Abstract
Thyroid and pituitary disorders linked to the coronavirus SARS-CoV-2, responsible for the COVID-19 epidemic, are mainly due to direct infection of the endocrine glands by the virus and to cell damage induced by the immune response. The two most frequent thyroid complications of COVID-19 are low T3 syndrome, or “non-thyroidal illness syndrome” (NTIS), and thyroiditis. Studies among in-patients with COVID-19 have shown that between one out of six and half of them have a low TSH level, related to NTIS and thyroiditis, respectively, sometimes found in the same patient. In NTIS, the decrease in free T3 concentration correlates with the severity of the infection and with a poor prognosis. Assessment of thyroid function in patients after a COVID-19 infection, shows normalization of thyroid function tests. Thyroiditis linked to COVID-19 can be divided into two groups, which probably differ in their pathophysiology. One is “destructive” thyroiditis occurring early in infection with SARS-CoV-2, with a severe form of COVID-19, usually observed in men. It is often asymptomatic and associated with lymphopenia. The other is subacute thyroiditis occurring, on average, one month after the COVID-19 episode, usually in clinically symptomatic women and associated with moderate hyperleukocytosis. Post-infection, one quarter to one third of patients remain hypothyroid. An Italian study demonstrated that low TSH in patients hospitalized for COVID-19 was associated with prolonged hospitalization and a higher mortality risk. Pituitary diseases associated with SARS-CoV-2 infection are much rarer and the causal relationship more difficult to ascertain. Several cases of pituitary apoplexy and diabetes insipidus during COVID-19 infection have been reported. Hyponatremia occurs in 20–50% of patients admitted to hospital for COVID-19. The prevalence of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) amongst these hyponatremic cases is difficult to determine. These endocrine complications may influence the prognosis of infection with SARS-CoV-2. Although they rarely require specific treatment, it is important that endocrinologists recognize them to ensure appropriate management, particularly in the acute phase.
Keywords: SARS-CoV-2, COVID-19, Thyroid, Pituitary, Low T3 syndrome, Thyroiditis, Graves’ disease, Pituitary apoplexy, Hypophysitis
Résumé
Les atteintes thyroïdiennes et hypophysaires liées au coronavirus SARS-CoV-2, responsable de l’épidémie de COVID-19, tiennent principalement à une infection directe de la glande endocrine par le virus et à des lésions cellulaires induites par la réponse immunitaire. Sur le plan thyroïdien, les deux complications les plus fréquentes du COVID-19 sont le syndrome de basse T3 ou « Non-Thyroidal Illness Syndrome (NTIS) » et des thyroïdites. Selon les études, un patient sur six à un patient sur deux hospitalisé pour un épisode de COVID-19 a une TSH basse, le NTIS et la thyroïdite étant parfois associés chez un même patient. Dans le NTIS, la diminution de la concentration de T3L est corrélée à la sévérité de l’infection et au mauvais pronostic. Une évaluation de la fonction thyroïdienne des patients à distance de l’épisode de COVID-19 montre une normalisation. Les thyroïdites associées au COVID-19 se divisent en deux groupes, dont la physiopathologie est probablement différente. D’une part, des thyroïdites destructrices, survenant précocement au cours de l’infection par le SARS-CoV-2, en général chez des hommes avec une forme sévère de COVID-19 ; elles sont souvent asymptomatiques, associées à une lymphopénie. D’autre part, des thyroïdites subaiguës, survenant en moyenne un mois après l’épisode de COVID-19, en général chez des femmes, symptomatiques sur le plan clinique, associées à une hyperleucocytose modérée. À distance de l’infection, un quart à un tiers des patients sont en hypothyroïdie selon les études. Une étude italienne a montré qu’une TSH basse était associée à des hospitalisations plus longues et à un plus grand risque de mortalité chez les patients hospitalisés pour un épisode de COVID-19. Sur le plan hypophysaire, les atteintes au cours d’une infection par le SARS-CoV-2 sont bien plus rares et le lien de cause à effet plus difficile à établir. Quelques cas d’apoplexie hypophysaire et de diabète insipide survenant au cours d’un épisode de COVID-19 ont été rapportés. Une hyponatrémie est présente chez 20 à 50 % des patients hospitalisés pour un épisode de COVID-19. La prévalence du SIADH (syndrome de sécrétion inappropriée d’hormone anti-diurétique) parmi ces hyponatrémies est difficile à déterminer. Ces complications endocriniennes peuvent influencer le pronostic de l’infection par le SARS-CoV-2. Bien qu’elles nécessitent rarement un traitement spécifique, il est important que les endocrinologues les reconnaissent, pour assurer une prise en charge adaptée, en particulier à la phase aiguë.
Mots clés: SARS-CoV-2, COVID-19, Thyroïde, Hypophyse, Syndrome de basse T3, Thyroïdite, Maladie de Basedow, Apoplexie hypophysaire, Hypophysite
1. Introduction
In March 2020, the World Health Organization (WHO) declared COVID-19, an infection caused by the coronavirus SARS-CoV-2, a pandemic. The virus is responsible for acute pneumonia, the severity of which determines prognosis of the disease. However, it is also responsible for extra-pulmonary condition, especially in endocrine organs.
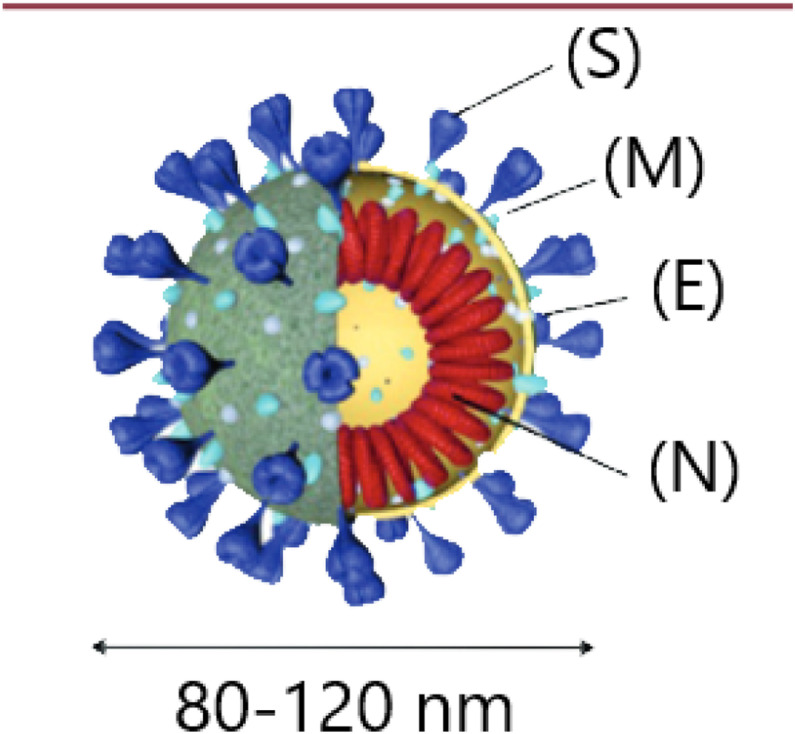
Like SARS-CoV-1 and MERS-CoV, responsible for epidemics in 2002 and 2013, SARS-CoV-2 is a member of the coronavirus family [1] and some of the data on SARS-CoV-2 derives from knowledge acquired from SARS-CoV-1. Coronaviruses consist of an enveloped single strand of RNA. They contain four structural proteins (Fig. 1 ): the envelope protein (E), membrane protein (M), nucleocapsid protein (N) and spike protein (S). This last protein consists of three monomers that form a trimer. The distal subunit of the trimer is essential for binding of the virus to receptors on the host cell surface. The proximal subunit contains both an ectodomain and a transmembrane domain and is responsible for fusion of the virus with the host cell membrane [2], [3], [4].
Fig. 1.
Coronavirus structure. Reproduced from an article in Médecine/Sciences by Juckel et al., 2020 [3]. S: spike protein; M: membrane protein; E: envelope protein; N: nucleocapsid protein associated with genomic RNA.
2. Pathophysiology of endocrine gland diseases (other than diabetes mellitus) in COVID-19 patients
Endocrine gland diseases linked to SARS-CoV-2 infection are mainly caused by three mechanisms: direct infection of the endocrine gland by the virus, activation of the hypothalamo-pituitary axis by inflammation mediators, and cell lesions induced by the immune response.
In the case of SARS-CoV-2, the S protein recognizes angiotensin converting enzyme 2 (ACE2) as its receptor on the host cell. ACE2 is a homolog of the ACE that plays a key role in the renin-angiotensin-aldosterone system [4], [5] and is expressed by pneumocytes but also by cells in the thyroid, pituitary, adrenal, pancreas, testis and ovary [6], [7], [8], [9]. In endocrine glands, ACE2 expression is responsible for direct cellular damage, due to the entry of the virus and its replication in cells of these organs. As with SARS-CoV-1 and MERS, the pathogenicity of SARS-CoV-2 is characterized by a “cytokine storm”, frequently associated with lymphopenia, leading to vasculitis, arteriolar and venular thrombosis, hypoxia and cellular damage due to the immune response [10], [11], [12]. The “cytokine storm” is linked to overactivity of the innate immune response, involving Th1/Th17 lymphocytes, causing an influx of neutrophils, monocytes/macrophages and overproduction of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) [13]. In addition, SARS-CoV-2 appears to inhibit the action of ACE2, thus decreasing the breakdown of angiotensin II to angiotensin (1-7), which normally has anti-inflammatory and anti-fibrotic effects [14], [15].
3. Thyroid diseases
In patients with COVID-19, two types of thyroid diseases are generally encountered: low T3 syndrome known as “non-thyroidal illness syndrome” (NTIS), and thyroiditis.
These thyroid diseases are seen frequently: an Italian study by Lania et al. in 2020, focused on thyroid function in 287 patients hospitalized with COVID-19. In their cohort, 20% had low plasma TSH and 62% of these fit the criteria for thyrotoxicosis (increased free T4). The authors did not rule out that among those patients with a biochemical profile of thyrotoxicosis, some had concomitant NTIS, as the concentration of free T3 was moderately increased compared to that of free T4, consistent with “T4 thyrotoxicosis”. In contrast, hypothyroidism was seen in 5% of patients [16]. In studies carried out in China by Chen et al., Gao et al. and Lui et al. in 2021, a free T3 concentration below the normal limit was found in approximately 7% of patients presenting with mild to moderate COVID, and in 39% of patients with severe COVID or who were in intensive care units. When all degrees of severity were combined, TSH was found to be lower than normal in between 7 and 56% of patients hospitalized for COVID-19 [17], [18], [19]. Muller et al. (2002) also noted, that in patients admitted in intensive care unit in their hospital, compared to the 1% incidence of low TSH related to thyroiditis more or less associated with NTIS in 2019 (prior to SARS-CoV-2), incidence in COVID-19 patients in 2020 was 15%. Hence, a greater prevalence of thyroiditis was seen in COVID-19 patients compared with patients admitted to intensive care for other pathologies. This is especially the case when SARS-CoV-2 infection is severe, as the same group also observed that only 2% of hospitalized COVID-19 patients who were not in intensive care unit, had decreased TSH [20].
3.1. Euthyroid Sick Syndrome or Non-Thyroidal Illness Syndrome (NTIS)
NTIS is likely a body defence mechanism and is characterized by a decrease in circulating free T3 (in the most severe cases TSH and free T4 are also decreased) in severely ill patients or patients with severe nutritional deficiency, but in whom thyroid function is normal. The pathophysiology of NTIS involves changes in the function of deiodinases, alterations in thyroid hormone transport proteins and secretion of TSH from the anterior pituitary, leading to low free T3. Supplementation with levothyroxine or T3 treatment has no beneficial effect in patients with NTIS [21].
As previously noted, NTIS can be seen during SARS-CoV-2 infection. Low circulating free T3 correlates with the severity of the infection and with a poorer prognosis, as well as increased concentration of IL-6 [17], [18], [19], [22]. Concentrations of TSH and total T3 are significantly lower in patients with COVID-19 than in patients presenting with pneumonia of other etiologies. Despite equal severity of illness, patients with COVID-19 have lower TSH concentrations than patients with pneumonia not caused by SARS-CoV-2 [17]. Following recovery, thyroid function in patients who presented with NTIS returns to normal [17].
3.2. Thyroiditis
Among reported cases of thyroiditis during COVID-19 infection, “destructive” or inflammatory thyroiditis appears early in the course of disease. It correlates with infection severity, and is clinically asymptomatic but lymphopenia is present. Subacute thyroiditis occurs later in infection and is associated with pain in the anterior cervical region and hyperleukocytosis detected in total blood counts.
In the Italian study by Lania et al. (2020), 11% of patients hospitalized for COVID-19 displayed thyrotoxicosis [16]. The levels of TSH were inversely correlated with age of the patients and plasma concentration of IL-6, implying that thyroiditis was due to cytokine storm induced by the SARS-CoV-2 infection. Histopathology of samples from patients infected with SARS-CoV-1, found destruction of follicular and parafollicular cells in the thyroid, with extensive apoptosis without lymphocytic infiltrate [23]. Similar lesions were also described in various organs infected with SARS-CoV-2 [12], [24]. It is reasonable to assume therefore, that in the acute phase of infection by SARS-CoV-2, the cytokine storm is responsible for thyroiditis resulting from destruction of thyroid follicular cells. In the study by Muller et al. (2020), thyrotoxicosis occurred in 15% of patients with COVID-19 who were in intensive care units. The patients were predominantly males (68%) with an average age of 65 years [20]. Thyrotoxicosis was associated with lymphopenia. Anti-thyroid antibodies were negative, and thyroid ultrasound was either normal or showed hypoechoic and heterogeneous thyroid gland. It was also commonly associated with NTIS. Eight patients followed-up in the weeks after their hospitalization, showed that after an average of 55 days, six had regained normal thyroid function and two remained hypothyroid [20]. In the study by Lania et al. (2020), decreased TSH correlated with longer admission and a greater risk of mortality among in-patients with COVID-19 [16]. They also found an increased risk of atrial fibrillation (AF) and arterial or venous thromboembolic episodes in patients who had thyrotoxicosis during COVID-19 infection (AF in 32% and thromboembolism in 16%) [16].
In addition, a review of the literature by Christensen et al., published in June 2021, described 17 cases of subacute thyroiditis after an episode of COVID-19. Most patients were female (82%), with an average age of 39 years (18 to 69 years). The delay between respiratory symptoms and diagnosis of subacute thyroiditis varied from 5 to 49 days (average 26 days). In 84% of cases, thyroiditis was diagnosed at least 14 days after the onset of respiratory symptoms [25]. Most patients were symptomatic for thyrotoxicosis (tachycardia 47%) or thyroiditis (neck pain 82%). Biochemistry showed CRP (C-reactive protein) values between 4.5 and 176 mg/L (mean 41 mg/L), total blood count was normal or demonstrated hyperleukocytosis with neutrophils, anti-thyroid antibodies were usually negative (only one patient had anti-thyroglobulin antibodies). Thyroid ultrasound of patients often showed an enlarged, heterogeneous and hypoechoic thyroid with reduced vascularisation. Thyroid radio-iodine scan performed in these patients showed low thyroid uptake, indicative of subacute thyroiditis. Treatment for thyrotoxicosis was mainly symptomatic, using beta-blockers, but 70% of patients also received steroids initially at low dose for few weeks, while others were treated with non-steroidal anti-inflammatory drugs [25]. Most patients had recovered normal thyroid function at follow-up several weeks after the acute episode of thyroiditis. Five (29%) were receiving levothyroxine at the time of publication of the results [25]. On the other hand, in the study by Brancatella et al. (2021), at 3 months following COVID-19 infection related thyroiditis, 87% of patients remained hypothyroid [26]. This study highlights the probable link between infection by SARS-CoV-2 and subacute thyroiditis. Finally, whereas between 2016 and 2019 subacute thyroiditis occurred usually during the summer months, in 2020 subacute thyroiditis incidence peaked in spring and at the end of the year, concomitantly with peak mortality linked to COVID-19 in the region [26].
3.3. Graves’ disease
A few patients with primary or recurrent Graves’ disease have been described following COVID-19 infection [27], [28], [29], [30]. It is difficult to confirm causal relationship but multiple factors point towards an association. Recurrent Graves’ disease cases occurred in patients who had been in remission for several years (more than 30 years in one case) and occurred in the month following COVID-19 illness. In one patient, the anti-TSH receptor antibody titer was significantly increased following COVID-19 illness compared to a test performed two months earlier. Given environmental factors that contribute to the pathogenesis of autoimmune disease includes viral infections, a pathophysiological effect of COVID-19 is consistent in this regard. As mentioned above, patients with subacute thyroiditis appearing after an episode of COVID-19 have been published.
3.4. Long-term evolution
As has been previously described, the evolution of thyroid function over time in patients who presented with NTIS appears favorable, patients who developed thyroiditis present a hypothyroidism in one-quarter to one-third of cases [17], [20], [25]. Autoimmune hypothyroidism or Hashimoto's thyroiditis can appear during the months or years following an episode of subacute viral thyroiditis. It would therefore be interesting to continue to follow-up thyroid function in these patients [19].
Clarke et al. (2021), analysed thyroid function in 68 patients with no history of thyroid disease, who presented with SARS-CoV-2 infection requiring or not standard hospitalisation or intensive care, at least three months after infection, and who had persistent weakness and fatigue. All patients were found to have normal thyroid parameters, regardless of their symptoms [31].
3.5. Thyroid disorders and vaccination against COVID
As has previously been reported with other vaccines, mainly against influenza [32], [33], [34], [35], [36] or hepatitis B, subacute thyroiditis following vaccination against SARS-CoV-2 has also been observed, usually in women with no history of thyroid disorders. Subacute thyroiditis occurred a few days or weeks after the first or second dose of the vaccine. Anti-thyroperoxidase, anti-thyroglobulin and anti-TSH receptor antibodies were all negative while ultrasound imaging indicated thyroiditis. The patients had received various vaccines [37], [38], [39], [40]. Four cases of Graves’ disease occurred within days or weeks of vaccination with mRNA vaccine [41], [42]. It should be noted that none of these patients presented signs of orbitopathy or dermopathy.
Multiple factors, sometimes combined, could contribute to the onset of subacute thyroiditis in these patients. For example genetic predisposition, background autoimmunity, and cross-reactivity between the coronavirus spike protein (the target of some vaccines) and thyroid cell antigens [37].
4. Pituitary diseases
Expression of ACE2 on hypothalamic and pituitary cells and the presence of neurologic symptoms in patients infected with SARS-CoV2 suggest that coronaviruses infect the central nervous system and consequently the hypothalamus and pituitary. The presence of SARS-CoV-2 has also been demonstrated in cerebrospinal fluid [43]. Infection could occur through the blood, lymphatics or via peripheral nerves, particularly the olfactory nerves [44], [45]. Coronavirus-induced hypoxemia and “cytokine storm” may also contribute to the pathophysiology of hypothalamic-pituitary damage [44], [46].
4.1. Pituitary apoplexy
Pituitary apoplexy is defined as hemorrhage and necrosis within a pituitary adenoma, either spontaneous or promoted by various factors: head trauma, hypertension, diabetes mellitus, sickle cell anemia, hypovolemic shock, surgery, pregnancy, radiation, anticoagulants or blood clotting disorders, administration of TRH, GnRH agonists or treatment with dopaminergic agonists [47].
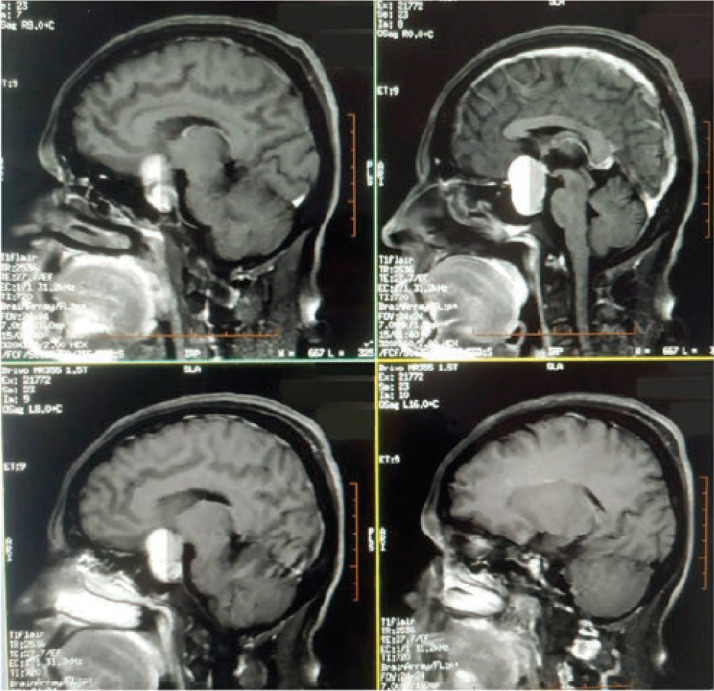
Several cases of pituitary apoplexy have been described in patients with COVID-19 infections. The clinical picture in these patients was consistent with or suggestive of apoplexy: headache, visual disturbances, sometimes associated with neurological signs or vomiting. Both males and females younger than 50 years were affected. The involvement of different pituitary functions was variable, ranging from normal anterior pituitary function to insufficiency in one or more pituitary functions. One patient presented with hyponatremia of 126 mmol/L and MRI showed a mixed intrasellar cystic and tissular lesion (Fig. 2 ).
Fig. 2.
T1 MRI imaging in a patient with pituitary apoplexy following COVID-19 infection. Presence of a mixed (tissue and cystic) supra-sellar lesion measuring 24 × 25 × 31 mm. Reproduced from Ghosh et al., 2021 [50], A Rare Case of SARS-CoV-2 Infection Associated With Pituitary Apoplexy Without Comorbidities, published in the Journal of the Endocrine Society 2021 [50].
Patients received treatment with steroids and pituitary surgery was also suggested [48], [49], [50], [51]. A 38-week pregnant woman underwent surgery and pituitary apoplexy was confirmed by histopathology [48]. All patients were SARS-CoV-2 positive by PCR at the time of apoplexy and symptoms consistent with COVID-19 had appeared several days prior to the apoplexy episode [48], [49], [50], [51]. One of the patients, aged 27, died quickly after diagnosis. He had presented a decline in bilateral visual acuity for six months and had left-side exotropia. Six days before hospitalization, he was seen for asthenia and fever requiring non-steroidal anti-inflammatory drug treatment. Two days before hospitalization, a sudden headache and temporospatial disorientation occurred, followed by drowsiness and respiratory distress. The patient died from respiratory complications 12 h after admission to hospital [49].
As previously indicated, apoplexy can be a direct complication of SARS-CoV-2 infection in the central nervous system, probably associated with endothelial effects of the virus, leading to thrombocytopenia, coagulopathy and platelet dysfunction [50], [52]. It could also be simply a coincidence, given the frequency of SARS-CoV-2 infections.
4.2. Hypophysitis
In 2005, Leow et al. studied pituitary function in 61 patients who had recovered from infection with SARS-CoV-1. They were assessed approximately 3 months after hospital discharge. Ten had received steroid therapy during SARS infection. Hypocortisolism was observed in 39% of the patients, of central origin in 83% of these cases. The majority (62.5%) recovered normal adrenocortical function within a year. Hypothyroidism was present in four patients (6.7%), three of which had a central origin (of these, two additionally had corticotroph insufficiency). In these patients, thyrotroph insufficiency lasted 3–9 months and all recovered during the course of follow-up [53].
Several groups have investigated pituitary function in patients with SARS-CoV-2 infection and have published cases of diabetes insipidus that appeared in the weeks following the first signs of COVID-19 infection [54], [55]. One case was associated with corticotroph insufficiency [55]. These patients remain few in number, so it is difficult to establish a link between SARS-CoV-2 infection and the risk of hypophysitis or corticotroph insufficiency. Indeed, patients in critical care have, in any case, altered glucocorticoid metabolism, including reduced glucocorticoid clearance and decreased blood levels of corticosteroid-binding protein [56].
4.3. Syndrome of inappropriate anti-diuretic hormone secretion (SIADH)
Hyponatremia occurs in 20–50% of patients admitted for COVID-19 infection, though it is generally mild (130–134 mmol/L) [57], [58]. Several cases of SIADH in patients with COVID-19 have been reported, but the actual prevalence of SIADH is difficult to determine because in these patients, antibiotics, steroids, positive pressure ventilation, digestive ionic losses and sodium depletion can all contribute to development of hyponatremia [52], [58]. SIADH could also be caused by hypersecretion of IL-6, which stimulates vasopressin release [52], [58].
In these studies, hyponatremia was independently associated with age, male sex, and presence of comorbidities [52], [57], [58]. An association with a poorer prognosis for SARS-CoV-2 infection was also found, including higher rates of hospitalisation, more frequent admission in intensive care units, a more frequent requirement for mechanical ventilation and more deaths [58].
5. Corticotroph function post-COVID-19
It is currently recommended that patients with oxygen-dependent COVID-19 be prescribed dexamethasone at 6 mg/d for 10 days [59].
In patients with COVID-19, multiple factors can contribute to development of adrenal insufficiency. Although it is generally accepted that steroid therapy of short duration (< 3 weeks) does not cause corticotroph insufficiency, in some predisposed individuals, it can occur after only a few days of treatment at low dose. This individual variability is mostly explained by pharmacokinetic differences and glucocorticoid receptor sensitivity. In addition, as previously mentioned, patients in critical care units have altered glucocorticoid metabolism. Anti-retroviral drugs, such as ritonavir that may have been administered in COVID-19 patients, may increase plasma steroids by inhibiting cytochrome P450, leading to hypercortisolemia and through a negative feedback loop, to endogenous corticotroph insufficiency. Among the organs directly affected by SARS-CoV-2, we have already mentioned the thyroid, hypothalamus and pituitary, but adrenal damage has also been reported [56], [60]. It is therefore important to investigate adrenocortical function in patients with COVID-19.
A study from London was published in July 2021 in which corticotroph function was analysed in 70 patients, using Synacthen® test, at least 3 months after COVID-19 illness. Of these patients, 77% had been hospitalized, 22% of whom had required non-invasive ventilation or intensive care, and 31% had been treated with dexamethasone (for a median duration of 5 days). Samples were collected on average 210 days after COVID-19 diagnosis (after a mean of 95 days in those who received dexamethasone). Mean plasma cortisol at baseline was 233 nmol/L and ACTH was 14.8 ng/L. All patients showed a plasma cortisol peak in response to Synacthen® injection greater than 450 nmol/L (mean concentration 60 min after injection was 636 nmol/L), regardless of the severity of the COVID-19 illness and whether or not they had been treated with dexamethasone. There was no association of the average baseline or peak cortisol concentrations and time after diagnosis of SARS-CoV-2 infection (long COVID), with severity of fatigue [31].
6. Conclusion
The number of publications reporting studies about the endocrine complications related to COVID-19 is growing, particularly regarding the thyroid and hypothalamic-pituitary axis. Diseases of other endocrine organs have also been reported in the medical literature, including the adrenal, pancreas and gonads [29], [52]. More than a year and half after the beginning of the pandemic, it is now clear that these effects occur frequently, particularly at the level of the thyroid. They can influence the prognosis of SARS-CoV-2 infection. Thus understanding their pathophysiology and evolution is crucial. Even though these complications rarely require specific treatment, endocrinologists need to be able to recognize them as well as to support the physicians involved in the front-line care of COVID-19 patients, or in the detection and management of these complications, especially in the acute phase of COVID-19 disease.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgement
This paper was produced with institutional support provided by Ipsen Pharma, the first author having been a participant in Must de l'Endocrinologie 2021.
Footnotes
This paper was produced with institutional support provided by Ipsen Pharma, the first author having been a participant in Must de l’Endocrinologie 2021.
References
- 1.Chen K.Y., Cypess A.M., Laughlin M.R., Haft C.R., Hu H.H., Bredella M.A., et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 2016;24:210–222. doi: 10.1016/j.cmet.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juckel D., Dubuisson J., Belouzard S. Les coronavirus, ennemis incertains. Med Sci. 2020;36:633–641. doi: 10.1051/medsci/2020113. [DOI] [PubMed] [Google Scholar]
- 4.Kothandaraman N., Rengaraj A., Xue B., Yew W.S., Velan S.S., Karnani N., et al. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol Endocrinol Metab. 2021;320:E139–E150. doi: 10.1152/ajpendo.00480.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.058. [281–292.e6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazartigues E., Qadir M.M.F., Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's reliance on ACE2. Endocrinology. 2020:161. doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner A.J. ACE2 cell biology, regulation, and physiological functions. Prot Arm Renin Angiotensin Syst RAS. 2015:185–189. doi: 10.1016/B978-0-12-801364-9.00025-0. [DOI] [Google Scholar]
- 10.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y., Korteweg C., McNutt M.A., Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 13.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 14.Grassi T., Varotto E., Galassi F.M. COVID-19, a viral endocrinological disease? Eur J Intern Med. 2020;77:156–157. doi: 10.1016/j.ejim.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lania A., Sandri M.T., Cellini M., Mirani M., Lavezzi E., Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183:381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M., Zhou W., Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2021;31:8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
- 18.Gao W., Guo W., Guo Y., Shi M., Dong G., Wang G., et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44:1031–1040. doi: 10.1007/s40618-020-01460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106:e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8:739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner M.H., Beckett G.J. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 22.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei L., Sun S., Xu C.-H., Zhang J., Xu Y., Zhu H., et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol. 2007;38:95–102. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen J., O’Callaghan K., Sinclair H., Hawke K., Love A., Hajkowicz K., et al. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: a systematic review. Intern Med J. 2021 doi: 10.1111/imj.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brancatella A., Viola N., Rutigliano G., Sgrò D., Santini F., Latrofa F. Subacute thyroiditis during the SARS-CoV-2 pandemic. J Endocr Soc. 2021;5:bvab130. doi: 10.1210/jendso/bvab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez-Blanco S., Pla-Peris B., Marazuela M. COVID-19: a cause of recurrent Graves’ hyperthyroidism? J Endocrinol Invest. 2021;44:387–388. doi: 10.1007/s40618-020-01440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateu-Salat M., Urgell E., Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Invest. 2020;43:1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marazuela M., Giustina A., Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord. 2020;21:495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caron P. Thyroid disorders and SARS-CoV-2 infection: from pathophysiological mechanism to patient management. Ann Endocrinol. 2020;81:507–510. doi: 10.1016/j.ando.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke S.A., Phylactou M., Patel B., Mills E.G., Muzi B., Izzi-Engbeaya C., et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J Clin Endocrinol Metab. 2021;106:2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girgis C.M., Russo R.R., Benson K. Subacute thyroiditis following the H1N1 vaccine. J Endocrinol Invest. 2010;33:506. doi: 10.1007/BF03346633. [DOI] [PubMed] [Google Scholar]
- 33.Altay F.A., Güz G., Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccines Immunother. 2016;12:1033–1034. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiao J.-Y., Hsin S.-C., Hsieh M.-C., Hsia P.-J., Shin S.-J. Subacute thyroiditis following influenza vaccine (Vaxigrip®) in a young female. Kaohsiung J Med Sci. 2006;22:297–300. doi: 10.1016/S1607-551X(09)70315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passah A., Arora S., Damle N.A., Reddy K.S., Khandelwal D., Aggarwal S. Occurrence of subacute thyroiditis following influenza vaccination. Indian J Endocrinol Metab. 2018;22:713. doi: 10.4103/ijem.IJEM_237_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toft J., Larsen S., Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr J. 1998;45:135. [PubMed] [Google Scholar]
- 37.Oyibo S.O. Subacute Thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13:e16045. doi: 10.7759/cureus.16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franquemont S., Galvez J. Subacute thyroiditis after mRNA vaccine for Covid-19. J Endocr Soc. 2021;5:A956–A957. [Google Scholar]
- 39.Tekin MŞ, Şaylısoy S., Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccines Immunother. 2021 doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.İremli B.G., Şendur S.N., Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: post-vaccination ASIA syndrome. J Clin Endocrinol Metab. 2021:dgab373. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vera-Lastra O., Ordinola Navarro A., Cruz Domiguez M.P., Medina G., Sánchez Valadez T.I., Jara L.J. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021 doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 42.Zettinig G., Krebs M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2021:1–2. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piticchio T., Le Moli R., Tumino D., Frasca F. Relationship between betacoronaviruses and the endocrine system: a new key to understand the COVID-19 pandemic—A comprehensive review. J Endocrinol Invest. 2021;44:1553–1570. doi: 10.1007/s40618-020-01486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Liu T., Yang N., Han D., Mi X., Li Y., et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020;14:533–541. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg M.K., Gopalakrishnan M., Yadav P., Misra S. Endocrine involvement in COVID-19: mechanisms, clinical features, and implications for care. Indian J Endocrinol Metab. 2020;24:381–386. doi: 10.4103/ijem.IJEM_440_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briet C., Salenave S., Bonneville J.-F., Laws E.R., Chanson P. Pituitary apoplexy. Endocr Rev. 2015;36:622–645. doi: 10.1210/er.2015-1042. [DOI] [PubMed] [Google Scholar]
- 48.Chan J.L., Gregory K.D., Smithson S.S., Naqvi M., Mamelak A.N. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020;23:716–720. doi: 10.1007/s11102-020-01080-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solorio-Pineda S., Almendárez-Sánchez C.A., Tafur-Grandett A.A., Ramos-Martínez G.A., Huato-Reyes R., Ruiz-Flores M.I., et al. Pituitary macroadenoma apoplexy in a severe acute respiratory syndrome-coronavirus-2-positive testing: causal or casual? Surg Neurol Int. 2020;11:304. doi: 10.25259/SNI_305_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh R., Roy D., Roy D., Mandal A., Dutta A., Naga D., et al. A rare case of SARS-CoV-2 infection associated with pituitary apoplexy without comorbidities. J Endocr Soc. 2021;5:bvaa203. doi: 10.1210/jendso/bvaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaRoy M., McGuire M. Pituitary apoplexy in the setting of COVID-19 infection: a case report. Am J Emerg Med. 2021 doi: 10.1016/j.ajem.2021.02.045. [S0735-6757(21)00153-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leow M.K.-S., Kwek D.S.-K., Ng A.W.-K., Ong K.-C., Kaw G.J.-L., Lee L.S.-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol. 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misgar R.A., Rasool A., Wani A.I., Bashir M.I. Central diabetes insipidus (Infundibuloneuro hypophysitis): a late complication of COVID-19 infection. J Endocrinol Invest. 2021 doi: 10.1007/s40618-021-01627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheikh A.B., Javaid M.A., Sheikh A.A.E., Shekhar R. Central adrenal insufficiency and diabetes insipidus as potential endocrine manifestations of COVID-19 infection: a case report. Pan Afr Med J. 2021;38:222. doi: 10.11604/pamj.2021.38.222.28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferraù F., Ceccato F., Cannavò S., Scaroni C. What we have to know about corticosteroids use during Sars-Cov-2 infection. J Endocrinol Invest. 2021;44:693–701. doi: 10.1007/s40618-020-01384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez Martinez A., Barajas Galindo D., Ruiz Sanchez J. Management of hyponatraemia and hypernatraemia during the Covid-19 pandemic: a consensus statement of the Spanish Society for Endocrinology (Acqua Neuroendocrinology Group) Rev Endocr Metab Disord. 2021;22:317–324. doi: 10.1007/s11154-021-09627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frara S., Allora A., Castellino L., di Filippo L., Loli P., Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24:465–481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dexamethasone in hospitalized patients with Covid-19 — Preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [NEJMoa2021436] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berton A.M., Prencipe N., Giordano R., Ghigo E., Grottoli S. Systemic steroids in patients with COVID-19: pros and contras, an endocrinological point of view. J Endocrinol Invest. 2021;44:873–875. doi: 10.1007/s40618-020-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]