Abstract
Although originally implicated in appetite and sleep/wakefulness, the hypothalamic orexin (hypocretin) system has now been demonstrably linked with motivated behavior. This highly plastic system responds to reward-associated environmental stimuli and becomes pathologically overactive in addicted states. Here, we provide a brief overview of the roles of the orexin system in reward-seeking and addiction, as well as potential therapeutic opportunities for substance use disorders based on normalizing orexin function.
Introduction
Recently, the field of addiction neuroscience has dedicated considerable effort to exploring the roles of novel neuropeptides in reward and motivation. Among these, the hypothalamic orexin (also known as hypocretin) neuropeptides have emerged as a system of interest, with converging evidence pointing to plasticity within this system as one of the neural signatures of addiction to drugs of abuse. Despite orexins’ relatively nascent status as components of the reward system, recent studies indicate that orexins may represent a promising target for therapeutics designed to manage cravings and reduce relapse risk in substance use disorders (SUDs).
Orexin as a general motivation system
Orexin peptides (A and B) are produced by neurons in the lateral hypothalamus and signal via two receptors, orexin receptor 1 (Ox1R) and 2 (Ox2R), which are broadly distributed throughout the brain [1,2] (Figure 1). Orexins were initially shown to regulate appetite and arousal; their role in regulating arousal/sleep is exemplified by narcolepsy, a disorder characterized by inappropriate daytime sleepiness. This disorder is associated with reduced orexins in both the brain and cerebrospinal fluid, in some cases to nearly undetectable levels [3]. It has since become clear that orexin neurons regulate a seemingly disparate range of behavioral and physiological processes, including respiration, stress responsivity, and reward-seeking [4]. This diversity of function can be explained by a unified function for orexins in translating states of heightened motivation, such as during physiological need states or following exposure to reward opportunities, into organized goal-directed behaviors [4]. In addicted states, environmental stimuli that signal drug availability robustly activate orexin neurons, which in turn engage downstream circuits to facilitate behaviors directed towards obtaining the drug [4,5]. Thus, unlike most other classic reward systems, the orexin system does not modulate the primary reinforcing effects of drugs per se, but preferentially regulates the motivated drug seeking behavioral processes that characterize addiction [4,5].
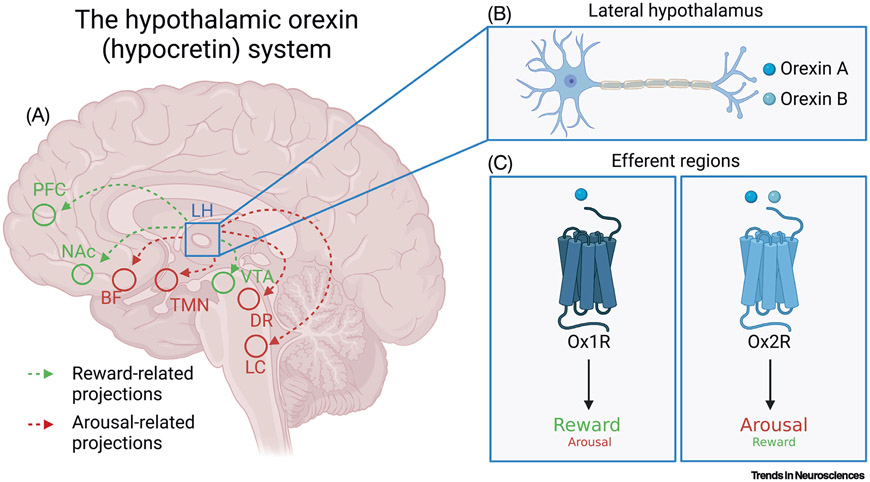
Figure 1. An overview of the orexin (hypocretin) system and its receptors.
(A) Orexin-producing neurons in lateral hypothalamus (LH) mediate a wide range of physiological processes via their broad projections throughout the neuroaxis. Of note, orexins promote reward behavior by influencing key reward centers, including ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC). Orexins also regulate sleep/wake states via inputs to arousal centers, including tuberomammillary nucleus (TMN), dorsal raphe nucleus (DR), and locus coeruleus (LC). (B) Orexin-producing neurons in lateral hypothalamus produce orexins A and B, which are cleaved from the common precursor prepro-orexin. (C) In efferent brain regions, orexins A and B signal via two G-protein-coupled receptors, orexin receptor 1 (Ox1R) and 2 (Ox2R); orexin A binds Ox1R and Ox2R with equal affinity, whereas orexin B preferentially binds Ox2R [2]. In general, although not exclusively, orexins mediate reward behaviors via their actions at Ox1R. The arousal and wake-promoting effects of orexin are primarily achieved via actions at Ox2R, but also rely on signaling at Ox1R.
Drug-induced plasticity of orexin system function
Central to the involvement of orexins in addiction is their susceptibility to exhibit plasticity of function with repeated drug exposure, as well as their ability to influence synaptic function in key reward regions of the brain. Major aspects of this plasticity are summarized in Figure 2 and include, as discussed next: (i) plasticity of orexin cell numbers; (ii) plasticity of orexin neuron excitability; and (iii) plasticity at projection sites.
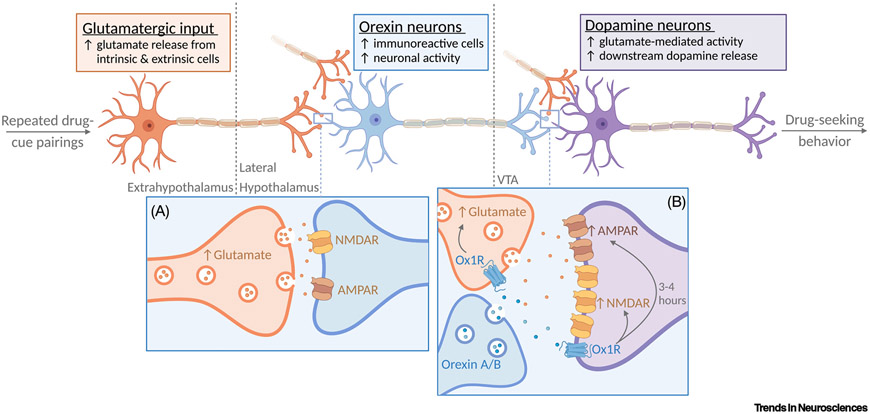
Figure 2. An overview of orexin system plasticity in addicted states.
Orexin neurons are preferentially recruited by drug-associated stimuli and act to translate this information into motivated behavior via outputs to reward centers, including the ventral tegmental area (VTA). (A) Hyperexcitability of this circuit reflects plasticity that occurs with repeated drug-cue pairings, whereby enhanced glutamatergic release (orange) onto orexin neurons (blue) increases neuronal activity [5]. Combined with drug-induced increases in orexin peptide production [6,7], this increased activity promotes heightened downstream orexin release in the VTA. (B) In the VTA, orexin promotes enhanced glutamate release onto dopaminergic (purple) neurons, as well as postsynaptic increases in first N-methyl-d-aspartate receptors (NMDAR) and later α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) to facilitate exaggerated dopamine release [9]. The net result of this plasticity is enhanced activity of the circuit in response to future presentations of drug-associated stimuli, resulting in the expression of hypermotivated, drug-seeking behaviors.
Plasticity of orexin cell numbers
A study examining five postmortem brains from individuals with heroin use disorder reported a 54% increase in the number of orexin-immunoreactive neurons in the hypothalamus compared with control brains from apparently neurologically normal individuals [6]. This difference appears to reflect drug-induced plasticity, rather than a pre-existing biological vulnerability, as increased orexin cell numbers are recapitulated in mice by morphine injections. Similar increases are observed in rats with a history of drug self-administration, and normalization of orexin cell numbers using a genetic knockdown strategy ameliorates drug motivation, suggesting a causal link [7]. Notably, increased numbers of orexin-producing neurons in addiction do not seem to be the result of neurogenesis [6], but rather appear to reflect an arrest of normal fluctuations in orexin neuropeptide production, although the mechanism underlying this process is not known at this point.
Plasticity of orexin neuron excitability
Exposure to environments previously paired with drug reward promotes activation of orexin neurons, indicating that drug-induced plasticity of orexin function also manifests as hyperexcitability of the neurons themselves [8]. This appears to be largely driven by both a strengthening and an increase of glutamatergic synaptic inputs onto the orexin neurons [5], presumably arising from brain regions that convey contextual information (Figure 2A). Importantly, the magnitude of orexin cell activation following exposure to drug stimuli is directly correlated with the extent to which an animal engages in drug-seeking behavior within that environment [5,8]. Concordantly, blocking orexin signaling using both pharmacological and genetic interventions prevents relapse to drug-taking elicited by drug cues in animal models [5].
Plasticity at projection sites
Orexin neurons innervate several key reward regions, including the ventral tegmental area (VTA), which harbors major clusters of dopaminergic (DA) neurons. Although orexin neurons provide little direct input onto VTA DA neurons themselves, orexin signaling in the VTA causes presynaptic release of glutamate, which enhances the probability of firing of VTA DA cells [9]. Postsynaptically, orexin A facilitates glutamatergic signaling in the VTA initially by promoting synaptic translocation of N-methyl-d-aspartate (NMDA) receptors, and later by facilitating signaling at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Figure 2B) [9]. Drug exposure similarly potentiates excitatory input onto VTA DA neurons, and local blockade of Ox1R signaling in the VTA prevents the locomotor-enhancing effects of repeated cocaine exposure [9], indicating that orexins are necessary for drug-induced upregulation of DA neuron output.
A new treatment option for substance use disorders?
Considering the research findings discussed above, there is a compelling case for targeting the orexin system for the clinical management of SUDs. Indeed, the National Institute on Drug Abuse (NIDA) recently listed orexin receptor antagonists and/or negative allostatic modulators on their list of priority targets for new medications to tackle the opioid crisis [10]. Animal studies have demonstrated that selective Ox1R antagonists (SORA-1s) are highly effective at reducing various drug-seeking behaviors, particularly those elicited by drug stimuli, including relapse [5]. These studies also indicate that SORA-1s lack the sedative properties associated with dual orexin receptor antagonists (DORAs), which were commercially developed for their sleep-promoting properties [5]. Notably, however, several DORAs have already gained regulatory approval for clinical use in insomnia, raising the question of whether these therapeutics can be repurposed to expand treatment options for SUDs on a shorter timeline. One of these compounds, suvorexant, is the subject of several ongoing clinical trials designed to assess its utility in clinical populations with alcohol use disorderi,ii, cocaine use disorderiii, opioid use disorderiv,v, and nicotine dependencevi.
Addressing knowledge gaps of orexin plasticity
Several fundamental questions remain regarding orexin system plasticity in addiction. Primary among these is how exposure to drugs of abuse promotes an upregulation of orexin-expressing neurons. Under normal conditions, orexin peptide production varies with arousal state [11], and pathological dysregulation of this variation may contribute to differences in cell numbers. Thus, orexin neurons should be profiled following drug exposure to identify potential molecular machinery (e.g., transcription factors) contributing to altered orexin peptide expression levels. Another outstanding question relates to the source and strength of inputs that drive plasticity of orexin neuron activity. Recent advances in tracing strategies have revealed major orexin afferents arising from the forebrain, hypothalamus, and amygdala [12]. Such work should be combined with functional mapping techniques to identify which of these afferents are engaged by drug stimuli and contribute to the recruitment of orexin neurons. Finally, although orexin neurons project broadly throughout the brain, their role in mediating plasticity at sites other than the VTA is not yet well characterized.
Concluding remarks
Over the last few years, the orexin system has progressed to being among the leading targets for new SUD medications. Despite this rapid advancement, current understanding of precisely how the orexin system is co-opted by drugs of abuse and related stimuli remains in its relative infancy. Often a sequential approach is advocated in drug development, wherein mechanistic understanding through basic research lays the groundwork for therapeutic approaches. The orexin system, we would argue, could make a case for a different path – at least in the context of SUDs. Given that drugs targeting the orexin system have already gained regulatory approval for sleep-related disorders, one could possibly ‘walk and chew gum’: that is, exploring the therapeutic potential of orexin-based compounds for SUDs in parallel to addressing knowledge gaps regarding the mechanistic underpinnings of the plasticity processes described above. Although the multifaceted nature of addiction means that targeting a single brain system is unlikely to offer a panacea, there is basis for optimism that orexin-based compounds will add considerably to what is currently a suboptimal range of treatment options for SUDs.
Acknowledgments
We thank Hannah Bowrey, Karina Kruth, and Annette Klomp for their helpful feedback and insights on this article. This work was supported by a National Institute on Drug Abuse (NIDA) R00 Award (R00 045765) to MHJ. Figures were created with BioRender.com
Footnotes
Declaration of interests
The authors declare no competing interests in relation to this work.
References
- 1.de Lecea L et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A 95, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC et al. (2000) Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahler SV et al. (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci 17, 1298–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James MH et al. (2017) A decade of orexin/hypocretin and addiction: where are we now? Curr. Top. Behav. Neurosci 33, 247–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal TC et al. (2018) Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci. Transl. Med 10, eaao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MH et al. (2019) Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol. Psychiatry 85, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris GC et al. (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559 [DOI] [PubMed] [Google Scholar]
- 9.Baimel C and Borgland SL (2012) Hypocretin modulation of drug-induced synaptic plasticity. Prog. Brain Res 198, 123–131 [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen K et al. (2019) NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44, 657–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGregor R et al. (2017) Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS One 12, e0178573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giardino WJ et al. (2018) Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci 21, 1084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]