Abstract
Background:
Necrotizing soft tissue infections (NSTIs) are typically characterized by extensive soft tissue destruction with systemic signs of toxicity, ranging from sepsis to septic shock. Our aim was to analyze the clinical characteristics, microbiological results, laboratory data, therapies, and outcome of patients with NSTIs admitted to an intensive care unit (ICU).
Methods:
A monocentric observational study of patients admitted to the ICU of a university hospital between January 2009 and December 2017. The demographic characteristics, comorbidities, clinical features, microbiology and laboratory results, organ dysfunctions, therapies, and outcome were retrospectively analyzed.
Results:
There were 59 patients and 70% males. The mean age (± SD) was 55 ± 18; type II (monomicrobial) NSTI was present in 36 patients (61%); the most common isolated pathogen was Streptococcus pyogenes in 28 patients (48%). Septic shock was diagnosed in 41 patients (70%). The most common organ dysfunctions were circulatory and renal in 42 (71%) and 38 patients (64%). The mean value (± SD) of serum lactate at admission to the ICU was 4.22 ± 5.42 mmol/l, the median SOFA score and SAPS II were 7 (IQR 4 - 10) and 46 (IQR 30.5 - 53). ICU mortality rate was 25%. Both SOFA score and serum lactate demonstrated a good prognostic value regarding ICU outcome (OR 1.29, 95%CI 1.07-1.57, P < 0.007 and OR 1.53, 95%CI 1.19-1.98, P < 0.001). A cut-off value for serum lactate of 6.55 mmol/L positively predicted mortality with 67% sensitivity and 97% specificity.
Conclusion:
NSTIs carry a high risk of septic shock and multiple organ dysfunction syndrome and thus are still associated with high mortality. In our study, the value of serum lactate at admission to the ICU correlated well with mortality. This easy-to-measure parameter could play a role in the decision-making process regarding prognosis and continuation of care.
Keywords: necrotizing soft tissue infection, sepsis, lactate, intensive care
Introduction
Necrotizing soft tissue infections (NSTIs) include necrotizing forms of fasciitis, myositis, and cellulitis and are divided into 2 groups, referring to microbiological findings: type I polymicrobial and type II monomicrobial.
Worldwide incidence varies from 0.3 to 5 cases in Europe 1,2 and up to 15.5 cases per 100,000 inhabitants in Asia. 3 The reported mortality rates range from 20% to 32%, 1,4 with an average of 20% within the past 20 years. 5
Necrotizing soft tissue infections are medical emergencies characterized by extensive soft tissue destruction. The clinical picture ranges from mild sepsis to septic shock. Various degrees of organ dysfunction are common during this period. 6 Early surgical exploration and extensive antibiotic therapy form the cornerstones of therapy. The first 24 hours after surgery in the intensive care unit (ICU) are critical, as the postoperative cytokine storm results in a global nonspecific inflammatory reaction characterized by vasodilation, microcirculatory disturbance, and capillary leak.
Previous studies demonstrated conflicting results concerning predictors for mortality in these patients. Furthermore, detailed information regarding the course of the disease in the ICU is lacking.
In the present study, we extensively describe the intensive care course of these patients and search for possible predictors for mortality through statistical analysis.
Material and Methods
Study Design
We retrospectively described a cohort of critically ill patients treated for an NSTI at one of the ICUs of our university hospital between January 2009 and December 2017.
Setting
The University Medical Centre Hamburg-Eppendorf is a tertiary-level metropolitan medical center, containing 1,738 hospital beds and reaching a volume of more than 100,000 in-patients per year. The Department of Intensive Care Medicine includes 12 ICUs, with a total capacity of 140 ICU beds. It serves the maximum level of treatment to medical and surgical patients.
Participants and Data Collection
We included all patients aged 18 or older, who were admitted to the ICU for NSTI. The follow-up period ended with the discharge from the hospital. Patients with non-necrotizing soft tissue infections (e.g. abscesses or phlegmons) were excluded. Data were provided by the electronic patient data management systems [Soarian, Siemens, Erlangen for non-ICU data and Integrated Care Manager (ICM), Dräger Medical, Lübeck, Germany for ICU data]. Disease characteristics (comorbidities, risk factors, localization, imaging, surgical interventions, and microbiologic characteristics) and ICU data (laboratory values at admission to the ICU, organ dysfunctions, shock parameters, and medical therapy) were extracted. The Laboratory Risk Indicator for Necrotising Fasciitis score (LRINEC score), a disease-specific score proposed by Wong et al 7 to distinguish NSTIs from other soft tissue infections, was computed retrospectively. The baseline laboratory values recorded at admission to the ICU were used to quantify the degree of organ dysfunction and in calculating the sepsis-related organ failure assessment (SOFA) score at admission. We employed the most recent definitions for sepsis and septic shock proposed by the 3rd Surviving Sepsis Campaign. 8,9 Circulatory dysfunction was defined by the use of vasopressors. Acute kidney injury was classified using the KDIGO Guidelines (“Kidney Disease—Improving Global Outcomes”). 10 Hematological, liver, neurological und pulmonary dysfunction were defined according to SOFA score definitions. The severity of illness was assessed using the Simplified Acute Physiology Score II (SAPS II) at 24 hours after admission to the ICU. Serum lactate, vasopressor use, and fluid balance were documented 24 hours after admission to the ICU, as well.
Statistics
Patients’ data were collected using Excel (Microsoft Software, Version 2013) and presented either as absolute numbers with percentages or as a median, range, interquartile range (IQR), or mean (± standard deviation), as appropriate. Student’s t-test was used for continuous variables, while Pearson’s chi-square or Fisher’s exact test was used for categorical variables, as appropriate. Parameters were considered significant in the univariate analysis if the 2-tailed P-value was less than 0.05. The endpoint was ICU mortality. For the clinically relevant variables, which demonstrated significant correlation to the selected endpoint, we computed receiver operating characteristic (ROC) curves. Statistical analysis was conducted using IBM SPSS Statistics Version 25.0.
Results
During the study period, 59 patients were treated for an NSTI in the ICUs of our hospital.
Demographic Data
The mean age (± SD, range) was 55 ± 18 years (19 to 89), and the proportion of male patients was 70%. The most frequent comorbidity was arterial hypertension, found in 21 patients (36%). Moreover, various types of autoimmune diseases were present in 12 patients (20%). Table 1 provides a detailed list of the patients’ demographic characteristics and comorbidities.
Table 1.
Demographic Characteristics and Comorbidities of the Patients With NSTI, Classified by Outcome.
| Variable | Patients (N = 59) | ICU survivors (N = 44) | ICU non-survivors (N = 15) | P value |
|---|---|---|---|---|
| Male gender (N, %) | 41 (70) | 30 (68) | 11 (73) | NS |
| Age, y.o. (mean ± SD) | 55 ± 18 | 54 ± 18 | 58 ± 18 | NS |
| Weight, kg (mean ± SD) | 85 ± 24 | 83 ± 21 | 90 ± 31 | NS |
| BMI, kg/m 2 (mean ± SD) | 27 ± 6 | 27 ± 6 | 28 ± 6 | NS |
|
Comorbidities (N, %) Arterial hypertension Immunosuppressive therapy Autoimmune disease History of cancer Diabetes mellitus Peripheral vasculopathy Coronary artery disease Hypercholesterolemia Liver disease COPD Alcoholism |
21 (36) 16 (27) 12 (20) 12 (20) 11 (19) 8 (14) 8 (14) 6 (10) 5 (8) 5 (8) 5 (8) | 14 (32) 11 (25) 6 (14) 10 (23) 7 (16) 4 (9) 6 (14) 4 (9) 0 2 (5) 4 (9) | 7 (47) 5 (33) 6 (40) 2 (13) 4 (27) 4 (27) 2 (13) 2 (13) 5 (33) 3 (20) 1 (7) | NS NS 0.02 NS NS NS NS NS NS NS |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; NS, not significant.
Admission Data
All patients arrived through the emergency department, 19 (32%) as an interhospital transfer. Chronic wounds were observed in 12 patients (20%), while 11 (20%) developed an NSTI postoperatively and 10 (17%) after a skin breach through injections or minor trauma. The classical triad of pain, swelling, and erythema was present in only 7 patients (12%). The major localization of the NSTI was the lower extremity in 41 patients (70%).
Diagnostic imaging studies were performed in 38 patients (64%). This was associated with a higher, but statistically not significant, percentage of patients receiving surgical exploration within the first 6 hours (71% versus 52%, P-value 0.08).
The median value (IQR) of the LRINEC score was 7 points (5-8), 6 points (6-8) for survivors and 7 points (5-8) for non-survivors. The proposed cut-off of 6 points was present in 42 patients (72%).
Surgical Therapy
All patients received at least one surgical debridement within the first 24 hours after establishing the diagnosis, 38 of whom (67%) within 6 hours after hospital admission. A subsequent debridement was necessary in 22 patients (44%). This was dictated by clinical worsening in 13 patients (59%) and planned second look surgery for the others. The median (IQR) number of surgical interventions including plastic surgery until discharge was 4 (2-6). Limb amputation and disarticulation was necessary in 8 patients (17%). The surgical intervention within the first 6 hours after admission did not influence the rate of amputation (P-value 0.84).
Antibiotic Therapy and Microbiological Data
There were 17 patients (29%) with type I and 36 patients (61%) with type II NSTI. In 6 patients (10%), no pathogen was isolated. The antibiotic regimen received at presentation or before admission was empirically modified in 35 patients (59%) within the first 24 hours. The antibiotic regimen has been changed for several reasons, often because the diagnosis of NSTI was not clear initially, or addition of a further antibiotic (vancomycin, clindamycin etc.) has been performed.
Complete data concerning these general aspects and therapy are provided in Table 2.
Table 2.
Medical Data of the Patients Classified by Outcome.
| Variable (N, %) | Patients (N = 59) | ICU survivors (N = 44) | ICU non-survivors (N = 15) | P value |
|---|---|---|---|---|
| Risk factors | ||||
| Chronic wounds | 13 (22) | 8 (18) | 5 (33) | NS |
| Skin breach | 10 (17) | 9 (21) | 1 (7) | NS |
| Trauma | 4 (7) | 3 (7) | 1 (7) | NS |
| Postoperative | 12 (20) | 10 (23) | 2 (13) | NS |
| Signs and symptoms at presentation | ||||
| Fever | 9 (15) | 9 (21) | 0 | NS |
| Pain | 31 (53) | 25 (57) | 6 (40) | NS |
| Swelling | 33 (56) | 25 (57) | 8 (53) | NS |
| Erythema | 25 (42) | 20 (46) | 5 (33) | NS |
| Gastrointestinal symptoms | 10 (17) | 7 (16) | 3 (20) | NS |
| Bullae and skin necrosis | 9 (15) | 5 (11) | 4 (27) | NS |
| NSTI Iocalisation | ||||
| Lower extremity | 41 (70) | 31 (71) | 10 (67) | NS |
| Upper extremity | 6 (10) | 5 (11) | 1 (7) | NS |
| Head and neck | 7 (12) | 4 (9) | 3 (20) | NS |
| Thoracoabdominal | 7 (12) | 5 (11) | 2 (13) | NS |
| Imaging studies | ||||
| Computer tomography | 22 (40) | 14 (34) | 8 (57) | NS |
| Magnetic resonance imaging | 16 (29) | 14 (34) | 2 (14) | NS |
| Surgical therapy | ||||
| Intervention within 6 hours after admission | 38 (66) | 28 (64) | 10 (71) | NS |
| Second exploration within the next 24 hours | 22 (42) | 14 (33) | 8 (80) | 0.007 |
| Limb amputation with disarticulation | 8 (17) | 5 (11) | 3 (20) | NS |
| Less than 4 surgical interventions | 21 (36) | 11 (25) | 10 (67) | 0.004 |
| Antibiotic therapy | ||||
| Initial combination therapya | 32 (54) | 21 (48) | 11 (73) | NS |
| Initial clindamycin | 36 (61) | 26 (59) | 10 (67) | NS |
| Positive blood cultures | 8 (15) | 5 (13) | 3 (21) | NS |
| Microorganism | ||||
| Streptococcus pyogenes | 28 (48) | 23 (52) | 5 (33) | NS |
| Streptococcus dysgalactiae | 6 (10) | 4 (9) | 2 (13) | NS |
| Staphylococcus aureus | 11 (19) | 7 (16) | 4 (27) | NS |
| E. coli | 9 (15) | 4 (9) | 5 (33) | 0.02 |
| Acinetobacter spp. | 3 (5) | 3 (7) | 0 | NS |
| Pseudomonas aeruginosa | 3 (5) | 3 (7) | 0 | NS |
| Enterobacter spp. | 3 (5) | 0 | 3 (20) | 0.01 |
Abbreviation: NS, not significant.
aCombination therapy - carbapenem/acyl-aminopenicillin plus a beta-lactamase inhibitor and a glycopeptide or linezolid.
ICU Data
The median SOFA score was 7 (IQR 2-10) for survivors and 9 (IQR 8-12) for non-survivors, while the median SAPS II after 24 hours was 42 (IQR 27-51) for survivors and 53.5 (IQR 50-59) for non-survivors.
Septic shock was present in 41 patients (70%). The most frequent organ dysfunctions were circulatory in 42 (71%) and renal in 38 patients (59%), respectively. The presence of more than 4 organ dysfunctions was significantly linked to a higher mortality (P-value 0.04). The details are presented in Table 3.
Table 3.
Distribution of Septic Shock and Organ Dysfunctions at Admission, Classified by Outcome.
| Variable (N, %) | Patients (N = 59) | ICU survivors (N = 44) | ICU non-survivors (N = 15) | P value |
|---|---|---|---|---|
| Septic shock | 41 (69) | 26 (59) | 15 (100) | 0.003 |
| Circulatory dysfunction | 42 (71) | 27 (61) | 15 (100) | 0.004 |
| Renal dysfunction | 38 (64) | 26 (59) | 12 (80) | 0.002 |
| Haematological dysfunction | 29 (49) | 21 (48) | 8 (53) | NS |
| Liver dysfunction | 16 (27) | 6 (14) | 10 (67) | < 0.001 |
| Neurological dysfunction | 8 (14) | 5 (11) | 3 (20) | NS |
| Pulmonary dysfunction | 7 (12) | 4 (9) | 3 (20) | NS |
Abbreviation: NS, not significant.
The relationship between laboratory values and mortality revealed an incongruous pattern. Serum lactate, C-reactive protein (CRP), and prothrombin time (PT) significantly differed between the 2 groups, as presented in Table 4. The CRP was considerably lower in the group of patients with 4 or more organ dysfunctions (11 patients—19%, P-value 0.05), however, this did not reach statistical significance.
Table 4.
Selected Laboratory Values at Admission, Classified by Outcome.
| Variable (mean ± SD) | Patients (N = 59) | ICU survivors (N = 44) | ICU non-survivors (N = 15) | P value |
|---|---|---|---|---|
| WBC count, x 10 3 /µL | 15 ± 11 | 13 ± 7 | 18 ± 16 | NS |
| Thrombocyte count, x 10 3 /µL | 182 ± 156 | 186 ± 171 | 170 ± 103 | NS |
| Lactate, mmol/L | 4.2 ± 5.4 | 2.1 ± 1.7 | 10.3 ± 7.6 | < 0.001 |
| CRP, mg/dl | 209 ± 100 | 226 ± 104 | 157 ± 69 | 0.02 |
| PCT, ng/dl | 24 ± 28 | 22 ± 26 | 31 ± 34 | NS |
| CK, IU/L | 3,284 ± 10,781 | 6,126 ± 12,926 | 2,132 ± 9,745 | NS |
| Prothrombin time, sec | 70 ± 26 | 74.41 ± 25.84 | 55.66 ± 21.96 | 0.015 |
| Creatinine, mg/dl | 2.29 ± 1.68 | 2.17 ± 1.64 | 2.64 ± 1.82 | NS |
Abbreviations: WBC, white blood cells; CRP, C-reactive protein; PCT, procalcitonin; CK, creatine kinase; NS, not significant.
Norepinephrine was the most frequently administered vasopressor, received by 40 patients (68%). An increase in the requirement for catecholamine support within the first 24 hours after admission to the ICU was observed in 19 patients (32%). Nine patients (15%) required additional inotropic therapy.
During the first 24 hours, 37 patients (63%) were invasively ventilated. Mechanical ventilation for more than 24 hours was necessary for 30 patients (51%).
An acute kidney insufficiency (AKI) Stage I was observed in 16 patients (27%), AKI Stage II in 13 patients (22%), and AKI Stage III in 9 patients (15%). Continuous renal replacement therapy was necessary for 26 patients (44%). Overall, there was a median positive fluid balance of approximately 5 liters over the first 24 hours. Non-survivors received in median 3.2 L more than survivors did (7.8 L versus 4.5 L). A higher positive fluid balance was directly proportional to mortality (P-value 0.003).
Corticosteroid therapy was administered to 24 patients (41%), and 6 patients (10%) received intravenous immunoglobulins.
A detailed presentation of the therapeutic interventions within the first 24 hours is provided in Table 5.
Table 5.
Medical Therapy in ICU During the First 24 h, Classified by Outcome.
| Variable (N, %) | Patients (N = 59) | ICU survivors (N = 44) | ICU non-survivors (N = 15) | P value |
|---|---|---|---|---|
| Vasopressor therapy | 40 (68) | 25 (61) | 15 (100) | 0.002 |
| Inotropic therapy | 9 (15) | 5 (11) | 4 (27) | NS |
| Invasive mechanical ventilation | 37 (63) | 23 (52) | 14 (93) | 0.005 |
| Renal replacement therapy | 20 (34) | 9 (20) | 11 (73) | < 0.001 |
| Packed red blood cells | 18 (31) | 11 (25) | 7 (47) | NS |
| Fresh frozen plasma | 21 (36) | 13 (30) | 8 (53) | NS |
| Platelet concentrates | 7 (12) | 4 (9) | 3 (20%) | NS |
| Corticosteroid therapy | 24 (41) | 12 (27) | 12 (80) | < 0.001 |
| Intravenous immunoglobulin therapy | 6 (10) | 5 (11) | 1 (7) | NS |
| Enteral/parenteral nutrition | 22 (37) | 15 (34) | 7 (47) | NS |
Abbreviation: NS, not significant.
Clinical Course
The median (IQR) length of stay in the ICU was 5 days (IQR 3-15). Fifteen patients died during ICU stay, resulting in a mortality rate of 25%. Death occurred within the first 48 hours in 8 patients (54%). In the survivor group, the median length of stay at the hospital was 35 days IQR (10-51). Three patients died in the normal ward, accounting for an in-hospital mortality rate of 30%. Table 6 presents the odds for ICU mortality (OR, 95%CI) for the significant variables after the univariate analysis.
Table 6.
Odds Ratio for Death After the Univariate Analysis of Statistically Significant Data of the Cohort.
| Variable | OR (95%CI) |
|---|---|
| Autoimmune disease
Second surgical intervention within the first 24 hours E. coli isolates Presence of septic shock SOFA Score SAPS II Score Lactate at admission Failure of lactate clearance at 24 hours Circulatory dysfunction at admission Rise in norepinephrine dosage at 24 hours Liver dysfunction Corticosteroid therapy within 24 hours Renal replacement therapy ≥ 4 Organ dysfunctions |
4.22 (1.1-16.2) 7.71 (1.44-41.33) 5.00 (1.13-22.10) 1.69 (1.32-2.16) 1.29 (1.07-1.57) 1.07 (1.01-1.13) 1.53 (1.19-1.98) 8.75 (2.06-37.08) 1.55 (1.24-1.94) 12.37 (3.12-49.05) 12.67 (3.19-50.15) 10.67 (2.56-44.50) 10.69 (2.74-41.61) 5.13 (1.74-15.11) |
Serum lactate at admission to the ICU was a good independent factor for predicting ICU mortality. A value equal to or greater than 2 mmol/L increased the risk of mortality tenfold (95%CI 2.07-51.52, P-value 0.001) and greater than 4 mmol/L twentyfold (95%CI 4.52-88.4, P-value < 0.001). Interhospital transfer or time to surgery did not influence the value of serum lactate.
Serum lactate achieved an area under the curve (AUC) slightly larger than the SOFA score (0.85 versus 0.75, P-value < 0.001 versus 0.003) for predicting mortality. A cut-off value for serum lactate was 6.55 mmol/L, demonstrating 67% sensitivity and 97% specificity in positively predicting ICU mortality (see Figure 1).
Figure 1.
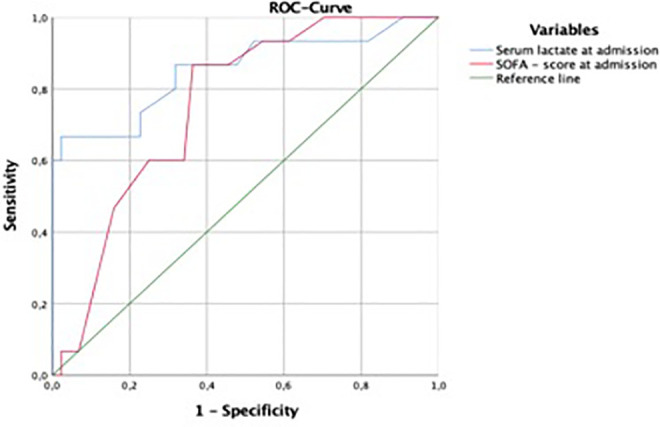
Area under the curve for serum lactate and SOFA score at admission against mortality in patients with NSTI.
Discussion
The present study analyzed the characteristics and outcomes of patients with an NSTI. Furthermore, the potential existence of risk factors linked to mortality was investigated. We identified serum lactate at admission as a robust parameter for predicting mortality. To our knowledge, our study is the first European study to link the value of serum lactate at admission to the ICU with mortality. In addition, we conducted a detailed analysis of intensive care therapy regarding, primarily, the first 24 hours after admission.
NSTIs are rare medical entities, especially in the Western world, so most monocentric studies in Europe and the US contain small numbers of patients over long periods. 4,11 –13 Multiple parameters predicting a negative outcome have been proposed.
Our cohort included 59 patients over a period of 9 years. In this time period 73,418 patients have been treated at our department of intensive care, resulting in a mean admission rate of 0.08%. The ICU and in-hospital mortality rates were 25% and 30%, respectively—comparable with those from previous studies conducted during the past 2 decades 1,4,7,11,13 –18 and slightly lower compared with earlier studies. A recently published metanalysis using 109 studies presented a mean mortality of 21%. 5 Our slightly higher mortality could be explained in that, being a university hospital, our unit receives the most critically ill patients from north Germany. One-third of the patients in our cohort were admitted by interhospital transfer.
Eight patients exhibited a fulminant course and died within the first 48 hours after admission to the ICU due to overwhelming septic shock with multiple organ dysfunction syndrome. Van Stigt et al reported a similar percentage, 13 again confirming the severity of this disease.
Our study contained the largest proportion of patients with autoimmune diseases to date. This was a significant risk factor for mortality in the univariate analysis, increasing the odds of dying by a factor of 4. We suppose that alterations of the immune system caused by the disease itself or by its therapy might favor NSTIs in such patients. The literature concerning this association is scarce and exists primarily in the form of case reports. 19
The statistical analysis revealed no statistically relevant association between the remaining demographic data, risk factors, clinical presentation or localization of the NSTI, and mortality compared with other studies. 4,20,21 Different settings and cohorts could be responsible for such discrepancies.
The LRINEC score helps to differentiate between necrotizing and non-necrotizing forms of soft tissue infections 7 but has been subject to criticism over the years. 22 This score is not routinely used in our hospital, so it was computed retrospectively for descriptive purposes. Its addition to a carefully recorded patient’s history with an unclear clinical presentation could be valuable in increasing diagnostic chances. 23
Surgical exploration was performed within the first 6 hours after hospital presentation in 38 patients (64%). Although our study did not reveal any statistically relevant reduction in mortality in these patients, it is our strong belief, in line with a recent meta-analysis, 5 that the surgical debridement is a treatment of highest priority in NSTI and should be performed without any delays. Some patients had already undergone a surgical intervention in the first hospital before being transferred, others were preoperatively admitted to the ICU for hemodynamic improvement, and others still had undergone a small debridement in the emergency department, which was not mentioned as a true surgical intervention in the documentation. Based on the small number of patients in each situation, it is not possible to draw a definite conclusion regarding such practices.
Regarding amputations in NSTIs, the recently published study by Chang et al had an amputation rate of 6% and classified amputations as early and late according to a cut-off time of 3 days. 24 This procedure was performed within 3 days in all patients in our study. There was no significant association between the timing of the first surgical exploration and the rate of amputation, as was demonstrated in a recent meta-analysis. 5
The presence of Escherichia coli or Enterobacter spp. in the wound isolates was associated with higher odds of increased mortality in the univariate analysis. To our best knowledge, studies that found significant correlations between different microorganisms and outcome are scarce. In a large Taiwanese study, infection with Aeromonas spp. was an independent factor for increased mortality. 21 In a recently published study, infection with Streptococcus pyogenes of Group A was associated with a significantly lower mortality. 25 Both E.coli and Streptococcus pyogenes were the most frequent microorganisms in a large study from Thailand, but there was no correlation to the outcome. 20 NSTIs with Enterobacter spp appear sporadically in the form of case reports. 26,27 The 2 bacteria typically appeared in type I (polymicrobial) NSTI, seen mostly postoperatively in patients with multiple comorbidities. Small numbers make it difficult to draw definite statements; however, it stresses once again the necessity for combination antibiotic therapy from the moment of NSTI suspicion.
Both the SOFA score at admission to the ICU and the SAPS II score at 24 hours after admission correlated well with mortality. Circulatory and renal dysfunction were the most common organ dysfunctions during the first 24 hours. We identified a statistically significant association between initial circulatory and liver dysfunction and mortality in the univariate analysis. The current literature is not consistent on this matter: a study by Krieg et al identified AKI as an independent prognostic parameter for mortality, 4 while Khamnuan et al revealed no correlation between organ dysfunction and mortality. 3 Demographic differences could explain these discrepancies; for example, the number of patients with diabetes mellitus in the first study was significantly higher compared to ours (42% versus 19%). The second study had a surprisingly low mortality rate (9.8%), and only 16% of patients had some form of organ dysfunction.
We interpreted the lower C-reactive protein levels in the subgroup of non-survivors more as a sign of deterioration of the liver function and synthesis than as an overall exhaustion of the immune response.
Signs of refractory septic shock with multiple organ dysfunctions as ongoing circulatory dysfunction, a necessity for renal replacement therapy or corticosteroid therapy and a positive fluid balance after the first 24 hours, were significantly associated with higher mortality, all of which were markers of disease severity. Septic shock is still associated with high mortality regardless of the cause, with a systematic review and meta-analysis demonstrating mortality at around 38% across Europe and North America. 28
Similar to the results of another study, 29 the present study could not establish a benefit of using intravenous immunoglobulin therapy. Since only a small number of patients received immunoglobulins, we cannot draw any definitive conclusion. At the moment, there is no recommendation concerning their utilization for the treatment of NSTIs. 6
The major finding of the present study was the identification of serum lactate at admission to the ICU as being a good predictor for mortality. This observation is consistent with recent studies linking severe hyperlactatemia in general critically ill patients to mortality. 30,31 The failure of serum lactate clearance after 24 hours was a negative predicting factor for survival in the univariate analysis, as well. Computing the ROC curves for serum lactate and the SOFA score, we observed better performance of the former in predicting mortality. Our results are similar to a recently published study by Chang et al. 32 In the present study, the serum lactate achieved a slightly larger AUC than the SOFA score.
Murphy et al demonstrated in a small study that serum lactate at presentation could serve as a marker to differentiate NSTIs from other non-necrotizing soft tissue infections, with lactate serving as a potential marker for tissue necrosis. 33
Linking hyperlactatemia to the prediction of mortality is an important finding in our study. Serum lactate is a simple parameter that can be easily measured by most point-of-care machines in the emergency department or in the ICU. A higher value could be helpful in the decision-making process, regarding further interventions or even discontinuation of care.
Our study has several limitations. It was a monocentric retrospective study with a limited number of patients over 9 years. The small number of patients and therefore small number of unfortunate events limited the use of advanced statistic studies, such as multivariate regression analysis. Concerning serum lactate, we used the first measured value upon admission to the ICU. As some of the patients were initially stable or were classified as having only an isolated dermatological problem, serum lactate was not routinely measured in the emergency department. We are aware that the admission value for serum lactate could have been influenced by a variety of factors. Nevertheless, and in line with recent studies, serum lactate continues to prove itself as a useful cellular marker, with higher dynamic values typically suggesting a greater threat to the patient’s vital status. Future prospective well-conducted studies should further explore the relationship between serum lactate at admission to the emergency department and mortality before any therapeutic actions are taken.
Conclusion
Necrotizing soft tissue infections are major surgical and intensive care emergencies. They progress rapidly to septic shock with multiple organ dysfunction syndrome and are still associated with high mortality. The fulminant course of the disease was evident in our study, with 8 patients dying within the first 48 hours after admission to the ICU. We identified serum lactate at admission as a good prognostic marker for ICU mortality. Its raised value should alert the clinician about the severity of the disease and the potential for higher mortality. Moreover, it can be used as a reference value in the decision-making process regarding prognosis and continuation of care.
Abbreviations
95%CI, 95% confidence interval; AKI, acute kidney injury; AUC, area under the curve; BMI, body mass index; CK, creatine kinase; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computer tomography; E. coli, Escherichia coli; ICM, integrated care manager; ICU, intensive care unit; IQR, interquartile ratio; KDIGO, Kidney Disease—Improving Global Outcomes; LRINEC, Laboratory Risk Indicator for Necrotising Fasciitis score; MRI, magnetic resonance imaging; N, number of patients; NSTI, necrotising soft tissue infection; OR, odds ratio; PCT, procalcitonin; PT, prothrombin time; ROC, receiver operating characteristic; SAPS II, Simplified Acute Physiology score II; SD, standard deviation; SOFA, sepsis-related organ failure assessment score; SPSS, statistical package for the social sciences; WBC, white blood cells.
Footnotes
Authors’ Note: AO collected the data. SK and GdH designed the study. GdH and HR verified the data. AO and CB performed the statistical analysis. AO and CB wrote the manuscript. All 5 authors read and corrected the final draft. The data set used and analyzed during the current study is available from the corresponding author upon reasonable request. Due to the retrospective character of the study, patients’ consent was not necessary according to local requirements (Decision WF-126/20 from 07.07.2020).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alexandru Ogica https://orcid.org/0000-0002-8934-9772
References
- 1. Glass GE, Sheil F, Ruston JC, Butler PE. Necrotising soft tissue infection in a UK metropolitan population. Ann R Coll Surg Engl. 2015;97(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naseer U, Steinbakk M, Blystad H, Caugant DA. Epidemiology of invasive group a streptococcal infections in Norway 2010-2014: A retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2016;35(10):1639–1648. [DOI] [PubMed] [Google Scholar]
- 3. Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A. Clinical predictors for severe sepsis in patients with necrotizing fasciitis: an observational cohort study in northern Thailand. Infect Drug Resist. 2015;8:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krieg A, Dizdar L, Verde PE, Knoefel WT. Predictors of mortality for necrotizing soft-tissue infections: a retrospective analysis of 64 cases. Langenbecks Arch Surg. 2014;399(3):333–341. [DOI] [PubMed] [Google Scholar]
- 5. Nawijn F, Smeeing DPJ, Houwert RM, Leenen LPH, Hietbrink F. Time is of the essence when treating necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg. 2020;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253–2265. [DOI] [PubMed] [Google Scholar]
- 7. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–1541. [DOI] [PubMed] [Google Scholar]
- 8. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552. [DOI] [PubMed] [Google Scholar]
- 9. Brunkhorst FM, Weigand MA, Pletz M, et al. [S3 Guideline Sepsis-prevention, diagnosis, therapy, and aftercare: Long version]. Med Klin Intensivmed Notfmed. 2020;115(Suppl 2):37–109. [DOI] [PubMed] [Google Scholar]
- 10. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184. [DOI] [PubMed] [Google Scholar]
- 11. Friederichs J, Hutter M, Hierholzer C, et al. Procalcitonin ratio as a predictor of successful surgical treatment of severe necrotizing soft tissue infections. Am J Surg. 2013;206(3):368–373. [DOI] [PubMed] [Google Scholar]
- 12. Ryssel H, Germann G, Kloeters O, Radu CA, Reichenberger M, Gazyakan E. Necrotizing fasciitis of the extremities: 34 cases at a single centre over the past 5 years. Arch Orthop Trauma Surg. 2010;130(12):1515–1522. [DOI] [PubMed] [Google Scholar]
- 13. van Stigt SF, de Vries J, Bijker JB, et al. Review of 58 patients with necrotizing fasciitis in the Netherlands. World J Emerg Surg. 2016;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. V K, Hiremath BV, V AI. Necrotising soft tissue infection-risk factors for mortality. J Clin Diagn Res. 2013;7(8):1662–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454–1460. [PubMed] [Google Scholar]
- 16. Jabbour G, El-Menyar A, Peralta R, et al. Pattern and predictors of mortality in necrotizing fasciitis patients in a single tertiary hospital. World J Emerg Surg. 2016;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulasegaran S, Cribb B, Vandal AC, McBride S, Holland D, MacCormick AD. Necrotizing fasciitis: 11-year retrospective case review in South Auckland. ANZ J Surg. 2016;86(10):826–830. [DOI] [PubMed] [Google Scholar]
- 18. Keung EZ, Liu X, Nuzhad A, Adams C, Ashley SW, Askari R. Immunocompromised status in patients with necrotizing soft-tissue infection. JAMA Surg. 2013;148(5):419–426. [DOI] [PubMed] [Google Scholar]
- 19. van de Sande MG, van Slobbe-Bijlsma ER. Necrotizing fasciitis in a rheumatoid arthritis patient treated with tocilizumab. Rheumatology (Oxford). 2012;51(3):577–578. [DOI] [PubMed] [Google Scholar]
- 20. Khamnuan P, Chongruksut W, Jearwattanakanok K, Patumanond J, Tantraworasin A. Necrotizing fasciitis: epidemiology and clinical predictors for amputation. Int J Gen Med. 2015;8:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang KF, Hung MH, Lin YS, et al. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma. 2011;71(2):467–473; discussion 73. [DOI] [PubMed] [Google Scholar]
- 22. Holland MJ. Application of the laboratory risk indicator in necrotising fasciitis (LRINEC) score to patients in a tropical tertiary referral centre. Anaesth Intensive Care. 2009;37(4):588–592. [DOI] [PubMed] [Google Scholar]
- 23. Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: clinical suspicion should trump scoring systems. J Emerg Med. 2013;44(5):928–931. [DOI] [PubMed] [Google Scholar]
- 24. Chang CP, Hsiao CT, Lin CN, Fann WC. Risk factors for mortality in the late amputation of necrotizing fasciitis: a retrospective study. World J Emerg Surg. 2018;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nawijn F, Wassenaar ECE, Smeeing DPJ, et al. Exhaustion of the immune system by Group A Streptococcus necrotizing fasciitis: the occurrence of late secondary infections in a retrospective study. Trauma Surg Acute Care Open. 2019;4(1):e000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choe YM, Park KM, Jeon YS, et al. Enterobacter cloacae-related necrotizing fasciitis after peritoneal dialysis in delayed graft function: a case report. Transplant Proc. 2017;49(5):1189–1191. [DOI] [PubMed] [Google Scholar]
- 27. Tyler K, Patterson A, Hebra A. Necrotizing fasciitis in a healthy pediatric patient caused by enterobacter cloacae and serratia marcescens: a discussion of diagnosis and management. Am Surg. 2016;82(9):e292–293. [PubMed] [Google Scholar]
- 28. Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadri SS, Swihart BJ, Bonne SL, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor-dependent shock: a propensity score-matched analysis from 130 US hospitals. Clin Infect Dis. 2017;64(7):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haas SA, Lange T, Saugel B, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016;42(2):202–210. [DOI] [PubMed] [Google Scholar]
- 31. Peschka AF, Kaestle S, Seidel F, et al. Hyperlactatemia on ICU admission: Comparison between direct admissions and inpatient transfers. Med Klin Intensivmed Notfmed. 2019;114(7):650–654. [DOI] [PubMed] [Google Scholar]
- 32. Chang CP, Fann WC, Wu SR, Lin CN, Hsiao CT. Lactate on emergency department arrival as a predictor of in-hospital mortality in necrotizing fasciitis: a retrospective study. J Orthop Surg Res. 2019;14(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy G, Markeson D, Choa R, Armstrong A. Raised serum lactate: a marker of necrotizing fasciitis? J Plast Reconstr Aesthet Surg. 2013;66(12):1712–1716. [DOI] [PubMed] [Google Scholar]


