Summary
Neurological complications are common in COVID-19. Although SARS-CoV-2 has been detected in patients’ brain tissues, its entry routes and resulting consequences are not well understood. Here, we show a pronounced upregulation of interferon signaling pathways of the neurovascular unit in fatal COVID-19. By investigating the susceptibility of human induced pluripotent stem cell (hiPSC)-derived brain capillary endothelial-like cells (BCECs) to SARS-CoV-2 infection, we found that BCECs were infected and recapitulated transcriptional changes detected in vivo. While BCECs were not compromised in their paracellular tightness, we found SARS-CoV-2 in the basolateral compartment in transwell assays after apical infection, suggesting active replication and transcellular transport of virus across the blood-brain barrier (BBB) in vitro. Moreover, entry of SARS-CoV-2 into BCECs could be reduced by anti-spike-, anti-angiotensin-converting enzyme 2 (ACE2)-, and anti-neuropilin-1 (NRP1)-specific antibodies or the transmembrane protease serine subtype 2 (TMPRSS2) inhibitor nafamostat. Together, our data provide strong support for SARS-CoV-2 brain entry across the BBB resulting in increased interferon signaling.
Keywords: SARS-CoV-2, COVID-19, blood-brain barrier, neurovascular unit, hiPSC, infection model
Graphical abstract
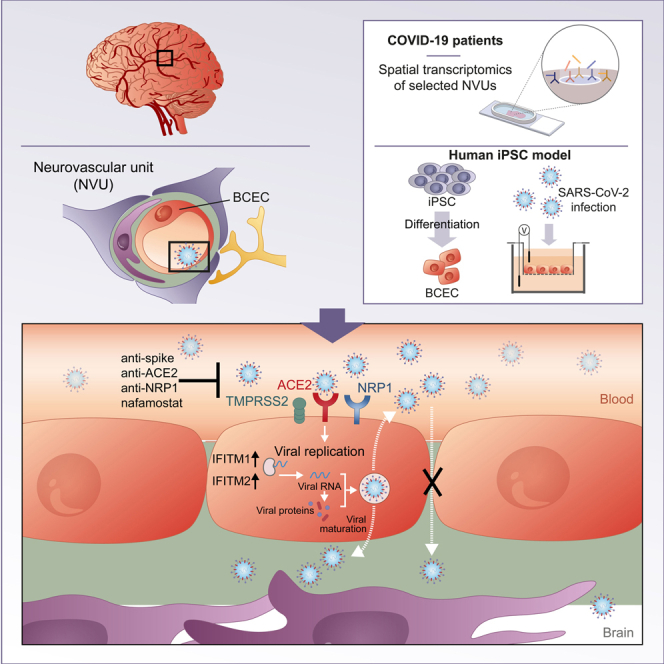
Highlights
-
•
IFNγ signaling is upregulated in COVID-19 human neurovascular unit
-
•
SARS-CoV-2-infected hiPS-BCECs display similar upregulation of IFNγ signaling
-
•
SARS-CoV-2 replicates in hiPS-BCECs and is released while barrier remains intact
-
•
SARS-CoV-2 infection of hiPS-BCECs is decreased by antibodies and protease inhibitors
In this article, Pless and colleagues show upregulation of IFNγ signaling in the neurovascular unit of the brain in fatal COVID-19. They show that an hiPSC-derived brain capillary endothelial cell model can be infected with SARS-CoV-2, resulting in similar expression changes, viral replication, and release while endothelial cell integrity is maintained. Infection can be prevented by antibodies or protease inhibitors.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had infected over 280 million people and contributed to over 5.4 million deaths worldwide by December 28, 2021 (World Health Organization, https://covid19.who.int/). Although the disease primarily affects the respiratory system, damage and dysfunction have also been found in other organs, including the kidney, heart, liver, and brain (Puelles et al., 2020). Neurological and neuropsychiatric complications, such as cerebrovascular injury, encephalitis, encephalopathy, dizziness, headache, hypogeusia, and hyposmia, but also psychosis, neurocognitive syndrome, and affective disorders, have been reported in a significant number of patients (Mao et al., 2020; Woo et al., 2020). SARS-CoV-2 RNA and proteins have been detected in the brain and cerebrospinal fluid (CSF) of COVID-19 patients, but viral loads are comparatively low and findings remain controversial (Matschke et al., 2020; Meinhardt et al., 2021; Puelles et al., 2020; Song et al., 2021). Despite numerous reports of neurological symptoms in COVID-19, it remains unclear whether these are a consequence of direct neural infection, parainfectious or post-infection immune-mediated disease, or sequelae of systemic disease (Ellul et al., 2020; Iadecola et al., 2020; Mao et al., 2020). Studies on brain tissue from deceased COVID-19 patients cannot address disease kinetics and underlying mechanisms, thus emphasizing the need for accessible and tractable experimental models to investigate SARS-CoV-2 cellular tropism, its functional impact, and therapeutic strategies.
Classic animal models are limited in their ability to recapitulate human COVID-19 symptoms and require non-physiological transgene-mediated overexpression of the human SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) (Bao et al., 2020; Sun et al., 2020) or mouse-adapted virus strains to exhibit symptoms (Dinnon et al., 2020). Although cell lines have been used to study SARS-CoV-2 infection and test drug efficacy (Ellinger et al., 2021), they do not recapitulate human cell physiology and may lack key proteins required for viral entry, such as ACE2, transmembrane protease serine subtype 2 (TMPRSS2) (Hoffmann et al., 2020), or neuropilin-1 (NRP1) (Cantuti-Castelvetri et al., 2020; Daly et al., 2020). These limitations call for the development of human cellular models of SARS-CoV-2 infection that more faithfully recapitulate the function of individual tissues.
Human induced pluripotent stem cell (hiPSC)-based models provide a versatile platform to investigate the susceptibility of various cell types to viral infection and the resulting consequences. hiPSC-derived blood-brain barrier models were instrumental in studying infection with bacteria, viruses (including Zika virus), and fungal toxins (Alimonti et al., 2018; Kim et al., 2017; Kim and Schubert-Unkmeir, 2019; Martins Gomes et al., 2019; Patel et al., 2018). hiPSC-derived organoids have been used to model SARS-CoV-2 infection in many organs, including the vasculature (Monteil et al., 2020) and brain (Ramani et al., 2020). These experiments have shown that SARS-CoV-2 may infect and replicate within cells of multiple organs, leading to expression changes in genes linked to inflammatory responses and altered cellular functions.
Applying spatial transcriptomics, we show that transcriptional changes, in particular upregulation of interferon signaling pathways, are abundant in the neurovascular unit in COVID-19 patients. The neurovascular unit maintains the physiological function of the blood-brain barrier (BBB) and comprises brain capillary endothelial cells (BCECs) and includes other cell types, such as pericytes, astrocytes, neurons, and microglia (Hawkins and Davis, 2005). Key BBB functions are the maintenance of CNS homeostasis and the prevention of penetration of neurotoxic substances as well as pathogens, such as bacteria and viruses. To investigate the susceptibility of the BBB and specifically model SARS-CoV-2 infection in the endothelial cell layer of the neurovascular unit, we used hiPSC-derived brain capillary endothelial-like cells (hiPS-BCECs) in a transwell setup to mimic the interface between the two compartments: vessel (apical) and brain parenchyma (basolateral). After apical SARS-CoV-2 application, we observed entry into hiPS-BCECs, active replication, transcellular transport, and release of the virus at the basolateral side. Moreover, we present functional consequences at the cellular and molecular levels. Elucidating such mechanisms and assessing therapeutics in a readily accessible compartment might ameliorate disease severity and COVID-19-related CNS phenotypes.
Results
The vascular niche is dysregulated in patients’ brains in fatal COVID-19
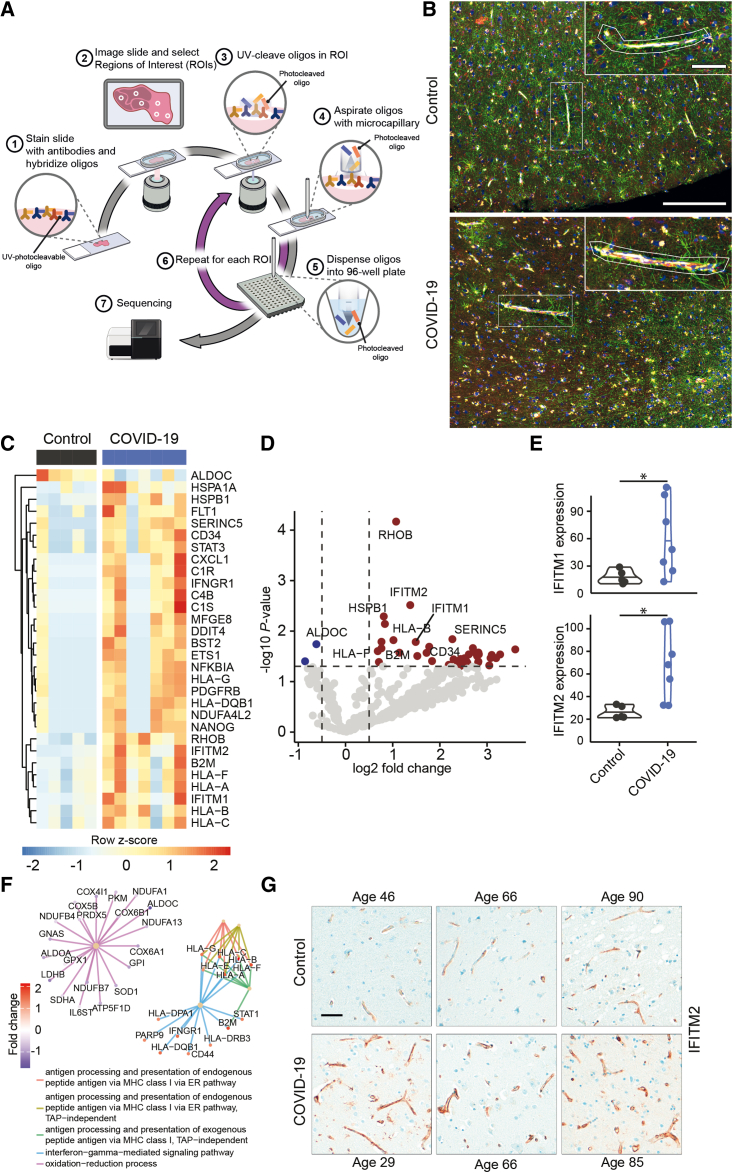
Histopathological studies have demonstrated the presence of SARS-CoV-2 in different cell types of the brain parenchyma (Matschke et al., 2020; Meinhardt et al., 2021; Song et al., 2021). Entry routes into the brain may include the olfactory mucosa (de Melo et al., 2021; Meinhardt et al., 2021), the vasculature (Meinhardt et al., 2021), the brain stem or vagal nerve (Matschke et al., 2020), or neuronal transport (Song et al., 2021), but molecular mechanisms underlying neuroinvasion of SARS-CoV-2 remain ill defined. To identify specific molecular alterations in the neurovascular unit in COVID-19, we performed spatial transcriptomics of selected neurovascular units of cortex gray matter tissue in COVID-19 patients and controls (Table S1) by using the Nanostring Digital Spacial Profiler (DSP) platform (Figure 1A). Here, specific regions of interest (ROIs) in a tissue section can be selected by cell-type-specific immunofluorescence staining (Figure 1A). A set of labeled probes is hybridized to the RNA on the entire tissue section. Hybridized probes are collected by UV-light cleavage specifically at the chosen ROI and then further analyzed by Illumina sequencing to determine differential expression of respective genes, enabling spatial transcriptomic analysis of human post mortem brain tissue (Figure 1A). In our approach, we specifically selected ROIs surrounding cortical vessels for sequencing (Figure 1B). This allowed us to specifically characterize SARS-CoV-2-induced transcriptional changes at the neurovascular unit. By immunofluorescent staining of the astrocyte-specific marker glial fibrillary acidic protein (GFAP), the endothelial marker CD31, and the leukocyte marker CD45, we chose brain regions with comparable glial activation and immune cell infiltration of control and COVID-19 patients (Figure 1B). We selected ROIs in five control and seven COVID-19 specimens, with three to five vessels per individual cortex tissue, in total 48 vessels. ROIs contained an average of 27 cells as measured by nucleus count (ranging from n = 8 to n = 67) surrounding CD31+ vessels, including mainly endothelial cells but also astrocyte endfeet and other cells (close up in Figure 1B). By using a conservative normalization and filtering approach and applying stringent cutoff criteria, we identified 30 differentially regulated genes (Figures 1C and 1D) in brain vessels of COVID-19 patients compared with controls. Of note, IFITM1 and IFITM2, which are necessary for SARS-CoV-2 infection in human lung cells (Shi et al., 2021), were significantly upregulated (∗p = 0.03 for IFITM1, ∗p = 0.01 for IFITM2) in COVID-19 patients (Figures 1D and 1E). Subsequent gene set enrichment analysis (GSEA) revealed an upregulation of mRNAs related to antigen processing and major histocompatibility complex (MHC) class I-dependent antigen presentation (Figure 1F), supporting the notion that SARS-CoV-2 directly affects brain endothelial cells. In addition, we found that interferon-γ signaling was enhanced in COVID-19 (Figure 1F). To validate our findings, we performed immunohistochemical staining of IFITM2 in COVID-19 and control brains. Here, we could show that IFITM2 expression is restricted to the neurovascular unit and upregulated in COVID-19 patients (Figure 1G). In contrast to these highly specific transcriptional changes, the brain endothelial marker CD31 revealed no significant differences between COVID-19 and control brains (Figures S1A and S1B). Assessment of the abundance of the SARS-CoV-2 entry factors ACE2 and NRP1 in human cortex brain vessels revealed that NRP1 was more abundant than ACE2 (Figure S1C).
Figure 1.
Transcriptional profiling of the neurovascular unit of COVID-19 and control brains
(A) Schematic representation of the spatial transcriptomic analysis of the neurovascular unit in human brain tissue with the Nanostring DSP platform. Brain tissue sections were stained for abundant cell populations (here: CD31, CD45, GFAP, and nuclei) and hybridized with a library of photocleavable probes for a specific gene panel. Regions of interest are chosen and illuminated, and the hybridized probes are collected only there. Downstream analyses of the collected probes provide a representative picture of RNA expression of genes of interest in this specific location, here, the neurovascular unit.
(B) Representative images of cortical regions of control (top) and COVID-19 brains (bottom), stained for GFAP (green), CD31 (yellow), CD45 (red), and DNA (blue). Two representative ROIs that were used for transcriptional analyses are shown. Scale bar, 250 μm; close up, 75 μm.
(C) Heatmap summarizing all differentially regulated genes. Gene-dependent h-clustering was performed. Color shows row Z score.
(D) Volcano plot showing differentially up- (red) and downregulated (blue) genes. Top differentially regulated genes are labeled.
(E) Normalized expression of IFITM1 (top) and IFITM2 (bottom). Shapiro-Wilk tests followed by Mann-Whitney U tests were performed. ∗p = 0.03 for IFITM1, ∗p = 0.01 for IFITM2.
(F) Gene set enrichment analysis of all detected genes. Color shows log2 fold change.
(G) Representative images for IFITM2 staining in brain tissue of control and COVID-19 patients. Images of age-matched pairs are displayed, demonstrating expression of IFITM2 in the neurovascular unit and its upregulation in fatal COVID-19. Scale bar, 50 μm.
SARS-CoV-2 infects hiPS-BCECs
Since we detected a specific interferon signature in the neurovascular unit in COVID-19 patients, we wondered whether this could be due to direct infection and contact with SARS-CoV-2 or a more general immune phenotype. Thus, to determine whether the brain vasculature could be a potential entry point for SARS-CoV-2 into the brain, we aimed to investigate the susceptibility of human BCECs to SARS-CoV-2 infection. hiPSCs provide an effective cell source to generate functional BCECs, thereby enabling mechanistic studies. We applied existing protocols to generate hiPS-BCECs in transwell monolayer cultures (Appelt-Menzel et al., 2017; Lippmann et al., 2012, 2014) and could confirm expected morphology (Figure S2A), transcriptional profiles (Figure S2B), BCEC-specific marker expression (Figure S2C), an intact tight-junction (TJ) network (Figure S2D), transendothelial electrical resistance (TEER) values >1,000 Ω·cm2 (Figure S2E), and permeability coefficients for paracellular tracer molecules like fluorescein (Figure S2F). Of note, we performed correlation analyses of transcripts derived from freshly prepared brain vessels of human brain biopsies and hiPS-BCECs and found a strong and significant correlation between the 100 highest and the 100 lowest expressing genes (Figures S3A and S3B).
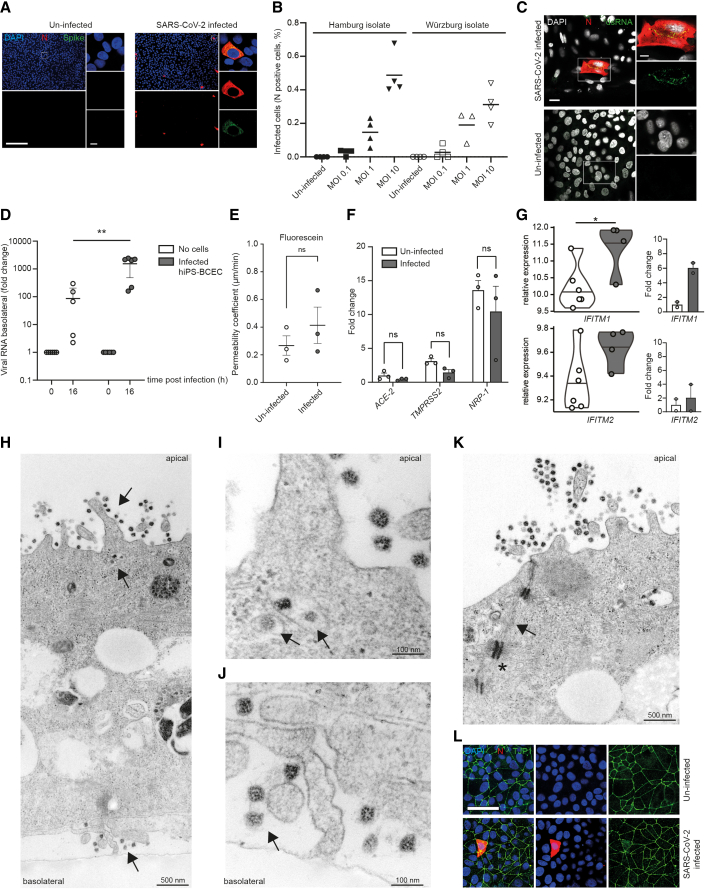
We infected hiPS-BCECs from the apical side of the transwell model, mimicking an infection from the endovascular compartment. One day post-infection, we detected SARS-CoV-2 N and spike proteins in infected hiPS-BCECs (Figure 2A). Infection with different MOIs (MOI 0.1, 1, and 10) using two independent SARS-CoV-2 isolates (Pfefferle et al., 2020a; Zimniak et al., 2021) resulted in a dose-dependent increase in the number of infected cells (Figure 2B). Of note, a human brain endothelial cell line, hCMEC/D3, could not be infected with SARS-CoV-2 (Figure S4), explicable by the ectopic ACE2 expression required to infect that model (Wenzel et al., 2021). In hiPS-BCECs, we could stain for double-stranded RNA (dsRNA) in a subset of N-protein-positive infected cells, indicating active virus replication (Figure 2C). Already after 16 h post-infection we detected a significant increase (∗p = 0.027) of SARS-CoV-2 RNA by qRT-PCR in the basolateral compartment compared with a transwell setting without the BCEC layer (Figure 2D), without compromising the integrity of the BBB with regard to paracellular permeability to fluorescein (Figure 2E). We therefore speculate that the basolateral abundance of viral particles could be a consequence of active virus production, transcellular transport, and release from the hiPS-BCECs. The SARS-CoV-2 entry receptor ACE2 and co-factor/alternative receptor TMPRSS2 and NRP1 are all expressed in hiPS-BCECs as detected by qRT-PCR, with NRP1 being the most abundant (Figure 2F), similar to the situation in human brain tissue (Figure S1C). A non-significant decrease of mRNA expression was observed for all three genes after SARS-CoV-2 exposure, a hallmark of infection (Glowacka et al., 2010). Importantly, the increase of IFITM1 and IFITM2 expression observed in tissue infected with SARS-CoV-2 could be recapitulated in our model by both mRNA sequencing (∗p = 0.031 for IFITM1, p = 0.403 for IFITM2) and qRT-PCR (Figure 2G). In transmission electron microscopy (TEM) cross sections of SARS-CoV-2-treated hiPS-BCECs, we could confirm attachment and apical uptake as well as basolateral shedding of the virus (Figures 2H–2J). After infection, neighboring hiPS-BCECs remained connected by complex TJs constricting the paracellular space (Figure 2K). Furthermore, adhesion points anchored within the actin filament network were detected, indicating the integrity of cell-cell contacts (Figure 2K). As reference, TEM images of uninfected hiPS-BCECs are shown in Figure S2D. The overall integrity of the hiPS-BCECs was further demonstrated by intact localization and unaltered expression of TJP1/ZO-1, a TJ marker, after infection (Figure 2L).
Figure 2.
SARS-CoV-2 infects and replicates in hiPS-BCECs
(A) Representative overview images that were used for subsequent quantification and respective close ups of N and spike protein double staining after infection with SARS-CoV-2 for 24 h (MOI 10) are shown. In the overview images, N protein is oversaturated to enable easy counting of infected cells; the close ups display the subcellular localization of N and spike protein in infected cells. Uninfected cells served as control and did not show any staining with SARS-CoV-2-specific antibodies. SARS-CoV-2 N protein (red), SARS-CoV-2 spike protein (green), counterstained by DAPI (blue). Scale bar, 200 μm; close up, 7.5 μm.
(B) Infected hiPS-BCECs (MOI 0.1, 1, and 10 each for Hamburg and Würzburg isolates) stained for N protein, indicating a dose-dependent rate of infection; n = 2 independent experiments, three or four technical replicates per condition.
(C) Representative immunofluorescence of SARS-CoV-2-infected hiPS-BCECs stained for N protein (red) and double-stranded RNA (dsRNA, green) (MOI 10), counterstained by DAPI (white). Uninfected hiPS-BCECs served as control. Scale bar, 25 μm; close up, 10 μm.
(D) In transwell assays, SARS-CoV-2 is applied from the apical side to infect hiPS-BCECs (MOI 10). Mean ± SEM of n = 3 independent experiments, three technical replicates per condition. A significant increase in viral RNA was detected in the basolateral compartment by qRT-PCR. Two-way ANOVA with post hoc Sidak's multiple comparison test, ∗∗p = 0.001.
(E) Fluorescein transport study. The permeability coefficient is comparable 24 h post-infection for SARS-CoV-2 (MOI 10) or control-treated samples. Mean ± SEM from n = 3 independent experiments. Unpaired Student’s t test, p > 0.05.
(F) Host factors required for SARS-CoV-2 uptake are expressed in hiPS-BCECs and are diminished 24 h post-infection (MOI 10) compared with uninfected cells. Normalized to ACE2 in uninfected cells. Mean ± SEM from n = 3 individual experiments. Two-way ANOVA with post hoc Sidak's multiple comparison test, p > 0.05.
(G) Normalized expression of IFITM1 and IFITM2. Left: differential expression analysis of RNA-sequencing data with false discovery rate (FDR) correction for multiple comparisons. ∗p = 0.031 for IFITM1, p = 0.403 for IFITM2. Violin plots and mean of n = 6 independent experiments (uninfected) and n = 4 independent experiments (infected) are shown. Right: differential expression analysis of qRT-PCR data. Mean ± SEM of n = 2 experiments with independent virus isolates, three technical replicates per condition.
(H–K) TEM micrographs of SARS-CoV-2-infected hiPS-BCECs. (H) Overview of a TEM cross section of a hiPS-BCEC monolayer. After infection (MOI 10) from the apical side (top black arrow), virus is taken up, is evident in intracellular vesicles (middle black arrow), and is released from the cells on the basolateral side (bottom black arrow). (I and J) Detailed areas in higher resolution from (H). (I) Virus is evident in intracellular vesicles (black arrows). (J) Virus is released from the cells on the basolateral side (black arrow). (K) Neighboring hiPS-BCEC monocultures are connected by complex TJs constricting the paracellular space (black arrows). Furthermore, adhesion points (punctum adherens, black asterisk in K) anchored within the actin filament network were detected, indicating the integrity of cell-cell contacts. Scale bars as indicated.
(L) Representative immunofluorescence of SARS-CoV-2-infected hiPS-BCECs stained for SARS-CoV-2 N (red) and TJP1 (green) proteins show intact cell connectivity 24 h post-infection (MOI 10), counterstained by DAPI (blue). Scale bar, 50 μm.
Virus-related transcriptional dysregulation in hiPS-BCECs mirrors in vivo findings
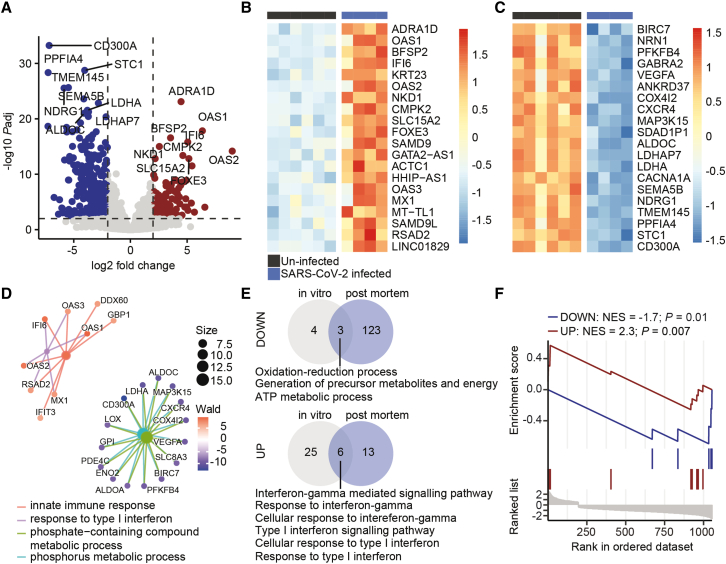
To compare the transcriptional response of the neurovascular unit in COVID-19 patients with that of SARS-CoV-2-infected hiPS-BCECs, we mock infected hiPS-BCECs or applied SARS-CoV-2 virus for 24 h and performed mRNA sequencing (Figures 3A and S3C). In line with our findings from spatial transcriptomics of brain vessels and their microenvironment in fatal COVID-19 (Figure 1), we detected that genes responsible for the innate immune response and type I interferon response were significantly upregulated. Moreover, genes that regulate phosphorus-dependent metabolic and ATP-generating pathways were significantly downregulated (Figures 3B–3D), which is in accord with studies in SARS-CoV-2-infected lung organoids (Pei et al., 2020). To analyze the translatability to humans and further support the validity of our model, we compared the biological themes of SARS-CoV-2-infected hiPS-BCECs with our spatial transcriptomics data from COVID-19 brain vasculature. Intriguingly, SARS-CoV-2 infection of endothelial cells consistently resulted in downregulation of metabolic pathways and upregulation of a cellular interferon response (Figure 3E). Of note, upregulated biological themes in COVID-19 brain vasculatures were significantly enriched (normalized enrichment score [NES] = 2.3, ∗∗p = 0.007), whereas downregulated biological themes were significantly de-enriched (NES = −1.7, ∗p = 0.01), in our SARS-CoV-2-infected hiPS-BCECs (Figure 3F). We thus show that brain endothelial cells show intrinsic inflammatory profiles upon contact with SARS-CoV-2, independent of immune cells. In summary, this highlights the usefulness of our in vitro model to study the BBB alterations in COVID-19, which is key to prospective mechanistic and pharmacological studies.
Figure 3.
Transcriptional profiling of SARS-CoV-2-infected hiPS-BCECs
(A) Volcano plot depicting differentially up- (red) and downregulated (blue) genes. Horizontal line shows −log10 of 0.01; vertical lines show log2 fold change of −1 and 1.
(B) Heatmap depicting top 20 upregulated genes in SARS-CoV-2-infected hiPS-BCECs (MOI 10). Color shows row Z score.
(C) Heatmap depicting top 20 downregulated genes in SARS-CoV-2-infected hiPS-BCECs. Color shows row Z score.
(D) GSEA of top 200 upregulated and top 200 downregulated genes. Color shows results of Wald statistics; size shows number of identified genes for each gene ontology (GO) term.
(E) Overlap of downregulated (top) and upregulated (bottom) biological themes of SARS-CoV-2-infected hiPS-BCECs (MOI 10) and blood vessels from COVID-19 brains.
(F) Enrichment analysis of upregulated (NES = 2.3, ∗∗p = 0.007) and downregulated (NES = −1.7, ∗p = 0.01) biological themes of blood vessels from COVID-19 brains in SARS-CoV-2-infected blood hiPS-BCECs. NES, normalized enrichment score. Data from n = 3 independent differentiation experiments, one or two replicates each.
SARS-CoV-2 infection of hiPS-BCECs can be pharmacologically inhibited
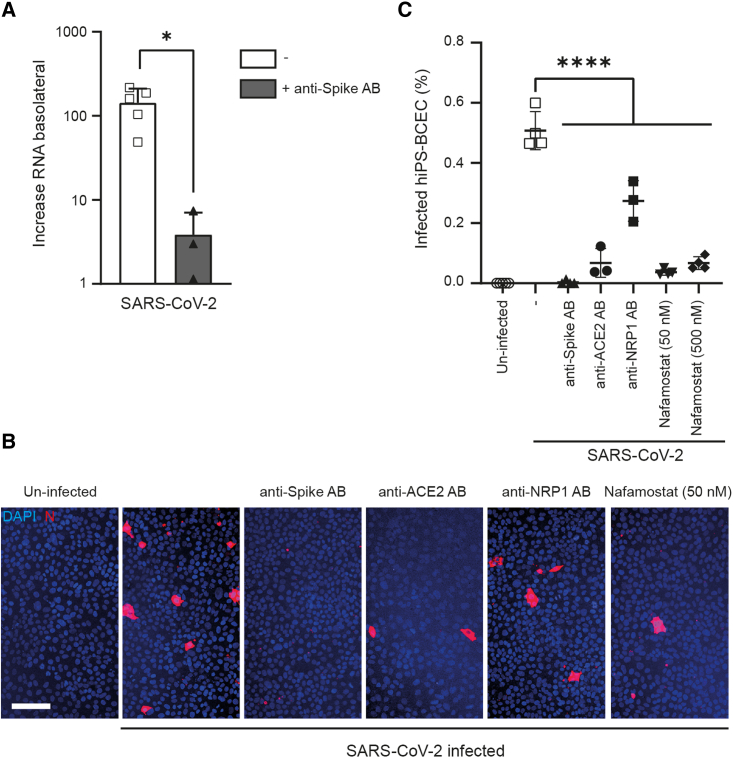
To investigate whether our hiPS-BCEC transwell system provides a suitable model to probe therapeutic intervention strategies, we went on to investigate the ability of blocking antibodies to prevent hiPS-BCEC infection by SARS-CoV-2. We infected the hiPS-BCEC transwell model from the apical (lumen-facing) side in either the presence or the absence of anti-spike antibodies targeting the receptor-binding domain (RBD) required for viral entry into the cell. After 24 h, SARS-CoV-2 RNA was quantified in the apical and basolateral compartments by qRT-PCR. SARS-CoV-2 RNA was significantly increased (∗p = 0.0132) in the basolateral compartment in untreated cells, an effect that could be mitigated by anti-spike-specific antibodies (Figure 4A). In addition, we tested reagents targeting viral uptake, including anti-spike, anti-ACE2, and anti-NRP1 antibodies and the protease inhibitor nafamostat, which inhibits TMPRSS2 with nanomolar potency in cell-based assays (Ellinger et al., 2021). Here, we infected the apical side with SARS-CoV-2 in the absence or presence of respective inhibitory molecules. Immunofluorescence staining of N-protein-positive cells compared with all cells was used to quantify SARS-CoV-2 infection (Figure 4B). Of note, all antibodies and nafamostat could significantly (∗∗∗∗p < 0.0001) diminish virus uptake by hiPS-BCECs (Figure 4C).
Figure 4.
SARS-CoV-2 infection of hiPS-BCECs can be diminished by blocking antibodies and small-molecule protease inhibitors
(A) In transwell assays, SARS-CoV-2 was used to infect hiPS-BCECs from the apical side (MOI 10). An increase in viral RNA was detected in the basolateral compartment by qRT-PCR. This effect could be significantly diminished by administration of anti-spike antibodies. Mean ± SEM of n = 3 independent experiments, one or two technical replicates each. Unpaired Student’s t test, ∗p = 0.0132.
(B) Image-based assessment of hiPS-BCECs after SARS-CoV-2 infection (MOI 10). Anti-spike, anti-ACE2, and anti-NRP1 antibodies and nafamostat (50 and 500 nM) were applied to counteract infection. Cell counting was performed using ImageJ software after staining SARS-CoV-2 N-protein-positive cells (red), counterstained by DAPI (blue). Scale bar, 100 μm.
(C) Quantification of (B). Mean ± SEM of n = 3 independent experiments, one or two technical replicates each. One-way ANOVA followed by Dunnett’s multiple comparisons test, ∗∗∗∗p < 0.0001.
Discussion
A substantial number of COVID-19 patients exhibit neurological symptoms, which may be further influenced by different host factors such as age, sex, comorbidities, disease progression, and others (Helms et al., 2020; Puelles et al., 2020; Solomon et al., 2020). The exact mechanism of how SARS-CoV-2 may enter the brain is currently unknown. The main hypotheses are neuron-to-neuron spread via bipolar cells located in the olfactory epithelium (de Melo et al., 2021; Meinhardt et al., 2021), a hematogenous route across the blood-CSF barrier (Desforges et al., 2014; Song et al., 2021), or transport via the vagal nerve to the brain stem (Matschke et al., 2020). Other routes of migration across the BBB, for example, transmigration of SARS-CoV-2-carrying leukocytes, cannot be excluded. Shortcomings of these studies relate to their non-mechanistic approaches or non-physiological animal models, which have been summarized recently (Butowt et al., 2021). In the work presented here, we systematically studied the brain vasculature as a potential entry site for SARS-CoV-2: initially, we performed spatial transcriptomics on post mortem COVID-19 and control brain tissue, focusing on the transcriptional changes in the neurovascular unit. We identified a significant upregulation of interferon-γ-mediated signaling pathways, including those coding for IFITM1 and IFITM2, which have been reported to potentially restrict SARS-CoV-2 infections (Shi et al., 2021). Second, we applied a transwell model of hiPS-BCECs that resembles human BCECs in their morphology, transcriptome, marker expression, and functional properties. In this model, we could observe entry and replication of SARS-CoV-2. Detailed analysis of these cells by electron microscopy showed virus uptake on the apical, lumen-facing side of the transwell model, active replication within the cells, transcellular transport, and shedding of viral particles on the basolateral, brain-mimicking side. This is in line with previous reports of BBB crossing of isolated spike protein in mice (Rhea et al., 2021) and in BBB in vitro models (Buzhdygan et al., 2020). Of note, the transcriptional changes observed in the neurovascular unit in COVID-19 patients, in particular an upregulation of interferon-γ-mediated signaling pathways and a downregulation of metabolic processes, could be recapitulated in this model. Thus, our study on one hand defines the molecular consequences of SARS-CoV-2 infection for the neurovascular unit but on the other hand also provides a means to investigate the cellular susceptibility, pathophysiology, and treatment strategies for SARS-CoV-2 infection of BCECs and subsequent brain infection and inflammation.
Building upon previous findings (Lippmann et al., 2012, 2014), we utilized an hiPS-BCEC model that is simple, robust, and reproducible with the initial goal to study the impact of SARS-CoV-2 infection and its molecular consequences on the BBB. The hiPS-BCECs exhibit a transcriptome that is highly comparable to that of freshly isolated brain vessels from human brain biopsies. Upon infection, the hiPS-BCEC model develops SARS-CoV-2-induced pathophysiological hallmarks at cellular and molecular levels, in particular a significant upregulation of genes involved in interferon signaling pathways. Of note, our findings indicate that endothelial cells might show an upregulation of interferon signaling after contact with SARS-CoV-2 that is independent of an immune cell contribution.
ACE2 has been identified as a key cell entry receptor for SARS-CoV-2 (Hoffmann et al., 2020). Low to moderate ACE2 expression has been detected in various brain regions, including the choroid plexus in humans and mice (Chen et al., 2020). Recent studies suggest that other proteins, such as NRP1, facilitate SARS-CoV-2 entry (Cantuti-Castelvetri et al., 2020; Daly et al., 2020). We here report expression of ACE2, confirming previous data (Matschke et al., 2020), but a higher relative abundance of NRP1 mRNA and protein in BCECs and hiPS-BCECs. Furthermore, our pharmacological blocking experiments confirm that both TMPRSS2 and NRP1 are involved in the entry of SARS-CoV-2 and may serve as promising intervention targets. Interestingly, although ACE2 expression was comparatively low in our BCEC model, incubation with an anti-ACE2 antibody almost completely blocked infection, highlighting the importance of ACE2 as a SARS-CoV-2 entry factor.
Limitations of the study
Our study demonstrates the susceptibility of hiPS-BCECs to SARS-CoV-2 infection in vitro. Moreover, we provide a thorough analysis of brain samples from individuals with COVID-19 suggesting that SARS-CoV-2 contact or viral entry via the BBB results in significant changes that might at least partially explain neurological symptoms in COVID-19. Direct evidence of SARS-CoV-2-infected brain endothelial cells in vivo by staining of viral antigens, however, is not presented here and is still under debate, although respective data have been published (Schwabenland et al., 2021). Our methodology has a number of constraints that may limit overall result interpretation. Direct exposure of hiPS-BCEC cultures to SARS-CoV-2 in the culture medium may not accurately recapitulate the physiological entry process in humans. Exposure of cells to large amounts of virus might possibly result in forced uptake with an entry via alternative routes. Our pharmacological experiments provide evidence of receptor-mediated entry in our model, since it could be efficiently and specifically blocked by specific antibodies despite high virus titers. Moreover, we showed that low MOI (as low as MOI 0.1) still leads to virus entry in hiPS-BCECs.
Also, given the comparatively low viral load (<1,000 SARS-CoV-2 RNA copies/mL) in the basolateral compartment, assessment of infectivity (e.g., via plaque assays) has not been possible in our model and experimental setting. For this, we would have to (1) significantly upscale our model or (2) incubate it for a much longer period of time, which is impossible due to a reported breakdown of barrier integrity at day 11 of the protocol (independent of virus) (Hollmann et al., 2017) and resulting leakage of virus to the basolateral compartment.
Regarding cellular identity, a recent study (Lu et al., 2021) challenges the hiPS-BCEC model applied here (Lippmann et al., 2012), which was further refined, evaluated, and widely applied over the last decade, including for the study of the biology of infection. Lu et al. claim that this differentiation protocol does not generate cells of endothelial but of epithelial origin. Opposed to the findings by Lu et al., we here report an increase in endothelial transcripts during the course of differentiation (e.g., VWF, CDH5, ABCG2, ABCB1) for two independent hiPSC lines (WISCi004-B and ZIPi013-B) and specific staining of key BCEC marker proteins (including CLDN5) after completion of the differentiation protocol, in line with other publications in the field (Appelt-Menzel et al., 2017; Lim et al., 2017; Lippmann et al., 2020). It is well appreciated that hiPSC-based models can show a lack of maturity in expression signatures and function. Therefore, epithelial expression signatures from pluripotent stem cells might be maintained after differentiation, as shown here for CLDN3, CLDN6, and CLDN7, which Lu et al. describe as epithelial markers. Having said this, several studies using human primary BCECs and hiPS-BCECs have been published over the past few years reporting the expression of almost all claudins in human BBB in vitro models (Delsing et al., 2018; Lim et al., 2017; Vatine et al., 2017). Of note, the alternative protocol suggested by Lu et al. cannot achieve a high paracellular tightness comparable to that of the physiological situation in vivo, which is of importance for our SARS-CoV-2 model to reliably test the molecular and functional consequences of infection and pharmacological treatment strategies. All our data were generated with hiPS-BCEC transwell inserts of TEER values >1,000 Ω·cm2 on day 10 of differentiation; inserts with lower values were not used for subsequent analysis.
Interestingly, the established hCMEC/D3 model could not be infected in our hands, pointing toward the suitability of the hiPS-BCEC model to study SARS-CoV-2-related phenomena.
The hiPS-BCEC transwell model applied here lacks additional cell types of the neurovascular unit, such as pericytes, astrocytes, and microglia, which may contribute to disease pathogenesis in vivo. Moreover, the model lacks immune cells, such as T cells and monocytes, which have been shown to mediate host responses to SARS-CoV-2 infection, including tissue-specific inflammation (Tay et al., 2020). Of note, our BCEC model displayed an intrinsic inflammatory profile after contact with SARS-CoV-2. Since endothelial cells are major targets of SARS-CoV-2, they may be the primary cause of SARS-CoV-2-related effects in the brain, with neurological symptoms being secondary to vascular changes and hypoxia (Ellul et al., 2020).
We here report that interferon signaling was increased in brain vessels in fatal COVID-19 when assessed by Nanostring DSP analysis. To enable expression analyses in post mortem tissue, we specifically sampled COVID-19 brain tissue with short post mortem and formalin fixation time. However, control tissue of the same quality was not available for Nanostring DSP analysis in required quantities; thus, we also included brain biopsies with short fixation times. Those tissues might not be an optimal control; however, over-fixed control tissues would likewise have been suboptimal. To overcome these limitations, we performed IFITM2 staining in our full panel of COVID-19 and control tissues, including biopsies and autopsies. With this approach, we could show that changes in IFITM2 are restricted to the neurovascular unit and specifically upregulated in COVID-19.
It is currently unclear whether neurological symptoms in COVID-19 are a direct result of neural infection or secondary to endothelial cell infection, hypoxia, or circulating pro-inflammatory cytokines. Future studies of SARS-CoV-2 susceptibility can extend to a more complex in vitro model of the neurovascular unit. Our in vitro studies provide useful information about specific cell types of focus for future human studies and offer a simple, accessible, and tractable human cell platform to investigate cellular susceptibility, disease mechanisms, and treatment strategies for SARS-CoV-2 infection of the human brain.
Experimental procedures
Human samples
Autopsies were performed at the Institute of Legal Medicine of the University Medical Center, Hamburg-Eppendorf, Germany. Use of post mortem human tissue and use of surgically removed brain specimens after conclusion of diagnostic procedures were reviewed and approved by the institutional review board of the independent ethics committee of the Hamburg Chamber of Physicians (protocol nos. PV7311, 2020-10353-BO-ff, and PV5034). Frontal/temporal cortex brain tissues were used for this study. For COVID-19, post mortem tissue samples were used; for non-COVID-19 controls, we added biopsy samples from patients undergoing neurosurgery for epilepsy. qPCR analysis of SARS-CoV-2 expression was already published elsewhere (Matschke et al., 2020) but is included in Table S1 (epidemiological and clinical information of COVID-19 patients and controls). For Nanostring DSP expression analysis, only samples with short formalin fixation times (24–96 h) were selected, to ensure high RNA quality. Cortical regions selected for Nanostring DSP were free of apparent inflammatory changes and displayed normal and non-activated morphology.
The use of human cells for the generation of hiPSCs was approved by the ethics committee of the Universitätsklinikum Schleswig-Holstein, Campus Kiel, Germany (A145/11) and is further described at https://www.sciencellonline.com/technical-support/ethical-statement.html.
Nanostring digital spatial profiling
Individuals selected for Nanostring DSP are labeled by # in Table S1. Mean age for Nanostring DSP expression analysis was 72 years for COVID-19 patients (29–89 years) and 57 years for controls (46–66 years). Regions of gray matter were selected from existing paraffin blocks of formalin-fixed cortex brain tissues from COVID-19 patients or controls; 5 mm tissue punches were prepared, and two new paraffin blocks were poured, each containing six different tissue punches. Tissue sections (5 μm) were mounted on slides, deparaffinized, and processed according to published protocols (Merritt et al., 2020). RNA-preserving antigen retrieval was performed, followed by RNA target exposure with proteinase K. In situ hybridization of the probe panel (GeoMx Cancer Transcriptome Atlas [CTA] panel [1,825 targets] + COVID-19 targets spike-in) on the tissue sections was performed overnight at 37°C, followed by stringent washes to remove off-target probes. Subsequently, the morphology markers CD31 (vessel endothelium, 1:50; #ab212712, Abcam), GFAP (astrocytes, 1:400; #53-9892-82, Invitrogen), CD45 (leukocytes, 1:100; #13917BF, CST), and DNA (SYTO83, 1:25; Thermo Fisher Scientific) were used to visualize target regions. Three to five vessels per individual cortex tissue were selected with the custom polygon ROI tool. These selected areas were illuminated individually via UV light on a GeoMx digital spatial profiler, resulting in photocleavage of oligonucleotides present within each ROI. The oligonucleotides were collected in a 96-well microwell plate. Each GeoMx DSP aspirate in the plate contained photocleaved DNA oligos comprising an analyte identifier, a unique molecular identifier (UMI) barcode, and a primer binding site. When PCR was performed on the aspirates, Illumina adapter sequences and unique dual-sample indices were added. The final library was used for sequencing on an Illumina NGS platform using a dual-index workflow after a pooling and quality control process. Signal-based normalization was performed by normalizing the signal of each probe against the 75th percentile of the cumulative signal of the respective ROI. For subsequent analysis, we calculated the average normalized expression value for each gene of all ROIs of each individual. We compared control and COVID-19 by a mixed linear model using the limma package within the R environment (Ritchie et al., 2015). GSEA (Merico et al., 2010) was performed using the clusterprofiler package (Yu et al., 2012).
SARS-CoV-2 molecular diagnostics and qRT-PCR of host gene expression
Detection and quantification of SARS-CoV-2 RNA were performed as described previously (Norz et al., 2020; Pfefferle et al., 2020b). For verification of mRNA expression of the SARS-CoV-2 receptor genes, cellular RNA was extracted using TRIzol reagent (Thermo Fisher Scientific) as recommended by the manufacturer. Commercially available TaqMan assays were performed (ACE2 [assay Hs01085333_m1], TMPRSS2 [assay Hs01122322_m1], NRP1 [assay Hs00826128_m1], IFITM1 [Hs01652522_g1], IFITM2 [Hs04194297_g1], all Thermo Fisher Scientific) using the RNA process control kit (Roche) on a Light Cycler 480 II instrument (Roche). We calculated gene expression as 2−ΔCt relative to GAPDH (human, assay Hs02786624_g1, Thermo Fisher Scientific) as the endogenous control.
Vero cell culture and SARS-CoV-2 isolates
Vero cells (ATCC CRL-1006) were cultivated and maintained under standard conditions (Pfefferle et al., 2020a). The MOI was determined by plaque assays. In all infection experiments, SARS-CoV-2 isolate HH-1 (Pfefferle et al., 2020a) was used at an MOI of 10 unless stated otherwise. In addition, SARS-CoV-2 isolate Würzburg (Zimniak et al., 2021) was used for experiments in Figure 2B. Furthermore, the MOIs for individual experiments are indicated in the respective figure legends.
hiPS-BCEC differentiation and establishment of the in vitro BBB model
The differentiation protocol was adapted from previously published protocols (Appelt-Menzel et al., 2017; Lippmann et al., 2012, 2014). A graphical overview of the procedure is provided in Figure S2A. See supplemental information for details.
SARS-CoV-2 infection of hiPS-BCEC and pharmacological treatment
hiPS-BCECs were cultivated as described above. At day 10 of differentiation, cells were prepared for SARS-CoV-2 infection. For this, virus was mixed in EC medium with the respective antibody or small-molecule inhibitor. Anti-spike antibody (NI-607.531_C8, Neurimmune) was used at 1 nM, anti-ACE2 antibody at 2 μg/mL (AF933; R&D Systems [Hoffmann et al., 2020]), and anti-NRP1 at 2.5 μg/mL (MSB178289, MyBioSource) combined with anti-NRP1 at 10 μg/mL (HPAB-0514-CN; Creative Biolabs) or the serine protease (TMPRSS2) inhibitor nafamostat at 50 or 500 nM. The mixtures were added to the apical transwell compartment. Experiments were stopped after 24 h by fixation of cells with 4% formalin, and medium was harvested for qRT-PCR. The number of infected cells was quantified by fluorescence staining of SARS-CoV-2 N protein and ImageJ.
mRNA sequencing
RNA sequencing libraries were prepared using the TruSeq stranded mRNA Library Prep Kit (Illumina) according to the manufacturer’s manual (document 1000000040498 v00) with a minimum total RNA input of 150 ng per sample. Libraries were pooled and sequenced on a NovaSeq 6000 sequencer (Illumina) generating 50 bp paired-end reads. The reads were aligned to the Ensembl human reference genome (GRCh38) using STAR v.2.4 (Dobin et al., 2013) with default parameters. The overlap with annotated gene loci was counted with featureCounts v.1.5.1 (Liao et al., 2014). Differential expression analysis was performed with DESeq2 (v.3.12) (Love et al., 2014) calling genes with a minimal 2-fold change and false discovery rate (FDR)-adjusted p < 0.05 differentially expressed. Gene lists were annotated using biomaRt (v.4.0) (Durinck et al., 2009). GSEA was performed using the clusterprofiler package (Yu et al., 2012).
Statistical analysis
The statistical analyses were carried out using GraphPad Prism (v.9.0.2, Windows 10 Enterprise). Images were analyzed using the PerkinElmer Columbus Image Data Storage and Analysis System (PerkinElmer) or ImageJ (NIH). Transcriptional data were analyzed within the R environment (v.1.2.5001) on a Mac OS X. All data were checked for normality before further analysis and means ± SEM were plotted. Depending on the data analyzed, differences between experimental groups were determined as indicated in the respective sections. Significant results are indicated by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Author contributions
S.K. conducted most staining experiments and related analysis of brain and hiPSC-derived tissues with support from E.T.; S.K., J.M., and M.G. performed neuropathological analysis; U.H., A.A.M., A.C., J.B., J.L., A.G., A.B., and W.N. established and characterized the hiPS-BCEC transwell model; S.P., N.G., E.-M.K., and J.B. performed all SARS-CoV-2 infections and related experiments; M.S.W. analyzed and visualized transcriptomic data; M.S. performed TEM experiments and analyzed the data; K.H., J.L.L., and D.S.F. assisted with the histological assessment of brain vessels; F.H., J.M., F.R., T.S., J.S., A.F., and B.O. autopsied the cases and provided autopsy or biopsy samples from COVID-19 patients and controls; S.F. and A.F. performed mRNA-sequencing experiments; S.M. provided anti-spike antibodies; F.J.M. assisted with the PluriTest analysis; S.K., A.A.M., J.B., G.G., C.C., A.K., P.G., B.O., M.A.F., M.G., and O.P. provided funding for the study; U.H., S.M., A.K., A.Z., and P.G. assisted with pharmacological experiments with antibodies and small molecules; S.K., U.H., S.P., M.S.W., M.A.F., M.G., and O.P. designed experiments for the study and analyzed the data; O.P. wrote the initial version of the manuscript; S.K., U.H., S.P., M.S.W., M.A.F., and M.G. revised the manuscript; S.K. and O.P. conceived and supervised the study. All co-authors contributed to the editing and discussion of the manuscript and approved the final version.
Conflict of interests
The authors declare no competing interests.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) for the projects HiPSTAR (01EK1608A to A.A.M. and 01EK1608B to O.P.) and DEFEAT PANDEMIcs (01KX2021), by the internal “Anti-Corona” programs of the Fraunhofer-Gesellschaft (DRECOR, 840260, and iCARE, 602015), by the Deutsche Forschungsgemeinschaft (FR1720/18-1 to M.A.F. and GL589/10-1 to M.G.), and by the Hamburg Ministry of Labour, Health, Family Affairs and Integration. The Nanostring DSP work was supported by a COVID-19 grant from Nanostring to S.K. We thank the UMIF of the UKE for access to the microscope facility. We thank Emanuela Szpotowicz (Morphology and Electron Microscopy Core Facility) for excellent technical assistance. We thank Philine Lange (Institute of Legal Medicine) for helpful assistance. Our condolences to the families and all those who have lost their loved ones during the pandemic.
Published: January 20, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.12.011.
Contributor Information
Susanne Krasemann, Email: s.krasemann@uke.de.
Ole Pless, Email: ole.pless@itmp.fraunhofer.de.
Supplemental information
Data and code availability
The code generated to analyze spatial transcriptomics is available from the corresponding author upon request. The accession number for the mRNA-sequencing data reported in this paper is GEO: GSE179923 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179923).
References
- Alimonti J.B., Ribecco-Lutkiewicz M., Sodja C., Jezierski A., Stanimirovic D.B., Liu Q., Haqqani A.S., Conlan W., Bani-Yaghoub M. Zika virus crosses an in vitro human blood brain barrier model. Fluids Barriers CNS. 2018;15:15. doi: 10.1186/s12987-018-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelt-Menzel A., Cubukova A., Gunther K., Edenhofer F., Piontek J., Krause G., Stuber T., Walles H., Neuhaus W., Metzger M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Rep. 2017;8:894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- Butowt R., Meunier N., Bryche B., von Bartheld C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol. 2021;141:809–822. doi: 10.1007/s00401-021-02314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., Razmpour R., Hale J.F., Galie P.A., Potula R., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Shen W., Rowan N.R., Kulaga H., Hillel A., Ramanathan M., Jr., Lane A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Anton-Plagaro C., Shoemark D.K., Simon-Gracia L., Bauer M., Hollandi R., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsing L., Donnes P., Sanchez J., Clausen M., Voulgaris D., Falk A., Herland A., Brolen G., Zetterberg H., Hicks R., Synnergren J. Barrier properties and transcriptome expression in human iPSC-derived models of the blood-brain barrier. Stem Cells. 2018;36:1816–1827. doi: 10.1002/stem.2908. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Stodola J.K., Meessen-Pinard M., Talbot P.J. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K.H., 3rd, Leist S.R., Schafer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Jr., Hou Y.J., Adams L.E., et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S., Spellman P.T., Birney E., Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger B., Bojkova D., Zaliani A., Cinatl J., Claussen C., Westhaus S., Keminer O., Reinshagen J., Kuzikov M., Wolf M., et al. A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci. Data. 2021;8:70. doi: 10.1038/s41597-021-00848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann E.K., Bailey A.K., Potharazu A.V., Neely M.D., Bowman A.B., Lippmann E.S. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS. 2017;14:9. doi: 10.1186/s12987-017-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e11. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Bee O.B., McDonagh M.A., Stebbins M.J., Palecek S.P., Doran K.S., Shusta E.V. Modeling group B Streptococcus and blood-brain barrier interaction by using induced pluripotent stem cell-derived brain endothelial cells. mSphere. 2017;2 doi: 10.1128/mSphere.00398-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Schubert-Unkmeir A. In vitro models for studying the interaction of Neisseria meningitidis with human brain endothelial cells. Methods Mol. Biol. 2019;1969:135–148. doi: 10.1007/978-1-4939-9202-7_10. [DOI] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lim R.G., Quan C., Reyes-Ortiz A.M., Lutz S.E., Kedaigle A.J., Gipson T.A., Wu J., Vatine G.D., Stocksdale J., Casale M.S., et al. Huntington's disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep. 2017;19:1365–1377. doi: 10.1016/j.celrep.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E.S., Al-Ahmad A., Azarin S.M., Palecek S.P., Shusta E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E.S., Azarin S.M., Kay J.E., Nessler R.A., Wilson H.K., Al-Ahmad A., Palecek S.P., Shusta E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E.S., Azarin S.M., Palecek S.P., Shusta E.V. Commentary on human pluripotent stem cell-based blood-brain barrier models. Fluids Barriers CNS. 2020;17:64. doi: 10.1186/s12987-020-00222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T.M., Houghton S., Magdeldin T., Duran J.G.B., Minotti A.P., Snead A., Sproul A., Nguyen D.T., Xiang J., Fine H.A., et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2016950118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins Gomes S.F., Westermann A.J., Sauerwein T., Hertlein T., Forstner K.U., Ohlsen K., Metzger M., Shusta E.V., Kim B.J., Appelt-Menzel A., Schubert-Unkmeir A. Induced pluripotent stem cell-derived brain endothelial cells as a cellular model to study Neisseria meningitidis infection. Front. Microbiol. 2019;10:1181. doi: 10.3389/fmicb.2019.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lutgehetmann M., Hagel C., Sperhake J.P., Schroder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brunink S., Greuel S., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Merico D., Isserlin R., Stueker O., Emili A., Bader G.D. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C.R., Ong G.T., Church S.E., Barker K., Danaher P., Geiss G., Hoang M., Jung J., Liang Y., McKay-Fleisch J., et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020;38:586–599. doi: 10.1038/s41587-020-0472-9. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913 e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norz D., Frontzek A., Eigner U., Oestereich L., Wichmann D., Kluge S., Fischer N., Aepfelbacher M., Pfefferle S., Lutgehetmann M. Pushing beyond specifications: evaluation of linearity and clinical performance of the cobas 6800/8800 SARS-CoV-2 RT-PCR assay for reliable quantification in blood and other materials outside recommendations. J. Clin. Virol. 2020;132:104650. doi: 10.1016/j.jcv.2020.104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hossain M.A., German N., Al-Ahmad A.J. Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res. 2018;34:257–268. doi: 10.1007/s12550-018-0320-7. [DOI] [PubMed] [Google Scholar]
- Pei R., Feng J., Zhang Y., Sun H., Li L., Yang X., He J., Xiao S., Xiong J., Lin Y., et al. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell. 2020;12:717–733. doi: 10.1007/s13238-020-00811-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Huang J., Norz D., Indenbirken D., Lutgehetmann M., Oestereich L., Gunther T., Grundhoff A., Aepfelbacher M., Fischer N. Complete genome sequence of a SARS-CoV-2 strain isolated in Northern Germany. Microbiol. Resour. Announc. 2020;9 doi: 10.1128/MRA.00520-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Reucher S., Norz D., Lutgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.9.2000152. 2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Muller L., Ostermann P.N., Gabriel E., Abida-Islam P., Muller-Schiffmann A., Mariappan A., Goureau O., Gruell H., Walker A., et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39:e106230. doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K., Holden S.J., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabenland M., Salie H., Tanevski J., Killmer S., Lago M.S., Schlaak A.E., Mayer L., Matschke J., Puschel K., Fitzek A., et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54:1594–1610.e11. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Kenney A.D., Kudryashova E., Zani A., Zhang L., Lai K.K., Hall-Stoodley L., Robinson R.T., Kudryashov D.S., Compton A.A., Yount J.S. Opposing activities of IFITM proteins in SARS-CoV-2 infection. EMBO J. 2021;40:e106501. doi: 10.15252/embj.2020106501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Jr., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., Liu S.S., Zhang N.N., Li X.F., Xiong R., et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133.e4. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine G.D., Al-Ahmad A., Barriga B.K., Svendsen S., Salim A., Garcia L., Garcia V.J., Ho R., Yucer N., Qian T., et al. Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell. 2017;20:831–843 e835. doi: 10.1016/j.stem.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J., Lampe J., Muller-Fielitz H., Schuster R., Zille M., Muller K., Krohn M., Korbelin J., Zhang L., Ozorhan U., et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 2021;24:1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M.S., Malsy J., Pottgen J., Seddiq Zai S., Ufer F., Hadjilaou A., Schmiedel S., Addo M.M., Gerloff C., Heesen C., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak M., Kirschner L., Hilpert H., Geiger N., Danov O., Oberwinkler H., Steinke M., Sewald K., Seibel J., Bodem J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021;11:5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code generated to analyze spatial transcriptomics is available from the corresponding author upon request. The accession number for the mRNA-sequencing data reported in this paper is GEO: GSE179923 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179923).