Abstract
Antibody–drug conjugates (ADCs) are conjugates of a monoclonal antibody and a cytotoxic drug that induce tumor apoptosis. The evaluation of ADC-induced tumor apoptosis is crucial for the development of ADCs for cancer therapy. To evaluate the efficacy of ADCs, we present in vitro and in vivo fluorescence imaging techniques for ADC-induced tumor apoptosis using annexin V–EGFP (EGFP: enhanced green fluorescent protein) conjugated quantum dots (annexin V–EGFP–QDs). This probe emits visible (VIS) and near-infrared (NIR) dual fluorescence at 515 nm (EGFP emission) and 850 nm (QD emission), which can be used for the detection of tumor apoptosis at the cellular and whole-body levels. By using annexin V–EGFP–QDs, we achieved VIS and NIR fluorescence imaging of human epidermal growth factor receptor 2-positive breast tumor apoptosis induced by an ADC, Kadcyla (trastuzumab emtansine). The results show that the in vitro and in vivo fluorescence imaging of ADC-induced tumor apoptosis using annexin V–EGFP–QDs is a useful tool to evaluate the efficacy of ADCs for cancer therapy.
1. Introduction
Antibody–drug conjugates (ADCs) are conjugates composed of a monoclonal antibody and a cytotoxic drug via a chemical linker.1−3 ADCs are used as the antitumor drugs for cancer therapy. To date, ADCs have received market approval and over 70 are being investigated in various stages of clinical development. For the development of ADCs for cancer treatment, the examination of the efficacy of ADCs that induce tumor apoptosis is crucial.4,5 For the apoptosis detection, we can employ several imaging modalities such as single photon emission computed tomography, positron emission tomography, magnetic resonance imaging, and optical imaging.6−11 Among these imaging modalities, fluorescence imaging has a high sensitivity and a spatial resolution (∼μm) sufficient to perform molecular imaging at the cellular and whole-body levels.11 Herein, we present visible (VIS) and near-infrared (NIR) fluorescence imaging techniques for the visualization of ADC-induced tumor apoptosis in vitro and in vivo.
In the early stages of apoptosis, the morphological change in the cancer cell membrane occurs due to the externalization of phosphatidylserine (PS).12,13 To detect PS molecules on the surface of apoptotic cells, annexin V,14 an endogenous protein with a binding ability to PS, is widely used as an apoptosis detection probe.14−17 Although various types of apoptosis detection optical probes18−36 [e.g., fluorescein isothiocyanate (FITC)-annexin V,31−34 Cy5-annexin V,35,36 and 800CW-annexin V29 have been developed, there are no robust fluorescent probes that enable the long-term optical imaging of apoptosis in vivo. The disadvantage of fluorescent-dye-labeled annexin V probes is the instability of photobleaching caused by the irradiation of excitation lights. To overcome this disadvantage, we synthesized a quantum dot (QD)-based robust probe (annexin V–EGFP–QD) that consists of a fusion protein, annexin V and enhanced green fluorescent protein (annexin V–EGFP),37 and an NIR-emitting CdSeTe/CdS QD.38,39 This probe emits VIS and NIR dual fluorescence at 515 nm (EGFP emission) and 850 nm (QD emission), which can be used for the detection of tumor apoptosis at both the cellular and whole-body levels.
In this paper, we present in vitro and in vivo fluorescence imaging of tumor apoptosis induced by an ADC, Kadcyla (ado-trastuzumab emtansine or T-DM1),40,41 using annexin V–EGFP–QD. Kadcyla is a conjugate of a humanized monoclonal anti-HER2 (human epidermal growth factor receptor 2)42 antibody and emtansine, a highly potent microtubule polymerization inhibitor.40,41 Kadcyla is used as an anticancer drug against HER2-positive solid tumors.43−45 For HER2-positive breast tumor cells (KPL-446,47), we detected Kadcyla-induced apoptosis by the VIS fluorescence of EGFP. We also detected the Kadcyla-induced apoptosis of the HER2-positive breast tumor in mice by the NIR fluorescence of QDs. The NIR fluorescence imaging enabled a long-term (24 days) observation of the tumor shrinking induced by Kadcyla-induced apoptosis. We demonstrate the utility of VIS and NIR fluorescence imaging using annexin V–EGFP–QDs for the detection of ADC-induced tumor apoptosis in vitro and in vivo.
2. Results and Discussion
2.1. Design of the VIS and NIR Emitting Apoptosis Detection Probe
To detect apoptotic cells in vitro, VIS fluorescence imaging is usually used because of the high sensitivity of the VIS light (400–700 nm) in conventional fluorescence microscopies.31−34 However, VIS fluorescence imaging is not suitable for the in vivo detection of apoptosis because of the strong absorption and scattering of VIS light. In contrast, NIR light (700–900 nm) shows high permeability and low scattering in living tissues. Thus, we employ EGFP and NIR-emitting QDs for apoptosis detection at the cellular and whole-body levels, respectively.29,35,36
For in vitro and in vivo apoptosis detection, we designed a QD-based VIS and NIR dual emitting probe, annexin V–EGFP–QD (Figure 1a). Although several types of QD-based apoptosis detection probes have been developed,25,28,48−52 there are no dual-emitting QD probes for the fluorescence imaging of apoptosis at the cellular and whole-body levels. Annexin V–EGFP is a histidine-tagged fused protein and directly binds to the surface of the Cd–S layer of glutathione-coated CdSeTe/CdS QDs (GSH–QDs) via the histidine tags of the protein.53−55 This is due to the high affinity of histidine molecules to Cd2+ ions at the surface of a GSH–QD.53−55 Annexin V molecules presented at the QD surface can bind the PS molecules of the apoptotic cell membrane in the presence of Ca2+ ions (Figure 1b). Since annexin V–EGFP–QD emits VIS (EGFP) and NIR (QD) dual fluorescence, in vitro and vivo fluorescence imaging of apoptotic cells can be performed using a single fluorescent probe, annexin V–EGFP–QD.
Figure 1.

(a) Synthetic method for the preparation of an annexin V–EGFP–QD by conjugation of a GSH-coated CdSeTe/CdS QD (GSH–QD) with a recombinant protein, annexin V–EGFP. (b) Schematic representation for the binding of an annexin V–EGFP–QD to PS molecules at the surface of the apoptotic cell membrane in the presence of Ca2+ ions.
2.2. Characterization of Annexin V–EGFP–QDs
The binding of annexin V–EGFP protein to GSH–QDs can be confirmed by agarose gel electrophoresis (Figures 2 and S1). The mobility of an NIR-emitting QD band decreased with the increasing amount of annexin V–EGFP, indicating the formation of an annexin V–EGFP–QD conjugate. The image of agarose gel electrophoresis for the mixture of annexin V–EGFP and GSH–QDs indicates that ca. five molecules of annexin V–EGFP bind to one QD particle (Figure 2). Annexin V–EGFP–QDs can be purified by dialysis (membrane, MWCO: 300,000) to remove unconjugated annexin V–EGFP (66.7 kDa).
Figure 2.

Agarose gel electrophoresis of GSH–QDs and mixtures of the QDs and annexin V–EGFP. The molar ratio of annexin V–EGFP–QD was changed from 0 to 7.5. The fluorescence bands of QDs were detected at the wavelength of 830 nm.
The fluorescence spectrum of annexin V–EGFP–QDs showed VIS and NIR dual emission resulting from EGFP and QDs, respectively (Figure 3a). The fluorescence quantum yields of annexin V–EGFP and CdSeTe/CdS QDs were 75 and 59%, respectively. The intensity of EGFP emission in annexin V–EGFP–QDs decreased by 6 times compared to that in annexin V–EGFP (Figures 3a and S2). This decrease in the fluorescence intensity of the EGFP emission can be explained by the intramolecular fluorescence resonance energy transfer (FRET) from EGFP to the QD in an annexin V–EGFP–QD conjugate. The fluorescence decay measurements confirmed the FRET from EGFP to the QD in the conjugate. The average fluorescence lifetime56 (τ) of EGFP in annexin V–EGFP was significantly decreased from 2.7 to 0.53 ns by complexation with GSH–QDs (Figure 3b). The FRET efficiency from EGFP to the QD was determined to be 76 and 80% from the fluorescence intensities and lifetimes of EGFP, respectively.57
Figure 3.

(a) Fluorescence spectra of annexin V–EGFP (green line) and annexin V–EGFP–QDs (red line) in PBS. A black dotted line shows the absorption spectrum of GSH–QDs. The inset shows the TEM image of annexin V–EGFP–QDs. Scale bar: 20 nm. (b) Fluorescence decay curves of EGFP emissions in annexin V–EGFP and annexin V–EGFP–QDs in PBS. An excitation pulse (at 475 nm) is shown as a black line.
Transmission electron microscopy (TEM) showed that annexin V–EGFP–QDs are monodispersed nanoparticles with a diameter of 4.4 ± 0.68 nm (inset in Figures 3a and S3). The hydrodynamic diameter of annexin V–EGFP–QDs was evaluated by using fluorescence correlation spectroscopy (FCS).56 Since the diffusion times (DTs) of GSH–QDs and annexin V–EGFP–QDs were calculated to be 0.35 ± 0.031 and 0.51 ± 0.045 ms, respectively (Figure 4a), the hydrodynamic diameters of GSH–QDs and annexin V–EGFP–QDs were estimated to be 5.4 ± 1.5 and 7.9 ± 2.2 nm, respectively.58−60 This result shows that the diameter of annexin V–EGFP–QDs increased 1.5 times compared to that of GSH–QDs after the binding of annexin V–EGFP to the surface of the QD.
Figure 4.

(a) Fluorescence autocorrelation curves for GSH–QDs, annexin V–EGFP–QDs, and fluorescent beads (14 nm in diameter) in 10 mM Na2CO3. The inset graph shows the average DT of the particles: (1) GSH–QDs, (2) annexin V–EGFP–QDs, and (3) fluorescent beads. (b) Size-exclusion column chromatography for GSH–QDs (red line) and annexin V–EGFP–QDs (green line). An inset plot shows the relationship between the molecular weights of standard proteins and their retention times. Molecular weights: 670 kDa for thyroglobulin, 450 kDa for ferritin, 145 kDa for Herceptin, 79.5 kDa for transferrin, and 66 kDa for bovine serum albumin.
The size of GSH–QDs and annexin V–EGFP–QDs was also evaluated by using size-exclusion column chromatography (Figure 4b). Using the relationship between the molecular weights of standard proteins and their retention times (inset in Figure 4b), apparent molecular weights of GSH–QDs and annexin V–EGFP–QDs were determined to be 200 and 563 kDa, respectively. As the molecular weight of annexin V–EGFP is 66.7 kDa, the number of annexin V–EGFP protein bound to one QD particle was calculated to be 5.4. This finding is consistent with the result obtained from the agarose gel electrophoresis experiment (Figure 2).
2.3. Binding Activity of the Annexin V–EGFP–QD to PS
The binding activity of the annexin V–EGFP–QD to PS was examined using FCS. FCS is a very sensitive method to detect changes in the hydrodynamic diameter of fluorescent particles.57−60 We measured the DTs of annexin V–EGFP–QDs before and after the addition of liposomes containing 10% PS [liposome (PS+)], whose size was 43 nm in diameter (Figure S4). When the liposome (PS+) was added to the solution of annexin V–EGFP–QDs, the DT of annexin V–EGFP–QDs increased from 0.65 to 2.9 ms (Figures S5 and 5a). This change indicates that the hydrodynamic size of annexin V–EGFP–QDs increased 4.5-fold upon the binding of the PS liposomes. In contrast, the addition of liposomes containing no PS [liposome (PS−)] to the solution of annexin V–EGFP–QDs did not change the DT of annexin V–EGFP–QDs (Figures S5 and 5a).
Figure 5.

Fluorescence autocorrelation curves for (a) annexin V–EGFP–QDs and (b) GSH–QDs in the absence and presence of soybean lecithin liposomes containing 0% PS (liposome PS−) and 10% PS (liposome PS+). To 10 μL of 10 nM annexin V–EGFP–QD or 10 nM GSH–QD solutions, 5 μL of a liposome solution (0.1 mg/mL, 0.1 mM Ca2+) was added.
A control experiment using GSH–QDs showed that the DT of GSH–QDs did not change upon the addition of liposomes (PS+) and (PS−) to the solutions of GSH–QDs (Figures S5 and 5b). This finding shows that there is no specific binding between GSH–QDs and liposomes (PS+) and (PS−). Thus, the change in the DT of annexin V–EGFP–QDs in the presence of liposome (PS+) indicates the specific binding of annexin V–EGFP–QDs to the PS molecules of liposomes via the annexin V moiety of the QDs.
2.4. In Vitro Imaging of Tumor Apoptosis
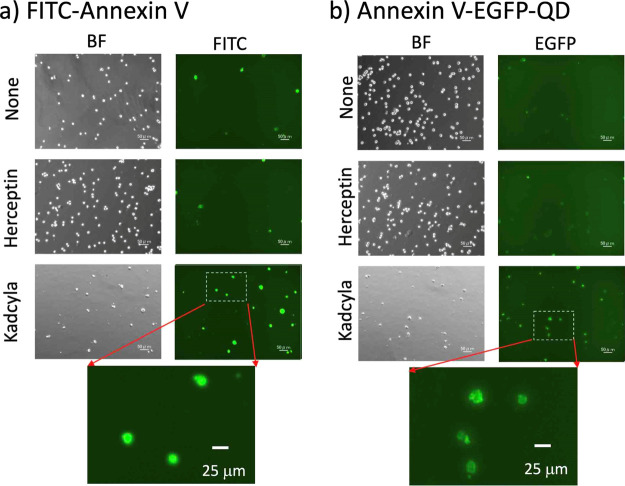
For fluorescence imaging of tumor cell apoptosis, we used a human breast tumor cell line, KPL-4, that overexpresses HER245,46 on the cell surface. The apoptosis of KPL-4 cells was induced by the treatment with an ADC, Kadcyla (a conjugate of Herceptin and emtansine).40,41 Kadcyla acts as an anticancer drug against HER2-positive cancer.42−44 Since Herceptin is ineffective to the treatment of KPL-4 cells,62 we used Herceptin as a control drug to confirm the cytotoxic effect of Kadcyla.
To detect the tumor apoptosis induced by Kadcyla, we first used a traditional apoptosis-detection probe, FITC-annexin V. FITC-annexin V is a widely used a VIS-emitting fluorescent probe for the detection of apoptosis.31−34 We observed that the KPL-4 cells treated with Kadcyla for 72 h emit intense VIS fluorescence from FITC-annexin V (lower image in Figure 6a). The image of the control cells treated with no drugs shows only weak emissions from a few cells (upper image in Figure 6a). The KPL-4 cells treated with an anti-HER2 monoclonal antibody (Herceptin)61 also show weak emissions from a few cells (middle image in Figure 6a). These findings indicate that Kadcyla can induce the apoptosis of KPL-4 cells, while Herceptin cannot induce the apoptosis of the KPL-4 cells.62,63
Figure 6.
Fluorescence imaging of breast tumor cells (KPL-4) treated with and without Herceptin and Kadcyla. Herceptin and Kadcyla (10 nM) were added to cell culture dishes and maintained at 37 °C for 72 h. The cell suspensions were dispensed and incubated with (a) FITC-annexin V (48 nM) or (b) annexin V–EGFP–QDs (33 nM) for 15 min at room temperature. Fluorescence emissions of FITC and EGFP were observed at 525 nm. Scale bar: 50 and 25 μm for magnified images.
As annexin V–EGFP–QDs emit VIS fluorescence from EGFP, we tested whether annexin V–EGFP–QDs can be used as an apoptosis detection probe similar to FITC-annexin V. For the KPL-4 cells treated with Kadcyla, we observed the VIS emission of EGFP (lower image in Figure 6b). For the control KPL-4 cells (upper and middle images in Figure 6b), we could not observe significant EGFP fluorescence emissions resulting from the apoptosis of the cells. These imaging patterns were similar to those obtained by using FITC-annexin V (Figure 6a). This finding indicates that annexin V–EGFP–QDs can be used as an apoptosis detection probe similar to FITC-annexin V for the detection of apoptotic cells.
2.5. In Vivo Imaging of Tumor Apoptosis
We conducted in vivo NIR fluorescence imaging of tumor apoptosis using breast tumor-bearing mice. The tumor-bearing mice were prepared by the transplantation of KPL-4 cells to a ventral region of nude mice. Annexin V–EGFP–QDs were intravenously injected to the tumor mice, and then, the NIR fluorescence of the mice was detected. The VIS fluorescence of EGFP could not be detected owing to the strong tissue absorption and autofluorescence (Figure S6).
For the control mouse treated with no antitumor drugs, NIR fluorescence resulting from the tumor accumulation of annexin V–EGFP–QDs was not observed (Figure 7a). For the mouse treated with Herceptin, NIR fluorescence from the breast tumor was also not observed (Figure 7b). In contrast, the mouse treated with Kadcyla showed intense NIR fluorescence emission from the breast tumor (Figure 7c),63 indicating the accumulation of annexin V–EGFP–QDs to the tumor. These results were consistent with the results obtained by the cellular imaging using annexin V–EGFP–QDs (Figure 6b). The cellular and whole-body fluorescence imaging (Figures 6 and 7) shows that Kadcyla (10 nM for the cell and 100 μL of 1 mg/mL for the mouse) induces the apoptosis of KPL-4 cells, and the resulting apoptotic cells can be detected by VIS and NIR fluorescence using annexin V–EGFP–QDs.
Figure 7.
In vivo NIR fluorescence imaging (a–c) of breast-tumor-bearing mice. Ex vivo images are shown at the right side of each image. Three days after the injection of Herceptin and Kadcyla, annexin V–EGFP–QDs (100 μL of 1 μM solution) were intravenously injected into the mice via the tail vein. The dotted white circles show the positions of tumors. (a) Control mouse: no antitumor drug was injected. (b) Herceptin-injected mouse: 100 μL of Herceptin (1 mg/mL) was injected. (c) Kadcyla-injected mouse: 100 μL of Kadcyla (1 mg/mL) was injected. The fluorescence emissions at 830 nm were detected 3, 4, and 7 days after the injection of Herceptin and Kadcyla. Seven days after the injection of tumors and organs were isolated from the mice. 1: heart, 2: spleen, 3: kidney, and 4: liver. Scale bar: 10 mm.
2.6. Long-Term In Vivo Imaging of Tumor Apoptosis
Long-term imaging for a Kadcyla-treated breast tumor-bearing mouse (100 μL of 1 mg/mL Kadcyla injection) was performed to observe the shrinking of a breast tumor. Annexin V–EGFP–QDs were intravenously injected to the Kadcyla-treated mouse 3 days after the injection of Kadcyla (Figure 8a). NIR fluorescence images of the mouse were taken at 4, 6, 13, and 24 days after the injection of Kadcyla. The accumulation of annexin V–EGFP–QDs to the breast tumor was observed for the tumor-bearing mouse one day after the injection of annexin V–EGFP–QDs (Figure 8b). The change in the tumor size was evaluated from the NIR fluorescence image of the tumor. The size of the breast tumor was decreased by 4 times during the imaging experiment, showing the effect of Kadcyla on the shrinking of the tumor (Figures 8c and S7). Twenty-four days after the injection of Kadcyla, the significant shrinking of the tumor (from 10 to 3 mm in diameter) was observed (Figure 8b). The average NIR fluorescence intensity of the breast tumor was almost constant 6–24 days after the injection of annexin V–EGFP–QDs (Figure 8d), although the size of the tumor gradually decreased with time. The stable NIR emission from the tumor can be attributed to the robustness of QDs, which have high resistance to photobleaching.
Figure 8.

(a) Time course of the experimental procedure for the administration of Kadcyla and annexin V–EGFP–QDs to a breast-tumor-bearing mouse. One hundred microliters of Kadcyla (1 mg/mL) and 200 μL of annexin V–EGFP–QDs (1 μM) were injected into the mouse. NIR fluorescence imaging was performed 4, 6, 13, and 24 days after the injection of Kadcyla. (b) NIR fluorescence images (at 830 nm) of a tumor-bearing mouse. The white dotted circles show the positions of tumors. (c) Time course of the change in the tumor size in a breast-tumor-bearing mouse treated with Kadcyla. The size was determined from the NIR fluorescence images of the breast tumors. (d) Time course of the average NIR fluorescence intensity of a breast tumor after the injection of annexin V–EGFP–QDs.
Finally, we checked the effect of the cytotoxicity of annexin V–EGFP–QDs on the viability of KPL-4 cells. The result showed that the annexin V–EGFP–QDs under the concentration of less than 1 nM did not affect the viability of KPL-4 cells (Figure S8). From the NIR fluorescence image of the mouse injected with annexin V–EGFP–QDs, we observed that the average concentration of annexin V–EGFP–QDs in the mouse was not higher than 1 nM (Figure S9). This finding indicates that the cytotoxicity of annexin V–EGFP–QDs did not affect the apoptosis of KPL-4 cells during the in vivo imaging in this study.
3. Conclusions
In this paper, we present in vitro and in vivo dual-fluorescence imaging of tumor apoptosis by using a conjugate of a recombinant protein, annexin V–EGFP, and a CdSeTe/CdS QD. We demonstrate that the conjugate, annexin V–EGFP–QDs, can be used for VIS and NIR dual-fluorescence imaging of tumor cell apoptosis. Since the NIR fluorescence emission of annexin V–EGFP–QDs is very bright and stable, long-term imaging of ADC-induced tumor cell apoptosis can be achieved. Although many types of fluorescence-labeled annexin V have been reported, there are a very few probes that can be used for the fluorescence imaging of tumor apoptosis in vitro and vivo. We demonstrate a long-term NIR fluorescence imaging technique of Kadcyla-induced tumor apoptosis using annexin V–EGFP–QDs. The presented imaging technique using annexin V–EGFP–QDs will greatly contribute to the study of the action of ADCs at the cellular level as well as the whole-body level.
4. Experimental Section
4.1. Materials
Glutathione-coated CdSeTe/CdS QDs (GSH–QDs) and annexinV–EGFP were prepared according to the previously reported methods (Supporting Information).64,65 The anti-HER2 monoclonal antibody (Herceptin) and Kadcyla (trastuzumab emtansine) were purchased from Chugai Pharmaceutical Co. Ltd. (Japan). The FITC-annexin V apoptosis detection kit and MTT cell counting kit were purchased from Nacalai Tesque (Japan). Fluorescent beads (size: 14 nm in diameter, latex, FluoSpheres, carboxylate-modified and red fluorescent) were purchased from Molecular Probes, Inc. Soybean lecithin was purchased from Nacalai Tesque. l-α-Phosphatidyl-l-serine (soybean) was purchased from Sigma. All other regents were of analytical grade and were used as received without further purification. Breast tumor cells (KPL-4) were kindly provided by Dr. J. Kurebayashi (Kawasaki Medical School). Nude mice (5 week old female BALB/c nu/nu) were purchased from Nihon SLC Inc. (Japan).
4.2. Preparation of Annexin V–EGFP–QDs
One hundred microliters of annexin V–EGFP [1 mg/mL, phosphate-buffered saline (PBS)] was added to 200 μL of an aqueous solution of GSH–QDs (1 μM, 10 mM Na2CO3 solution). Then, the solution buffer was dialyzed (MWCO: 300,000) for 1 h using 10 mM Na2CO3 to remove unconjugated EGFP–annexin V. The purified annexin V–EGFP–QDs were preserved at 4 °C.
4.3. Preparation of the Liposome
Liposome PS (−): 10 mg of soybean lecithin in 10 mL of PBS was sonicated with a tip-type sonicator (Branson Sonifier-150) for 5 min. Then, the liposome suspension was passed through a 0.45 μm membrane filter. The average diameter of the resulting liposomes was 32 nm. Liposome PS (+): 9 mg of soybean lecithin, 1 mg of l-α-phosphatidyl-l-serine (soybean), and 10 mL of PBS were added to a glass tube, and the mixture was sonicated with a tip-type sonicator for 5 min. Then, the liposome suspension was passed through a 0.45 μm membrane filter. The average diameter of the resulting liposomes was 43 nm.
4.4. Agarose Gel Electrophoresis
Ten microliters of aqueous solutions of GSH–QDs and annexin V–EGFP was processed with 1% agarose gel in Tris-acetate buffer (pH 8.0) at 100 V for 20 min. Fluorescence emissions of QDs and EGFP were monitored at 830 and 525 nm, respectively.
4.5. Optical Measurements
The absorption spectra were recorded with a spectrophotometer (V-670, Jasco). The fluorescence spectra were recorded using a photonic multi-channel analyzer (C10027, Hamamatsu Photonics). The fluorescence decay curves of EGFP emissions were measured by excitation at 485 nm using a time-correlated single-photon counting kit (Horiba Fluoro Cube). The fluorescence autocorrelation curves were measured on a compact FCS system (C9413-01MOD, Hamamatsu Photonics, Japan) at an excitation of 473 nm using a laser-diode-pumped solid-state laser. The size of the pinhole was 25 μm, and the spectral range of the detection wavelengths was 500–900 nm. For the determination of the concentration of GSH–QDs, the number of QD particles in a 10 μL solution was measured by using FCS, and the QD concentration was estimated by using a 20 nM solution of rhodamine 6G as a reference. Fluorescence quantum yields were measured using an absolute quantum yield measurement system (C9920, Hamamatsu Photonics). Excitation wavelengths were set to be 475 nm for annexin V–EGFP and 488 nm for GSH–QDs.
4.6. Transmission Electron Microscopy
The morphologies of GSH–QDs and annexin V–EGFP–QDs were observed by TEM using a Hitachi H-800 microscope operating at an acceleration voltage of 200 kV. The TEM sample (ca. 1 μM QDs in PBS) was prepared by dropping the sample solution onto a copper grid.
4.7. Size-Exclusion Column Chromatography
Size-exclusion column chromatography using a high-performance liquid chromatography (HPLC) system (Elite LaChrom, Hitachi) was performed using a TSK-gel G4000SW column (7.8 mm × 30 cm, Tosoh). The mobile phase was 10 mM PBS (pH 7.2–7.4), and the flow rate was adjusted to 1 mL/min. Standard proteins of thyroglobulin (670 kDa), ferritin (450 kDa), Herceptin (145 kDa), transferrin (79.5 kDa), and bovine serum albumin (66 kDa) were used to draw a calibration curve. The HPLC chromatographs of GSH–QDs and annexin V–EGFP–QDs were obtained by monitoring the absorption at 600 nm.
4.8. Cellular Imaging of Tumor Apoptosis
KPL-4 cells were seeded to 35 mm cell culture dishes (3 × 105 cells per dish) and incubated in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum overnight at 37 °C. Then, the cells were exposed to Herceptin and Kadcyla (10 nM or none) for 72 h at 37 °C. For each dish, the cells floating in the medium and the cells detached during PBS washing were collected, and the cells attached to the dish were carefully detached by trypsin treatment to combine all the cell suspensions into one. The cells were washed once with PBS and then resuspended in 1 mL of the annexin V binding buffer (Nacalai Tesque). The cell suspensions were dispensed in 100 μL volumes and incubated with FITC-annexin V (5 μL; FITC-annexin V apoptosis detection kit, Nacalai Tesque) or annexin V–EGFP–QDs (final concentration, 33 nM) for 15 min at room temperature. The stained cell suspensions were then diluted with 400 μL of binding buffer, from which 100 μL was transferred to a plastic dish for the observation of cells. Fluorescence images were acquired with a fluorescence microscope (BZ-X700, Keyence Corp., Japan) with emission filters. The emission filter for FITC and EGFP was 525 ± 25 nm. The emission filter for QDs was 832 ± 18 nm.
4.9. Preparation of Tumor-Bearing Mice
A suspension of KPL-4 cells (107 cells per mouse) was transplanted to the ventral side of 5 week old female BALB/c nu/nu mice. After several weeks, we selected a mouse bearing a tumor less than 10 mm in diameter for imaging. Mice maintenance and animal experiments were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of RIKEN and approved by the Animal Ethics Committee of RIKEN (QA2015-01-8).
4.10. In Vivo Imaging of Tumor Cell Apoptosis
NIR fluorescence images were obtained using an in vivo fluorescence imaging system (Bruker, MS FX PRO). An aqueous solution (100 μL) of Kadcyla (1 mg/mL) was intravenously injected via a tail vein of a tumor-bearing mouse. After 3 days, 100 μL of annexin V–EGFP–QDs was injected into the mouse. In vivo NIR fluorescence images of the tumor were obtained after the injection of annexin V–EGFP–QDs. Four days after the injection of the probe, ex vivo images of the breast tumor and organs were taken. In long-term in vivo imaging, images were obtained up to 24 days after the injection for Kadcyla. The NIR fluorescence of annexin V–EGFP–QDs was observed at 830 nm by the excitation at 760 nm. The exposure time was 30–60 s, and the excitation light (400 W xenon lamp) power was 30 μW/cm2 at the ventral side of the mouse.
Acknowledgments
The authors thank Sayumi Yamada and Satoko Masa for their help with animal experiments and manuscript preparations. This work was partly supported by the Ministry of Education, Science, Sport, and Culture of Japan [Grant-in-Aid for Scientific Research (B): 19H04459 to T.J.].
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05636.
Experimental details; agarose gel electrophoresis of GSH–QDs and a mixture of QDs and annexin V–EGFP; titration of the fluorescence spectrum of annexin V–EGFP with QDs; TEM images of GSH–QDs and annexin V–EGFP–QDs; hydrodynamic diameters of liposomes PS(+) and PS(−); DTs of annexin V–EGFP–QDs and GSH–QDs; NIR and VIS dual-color fluorescence imaging a breast-tumor-bearing mouse; long-term NIR fluorescence imaging of a breast-tumor-bearing mouse; viability of KPL-4 cells; and comparison of NIR fluorescence images of annexin V–EGFP–QDs in water and in a mouse (PDF)
Author Contributions
T.J. supervised this research. T.J. designed the probe. S.T. and T.J. performed the experiments and the analysis of data. All authors wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Junutula J. R.; Gerber H.-P. Next-Generation Antibody-Drug Conjugates (ADCs) for Cancer Therapy. ACS Med. Chem. Lett. 2016, 7, 972–973. 10.1021/acsmedchemlett.6b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. M.; Berkenblit A. Antibody-Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018, 69, 191–207. 10.1146/annurev-med-061516-121357. [DOI] [PubMed] [Google Scholar]
- Chau C. H.; Steeg P. S.; Figg W. D. Antibody-Drug Conjugates for Cancer. Lancet 2019, 394, 793–804. 10.1016/s0140-6736(19)31774-x. [DOI] [PubMed] [Google Scholar]
- Giddabasappa A.; Gupta V. R.; Norberg R.; Gupta P.; Spilker M. E.; Wentland J.; Rago B.; Eswaraka J.; Leal M.; Sapra P. Biodistribution and Targeting of Anti-5T4 Antibody–Drug Conjugate Using Fluorescence Molecular Tomography. Mol. Cancer Ther. 2016, 15, 2530–2540. 10.1158/1535-7163.mct-15-1012. [DOI] [PubMed] [Google Scholar]
- Cahuzac H.; Devel L. Analytical Methods for the Detection and Quantification of ADCs in Biological Matrices. Pharmaceuticals 2020, 13, 462. 10.3390/ph13120462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; McKinley E. T.; Xie J.; Li H.; Xu J.; Gore J. C. In Vivo Magnetic Resonance Imaging of Treatment-Induced Apoptosis. Sci. Rep. 2019, 9, 9540. 10.1038/s41598-019-45864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W.; Wang X.; Xu P.; Liu G.; Eden H. S.; Chen X. Molecular Imaging of Apoptosis: From Micro to Macro. Theranostics 2015, 5, 559–582. 10.7150/thno.11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangestel C.; Peeters M.; Mees G.; Oltenfreiter R.; Boersma H. H.; Elsinga P. H.; Reutelingsperger C.; Van Damme N.; De Spiegeleer B.; Van de Wiele C. In Vivo Imaging of Apoptosis in Oncology: an Update. Mol. Imaging 2011, 10, 340–358. 10.2310/7290.2010.00058. [DOI] [PubMed] [Google Scholar]
- Blankenberg F. G. In Vivo Detection of Apoptosis. J. Nucl. Med. 2008, 49, 81S–95S. 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- Blankenberg F. G. In Vivo Imaging of Apoptosis. Cancer Biol. Ther. 2008, 7, 1525–1532. 10.4161/cbt.7.10.6934. [DOI] [PubMed] [Google Scholar]
- Kherlopian A. R.; Song T.; Duan Q.; Neimark M. A.; Po M. J.; Gohagan J. K.; Laine A. F. A Review of Imaging Techniques for Systems Biology. BMC Syst. Biol. 2008, 2, 74. 10.1186/1752-0509-2-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen H. O.; Kenis H.; Hofstra L.; Narula J.; Reutelingsperger C. P. M. Extracellular Annexin A5: Functions of Phosphatidylserine-Binding and Two-Dimensional Crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Lizarbe M.; Barrasa J.; Olmo N.; Gavilanes F.; Turnay J. Annexin-Phospholipid Interactions. Functional Implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. 10.3390/ijms14022652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari M.; Minai-Tehrani A.; Emamzadeh R. Comparison of Different Probes Based on Labeled Annexin V for Detection of Apoptosis. RSC Adv. 2014, 4, 45128–45135. 10.1039/c4ra07577c. [DOI] [Google Scholar]
- Ruan L.; Ge M.; Huang X.; Ren J. Assay of Single-Cell Apoptosis by Ensemble and Single-Molecule Fluorescence Methods: Annexin-V/Polyethylene Glycol-Functionalized Quantum Dots as Probes. Langmuir 2018, 34, 10040–10047. 10.1021/acs.langmuir.8b01749. [DOI] [PubMed] [Google Scholar]
- Cal P. M. S. D.; Sieglitz F.; Santos F. M. F.; Carvalho C. P.; Guerreiro A.; Bertoldo J. B.; Pischel U.; Gois P. M. P.; Bernardes G. J. L. Site-Selective Installation of BASHY Fluorescent Dyes to Annexin V for Targeted Detection of Apoptotic Cells. Chem. Commun. 2017, 53, 368–371. 10.1039/C6CC08671C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head T.; Dau P.; Duffort S.; Daftarian P.; Joshi P. M.; Vazquez-Padron R.; Deo S. K.; Daunert S. An Enhanced Bioluminescence-Based Annexin V Probe for Apoptosis Detection in Vitro and in Vivo. Cell Death Dis. 2017, 8, e2826 10.1038/cddis.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T.; Zhang T.; Wei W.; Li Z.; Wu D.; Wang L.; Guo J.; He X.; Ma N. Compact, Programmable, and Stable Biofunctionalized Upconversion Nanoparticles Prepared through Peptide-Mediated Phase Transfer for High-Sensitive Protease Sensing and in Vivo Apoptosis Imaging. ACS Appl. Mater. Interfaces 2015, 7, 11849–11856. 10.1021/acsami.5b01446. [DOI] [PubMed] [Google Scholar]
- Jung H.-K.; Wang K.; Jung M. K.; Kim I.-S.; Lee B.-H. In Vivo Near-Infrared Fluorescence Imaging of Apoptosis Using Histone H1-Targeting Peptide Probe After Anti-Cancer Treatment with Cisplatin and Cetuximab for Early Decision on Tumor Response. PLoS One 2014, 9, e100341 10.1371/journal.pone.0100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A.; Gammon S. T.; Xiao S.; Wang W.; Chapman S.; McDermott R.; Suckow M. A.; Johnson J. R.; Piwnica-Worms D.; Gokel G. W.; Smith B. D.; Leevy W. M. In Vivo Optical Imaging of Acute Cell Death Using a Near-Infrared Fluorescent Zinc-Dipicolylamine Probe. Mol. Pharm. 2011, 8, 583–590. 10.1021/mp100395u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Lee S.; Chen X. Design of “Smart” Probes for Optical Imaging of Apoptosis. Am. J. Nucl. Med. Mol. Imaging 2011, 1, 3–17. [PMC free article] [PubMed] [Google Scholar]
- Hickson J.; Ackler S.; Klaubert D.; Bouska J.; Ellis P.; Foster K.; Oleksijew A.; Rodriguez L.; Schlessinger S.; Wang B.; Frost D. Noninvasive Molecular Imaging of Apoptosis in Vivo Using a Modified Firefly Luciferase Substrate, Z-DEVD-Aminoluciferin. Cell Death Differ. 2010, 17, 1003–1010. 10.1038/cdd.2009.205. [DOI] [PubMed] [Google Scholar]
- Niu G.; Chen X. Apoptosis Imaging: Beyond Annexin V. J. Nucl. Med. 2010, 51, 1659–1662. 10.2967/jnumed.110.078584. [DOI] [PubMed] [Google Scholar]
- Wang K.; Purushotham S.; Lee J.-Y.; Na M.-H.; Park H.; Oh S.-J.; Park R.-W.; Park J. Y.; Lee E.; Cho B. C.; Song M.-N.; Baek M.-C.; Kwak W.; Yoo J.; Hoffman A. S.; Oh Y.-K.; Kim I.-S.; Lee B.-H. In Vivo Imaging of Tumor Apoptosis Using Histone H1-Targeting Peptide. J. Controlled Release 2010, 148, 283–291. 10.1016/j.jconrel.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Cheng P.; Li J.; Zhang Y.; Gu M.; Liu J.; Zhang J.; Zhu J.-J. Analysis of Nonadherent Apoptotic Cells by a Quantum Dots Probe in a Microfluidic Device for Drug Screening. Anal. Chem. 2009, 81, 7075–7080. 10.1021/ac901121f. [DOI] [PubMed] [Google Scholar]
- Edgington L. E.; Berger A. B.; Blum G.; Albrow V. E.; Paulick M. G.; Lineberry N.; Bogyo M. Noninvasive Optical Imaging of Apoptosis by Caspase-Targeted Activity-Based Probes. Nat. Med. 2009, 15, 967–973. 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinti L.; Weissleder R.; Tung C.-H. A Fluorescent Nanosensor for Apoptotic Cells. Nano Lett. 2006, 6, 488–490. 10.1021/nl0524694. [DOI] [PubMed] [Google Scholar]
- Le Gac S.; Vermes I.; van den Berg A. Quantum Dots Based Probes Conjugated to Annexin V for Photostable Apoptosis Detection and Imaging. Nano Lett. 2006, 6, 1863–1869. 10.1021/nl060694v. [DOI] [PubMed] [Google Scholar]
- Ohnishi S.; Vanderheyden J.-L.; Tanaka E.; Patel B.; De Grand A. M.; Laurence R. G.; Yamashita K.; Frangioni J. V. Intraoperative Detection of Cell Injury and Cell Death with an 800 nm Near-Infrared Fluorescent Annexin V Derivative. Am. J. Transplant. 2006, 6, 2321–2331. 10.1111/j.1600-6143.2006.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli S. M.; Prabhakar S.; Tang Y.; Shah K.; Cortes M. L.; Murthy V.; Weissleder R.; Breakefield X. O.; Tung C.-H. A Novel Method for Imaging Apoptosis Using a Caspase-1 Near-Infrared Fluorescent Probe. Neoplasia 2004, 6, 95–105. 10.1593/neo.03214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B.; Gibson D.; Tait J. Increased Erythrocyte Phosphatidylserine Exposure in Sickle Cell Disease: Flow-Cytometric Measurement and Clinical Associations. Blood 1996, 88, 1873–1880. 10.1182/blood.v88.5.1873.bloodjournal8851873. [DOI] [PubMed] [Google Scholar]
- Boersma A. W. M.; Nooter K.; Oostrum R. G.; Stoter G. Quantification of Apoptotic Cells with Fluorescein Isothiocyanate-Labeled Annexin V in Chinese Hamster Ovary Cell Cultures Treated with Cisplatin. Cytometry 1996, 24, 123–130. . [DOI] [PubMed] [Google Scholar]
- Vermes I.; Haanen C.; Steffens-Nakken H.; Reutellingsperger C. A Novel Assay for Apoptosis. Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Koopman G.; Reutelingsperger C.; Kuijten G.; Keehnen R.; Pals S.; van Oers M. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420. 10.1182/blood.v84.5.1415.1415. [DOI] [PubMed] [Google Scholar]
- Petrovsky A.; Schellenberger E.; Josephson L.; Weissleder R.; Bogdanov A. Jr. Near-Infrared Fluorescent Imaging of Tumor Apoptosis. Cancer Res. 2003, 63, 1936–1942. [PubMed] [Google Scholar]
- Schellenberger E. A.; Bogdanov A. Jr.; Petrovsky A.; Ntziachristos V.; Weissleder R.; Josephson L. Optical Imaging of Apoptosis as a Biomarker of Tumor Response to Chemotherapy. Neoplasia 2003, 5, 187–192. 10.1016/s1476-5586(03)80050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S.; Jin T. Dual-Colour (Near-Infrared/Visible) Emitting Annexin V for Fluorescence Imaging of Tumour Cell Apoptosis in Vitro and in Vivo. RSC Adv. 2020, 10, 38244–38250. 10.1039/d0ra06495e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S.; Sasaki A.; Sakata T.; Yasuda H.; Jin T. Immunoglobulin Binding (B1) Domain Mediated Antibody Conjugation to Quantum dots for In Vitro and In Vivo Molecular Imaging. Chem. Commun. 2017, 53, 9450–9453. 10.1039/c7cc04966h. [DOI] [PubMed] [Google Scholar]
- Tsuboi S.; Jin T. Bioluminescence Resonance Energy Transfer (BRET)-coupled AnnexinV-functionalized Quantum Dots for Near -Infrared Optical Detection of Apoptotic Cells. ChemBioChem 2017, 18, 2231–2235. 10.1002/cbic.201700486. [DOI] [PubMed] [Google Scholar]
- Menezes B.; Cilliers C.; Wessler T.; Thurber G. M.; Linderman J. J. An Agent-Based Systems Pharmacology Model of the Antibody-Drug Conjugate Kadcyla to Predict Efficacy of Different Dosing Regimens. AAPS J. 2020, 22, 29. 10.1208/s12248-019-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. M.; Chari R. V. J. Ado-Trastuzumab Emtansine (T-DM1): An Antibody-Drug Conjugate (ADC) for HER2-Positive Breast Cancer. J. Med. Chem. 2014, 57, 6949–6964. 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- Iqbal N.; Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Fan J.; Wang Z.; Zai W.; Song P.; Li Y.; Ju D. The Role of Autophagy in the Cytotoxicity Induced by Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer Cells. AMB Express 2020, 10, 107. 10.1186/s13568-020-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura O.; Minami T.; Kijima T.; Koyama S.; Otsuka T.; Kinehara Y.; Osa A.; Higashiguchi M.; Miyake K.; Nagatomo I.; Hirata H.; Iwahori K.; Takimoto T.; Takeda Y.; Kida H.; Kumanogoh A. Trastuzumab Emtansine Suppresses the Growth of HER2-Positive Small-Cell Lung Cancer in Preclinical Models. Biochem. Biophys. Res. Commun. 2017, 488, 596–602. 10.1016/j.bbrc.2017.05.090. [DOI] [PubMed] [Google Scholar]
- Nicoletti R.; Lopez S.; Bellone S.; Cocco E.; Schwab C. L.; Black J. D.; Centritto F.; Zhu L.; Bonazzoli E.; Buza N.; Hui P.; Mezzanzanica D.; Canevari S.; Schwartz P. E.; Rutherford T. J.; Santin A. D. T-DM1, a Novel Antibody-Drug Conjugate, is Highly Effective Against Uterine and Ovarian Carcinosarcomas Overexpressing HER2. Clin. Exp. Metastasis 2015, 32, 29–38. 10.1007/s10585-014-9688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J.; Otsuki T.; Tang C. K.; Kurosumi M.; Yamamoto S.; Tanaka K.; Mochizuki M.; Nakamura H.; Soono H. Isolation and Characterization of a New Human Breast Cancer Cell Line, KPL-4, Expressing the Erb B Family Receptors and Interleukin-6. Br. J. Cancer 1999, 79, 707–717. 10.1038/sj.bjc.6690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto-Ouchi K.; Sekiguchi F.; Tanaka Y. Antitumor Activity of Combinations of Anti-HER-2 Antibody Trastuzumab and Oral Fluoropyrimidines Capecitabine/5′-dFUrd in Human Breast Cancer Models. Cancer Chemother. Pharmacol. 2002, 49, 211–216. 10.1007/s00280-001-0401-7. [DOI] [PubMed] [Google Scholar]
- van Tilborg G. A. F.; Mulder W. J. M.; Chin P. T. K.; Storm G.; Reutelingsperger C. P.; Nicolay K.; Strijkers G. J. Annexin A5-Conjugated Quantum Dots with a Paramagnetic Lipidic Coating for the Multimodal Detection of Apoptotic Cells. Bioconjugate Chem. 2006, 17, 865–868. 10.1021/bc0600463. [DOI] [PubMed] [Google Scholar]
- Koeppel F.; Jaiswal J. K.; Simon S. M. Quantum Dot-Based Sensor for Improved Detection of Apoptotic Cells. Nanomedicine 2007, 2, 71–78. 10.2217/17435889.2.1.71. [DOI] [PubMed] [Google Scholar]
- Prinzen L.; Miserus R.-J. J. H. M.; Dirksen A.; Hackeng T. M.; Deckers N.; Bitsch N. J.; Megens R. T. A.; Douma K.; Heemskerk J. W.; Kooi M. E.; Frederik P. M.; Slaaf D. W.; van Zandvoort M. A. M. J.; Reutelingsperger C. P. M. Optical and Magnetic Resonance Imaging of Cell Death and Platelet Activation Using Annexin A5 -Functionalized Quantum Dots. Nano Lett. 2007, 7, 93–100. 10.1021/nl062226r. [DOI] [PubMed] [Google Scholar]
- Roy P.; Periasamy A. P.; Lin C.-Y.; Her G.-M.; Chiu W.-J.; Li C.-L.; Shu C.-L.; Huang C.-C.; Liang C.-T.; Chang H.-T. Photoluminescent Graphene Quantum Dots for In Vivo Imaging of Apoptotic Cells. Nanoscale 2015, 7, 2504–2510. 10.1039/c4nr07005d. [DOI] [PubMed] [Google Scholar]
- Montón H.; Parolo C.; Aranda-Ramos A.; MerkoçI A.; Nogués C. Annexin-V/Quantum Dot Probes for Multimodal Apoptosis Monitoring in Living Cells: Improving Bioanalysis Using Electrochemistry. Nanoscale 2015, 7, 4097–4104. 10.1039/C4NR07191C. [DOI] [PubMed] [Google Scholar]
- Medintz I. L.; Uyeda H. T.; Goldman E. R.; Mattoussi H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Lao U. L.; Mulchandani A.; Chen W. Simple Conjugation and Purification of Quantum Dot-Antibody Complexes Using a Thermally Responsive Elastin-Protein L Scaffold as Immunofluorescent Agents. J. Am. Chem. Soc. 2006, 128, 14756–14757. 10.1021/ja065343x. [DOI] [PubMed] [Google Scholar]
- Alam R.; Karam L. M.; Doane T. L.; Zylstra J.; Fontaine D. M.; Branchini B. R.; Maye M. M. Near Infrared Bioluminescence Resonance Energy Transfer from Firefly Luciferase-Quantum Dot Bionanoconjugates. Nanotechnology 2014, 25, 495606. 10.1088/0957-4484/25/49/495606. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R.Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, USA, 2006. [Google Scholar]
- Tsuboi S.; Jin T. Fluorescent, Recombinant-Protein-Conjugated, Near-Infrared-Emitting Quantum Dots for in Vitro and in Vivo Dual-Color Molecular Imaging. ChemBioChem 2019, 20, 568–575. 10.1002/cbic.201800506. [DOI] [PubMed] [Google Scholar]
- Tiwari D.; Tanaka S.-I.; Inouye Y.; Yoshizawa K.; Watanabe T.; Jin T. Synthesis and Characterization of Anti-HER2 Antibody Conjugated CdSe/CdZnS Quantum Dots for Fluorescence Imaging of Breast Cancer Cells. Sensors 2009, 9, 9332–9354. 10.3390/s91109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A.; Tsukasaki Y.; Komatsuzaki A.; Sakata T.; Yasuda H.; Jin T. Recombinant Protein (EGFP-Protein G)-Coated PbS Quantum Dots for in Vitro and in Vivo Dual Fluorescence (Visible and Second-NIR) Imaging of Breast Tumors. Nanoscale 2015, 7, 5115–5119. 10.1039/c4nr06480a. [DOI] [PubMed] [Google Scholar]
- de Thomaz A. A.; Almeida D. B.; Cesar C. L. Measuring the Hydrodynamic Radius of Quantum Dots by Fluorescence Correlation Spectroscopy. Methods Mol. Biol. 2014, 1199, 85–91. 10.1007/978-1-4939-1280-3_6. [DOI] [PubMed] [Google Scholar]
- Baselga J.; Norton L.; Albanell J.; Kim Y. M.; Mendelsohn J. Recombinant Humanized Anti-HER2 Antibody (Herceptin) Enhances the Antitumor Activity of Paclitaxel and Doxorubicin Against HER2/Neu Overexpressing Human Breast Cancer Xenografts. Cancer Res. 1998, 58, 2825–2831. [PubMed] [Google Scholar]
- Wilken J. A.; Maihle N. J. Primary Trastuzumab Resistance: New Tricks for an Old Drug. Ann. N.Y. Acad. Sci. 2010, 1210, 53–65. 10.1111/j.1749-6632.2010.05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandi N. J.; Merkul E.; Muns J. A.; Waalboer D. C. J.; Adamzek K.; Bolijn M.; Montserrat V.; Somsen G. W.; Haselberg R.; Steverink P. J. G. M.; Houthoff H.-J.; van Dongen G. A. M. S. A Novel Platinum(II)-Based Bifunctional ADC Linker Benchmarked Using 89Zr-Desferal and Auristatin F Conjugated Trastuzumab. Cancer Res. 2017, 77, 257–267. 10.1158/0008-5472.can-16-1900. [DOI] [PubMed] [Google Scholar]
- Tsuboi S.; Jin T. Shortwave-Infrared (SWIR) Fluorescence Molecular Imaging Using Indocyanine Green-Antibody Conjugates for the Optical Diagnostics of Cancerous Tumours. RSC Adv. 2020, 10, 28171–28179. 10.1039/d0ra04710d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi S.; Jin T. BRET Based Dual-Colour (Visible/Near-Infrared) Molecular Imaging Using a Quantum Dot/EGFP-Luciferase Conjugate. RSC Adv. 2019, 9, 34964–34971. 10.1039/c9ra07011g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.