface structure with enhanced performance for aqueous asymmetric supercapacitors. Dalton Trans. 2018, 47, 9241. 10.1039/c8dt01920g. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Jiang Y.; Liang S.; Gao F.; Xie L.; Chen L. Two-dimensional porous nickel oxalate thin sheets constructed by ultrathin nanosheets as electrode materials for high-performance aqueous supercapacitors. CrystEngComm 2020, 22, 2953. 10.1039/d0ce00268b. [DOI] [Google Scholar]
- Liu T.; Shao G.; Ji M.; Ma Z. Composites of olive-like manganese oxalate on graphene sheets for supercapacitor electrodes. Ionics 2014, 20, 145–149. 10.1007/s11581-013-1017-8. [DOI] [Google Scholar]