Abstract
QTc interval prolongation can lead to life-threatening complications such as Torsade de Pointes (TdP), ventricular tachycardia (VT) and sudden cardiac death (SCD). It can occur with tyrosine kinase inhibitors (TKIs) but comparative real-world analyses on the incidence and complication rates are scarce. We retrospectively reviewed all cancer patients treated with TKI therapy at Mayo Clinic between January 2005 and December 2018 and had at least two ECGs (before and after TKI). For each TKI type, we determined the administration rate and incidence of QTc prolongation. QTc prolongation was defined as a corrected QT interval (by Fridericia formula) ≥450 ms in men and ≥470 ms in women. A total of 618 cancer patients were included with 902 TKI administrations, of which 654 (72.5%) were accounted for by pazopanib, sunitinib, imatinib, nilotinib and dasatinib. QTc prolongation (any grade) was reported in 28.8%, most commonly with nilotinib (38.7%) and dasatinib (41.7%). A QTc interval ≥500 ms and a QTc increase ≥60 ms was documented in 46 and 63 administrations, respectively. Life-threatening toxicity was seen in 14 cases (5.4% of QTc prolongation cases) including VT in 9, SCD in 3 and TdP in two administrations. The response to QTc prolongation was: discontinuation in 68%, dose reduction in 13.5%, temporary hold in 8.1% and no action in 10.4%. In conclusion, QTc prolongation with TKI therapy is very common (~1/3 of cases) and in 5% (1.7% overall) associated with life-threatening complications. These data support recommendations for careful ECG monitoring in cancer patients undergoing TKI therapy.
Keywords: QTc prolongation, sudden cardiac death, torsade de pointes, tyrosine kinase inhibitors, ventricular tachycardia
1 |. INTRODUCTION
With the introduction of new therapies designed to target the molecular pathways involved in the malignant disease process, a remarkable reduction in cancer-related mortality has been seen over the past three decades.1 One of the key drug classes responsible for this revolutionary development are tyrosine kinases inhibitors (TKIs).2 These agents are designed to interfere with the transfer of a phosphate group from ATP to tyrosine residues of a protein substrate, thereby modulating its function.3 Integrated in a complex “omics” network, such modulation of proteins has multiple effects on cell function and survival.4,5 It is utilized for therapeutic advantage (ie, silencing cancer cell activity and viability) with TKI treatment.6,7 At present, over 30 TKIs have been approved by the FDA.
As successful as TKIs are, they are not without adverse effects. Cardiomyopathy and heart failure were the initial cardiovascular side effects gaining attention with TKI use.8 Subsequently, vascular toxicities were also recognized as well as arrhythmias including QTc prolongation.8,9–12 The latter are reportedly far more common with TKIs than with classical chemotherapeutics, but incidences in real-world clinical practice remain to be defined for many TKIs. This topic does deserve particular attention as QTc prolongation can lead to lethal arrhythmias including ventricular tachycardia and fibrillation, especially Torsades de Pointes (TdP), and sudden cardiac death (SCD).13–15 Of note, two TKIs, nilotinib and vandetanib, carry an FDA black box warning for QTc prolongation. Such warnings derive their name from the black border around the information on serious, permanent or fatal side effects. They are to alert not only the public but physicians and other prescribers alike with recommendations for monitoring and action. Some evidence, however, suggests that these warnings may go unnoticed.
The current study was performed to determine the incidence of QTc interval prolongation, related complications and provider practices at a large U.S. medical center. Additional goals of our study were to identify the malignancies and the TKIs most commonly associated with QTc prolongation. These analyses may provide clinicians with much needed nonclinical trial data for daily practice.
2 |. METHODS
2.1 |. Study design and population
All consecutive adult male and female patients above 18 years of age who received TKIs for the treatment of different types of cancer at Mayo Clinic, Rochester MN between January 2005 and December 2018 were considered for this retrospective cohort study. Sixty-seven patients had to be excluded from the study due to missing QTc readings after TKI administration, leaving a total of 618 patients (387 male and 231 female) for final analysis. Data that were abstracted from the medical records of these patients included: (a) demographic characteristics such as gender, age and BMI, (b) the presence of chronic diseases such as hypertension (HTN), hyperlipidemia, diabetes mellitus (DM), congestive heart failure (CHF) and coronary artery disease (CAD), (c) type(s) of cancer, (d) type(s) of TKI given and (e) QTc results after each TKI administration. The corrected QT interval (QTc) was calculated using both, the Fridericia formula (QTcf = QT/(RR0.33) and Bazett’s formula (QTcb = QT/√RR) where RR is the time elapsed between two successive R-waves of the QRS signal on ECG, and QT interval is the time from the start of the Q wave to the end of the T wave. As deemed to be more accurate, all analyses were based on QTcf; results of the analyses with QTcb were presented as secondary analyses. The upper limit normal for the QTc interval has been defined as 450 ms in men and 470 ms in women.16–18
As some patients received multiple TKIs, we decided to record the total number of TKI administrations in all patients and then evaluated the QTcf interval and any complications (VT, TdP, SCD) with each separate administration. Diagnosis of TKI-related QTcf prolongation was made if at least one ECG showed a QTcf ≥450 ms or 470 ms among male or female patients, respectively during TKI treatment.16–18 Detection of these complications was established while the patients were monitored inside the hospital.
Differences in (a) demographic characteristics such as gender, age and BMI, (b) the presence of chronic diseases such as HTN, hyperlipidemia, DM, CHF and CAD and (c) type(s) of cancer between patients with normal QTcf and those with prolonged QTcf were compared. For each TKI type, we mentioned the type(s) of treated cancer and identified how many times TKI treatment was initiated. In cases with QTcf interval prolongation only, we compared (a) the mean (SD) of pretherapeutic and posttherapeutic QTcf interval, (b) the incidence of QTcf interval prolongation, (c) the frequency of QTcf prolongation ≥500 ms, (d) the frequency of QTcf progression by ≥60 ms and (e) the development of complications such as VT, TdP and SCD, between different types of TKIs. For the most widely used TKIs in our study, we compared the daily TKI dose at the time of the first QTcf prolongation, and the duration from TKI initiation and the development of the first QTcf prolongation. In addition, we also recorded how providers reacted to QTcf prolongation caused by TKIs. The institutional review board (IRB) approved our study and waived the requirement for written informed consent.
2.2 |. Statistical analysis
Mean and SD were used to represent the data of continuous variables, whereas number and percentage expressed the data of categorical or dichotomous variables. Pearson Chi-square was utilized to compare between categorical or dichotomous variables whereas T test was applied for comparisons between continuous variables. A P value of less than 0.05 was adopted as an indicator of statistical significance for all comparisons. The statistical data analysis was performed using IBM SPSS statistical software.19
3 |. RESULTS
The demographics and clinical characteristics of the study cohort are detailed in Table 1. Of the 618 cancer patients in the study, 261 were diagnosed with renal cell carcinoma (RCC), 175 with chronic myeloid leukemia (CML), 18 with acute myeloid leukemia (AML), 41 with acute lymphocytic leukemia (ALL), 53 with thyroid cancer, 28 with gastrointestinal stromal tumor (GIST) and 42 with lung cancer. QTc prolongation (defined as ≥450 ms in male and ≥470 in female patients) was documented in 208 (33.7%) patients. Patients with long QTcf were significantly older, more commonly obese, and had higher rates of HTN, DM, hyperlipidemia and CHF than patients with normal QTcf interval (Table 1).
TABLE 1.
Demographics and clinical characteristics of the study cohort
| Patients with normal QTc | Patients with long QTc | P valuea | |
|---|---|---|---|
| Number | 410 | 208 | |
| Male gender | 251 (61.2%) | 136 (65.3%) | .312 |
| Age (years) | 59.2 ± 12.3 | 60.1 ± 13.4 | .028 |
| Body mass index (kg/m2) | 28.8 ± 6.5 | 32.8 ± 8.6 | <.001 |
| Hypertension | 253 (61.7%) | 155 (74.5%) | .001 |
| Diabetes mellitus | 105 (25.6%) | 96 (46.2%) | <.001 |
| Hyperlipidemia | 192 (46.8%) | 130 (62.5%) | <.001 |
| Heart failure | 48 (11.7%) | 61 (29.3%) | <.001 |
| Coronary artery disease | 139 (33.9%) | 81 (38.9%) | .216 |
| Tumor type | |||
| RCC | 190 (46.3%) | 71 (34.1%) | .003 |
| CML | 112 (27.3%) | 63 (30.3%) | .438 |
| AML | 7 (1.7%) | 11 (5.3%) | .124 |
| ALL | 20 (4.9%) | 21 (10.1%) | .014 |
| Thyroid | 31 (7.6%) | 22 (10.6%) | .206 |
| GIST | 19 (4.6%) | 9 (4.3%) | .862 |
| Lung | 31 (7.6%) | 11 (5.3%) | .288 |
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; GIST, gastrointestinal stromal tumor; CML, chronic myeloid leukemia; RCC, renal cell carcinoma.
P value for comparisons between all patients with normal QTc vs all patients with long QTc interval.
A total of 18 different TKIs were used with 902 TKI administrations over the study period. The most frequently administered were imatinib (165), pazopanib (165), sunitinib (134), dasatinib (115) and niltotinib (75). These drugs accounted for 654 (72.5%) of the 902 total TKI administrations. QTcf prolongation was reported in 260 administrations (28.8%). Among these, QTcf interval ≥ 500 ms and QTcf progression ≥60 ms were documented in 46/260 (17.7% of QTcf prolongation cases) and 63/240 (24.2% of QTcf prolongation cases) administrations, respectively (Table 2). Moreover, complications were detected in 14/260 administrations (5.4% of QTcf prolongation cases) including VT in 9 (3.5%), SCD in 3 (1.2%) and TdP in 2 (0.8%) administrations. Among these cases, QTcf prolongation was correlated with QTcf ≥500 ms in seven cases, other QTcf prolonging drugs in seven cases, chronic kidney disease (CKD) in seven cases, CAD in four cases, CHF with low ejection fraction in three cases, electrolyte abnormalities (hypokalemia, hypocalcemia and hypomagnesemia) in two cases, premature ventricular contraction in four cases, atrial fibrillation in three cases and atrial flutter in three cases (Table S1).
TABLE 2.
Incidence of QTcf interval prolongation, frequency of QTcf prolongation ≥500 ms, the progression of QTcf interval by ≥60 ms and the development of complications (VT, TdP and SCD) for each TKI in all patients
| TKI administration with prolonged QTcf | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | ||||||||||
| Type of TKI | Type of treated cancer | Total number of TKI administration | TKI administration with normal QTcf | Total | Grade 1,450 ≥ QTcf ≤ 480 | Grade 2,480 < QTcf <500 | QTcf ≥500 | QTcf progression ≥60 | VT | SCD | TdP |
| Imatinib | CML, AML, ALL, GIST | 165 | 117 (70.9%) | 48 (29.1%) | 10 (20.8%) | 17 (35.4%) | 10 (20.8%) | 9 (18.8%) | 2 (4.2%) | ||
| Pazopanib | RCC, Thyroid cancer | 165 | 133 (80.6%) | 32 (19.4%) | 18 (56.3%) | 4 (12.5%) | 3 (9.3%) | 4 (12.5%) | 2 (6.3%) | 1 (3.1%) | |
| Sunitinib | RCC, GIST | 134 | 108 (80.6%) | 26 (19.4%) | 16 (61.5%) | 6 (23.1%) | 1 (3.8%) | 1 (3.8%) | 1 (3.8%) | 1 (3.8%) | |
| Dasatinib | CML, AML, ALL, ALL | 115 | 67 (58.3%) | 48 (41.7%) | 8 (16.7%) | 15 (31.3%) | 8 (16.7%) | 14 (29.2%) | 2 (4.2%) | 1 (2.1%) | |
| Nilotinib | CML, AML, ALL GIST | 75 | 46 (61.3%) | 29 (38.7%) | 1 (3.5%) | 2 (7%) | 9 (31.0%) | 17 (58.6%) | |||
| Axinitinb | RCC | 45 | 36 (80%) | 9 (20%) | 1 (11.1%) | 2 (22.2%) | 3 (30.0%) | 3 (30.0%) | |||
| Sorafenib | RCC, AML, Thyroid, Lung | 41 | 28 (68.3%) | 13 (31.7%) | 5 (38.5%) | 4 (30.7%) | 2 (15.4) | 2 (15.4) | |||
| Cabozantinib | RCC | 33 | 24 (72.7%) | 9 (27.3%) | 1 (11.1%) | 2 (22.2%) | 1 (11.1%) | 2 (22.2%) | 1 (11.1%) | 1 (11.1%) | 1 (11.1%) |
| Erlotinib | Lung | 29 | 22 (75.9%) | 7 (24.1%) | 2 (28.5%) | 1 (14.3%) | 2 (28.6%) | 2 (28.6) | |||
| Lenvatinib | Thyroid, Lung | 21 | 12 (57.1%) | 9 (42.9%) | 4 (44.4%) | 3 (33.3%) | 1 (11.1%) | 1 (11.1%) | |||
| Bosuotinib | CML | 21 | 15 (61.9%) | 8 (38.1%) | 5 (62.5%) | 3 (37.5%) | 1 (12.5%) | ||||
| Ponatinib | CML, AML, ALL | 17 | 11 (52.9%) | 8 (47.1%) | 1 (12.5%) | 3 (37.5%) | 4 (50.0%) | ||||
| Osimertinib | Lung | 16 | 12 (75%) | 4 (25%) | 1 (25%) | 1 (25%) | 1 (25.0%) | 1 (25.0%) | |||
| Regorafenib | GIST | 9 | 7 (77.8%) | 2 (22.2%) | 1 (50%) | 1 (50%) | |||||
| Afatinib | Lung | 8 | 6 (75%) | 2 (25%) | 1 (50.0%) | 1 (50.0%) | |||||
| Vandetanib | Thyroid cancer | 5 | 1 (20%) | 4 (80%) | 1 (25%) | 1 (25.0%) | 2 (50.0%) | ||||
| Crizotinib | Lung | 2 | 1 (50%) | 1 (50%) | 1 (100%) | ||||||
| Gefitinib | Lung | 1 | 1 (100%) | 1 (100%) | |||||||
| Total | 902 | 642 (71.2%) | 260 (28.8%) | 75 (30%) | 63 (24.2%) | 46 (17.7%) | 63 (24.2%) | 9 (3.5%) | 3 (1.2%) | 2 (0.8%) | |
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; GIST, gastrointestinal stromal tumor; RCC, renal cell carcinoma; SCD, sudden cardiac death; TdP, Torsade de Pointes; TKI, tyrosine kinase inhibitor; VT, ventricular tachycardia.
Figure 1 illustrates the mean (±SD) pretherapeutic and posttherapeutic QTcf interval for cases with QTc prolongation after different TKIs. The QTc interval change was 30 ± 9.7 ms for dasatinib, 35 ± 8 ms for imatinib, 27 ± 5.9 ms for nilotinib, 27 ± 4 ms for sunitinib and 23 ± 1.3 ms for pazopanib. Significant differences between pre-QTcf vs post-QTcf values were seen for dasatinib, imatinib, nilotinib, sunitinib and pazopanib (Figure 1).
FIGURE 1.
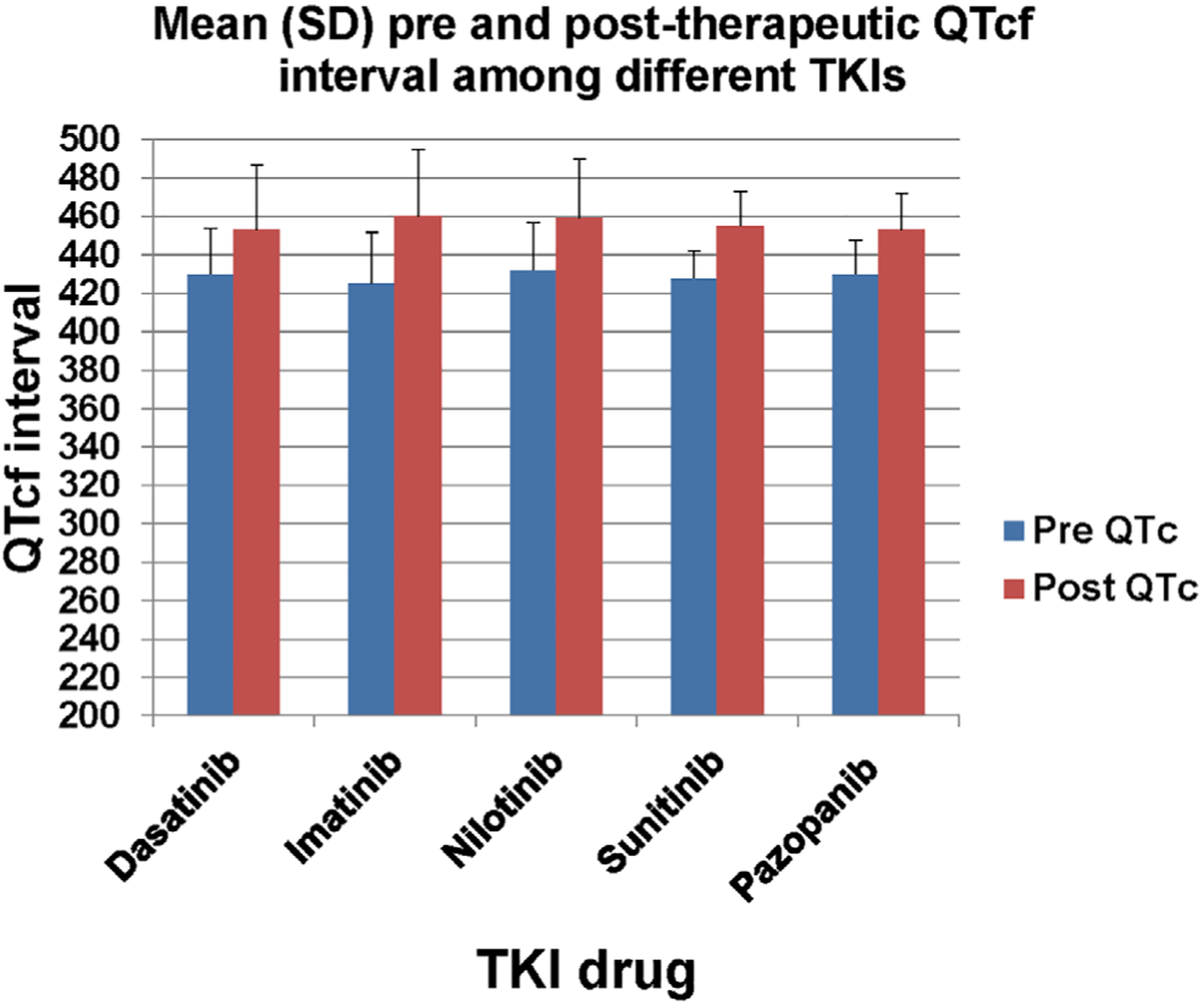
Mean (SD) of pretherapeutic and posttherapeutic QTcf interval among different TKIs. Significant differences were found in comparisons between pre-QTcf vs post-QTcf values for dasatinib (P value = .001), imatinib (P value = .001), nilotinib (P value = .001), sunitinib (P value = .001) and pazopanib (P value < .001)
QTcf prolongation was most frequently observed with dasatinib (41.7%), nilotinib (38.7%) and imatinib (29.1%). Among administrations with QTcf prolongation, we recognized that 10/48 (20.8%), 9/29 (31%) and 8/48 (16.7%) of imatinib, nilotinib and dasatinib administrations, respectively, caused prolongations of the QTcf interval ≥500 ms and 9/48 (18.8%), 17/29 (58.6%) and 14/48 (29.2%) of the same aforementioned drugs resulted in QTcf progression ≥60 ms. Collectively, these TKI types were responsible for 27/46 (58.7%) and 40/63 (63.5%) of administrations with QTcf interval ≥500 ms and QTcf progression ≥60 ms, respectively.
As outlined in Table 3, most QTcf prolongations (67.7%) were seen 1 to 6 months after therapy initiation and after at least 25 doses. QTcf prolongation was most commonly noted in the higher dose range for each TKI: (a) 45/48 (93.8%) of imatinib administrations were given at daily dose ≥400 mg, (b) 30/32 (93.8%) of pazopanib administrations were given at daily dose ≥400 mg, (c) 23/26 (88.5%) of sunitinib administrations were given at daily dose ≥37.5 mg, (d) 41/48 (85.4%) of dasatinib administrations were given at daily dose ≥70 mg and (e) 24/29 (82.8%) of nilotinib administrations were given at daily dose ≥600 mg.
TABLE 3.
Daily TKI dose at the time of the first QTcf prolongation and the duration from TKI initiation and the development of the first QTcf prolongation for the following TKIs: Imatinib, Dasatinib, Sunitinib, Pazopanib and Nilotinib
| Number ofTKI | Daily TKI dose at the time | Duration from TKI initiation and the first QTcf prolongation | |||||
|---|---|---|---|---|---|---|---|
| Type ofTKI | administrations that cause QTcf prolongation | of the first QTcf prolongation | <1 month | 1–3 months | 3–6 months | 6 months-1 year | >1 year |
| Imatinib | 48 | 200 mg: 1 (2.1%) 300 mg: 2 (4.2%) 400 mg: 35 (72.9%) 500 mg: 1 (2.1%) 600 mg: 6 (12.5%) 800 mg: 3 (6.3%) |
10 (20.8%) | 16 (33.3%) | 15 (31.3%) | 3 (6.3%) | 4 (8.3%) |
| Pazopanib | 32 | 200 mg: 2 (6.3%) 400 mg: 7 (21.9%) 600 mg 10 (31.3%) 800 mg: 13 (40.6%) |
6 (18.8%) | 12 (37.5%) | 10 (31.3%) | 2 (6.3%) | 2 (6.3%) |
| Sunitinib | 26 | 25 mg: 3 (11.5%) 37.5 mg: 8 (30.8%) 50 mg: 15 (57.7%) |
5 (19.2%) | 9 (34.6%) | 11 (42.3%) | 0 (0%) | 1 (3.9%) |
| Dasatinib | 48 | 20 mg: 3 (6.3%) 50 mg: 6 (12.5%) 70 mg: 8 (16.7%) 100 mg: 20 (41.2%) 140 mg: 11 (22.9%) |
10 (20.8%) | 16 (33.3%) | 17 (35.4%) | 3 (6.3%) | 2 (4.2%) |
| Nilotinib | 29 | 300 mg: 2 (6.9%) 400 mg: 3 (10.3%) 600 mg: 5 (17.2%) 800 mg: 19 (65.5%) |
7 (24.1%) | 10 (34.5%) | 7 (24.1%) | 3 (10.3%) | 2 (6.9%) |
| Total | 183 | 38 (20.8%) | 63 (34.4%) | 60 (32.8%) | 11(6.0%) | 10 (5.5%) | |
In regards to the response to QTcf prolongation on provider level, TKI therapy was discontinued in 68% of administrations, including switching to a different TKI in 57 (21.9%) or a different type of treatment in 120 (46.1%; different class of chemotherapy or radiotherapy or bone marrow transplant). TKI therapy was continued in 32% of administrations with a dose reduction in 35 (13.5%), temporarily held in 21 (8.1%) and not modified in 27patients (10.4%; in these cases, providers either monitored the patient with weekly ECG tests, corrected other problems such as electrolyte imbalances and discontinued any concurrent QTcf prolonging medications).
In terms of comparison of formulas most commonly used for automatic reporting of the QT interval, the incidence of QTc prolongation was 32% using the Bazett’s vs 28.8% using the Fridericia’s formula. Frequencies of QTc prolongation using the Bazett’s vs the Fridericia’s formula with the different TKIs were: 50% vs 42% for dasatinib, 44% vs 39% for nilotinib, 33% vs 29% for imatinib, 23% vs 19% for sunitinib and 22% vs 19% for pazopanib. QTc prolongation to a QTc interval ≥500 ms and by ≥60 ms were documented in 18.3% vs 18.1% and 25% vs 25% (Bazett’s vs Fridericia’s), respectively. Stratified by TKI, QTc prolongation to ≥500 ms was seen in 24.1% vs 29.1% with imatinib, 30.3% vs 31% for nilotinib and 17.2% vs 16.7% for dasatinib, and QTc prolongation by ≥60 ms in 18.5% vs 18.8% with imatinib, 57.6% vs 58.6% with nilotinib and 27.6% vs 29.2% with dasatinib, using Bazett’s and Friderica’s formula, respectively. There were no significant differences comparing the results obtained with either formula.
4 |. DISCUSSION
Our study shows that (a) one-third of TKI administrations develop QTc prolongation (20% of them had Grade 1 QTc prolongation at baseline), (b) 41.9% of QTc prolongations are of high-grade and (c) 5.4% of QTc prolongations are life-threatening. BCR-ABL inhibitors and VEGF inhibitors were the two types of TKIs most commonly associated with QTc prolongation and complications, especially when used at higher dose ranges. Approximately 32% of providers did not change therapy in response to QTc prolongation and another 10% of patients did not even undergo ECG monitoring. Most cases of QTc prolongation were noted during the first 6 months of therapy. Collectively these data define the need and windows of opportunity for serial ECG monitoring of patients on TKI therapy.
A recent extensive meta-analysis of available studies up to 2017 found wide variations in the incidence of QTc prolongation among different TKIs and even among studies on the same TKI.20 The TKIs most commonly identified to prolong the QTc interval in the meta-analysis were dasatinib (range, 1.6%–73%; weighted incidence rate of QTc prolongation in 8%; weighted incidence rate of QTc >500 ms in 1%), vandetanib (0%–66.7%; weighted incidence rate of QTc prolongation = 8.5; weighted incidence rate of QTc >500 ms in 2.7%), nilotinib (0%–24%; weighted incidence rate of QTc prolongation in 2.7; weighted incidence rate of QTc >500 ms in 0.2%), and sorafenib or sunitinib (0%–17.8%; weighted incidence rate of QTc prolongation = 8.5; weighted incidence rate of QTc >500 ms in 1.9%). In comparison, incidence rates of QTc prolongation reported in the current study were within the prior range for sunitinib (range, 0%–17.8%) and dasatinib (range, 1.6%–73%) but higher than previously reported for nilotinib (range, 0%–24%), imatinib (range, 0.5%–6.9%) and pazopanib (range, 0%–5.9%).20
An important observation of the current study is the high incidence of QTc prolongation and complications in patients on BCR-ABL and VEGF inhibitors other than the ones carrying a black box warning, that is, nilotinib and vandetinib. Of note, in patients on nilotinib we did not see a lower incidence of QTc prolongation ≥500 or >−60 milliseconds. This observation argues against the notion that providers are following prescription recommendations and are implementing therapy interruptions and dose adjustments in response to a QTc value >480 milliseconds (thus aiming to maintain a QTc <500 milliseconds at all times). Interestingly enough, however, nilotinib was not associated with VT, TdP or SCD. Rather, imatinib and dasatinib, two BCR-ABL inhibitors considered safe were associated with VT.
GI side effects, edema and effusions are noted with the use of TKIs, especially BCR-ABL TKIs,21 and one could argue that electrolyte shifts related to diarrhea and/or diuresis could predispose to QTc prolongations with these agents. However, in our analysis of the five cases with complications, electrolyte abnormalities were seen only in one patient. Most common was the combination with other QTc prolonging drugs in three cases and CKD was present in two cases.
Intriguingly, all cases (n = 3) of SCD occurred in patients receiving VEGF inhibitors. The sample size though is too small to draw final conclusions. The same holds true for any other correlative analyses. All patients with SCD were on drugs that cause QTc prolongation in addition to a TKI, two of them had CKD, and VT was documented in one patient. TdP typical for QTc prolongation occurred at a much lower incidence. This may suggest that mechanisms other than QTc prolongation account for potentially lethal arrhythmias and cardiac arrests in these patients. Along these lines, it is of interest that patients with complications from QTc prolongation more commonly had CV risk factors and CVD, that is, either heart failure (3 patients), CAD (4 patients), atrial fibrillation (3 patients) or atrial flutter (3 patients).
Accordingly, patients with CV risk factors and CVD should be evaluated carefully before therapy initiation and should be monitored closely during TKI administration by serial ECGs. One difficulty is the chronicity of treatment, often over the years. In the current study, howeverm, we found that twothirds of QTc prolongations occurred in the earlier time period, that is, 1 to 6 months after the start of therapy. A commonly recommended surveillance scheme is an ECG at baseline, 2, 4, 8, 12 weeks and then every 3 months. Whether a universal screening recommendation applies to all patients remains to be seen.
Providers should be aware of potential symptoms of QTc prolongation and its complications including palpitations, shortness of breath, chest pain, dizziness, hypotension, light-headedness, syncope and seizure.22 One may want to highlight that 1 in 5 patients with QTc prolongation already had an abnormal QTc interval at baseline, underscoring the need for baseline ECGs in all patients undergoing TKI therapy (especially in those with highest QTc prolonging risk). The emergence of mobile ECGs may make these screening efforts easier in the future.23–25 Such techniques may furthermore allow for monitoring of declines in cardiac function as currently tested in the TACTIC trial.20,26
In addition to baseline screening for predisposing conditions and serial ECGs during therapy, providers should also always monitor for additional reversible factors that can prolong the QT interval. These include electrolyte disturbances (hypocalcemia, hypokalemia and hypomagnesemia) and the additional use of QTc prolonging drugs.27,28 Scoring systems including the RISQ-PATH score,29 the Tisdale et al risk score30 or the Mayo Clinic pro-QTc score31 could be in place to flag patients at risk of QTc prolongation based on reported QTc intervals and QTc interval changes. We believe that a QTc interval ≥500 milliseconds, or a QTc progression ≥60 milliseconds should prompt providers to decrease the dose or even stop TKI treatment in view of an increased risk for TdP, VT and SCD. Our findings showed that TKI administrations with daily doses below 400 mg for imatinib, 400 mg for pazopanib, 37.5 mg for sunitinib, 70 mg for dasatinib and 600 mg for nilotinib were significantly less likely to be associated with QTc prolongation. For this reason, we advise to maintain patients on the lowest effective treatment dose, and if not possible to be attentive, to consider alternatives and to maintain screening efforts. Importantly, not much deviation was seen with the two formulas most commonly used by ECG machines for automated reporting. The Fredericia formula is recommended though as considered more accurate with less overcorrection and undercorrection than the Bazett’s formula.18
Several limitations should be taken into consideration when interpreting our study. First, this was a retrospective cohort study in cancer patients treated with TKIs and QTcf interval measurements were not taken at the same time points for all patients. Second, since the data of exposure and outcome variables were collected before the initiation of the study, QTcf readings for some patients during TKI therapy were missed. Third, this was a single-arm study that only included patients who received TKI without comparing them with non-TKI control cancer patients. Fourth, patient selection bias cannot be excluded due to the retrospective nature of the study design. Fifth, possible effects from electrolyte disorders and other concomitant drugs on the QTc interval could not systematically studied. All of this being said, the current study is an important illustration of the landscape in clinical practice, reflecting what is being done and what is not in terms of vigilance towards a potentially lethal side effect of TKI therapy.
In summary, the current study shows that approximately 1 in 3 patients on TKI therapy in clinical practice experience QTc prolongation. Of these, nearly 20% were associated with high-grade QTc prolongations and 5% with life-threatening complications. Importantly, these toxicities were seen not only with drugs that carry a black box warning, but also with those usually considered safe and frequently used in clinical practice. These results call for vigilance among oncology, hematology and cardiology providers for all patients on TKI therapy, especially with BCR-ABL and VEGF inhibitors. QTc monitoring with serial ECGs should remain a universal standard and any factors that can contribute to QTc prolongation in these patients should be minimized. Taken current developments into account, mobile device-based ECGs may add to the screening and safety of cancer patients on TKI therapies in the future.
DATA ACCESSIBILITY
The data, analytic methods and study materials will be made available upon reasonable request and following approval by all authors.
Supplementary Material
What’s new?
While tyrosine kinase inhibitors (TKIs) are superior cancer therapies and increase the efficacy of classical chemotherapeutics, significant toxicities limit their use. Among these, QTc prolongation represents a major concern, though real-world clinical data are sparse. Here, among 618 cancer patients treated with TKIs at the Mayo Clinic, approximately one-third experienced QTc prolongation, and this was noted in particular with BCR-ABL and VEGF TKIs. Overall, high-grade QTc prolongations were observed in one-fifth of patients. About 5 percent of patients had life-threatening complications. These data highlight the importance of QTc interval monitoring and reduction of contributing factors during TKI therapy.
ACKNOWLEDGEMENT
Our study was supported by the National Cancer Institute (CA 233610), to J. H.
Funding information
National Cancer Institute, Grant/Award Number: CA 233610
Abbreviations:
- ALL
acute lymphocytic leukemia
- AML
acute myeloid leukemia
- BCR-ABL
breakpoint cluster region protein-Abelson murine leukemia
- BMI
body mass index
- BMT
bone marrow transplant
- CAD
coronary artery disease
- CHF
congestive heart failure
- CKD
chronic kidney disease
- CML
chronic myeloid leukemia
- DM
diabetes mellitus
- ECG
electrocardiogram
- GIST
gastrointestinal stromal tumor
- HTN
hypertension
- RCC
renal cell carcinoma
- SCD
sudden cardiac death
- TdP
Torsade de Pointes
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
- VT
ventricular tachycardia
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. [DOI] [PubMed] [Google Scholar]
- 3.Cohen P Protein kinases—the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Alvarez A, Garcia-Albeniz X, Esteve J, Rovira M, Bosch X. Cardiotoxicity of tyrosine-kinase-targeting drugs. Cardiovasc Hematol Agents Med Chem. 2010;8:11–21. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–439. [DOI] [PubMed] [Google Scholar]
- 6.Brown SA, Sandhu N, Herrmann J. Systems biology approaches to adverse drug effects: the example of cardio-oncology. Nat Rev Clin Oncol. 2015;12:718–731. [DOI] [PubMed] [Google Scholar]
- 7.Brown SA, Nhola L, Herrmann J. Cardiovascular toxicities of small molecule tyrosine kinase inhibitors: an opportunity for systems-based approaches. Clin Pharmacol Ther. 2017;101:65–80. [DOI] [PubMed] [Google Scholar]
- 8.Chen Ming H, Kerkelä R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. OncoTargets Ther. 2018;11:6227–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamore SD, Kohnken RA, Peters MF, Kolaja KL. Cardiovascular toxicity induced by kinase inhibitors: mechanisms and preclinical approaches. Chem Res Toxicol. 2020;33:125–136. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020. 10.1038/s41569-020-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann J Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020. 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Ramos LG. Drug-induced QT prolongation and Torsades de pointes. P & T. 2017;42:473–477. [PMC free article] [PubMed] [Google Scholar]
- 14.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25:3362–3371. [DOI] [PubMed] [Google Scholar]
- 15.Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013;36:295–316. [DOI] [PubMed] [Google Scholar]
- 16.Hunter JD, Sharma P, Rathi S. Long QT syndrome. BJA Educ. 2008;8: 67–70. [Google Scholar]
- 17.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. [DOI] [PubMed] [Google Scholar]
- 18.Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5:e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IBM Corp. Released. IBM SPSS Statistics for Windows VA. New York, NY: IBM Corp. In; 2017. [Google Scholar]
- 20.Porta-Sánchez A, Gilbert C, Spears D, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc. 2017;6:e007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid Leukemia. Curr Hematol Malig Rep. 2016;11:71–79. [DOI] [PubMed] [Google Scholar]
- 22.Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karacan M, Celik N, Gul EE, Akdeniz C, Tuzcu V. Validation of a smartphone-based electrocardiography in the screening of QT intervals in children. North Clin Istanb. 2019;6:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schram M, Shreibati JB, Bos M, et al. Prediction of the heart rate corrected QT interval (QTC) from a novel, MULTILEAD smartphone-enabled ECG using a DEEP neural network. J Am Coll Cardiol. 2019; 73:368. [Google Scholar]
- 25.Rotella JA, Taylor DM, Wong A, Greene SL. Accuracy of QT interval measurement on electrocardiographs displayed on electronic ‘smart’ devices. Emerg Med Australas. 2016;28:187–192. [DOI] [PubMed] [Google Scholar]
- 26.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat Med. 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 27.Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharmacol. 2017;39:16–25. [DOI] [PubMed] [Google Scholar]
- 28.Heemskerk CPM, Pereboom M, van Stralen K, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol. 2018;74:183–191. [DOI] [PubMed] [Google Scholar]
- 29.Vandael E, Vandenberk B, Vandenberghe J, Spriet I, Willems R, Foulon V. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharmacol. 2017;39:424–432. [DOI] [PubMed] [Google Scholar]
- 30.Tisdale James E, Jaynes Heather A, Kingery Joanna R, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods and study materials will be made available upon reasonable request and following approval by all authors.


