Abstract
The methods currently available for the identification of the pathogenic yeast Candida dubliniensis all have disadvantages in that they are time-consuming, expensive, and/or, in some cases, unreliable. In a recent study (P. Staib and J. Morschhäuser, Mycoses 42:521–524; 1999) of 14 C. dubliniensis and 11 C. albicans isolates, it was suggested that the ability of C. dubliniensis to produce rough colonies and chlamydospores (chlamydoconidia) on Staib agar (SA) provided a simple means of differentiating it from its close relative C. albicans. In the present investigation, we examined the colony morphology and chlamydospore production of 130 C. dubliniensis and 166 C. albicans isolates on SA and on the related defined medium caffeic acid-ferric citrate agar (CAF). All of the C. dubliniensis and C. albicans isolates produced chlamydospores on the control medium, i.e., rice-agar-Tween agar. However, while none of the C. albicans isolates produced chlamydospores on either SA or CAF, 85.4 and 83.8% of the C. dubliniensis isolates produced chlamydospores on SA and CAF, respectively. All of the C. albicans isolates grew as smooth, shiny colonies on SA after 48 to 72 h of incubation at 30°C, while 97.7% of the C. dubliniensis isolates grew as rough colonies, many (65%) with a hyphal fringe. In contrast, 87.4% of the C. albicans and 93.8% of the C. dubliniensis isolates yielded rough colonies on CAF. Although the results of this study confirm that SA is a good medium for distinguishing between C. dubliniensis and C. albicans, we believe that discrimination between these two species is best achieved on the basis of colony morphology rather than chlamydospore production.
Due to the increasing incidence of fungal infections and the recent emergence of novel opportunistic fungal pathogens, there is a growing need for the development of simple, rapid, and accurate identification methods for potential fungal pathogens recovered in the clinical microbiology laboratory (18). This is particularly true of the newly described yeast species Candida dubliniensis. Although first associated with oral candidiasis in human immunodeficiency virus (HIV)-infected patients (25), it has more recently been recognized as a cause of superficial and systemic disease in HIV-negative individuals (3, 11, 16, 17, 24, 26). The close genotypic relationship between C. dubliniensis and C. albicans results in their sharing a broad range of phenotypic characteristics, which hampers the accurate and rapid differentiation of the two species (23). Although the majority of C. dubliniensis isolates are susceptible to currently used antifungal drugs, it has been shown that isolates of this species, unlike C. albicans, can rapidly develop stable resistance to fluconazole upon exposure in vitro (12, 13). This ability, the emergence of C. dubliniensis worldwide, its growing importance as a cause of systemic disease, and the introduction of novel antifungal agents all indicate that a thorough investigation of the incidence and epidemiology of C. dubliniensis is required. In order to be able to achieve this, simple and reliable tests for differentiating C. dubliniensis from C. albicans need to be developed. The “gold standard” methods for the identification of C. albicans are based on its ability to produce germ tubes and chlamydospores (chlamydoconidia) on appropriate nutrient media. However, since C. dubliniensis also produces these structures, many isolates of C. dubliniensis have been misidentified as C. albicans (4, 14, 15). Therefore, in order to perform urgently required epidemiological studies of C. dubliniensis infections, there is a need to develop inexpensive, accurate, and easy to perform tests that will allow the differentiation of isolates of the two species which have been recovered from clinical samples. In this regard, a variety of procedures have been developed and assessed in laboratories around the world, including, among others, colony color on CHROMagar Candida medium (19), lack of growth at 45°C (16), immunofluorescence (1), carbohydrate assimilation profiles (15), β-glucosidase activity (2), coaggregation with Fusobacterium nucleatum (8), and PCR tests (5). Some of these tests (e.g., PCR) are very reliable but are not yet used routinely by many clinical microbiology laboratories, while others rely on reagents which are not widely available (e.g., immunofluorescence with anti-C. dubliniensis antibodies) and yet others (e.g., colony color on CHROMagar Candida medium) are unreliable. In the original description of C. dubliniensis, it was noted that this species produces much higher numbers of chlamydospores than C. albicans when grown on rice-agar-Tween agar (RAT; 25) but subsequent studies have shown that this trait does not provide a definitive means of differentiating between the two species (9). In a recent study of 14 C. dubliniensis and 11 C. albicans strains, Staib and Morschhäuser (21) reported that colony morphology and chlamydospore production by C. dubliniensis on Staib agar (SA; a medium originally developed for the identification of Cryptococcus neoformans) could form the basis of a simple and accurate test for distinguishing this species from C. albicans.
In the present study, we evaluated the usefulness of colony morphology and chlamydospore production on SA and on caffeic acid-ferric citrate agar (CAF; a defined medium also developed to aid in the identification of C. neoformans) as a means of differentiating C. albicans from C. dubliniensis using a large collection of C. dubliniensis isolates recovered from individuals in 18 different countries around the world.
MATERIALS AND METHODS
Yeast isolates.
The yeast isolates and reference strains used in this investigation are listed in Table 1. A total of 296 isolates were studied, including 130 C. dubliniensis isolates and 166 C. albicans isolates. All isolates were from the culture collection of the Microbiology Research Laboratory, Department of Oral Medicine and Oral Pathology, School of Dental Science, Trinity College, University of Dublin, Dublin, Republic of Ireland. Each isolate was originally recovered from one or more specimens from a separate individual, and its identity was confirmed using the API ID 32C (bioMérieux, Marcy l'Étoile, France) yeast identification system, growth at 45°C, and PCR analysis with C. dubliniensis-specific primers (5, 15, 16).
TABLE 1.
C. dubliniensis and C. albicans isolates used in this study
| Yeast species and country of isolation | No. of isolates | Chlamydospore production (no. of isolates)
|
Source or reference | |||||
|---|---|---|---|---|---|---|---|---|
| SA
|
CAF
|
RAT
|
||||||
| + | − | + | − | + | − | |||
| C. dubliniensisa | ||||||||
| Argentina | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 24 |
| Australia | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 25 |
| Belgium | 5 | 5 | 0 | 5 | 0 | 5 | 0 | 16 |
| Canada | 6 | 4 | 2 | 5 | 1 | 6 | 0 | 16 |
| Finland | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 16 |
| France | 9 | 5 | 4 | 8 | 1 | 9 | 0 | This study |
| Germany | 4 | 4 | 0 | 3 | 1 | 4 | 0 | 16 |
| Greece | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 16 |
| India | 1 | 1 | 0 | 1 | 0 | 1 | 0 | This study |
| Israel | 3 | 1 | 2 | 3 | 0 | 3 | 0 | 17 |
| Ireland | 41 | 36 | 5 | 36 | 5 | 41 | 0 | 16, 25, this study |
| Japan | 1 | 1 | 0 | 1 | 0 | 1 | 0 | This study |
| Malta | 3 | 3 | 0 | 3 | 0 | 3 | 0 | This study |
| Norway | 5 | 4 | 1 | 0 | 5 | 5 | 0 | This study |
| Spain | 5 | 5 | 0 | 4 | 1 | 5 | 0 | 1 |
| Switzerland | 3 | 3 | 0 | 2 | 1 | 3 | 0 | 23 |
| United Kingdom | 18 | 16 | 2 | 16 | 2 | 18 | 0 | 16 |
| United States | 22 | 20 | 2 | 19 | 3 | 22 | 0 | 15, this study |
| Total | 130 | 111 | 19 | 109 | 21 | 130 | 0 | |
| C. albicansa | ||||||||
| Australia | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 16 |
| Hong Kong | 6 | 0 | 6 | 0 | 6 | 6 | 0 | 16 |
| Ireland | 61 | 0 | 61 | 0 | 61 | 61 | 0 | 16, this study |
| United Kingdom | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 16 |
| United States | 96 | 0 | 96 | 0 | 96 | 96 | 0 | 15 |
| Total | 166 | 0 | 166 | 0 | 166 | 166 | 0 | |
Chemicals, enzymes, and oligonucleotides.
Analar-grade or molecular biology grade chemicals were purchased from Sigma-Aldrich or BDH (Poole, Dorset, United Kingdom). Enzymes were purchased from Roche Diagnostics Ltd. (Lewes, East Sussex, United Kingdom) or the Promega Corporation (Madison, Wis.) and used in accordance with the manufacturer's instructions. Custom-synthesized oligonucleotides were purchased from Sigma-Genosys Biotechnologies (Pampisford, Cambridge, United Kingdom).
Culture media and growth conditions.
Stock cultures of yeast isolates were maintained on plastic beads in Protect cryovials (Technical Service Consultants Ltd., Lancashire, United Kingdom) at −80°C. For each isolate, two or three plastic beads were removed from their respective cryovials using a sterile plastic loop, allowed to thaw, and then used to inoculate potato dextrose agar (PDA; Oxoid) medium. Forty-eight-hour-old PDA medium cultures grown at 37°C were used as the source of inoculua for subsequent experiments with SA and CAF. SA (20) and CAF (7) were prepared fresh as described previously and used immediately. SA was prepared by first making an aqueous extract of Guizotia abyssinica seed (Power Seeds, Kildare, Republic of Ireland) by pulverizing 50 g of seed in a Moulinex B57 domestic blender for 2.5 min and then adding the ground seeds to 1 liter of distilled water, followed by boiling for 30 min. The seed extract was cooled and filtered, and the following ingredients were added: glucose, 1 g; KH2PO4, 1 g; creatinine, 1 g. The pH was adjusted to 5.5, the volume was readjusted to 1 liter, and 15 g of agar (Difco) was then added before autoclaving. The composition of CAF (per liter) was as follows: NHSO4, 5 g; glucose, 5 g; yeast extract, 2 g; K2HPO4, 0.8 g; MgSO4 · 7H2O, 0.7 g; caffeic acid, 0.18 g; chloramphenicol, 0.05 g; ferric citrate, 0.002 g.
A 48-h-old single colony from a PDA medium plate culture of each isolate to be tested was separately streak inoculated with a sterile wire loop onto SA and CAF, respectively, contained in 90-mm-diameter petri dishes (25 ml of agar per plate) and incubated at 30°C for 72 to 120 h. Gross colony morphologic features were examined visually on both media at 24-h intervals, and the data were recorded. Yeast colonies were also evaluated microscopically to detect the presence or absence of chlamydospore formation following 48 to 120 h of incubation on SA and on CAF. For each isolate, 10 well-separated single colonies were chosen at random and stained by the addition of 1 drop of 1% (wt/vol) lactophenol cotton blue stain (10) to enhance the detection of chlamydospores. Lactophenol cotton blue preferentially stains chlamydospores more intensely than suspensor cells, pseudomycelium, and blastospores (blastoconidia; 25). Colonies were allowed to stain for 5 min and then covered with sterile glass coverslips and examined microscopically under bright-field illumination using a ×40 objective. Plates containing isolates that did not exhibit detectable chlamydospore formation within 120 h were re-examined following further incubation at intervals of 24 h for up to 3 weeks in total. All isolates were also tested for chlamydospore formation on RAT (bioMérieux) as described previously (25).
All of the isolates included in this study were examined on SA and CFA on at least two separate occasions with different batches of medium prepared from different lots of reagents.
RESULTS AND DISCUSSION
Growth of Candida isolates on SA and CFA.
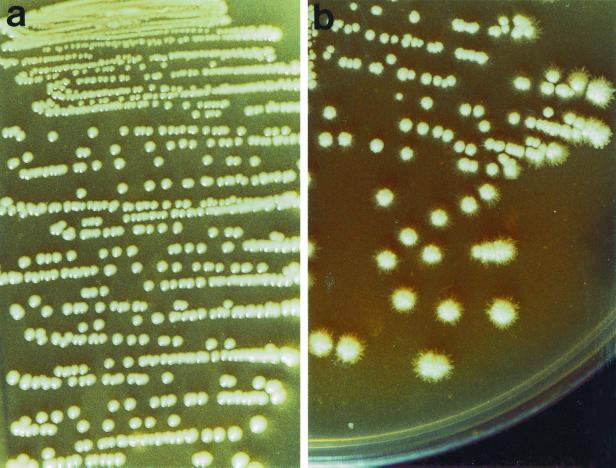
All 296 yeast isolates grew on both SA and CAF and yielded grey-white colonies. On SA, all of the 166 C. albicans isolates tested produced smooth, shiny colonies after 48 h of incubation (Fig. 1a). In all but three isolates, the colonies were found upon microscopic examination to be composed only of blastospores. Colonies of the three isolates consisted mainly of blastospores with a few pseudohyphal elements after 48 and 72 h of incubation. Similar findings were observed after 96 and 120 h of incubation.
FIG. 1.
Morphological appearance of C. dubliniensis and C. albicans colonies on SA following 72 h of incubation at 30°C. (a) Smooth, shiny colonies exhibited by C. albicans strain 132A (6). (b) Rough colonies exhibited by C. dubliniensis strain CD36 (25) displaying a hyphal fringe or halo.
In contrast, 127 (97.7%) of the 130 C. dubliniensis isolates tested on SA yielded rough colonies, the majority (84; 64.6%) of which also exhibited a hyphal halo or fringe around the colonies visible to the naked eye after 72 h of incubation (Fig. 1b). The rough colonies were composed of mycelial forms (predominantly pseudohyphae) and blastospores. The three remaining C. dubliniensis isolates (two from Norway and one from Canada) produced smooth, shiny colonies on SA that were similar in appearance and composition to those of C. albicans isolates, even after 2 weeks of incubation. The identity of these isolates as C. dubliniensis was reconfirmed using PCR with C. dubliniensis-specific primers, by carbohydrate assimilation profile analysis with the API ID 32C system, and by absence of growth at 45°C. Apart from these three isolates, the difference between the C. dubliniensis and C. albicans isolates was particularly evident on SA after 48 h of incubation, and became further enhanced after 72 h of incubation, in the area of heavy culture growth where the primary inoculum was streaked (i.e., the C. dubliniensis culture growth appeared rough and the C. albicans culture growth appeared smooth and shiny). These findings indicated that the different colony morphologies exhibited by isolates of C. dubliniensis and C. albicans were due predominantly to the production of mycelial forms by C. dubliniensis isolates. These results confirm the findings recently reported by Staib and Morschhäuser (21) that SA can be used as a useful means to discriminate between isolates of C. dubliniensis and C. albicans. However, the method is not absolute, as a small minority (3 [2.3%]) of the 130 C. dubliniensis isolates tested were indistinguishable from C. albicans based on colony morphology on SA.
Several isolates each of C. tropicalis, C. glabrata, and C. parapsilosis were also tested on SA in order to determine whether they could be distinguished from isolates of C. albicans and C. dubliniensis. The five oral C. glabrata isolates tested yielded smooth, grey-white colonies similar to those of C. albicans on this medium. Furthermore, of the five C. tropicalis isolates tested, three yielded rough colonies similar to those of C. dubliniensis and two yielded smooth colonies similar to those of C. albicans. Of the six C. parapsilosis isolates tested, four yielded smooth, shiny colonies similar to those of C. albicans and two yielded rough colonies similar to those of C. dubliniensis. These findings indicated that it would not be possible to identify colonies of C. albicans and C. dubliniensis on SA based on colony morphology alone following primary isolation from a clinical specimen. Therefore, we propose that SA be used to assess clinical isolates which have been shown to be germ tube positive or to confirm the identity of C. dubliniensis isolates presumptively identified following primary isolation on CHROMagar Candida medium or by other means. The use of SA to differentiate C. dubliniensis and C. albicans isolates has a number of advantages over carbohydrate assimilation profile analysis. Firstly, the use of SA is considerably less expensive. Secondly, it is amenable to the analysis of large numbers of isolates. Finally, whereas the databases used with many of the commonly used commercial yeast identification systems (e.g., the bioMérieux API 20C AUX and ID 32C systems) have been updated in recent years to include C. dubliniensis profiles, they are far from comprehensive. In this regard, recent studies have highlighted the necessity to revise the databases to improve the accuracy of identification of C. dubliniensis (15).
SA was originally developed as a means of identifying colonies of C. neoformans, which, unlike other members of this genus, develops dark pigmentation on this medium. Strachan et al. (22) demonstrated that similar results could be obtained on a growth medium containing caffeic acid extracted from G. abyssinia seeds. Since SA was found to be excellent for distinguishing between C. dubliniensis and C. albicans, we investigated the usefulness of the more defined CAF to differentiate between isolates of these species. Of the 166 C. albicans isolates included in the study, 145 (87.4%) yielded rough colonies with a mycelial halo visible to the naked eye after 5 days incubation. The remaining 21 (12.6%) isolates yielded smooth, nonshiny colonies on this medium. In comparison, 122 (93.8%) of the 130 C. dubliniensis isolates, including the 3 isolates which exhibited a smooth-colony phenotype on SA, yielded rough colonies with a mycelial halo after 5 days of incubation. The remaining eight (6.2%) isolates yielded rough colonies without a mycelial halo. Colonies of the C. dubliniensis isolates were noticeably smaller (∼2 mm in diameter) than the C. albicans colonies (∼3 mm) after 5 days of incubation on CAF. These findings indicated that, unlike that on SA, colony morphology on CAF could not be used to differentiate between C. dubliniensis and C. albicans isolates.
Chlamydospore production on SA and CAF.
None of the 166 C. albicans isolates tested produced chlamydospores on SA or CAF, even after prolonged incubation periods of up to 3 weeks (Table 1). In contrast, all isolates produced chlamydospores within 48 to 72 h on RAT. Similarly, all 130 of the C. dubliniensis isolates formed chlamydospores on RAT but not all produced chlamydospores on either SA or CAF (Tables 1 and 2). A total of 111 (85.4%) of the 130 C. dubliniensis isolates formed chlamydospores on SA, and 100 (90.1%) of these 111 produced abundant chlamydospores within 72 h, whereas 11 (9.9%) of them produced relatively few chlamydospores, which were only detected following incubation periods of up to a week. Similarly, 109 (83.8%) of the 130 C. dubliniensis isolates produced chlamydospores on CAF, all but 5 within 120 h (Tables 1 and 2). Eighty-three (63.9%) of the C. dubliniensis isolates produced chlamydospores on both SA and CAF, whereas the remainder produced chlamydospores on one or the other medium only (Table 2).
TABLE 2.
Chlamydospore production by C. dubliniensis isolatesa on SA and CAF
| Medium and chlamydo- spore production | No. of isolates |
|---|---|
| SA | |
| + | 111c |
| − | 19d |
| CAF | |
| + | 109e |
| − | 21f |
| SA + CAFb | |
| + | 83 |
| − | 3 |
130 isolates were studied.
Refers to isolates that were chlamydospore positive on both SA and CAF or chlamydospore negative on both media.
28 isolates were negative on CAF.
16 isolates were positive on CAF.
26 isolates were negative on SA.
18 isolates were positive on SA.
On the basis of these data, we agree with Staib and Morschhäuser that growth on SA is an efficient means of discriminating between C. dubliniensis and C. albicans. However, our results suggest that colony morphology, rather than chlamydospore formation, is a more accurate criterion for species identification, since a significant proportion of C. dubliniensis isolates failed to produce chlamydospores on SA. The disparity between our data and that of Staib and Morschhäuser could be due to either different supplies of G. abyssinica seed, to seed of different ages, or to seed storage conditions. However, the most likely explanation lies in the larger and more diverse group of C. dubliniensis isolates examined in the present study.
SA is inexpensive and readily available in many clinical mycology laboratories and provides a simple test for the accurate differentiation of C. dubliniensis from C. albicans. We propose that colony morphology on SA is a reliable and inexpensive phenotypic test for confirming the identification of C. dubliniensis and will be of benefit for researchers interested in studying the incidence and epidemiology of this emerging pathogen. We suggest that germ tube-positive oral isolates from HIV-positive and AIDS patients, as well as germ tube-positive isolates from sterile sites from other immunocompromised groups, should be tested on SA in association with other phenotypic tests for C. dubliniensis.
The molecular basis of the phenotypic differences observed between C. dubliniensis and C. albicans following growth on these media has yet to be established. However, comparative analysis of gene expression on these media could prove helpful in increasing our understanding of dimorphism in these species.
ACKNOWLEDGMENTS
This study was supported by Irish Health Research Board grant 05.97. A. Al Mosaid was supported by the Ministry for Education, King Saud University, Saudi Arabia.
We thank all of our colleagues throughout the world who have sent us strains of C. dubliniensis.
REFERENCES
- 1.Bikandi J, San Millán R, Moragues M D, Cebas G, Clarke M, Coleman D C, Sullivan D J, Quindós G, Pontón J. Rapid identification of Candida dubliniensis by indirect immunofluorescence based on differential localization of antigens on C. dubliniensis blastospores and Candida albicans germ tubes. J Clin Microbiol. 1998;36:2428–2433. doi: 10.1128/jcm.36.9.2428-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerlin P, Boerlin-Petzold F, Durussel C, Addo M, Pagani J-L, Chave J-P, Bille J. Cluster of atypical Candida isolates in a group of human immunodeficiency virus-positive drug users. J Clin Microbiol. 1995;33:1129–1135. doi: 10.1128/jcm.33.5.1129-1135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt M E, Harrison L H, Pass M, Sofair A N, Huie S, Li R-K, Morrison C J, Warnock D W, Hajjeh R H. Candida dubliniensis fungemia: the first four cases in North America. Emerg Infect Dis. 2000;6:46–49. doi: 10.3201/eid0601.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly S A, Sullivan D J, Shanley D B, Coleman D C. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology. 1999;145:1871–1882. doi: 10.1099/13500872-145-8-1871. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher P J, Bennett D E, Henman M C, Russell R J, Flint S R, Shanley D B, Coleman D C. Reduced azole susceptibility of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J Gen Microbiol. 1992;138:1901–1911. doi: 10.1099/00221287-138-9-1901. [DOI] [PubMed] [Google Scholar]
- 7.Hopfer R L, Blank F. Caffeic acid-containing medium for identification of Cryptococcus neoformans. J Clin Microbiol. 1975;2:115–120. [PMC free article] [PubMed] [Google Scholar]
- 8.Jabra-Rizk M A, Falkler W A, Merz W G, Kelley J I, Baqui A A, Meiller T F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J Clin Microbiol. 1999;37:1464–1468. doi: 10.1128/jcm.37.5.1464-1468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick W R, Revankar S G, McAtee R K, Lopez-Ribot J L, Fothergill A W, McCarthy D I, Sanche S E, Cantu R A, Rinaldi M G, Patterson T F. Dectection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar Candida screening and susceptibility testing of isolates. J Clin Microbiol. 1998;36:3007–3012. doi: 10.1128/jcm.36.10.3007-3012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larone D H. Staining methods. In: Larone D H, editor. Medically important fungi: a guide to identification. 2nd edition. Washington, D. C.: American Society for Microbiology; 1993. pp. 186–192. [Google Scholar]
- 11.Meis J F, Ruhnke M, De Pauw B E, Odds F C, Siegert W, Verweij P E. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg Infect Dis. 1999;5:150–153. doi: 10.3201/eid0501.990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran G P, Sanglard D, Donnelly S M, Shanley D B, Sullivan D J, Coleman D C. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odds F C, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998;6:2869–2873. doi: 10.1128/jcm.36.10.2869-2873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pincus D H, Coleman D C, Pruitt W R, Padhye A A, Salkin I F, Geimer M, Bassel A, Sullivan D J, Clarke M, Hearn V. Rapid identification of Candida dubliniensis with commercial yeast identification systems. J Clin Microbiol. 1999;37:3533–3539. doi: 10.1128/jcm.37.11.3533-3539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998;36:2093–2095. doi: 10.1128/jcm.36.7.2093-2095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polacheck I, Strahilevitz J, Sullivan D, Donnelly S, Salkin I F, Coleman D C. Recovery of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Israel. J Clin Microbiol. 2000;38:170–174. doi: 10.1128/jcm.38.1.170-174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss E, Tanaka K, Bruker G, Chazalet V, Coleman D, Debeaupuis J P, Hanazawa R, Latge J P, Lortholary J, Makimura K, Morrison C J, Murayama S Y, Naoe S, Paris S, Sarfati J, Shibuya K, Sullivan D, Uchida K, Yamaguchi H. Molecular diagnosis and epidemiology of fungal infections. Med Mycol. 1998;36(Suppl. 1):249–257. [PubMed] [Google Scholar]
- 19.Schoofs A, Odds F C, Colebunders R, Ieven M, Goosens H. Use of specialised isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 20.Staib F, Seibold M, Antweiler E, Frolich B, Weber S, Blisse A. The brown colour effect (BCE) of Cryptococcus neoformans in the diagnosis, control and epidemiology of C. neoformans infections in AIDS patients. Zentralbl Bakteriol Microbiol Hyg A. 1987;266:167–177. doi: 10.1016/s0176-6724(87)80030-5. [DOI] [PubMed] [Google Scholar]
- 21.Staib P, Morschhauser J. Chlamydospore formation on Staib agar as a species-specific characteristic of Candida dubliniensis. Mycoses. 1999;42:521–524. doi: 10.1046/j.1439-0507.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- 22.Strachan A A, Yu R J, Blank F. Pigment production of Cryptococcus neoformans grown with extracts of Guizotia abyssinica. Appl Microbiol. 1971;22:478–479. doi: 10.1128/am.22.3.478-479.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Lloyd S, Henman M, Coleman D. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J Clin Microbiol. 1997;35:960–964. doi: 10.1128/jcm.35.4.960-964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterisation of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 26.Willis A M, Coulter W A, Sullivan D J, Coleman D C, Hayes J R, Bell P M, Lamey P-J. Isolation of C. dubliniensis from insulin-using diabetes mellitus patients. J Oral Pathol Med. 2000;29:86–90. doi: 10.1034/j.1600-0714.2000.290206.x. [DOI] [PubMed] [Google Scholar]