Abstract
Background:
Several chemotherapy agents are associated with the development of non-ischemic cardiomyopathy (NIC). When chemotherapy-induced cardiomyopathy (CHIC) is associated with left bundle branch block (LBBB) and a left ventricular ejection fraction (LVEF) 35% or lower, cardiac resynchronization therapy (CRT) is often utilized to improve cardiac function and relieve symptoms.
Objective:
To determine the echocardiographic and clinical outcomes of CRT in patients with CHIC.
Methods:
The study included 29 patients with CHIC (CHIC group) and 58 patients with other types of NIC (control group) who underwent CRT implantation between 2004 and 2017. The primary endpoints were changes in LVEF, left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD) at 6–18 months after CRT. The secondary outcomes included changes in left ventricular global longitudinal strain (GLS), systolic strain rate (SRS), early diastolic strain rate (SRE), and overall survival.
Results:
Out of 29 patients with CHIC, 62.1% received chemotherapy for lymphoma, 13.7% for breast cancer, and 24.1% for sarcoma. The agent implicated in 93.1% of the patients was an anthracycline. Half of the patients had LBBB. The mean baseline LVEF was 28% ± 8%. The mean baseline QRS duration was 146 ± 26 ms. Twenty-eight patients had post-CRT follow-up data. CRT was associated with improvement in echocardiographic outcomes in the CHIC group and the control group. There was no difference in overall survival between the two groups (log-rank p = .148).
Conclusion:
CRT improves left ventricular function and reverses remodeling in patients with CHIC.
Keywords: cardiac remodeling, cardiac resynchronization therapy, cardiomyopathy, chemotherapy, ejection fraction, strain
1 |. INTRODUCTION
With the therapeutic advances in oncology, cancer has been transformed into a chronic disease with an aging population of cancer survivors and patients living with malignancy.1 Several chemotherapy agents, especially anthracyclines, are associated with the development of non-ischemic cardiomyopathy (NIC).2 Around 10% of patients treated with doxorubicin or its derivatives develop cardiomyopathy up to 10 years after the cessation of chemotherapy.2 When NIC is associated with left bundle branch block (LBBB) and a left ventricular ejection fraction (LVEF) of 35% or lower, cardiac resynchronization therapy (CRT) is often utilized to improve cardiac function and relieve symptoms. A clear benefit from CRT in patients with chemotherapy-induced cardiomyopathy (CHIC) has not been well established. Patients with CHIC have not been well represented in previous CRT studies in the literature. Therefore, the aim of our study is to assess the clinical and echocardiographic outcomes of CRT in patients with CHIC.
2 |. METHODS
2.1 |. Study design and study population
This retrospective cohort study included patients with CHIC who underwent CRT at Mayo Clinic (Minnesota, Arizona, and Florida) between 2004 and 2017. The Mayo Clinic CRT Database was initially screened for adult patients with CHIC using the following ICD 9 and 10 codes: I42.7 (CHIC), V58.11/Z51.11 (encounter for antineoplastic therapy), V58.12/Z51.12 (encounter for immunotherapy), V58.1 (encounter for antineoplastic therapy and immunotherapy), V87.41/Z92.21 (personal history of antineoplastic therapy), V66.2 (convalescence following chemotherapy), and V67.2 (follow-up examination, following chemotherapy). The diagnosis of CHIC prior to CRT implantation was subsequently manually verified by the study authors (Drs. FME and ANS, N = 29). This cohort was, in 1:2 ratio, compared to 58 adult patients with other forms of NIC (control group) who underwent CRT implantation between 2004 and 2017. Controls were randomly chosen from the Mayo Clinic CRT database. Only patients with NIC were included in this study to minimize confounding from other concomitant causes of cardiomyopathy. All demographic, clinical, and echocardiographic data were manually collected from the electronic health records.
2.1.1 |. Inclusion criteria
Patients included in this study had (i) a LVEF ≤35% and a wide QRS complex ≥120 ms or (ii) a narrow QRS with LVEF <50% and an indication for permanent pacing.3 Patients were on optimal medical therapy for at least 3 months prior to CRT implantation.
2.1.2 |. Exclusion criteria
Patients with CHIC and other concomitant etiologies of cardiomyopathy were excluded from the study to minimize confounding.
2.2 |. Definitions of variables and data collection
In our study, CHIC was defined as a symptomatic drop of >5% or asymptomatic drop of >10% in LVEF compared to baseline to <55% at any follow-up time in patients who were previously exposed to chemotherapy agents after exclusion of other causes. An ischemic workup, including stress testing and/or cardiac catheterization, was used to establish a non-ischemic cause of cardiomyopathy.
LVEF was analyzed as a continuous variable and as a categorical variable (LVEF 35% or less vs. LVEF greater than 35%). Baseline characteristics analyzed as continuous variables included age at time of CRT, baseline QRS interval (in ms), left ventricular end systolic diameter (LVESD), left ventricular end diastolic diameter (LVEDD), left ventricular end systolic volume (LVESV), left ventricular end diastolic volume (LVEDV), right ventricular systolic pressure (RVSP), LV global longitudinal strain (GLS), LV systolic strain rate (SRS), and LV early diastolic strain rate (SRE). Baseline characteristics analyzed as categorical variables included sex, hypertension, diabetes mellitus, atrial fibrillation, presence of LBBB, use of beta blockers, use of angiotensin receptor blockers or angiotensin converting enzyme inhibitors, use of hydralazine, use of nitrates, use of spironolactone, use of diuretics (other than spironolactone), and use of digoxin.
Right ventricular enlargement, right ventricular systolic dysfunction, and mitral regurgitation were evaluated as numeric variables. Right ventricular enlargement and right ventricular dysfunction were graded as absent (= 0), mild (= 1), moderate (= 2), or severe (= 3). Mitral regurgitation was graded as absent (= 0), mild (= 1), mild-moderate (= 2), moderate (= 3), moderate-severe (= 4), or severe (= 5). Those numeric variables were treated as continuous variables in the statistical analysis to facilitate clear and meaningful reporting and comparison between the different groups.
2.3 |. Definitions of outcomes
The primary outcomes were changes in LVEF, LVESD, and LVEDD at a target time point of 6–18 months after CRT. The secondary outcomes included changes in LV GLS, SRS, and SRE, and overall survival.
2.4 |. Echocardiographic studies
Two-dimensional (2D) echocardiography was interpreted by a cardiologist before and after CRT. Offline 2D strain imaging analysis was performed using speckle-tracking method from stored transthoracic echocardiography images (DICOM) using TomTec (TomTec Imaging Systems GmbH, Unterschleissheim, Germany). LV GLS, SRS, and SRE measurements were performed in the apical four-, three-, and two-chamber views. The endocardial border was traced manually at end-systole. Tracking was adjusted to include the entire myocardial wall from the endocardium to the myoepicardial border. All LV GLS, SRS, and SRE measurements were performed by a single investigator (Dr. VJ), with all the images reviewed and validated by a second reader (Dr. HRV). LV myocardial GLS, SRS, and SRE were calculated from the averaged strain curves generated from 16 segments (six basal, six mid-, and four apical segments).
2.5 |. Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were reported as percentages and were compared using the chi-square test among different categories. Continuous variables were compared using the independent or Student’s t-test. The Wilcoxon signed-rank test was used to compare differences between repeated measurements of continuous variables before and after CRT implantation. Multivariate linear regression models were created for continuous outcomes including change in LVEF and change in LVESD. Multivariate logistic regression was used to create a model to compare improvement in LVEF (an increase of more than 5%) between the control and CHIC groups. Cox proportional-hazards model was used to study the association between overall survival time and multiple predictor variables that are clinically or statistically relevant. Statistically significant variables included use of diuretics (other than spironolactone) and use of digoxin. All p values were two-sided with level of significance < .05. Overall survival outcomes were also compared using Kaplan-Meier survival analysis. A p value < .05 was considered statistically significant for the log-rank test. Statistical analysis was done using JMP 14.1.0 from Statistical Analysis System (SAS).
2.6 |. Ethical considerations
This study was approved by the Institutional Review Board at Mayo Clinic. This study was not funded by an external source.
3 |. RESULTS
3.1 |. Baseline patient characteristics
The baseline characteristics of the overall study population are described in Table 1. The CHIC and the control group characteristics were comparable at baseline except for digoxin and diuretic use. Diuretic use was more common in the control group (81.0% vs. 58.6%, p .025); digoxin use was more common in the CHIC group (41.4% vs. 13.8%, p .004). Out of 29 patients with CHIC, 18 (62.1%) received chemotherapy for lymphoma, 4 (13.7%) for breast cancer, and 7 (24.1%) for other malignancies (Table 2). The agent implicated in 27 (93.1%) patients was an anthracycline. Twenty-six patients received doxorubicin (323 ± 72 mg/m2) and one patient received daunorubicin with a cumulative dose of 540 mg/m2. A total of 18 (62.1%) patients with CHIC had received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for treatment of lymphoma. A total of 10 (34.5%) patients received radiation therapy to the chest. In the group of patients with CHIC, 16 (55.2 %) patients had a LBBB, and 4 (13.8%) patients had ventricular pacing. Among the 16 patients with LBBB, 9 (56%) patients had LBBB prior to the onset of CHIC while 7 (44%) patients had progression from non-specific intraventricular conduction delay to LBBB after being exposed to chemotherapy. The mean baseline LVEF was 28% ± 8%. The mean baseline QRS duration was 146 ± 26 ms.
TABLE 1.
Baseline characteristics of the study subjects
| Variable | CHIC group (n = 29) | Control group (n = 58) | p |
|---|---|---|---|
| Female sex (n, %) | 16 (55.2) | 28 (48.3) | .54 |
| Age at the time of CRT implantation, mean (years) | 66.3 ± 13.8 | 67.4 ± 11.3 | .36 |
| QRS duration, mean (ms) | 146 ± 26 | 154 ± 26 | .11 |
| Baseline LVESD, mean (mm) | 52 ± 8 | 54 ± 10 | .13 |
| Baseline LVEDD, mean (mm) | 60 ± 8 | 63 ± 9 | .10 |
| Baseline LVEF, mean (%) | 28 ± 8 | 28 ± 7 | .54 |
| Type of conduction abnormality, LBBB (n,%) | 16 (55.2) | 25 (43.1) | .29 |
| Hypertension (n,%) | 10 (34.5) | 24 (41.4) | .53 |
| Diabetes mellitus (n,%) | 4 (13.8) | 12 (20.7) | .43 |
| Atrial fibrillation (n,%) | 11 (37.9) | 31 (53.5) | .17 |
| Beta-blocker use (n,%) | 27 (93.1) | 54 (93.1) | 1.00 |
| Angiotensin-converting enzyme inhibitor use (n, %) | 23 (79.3) | 41 (70.7) | .39 |
| Spironolactone use (n, %) | 7 (24.1) | 14 (24.1) | 1.00 |
| Nitrate use (n,%) | 2 (6.9) | 1 (1.7) | .21 |
| Hydralazine use (n, %) | 1 (3.5) | 1 (1.72) | .61 |
| Diuretic use (n,%) | 17 (58.6) | 47 (81.0) | .025 |
| Digoxin use (n,%) | 12 (41.4) | 8 (13.8) | .004 |
Abbreviations: CHIC, chemotherapy-induced cardiomyopathy; CRT, cardiac resynchronization therapy; LVESD, left ventricular end systolic diameter; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LBBB, left bundle branch block.
TABLE 2.
Type of cancer, type of chemotherapy and radiation use in patients with CHIC
| n (%) | ||
|---|---|---|
| Type of cancer | Lymphoma | 18 (62.1) |
| Breast cancer | 4 (13.8) | |
| Other | 7 (24.1) | |
| Type of chemotherapy | Anthracycline (doxorubicin, daunorubicin) | 27 (93.1) |
| CHOP | 18 (62.1) | |
| Cyclophosphamide | 23 (79.3) | |
| 5-fluorouracil | 2 (6.9) | |
| Vinca alkaloids (vincristine, vinblastine) | 21 (72.4) | |
| Radiation | Chest | 10 (34.5) |
| Other body areas | 7 (24.1) | |
3.2 |. Comparative primary outcomes of CRT
Pre-implantation and post-implantation echocardiograms were available for 28 of 29 patients in the CHIC group and in 58 patients in the control group. Follow-up echocardiography was performed at a mean of 10.7 ± 4.7 months after CRT implantation. The median followup time for survival was 6.1 years (72.8 months). At 6–18 months of follow-up, CHIC group had an increase in mean LVEF from 28% ± 8% to 38% ± 10% (p < .001). LVEDD decreased from 60 ± 8 to 56 ± 8 mm (p = .006) and LVESD decreased from 52 ± 8 mm to 45 ± 8 mm (p = .002) (Table 3). Volumetric echocardiographic dimensions before and after CRT were available in 12 patients in the CHIC group and 27 patients in the control group. LVEDV decreased from 206.8 ± 76.4 to 165.3 ± 52.7 mL (p = .065) and LVESV decreased from 148.4 ± 64.7 to 94.8 ± 38.5 mL (p = .109). These favorable echocardiographic outcomes in the CHIC group were comparable to that seen in the control group (Table 3). The mean change in LVEF after CRT was similar between the CHIC and control groups (10.2 vs. 10.3, p = .985). The proportion of patients whose LVEF increased by more than 5% was similar between the two groups (62.1% in the control group vs. 57.1% in the CHIC group, p = .662). A total of 48.2% of patients with CHIC had decrease in their LVESD by more than 15% as compared to 36.8% in the control group (p = .308). Sixty-seven percent of patients with CHIC had decrease in their LVESV by more than 15% as compared to 63% in the control group (p = .82).
TABLE 3.
Echocardiographic outcomes before and after cardiac resynchronization therapy in patients with chemotherapy-induced cardiomyopathy and patients with other types of non-ischemic cardiomyopathy
| Control (n = 58) | CHIC (n = 28) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre CRT | After CRT | Mean difference | p | Pre CRT | After CRT | Mean difference | p |
| LVEF, mean (%) | 28 ± 7 | 38 ± 13 | 10 | < .001 | 28 ± 8 | 38 ± 10 | 10 | < .001 |
| LVEDD, mean (mm) | 63 ± 9 | 61 ± 11 | −2 | .065 | 60 ± 8 | 56 ± 8 | −4 | .006 |
| LVESD, mean (mm) | 54 ± 10 | 49 ± 13 | −5 | < .001 | 52 ± 8 | 45 ± 8 | −7 | .002 |
| LVEDV, mean (mL) | 202.4 ± 91.9 | 162.4 ± 75.3 | −40 | <.0001 | 206.8 ± 76.4 | 165.3 ± 52.7 | −41.5 | .065 |
| LVESV, mean (mL) | 144.4 ± 82.6 | 105.7 ± 68.2 | −38.7 | <.0001 | 148.4 ± 64.7 | 94.8 ± 38.5 | −53.6 | .109 |
| RVSP, mean (mmHg) | 40 ± 13 | 37 ± 11 | −2 | .216 | 37 ± 15 | 37 ± 11 | −0.2 | .962 |
| RVE, mean | 0.69 ± 0.88 | 0.73 ± 0.70 | 0.07 | .324 | 0.72 ± 0.92 | 0.78 ± 0.93 | 0.04 | .746 |
| RV dysfunction, mean | 0.91 ± 0.98 | 0.65 ± 0.82 | −0.18 | .236 | 0.68 ± 0.94 | 0.78 ± 0.97 | 0.07 | .252 |
| MR, mean | 1.54 ± 1.40 | 1.18 ± 1.40 | −0.315 | .077 | 1.14 ± 1.09 | 0.82 ± 0.86 | −0.29 | .183 |
Abbreviations: CHIC, chemotherapy-induced cardiomyopathy; CRT, cardiac resynchronization therapy; LVESD, left ventricular end systolic diameter; LVEDD, left ventricular end diastolic diameter; LVESV, left ventricular end systolic volume; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure; RVE, right ventricular enlargement; RV, right ventricular; MR, mitral regurgitation.
In the CHIC group, patients with LBBB appeared to have a trend of greater improvement in LVEF and LV structural reverse remodeling, yet they did not reach statistical significance (Figure 1). When assessing for sex differences, the response to CRT was similar between men and women.
FIGURE 1.
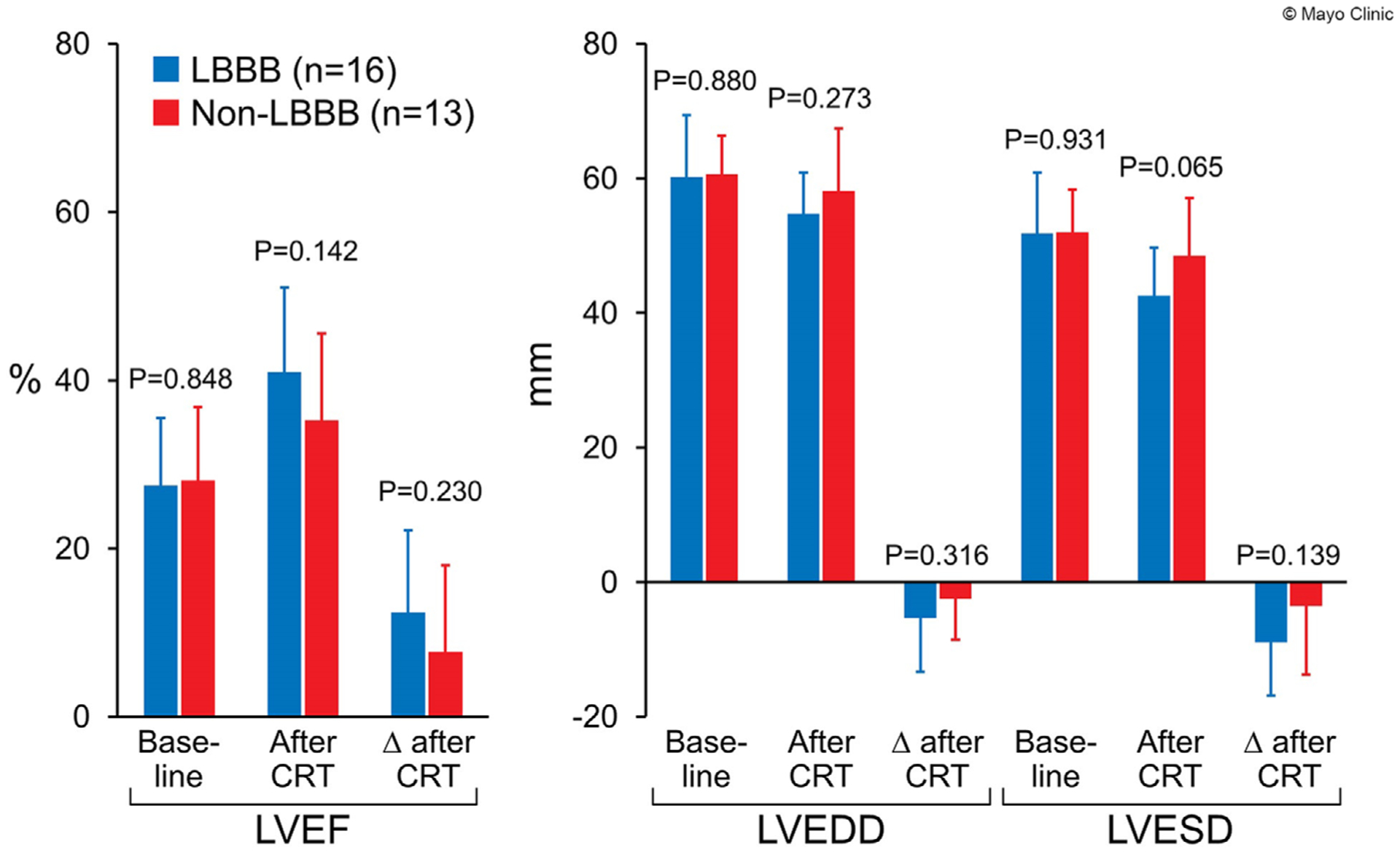
Comparison of echocardiographic outcomes between patients with left bundle branch block and non-left bundle branch block in patients with chemotherapy-induced cardiomyopathy. LVESD, left ventricular end systolic diameter; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LBBB, left bundle branch block
3.3 |. Left ventricular echocardiographic strain measurements
Transthoracic echocardiography images were available for LV strain measurements in 22 patients in the CHIC group and 44 patients in the control group. The average improvement in LVEF after CRT was similar between the CHIC and control groups (10.2% ± 10.2% vs. 10.3% ± 10.8%, p = .985) as was the proportion of patients whose LVEF increased by more than 5% was similar between the two groups (57.1% in the CHIC group vs. 62.1% in the control group, p = .662). No difference in the average change in GLS after CRT was noted between the two groups (−2.15 ± 4.32 vs. −3.57 ± 4.02, p = .19), even when confined to the CRT responders alone (−2.51 ± 1.13 vs. −5.16 ± 0.54, p = .12). CRT responders had a significant improvement in left ventricular GLS as compared to CRT non-responders in the control group, but not in the CHIC group (Table 4). In the control group, the average change in left ventricular GLS was −5.16 ± 0.54 in CRT responders as compared to 0.68 ± 0.88 in CRT non-responders (p < .0001). In the CHIC group, the average change in left ventricular GLS was −2.51 ± 1.13 in CRT responders as compared to −1.36 ± 1.66 in CRT non-responders (p = .57). The average changes in left ventricular myocardial systolic and early diastolic strain rates were similar between the CHIC and control groups. Mean SRS decreased by 0.13 ± 0.14 in the CHIC group and by 0.34 ± 1.28 in the control group (p = .45). Mean SRE increased by 0.06 ± 0.18 in the CHIC group and by 0.07 ± 0.22 in the control group (p = .86).
TABLE 4.
Echocardiographic strain outcomes before and after cardiac resynchronization therapy in patients with chemotherapy-induced cardiomyopathy and patients with other types of non-ischemic cardiomyopathy
| Variables | Baseline | Post-CRT | |||||
|---|---|---|---|---|---|---|---|
| Responders | Non responders | p | Responders | Non responders | p | ||
| CHIC | GLS | −11.45 (1.16) | −10.65 (1.69) | .70 | −14.45 (0.93) | −13.11 (1.32) | .41 |
| SRS | −0.44 (0.03) | −0.39 (0.05) | .47 | −0.59 (0.04) | −0.55 (0.06) | .60 | |
| SRE | 0.41 (0.04) | 0.40 (0.06) | .84 | 0.47 (0.04) | 0.48 (0.05) | .92 | |
| Control | GLS | −10.49 (0.52) | −9.61 (0.73) | .33 | −15.59 (0.60) | −8.11 (0.911) | <.0001 |
| SRS | −0.38 (0.02) | −0.38 (0.02) | .84 | −0.83 (0.20) | −0.34 (0.31) | .20 | |
| SRE | 0.39 (0.02) | 0.39 (0.03) | .97 | 0.51 (0.02) | 0.31 (0.04) | .0002 | |
Abbreviations: GLS, global longitudinal strain; SRS, systolic strain rate; SRE, early diastolic strain rate.
3.4 |. Survival outcomes
Overall unadjusted mortality was similar between the two groups at the end of follow-up (48.2% in the CHIC group vs. 34.5% in the control group, p = .214). The Cox proportional-hazards model analysis revealed that use of diuretics at baseline (HR = 1.58, p = .026) and CHIC (HR = 1.55, p = .028) were positive predictors of mortality. On Kaplan-Meier analysis, there was no statistically significant difference in overall survival with a median overall survival of 113.4 months (9.5 years) in the CHIC group, 62.5 months (5.2 years) in the control group, and 72.8 months (6.1 years) in the pooled population (log-rank p = .148 between the CHIC and control group) (Figure 2).
FIGURE 2.
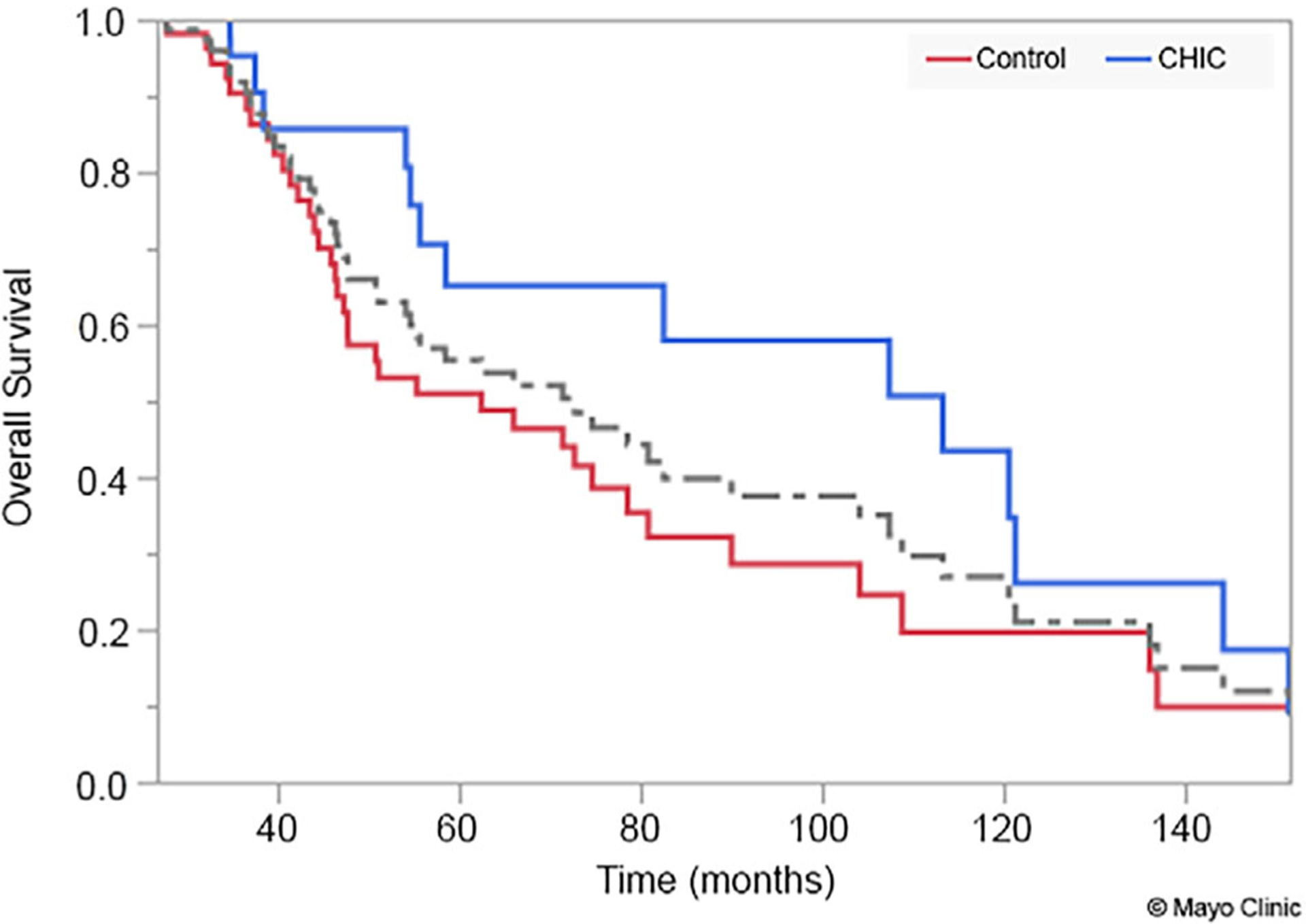
Kaplan-Meier curve demonstrating survival comparison between patients with the chemotherapy-induced cardiomyopathy (CHIC) and control groups
3.5 |. Predictors of CRT outcomes
By logistic regression analysis, older age (ORadjusted = 1.47, p = .034) was statistically significantly predictive of an improvement greater than 15% in LVESD. None of the baseline variables were predictive of an improvement greater than 5% in LVEF.
4 |. DISCUSSION
While many chemotherapy and immunotherapy agents are associated with cardiomyopathy, the anthracycline class is most commonly associated with the development of cardiomyopathy. This is reflected in our study where most patients were exposed to anthracyclines in the setting of lymphoma treatment. Anthracycline-induced cardiotoxicity is mainly due to free radical formation caused by doxorubicin metabolism.4 New onset LBBB has not been commonly reported in chemotherapy-related cardiac dysfunction. While 44% of patients with CHIC and LBBB in our study had progression from non-specific intraventricular conduction delay to LBBB after being exposed to chemotherapy, only a few cases in the literature have described new onset LBBB with cancer-directed therapy, particularly with trastuzumab and rituximab.5–8
Historically, anthracycline-induced cardiomyopathy seemed to be less responsive to conventional heart failure medical therapy as compared to other cardiomyopathies.9–11 This was attributed to the late detection of the disease which gets to an irreversible stage. In 2010, Cardinale et al. reported that early detection of anthracycline-induced cardiomyopathy and initiation of medical therapy was crucial to improve left ventricular function and clinical outcomes.12 Interestingly, our study showed that patients who did not respond or had an insufficient response to medical therapy were still able to reverse cardiac remodeling with CRT implantation.
The positive effects of CRT on cardiac remodeling in patients with CHIC were first reported by Jones et al. in a 9-years-old girl with acute doxorubicin-induced cardiomyopathy who had noticeable improvement with CRT.13 Ajijola et al. demonstrated similar results in a group of four patients with doxorubicin-induced cardiomyopathy.14 A few years later, Rickard et al. published their experience with CRT in 18 patients with doxorubicin-induced cardiomyopathy.15 They also showed that patients with CHIC derived clinical and echocardiographic benefits from CRT with significant improvement in LVEF, left ventricular end-diastolic and end-systolic diameters, mitral regurgitation, and New York Heart Association functional class.15
The MADIT-CHIC (Multicenter Automatic Defibrillator Implantation Trial–Chemotherapy-Induced Cardiomyopathy) study, an uncontrolled prospective cohort study, was recently published.16 It included 30 patients with CHIC from 12 centers with cardio-oncology programs. Similar to our study, it showed that CRT was associated with an improvement in LVEF and left ventricular dimensions at 6 months.
CRT response is defined as an increase in LVEF by 5% or more at 6 months after CRT implantation.17,18 Given that one-third of patients do not respond to CRT,19 appropriate patient selection has been crucial to determine who will benefit the most from this treatment. Previous studies showed that predictors of CRT response included age at the time of CRT implantation,20 vectorcardiography,20 echocardiographic dyssynchrony markers such as interventricular mechanical delay and apical rocking,20 non-ischemic etiologies of heart failure,21 and female sex.22 In this study, there was not a difference in CRT response between men and women. Furthermore, none of the baseline variables were predictive of an improvement greater than 5% in LVEF.
2D speckle-tracking echocardiography has been used to assess left ventricular dyssynchrony which may potentially predict response to CRT. This was first largely studied in the PROSPECT trial which failed to identify any echocardiographic parameter of dyssynchrony that can reliably predict CRT response.23 Subsequent studies showed that longitudinal strain can predict response to CRT.24,25 Our study showed that patients with NIC, including patients with CHIC, had improvement in left ventricular GLS after CRT. When compared to CRT non-responders, CRT responders had a significant improvement in left ventricular GLS in patients with NIC other than CHIC.
We believe that patients with CHIC meeting criteria for CRT derive benefit from CRT and the primary benefit is the reversal of mechanical dyssynchrony and cardiac remodeling over years. Despite its retrospective design, our study follows patients for a relatively longer period of time. Our data suggests that, despite the lack of data from randomized controlled studies, CHIC is reversible, even in its late phases, and CRT seems to offer both echocardiographic and clinical benefits.
The discrepancy in the predictive value of CHIC for mortality, when the Kaplan-Meier survival analysis or the Cox proportional hazards method is used, is partly explained by the nature of those statistical tests. Kaplan-Meier survival analysis cannot use multiple predictors. Cox regression, a semi-parametric procedure, can include both continuous and binary predictors. Our Kaplan-Meier analysis showed a prolongation of about 4.3 years in the CHIC group when compared with the control group. Although this result was not statistically significant by the log-rank test, this trend may be confirmed in future studies with larger sample sizes. Based on our different analyses, we cannot make a firm and definitive conclusion regarding the association between CHIC (vs. other types of NIC) and mortality after CRT.
4.1 |. Study limitations
The limitations of our study include the retrospective observational study design and the small number of patients. While every patient in our cohort had an ischemic evaluation, not every patient underwent MRI to further characterize their NIC. Therefore, although the diagnosis of CHIC was made by cardiology specialists after a negative ischemic evaluation, the definition of the term remains subject to provider subjectivity. Furthermore, additional echocardiographic data to substantiate mechanical dyssynchrony was not available. Another limitation for our study includes the lack of a control group which precludes a comparison of the CRT with medical therapy.
5 |. CONCLUSION
CRT improves left ventricular function and reverses remodeling in patients with CHIC. It should not be withheld in cancer patients who meet criteria for CRT implantation. Larger randomized trials are needed to validate our clinical observations.
Footnotes
CONFLICTS OF INTEREST
All authors have no relevant conflict of interest to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [FE], upon reasonable request.
REFERENCES
- 1.Cronin K, Lake AJ, Scott S, et al. Annual report to the Nation on the status of cancer, part I: National cancer statistics. Cancer. 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. [DOI] [PubMed] [Google Scholar]
- 3.Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. [DOI] [PubMed] [Google Scholar]
- 4.Volkova M Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro KB, Miranda CH, Andrade JM, et al. Trastuzumab-induced myocardiotoxicity mimicking acute coronary syndrome. Case Rep Oncol. 2012;5:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu CM, Chu KM, Yang SP, Cheng SM, Wang WB. Trastuzumab (Herceptin)-associated cardiomyopathy presented as new onset of complete left bundle-branch block mimicking acute coronary syndrome: a case report and literature review. Am J Emerg Med. 2009;27:903.e1–3. [DOI] [PubMed] [Google Scholar]
- 7.Tahir H, Bardia N, Bath K, et al. Trastuzumab-induced cardiomyopathy and intermittent left bundle branch block. Cardiol Res. 2019;10:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheikh M, Moza A, Grubb BP. Rituximab induced left bundle branch block. Heart Views. 2015;16:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabet JY, Meurin P, Ben Driss A, et al. Beta-blockade intolerance in anthracycline-induced cardiomyopathy. Int J Cardiol. 2006;106:132–134. [DOI] [PubMed] [Google Scholar]
- 10.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–828. [DOI] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–4522. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 13.Jones BO, Davis AM, Alison J, Weintraub RG, Butt W, Cheung MM. Cardiac re-synchronization therapy in a child with severe anthracycline-induced congestive heart failure and normal QRS duration. J Heart Lung Transplant. 2007;26:1333–1335. [DOI] [PubMed] [Google Scholar]
- 14.Ajijola OA, Nandigam KV, Chabner BA, et al. Usefulness of cardiac resynchronization therapy in the management of doxorubicin-induced cardiomyopathy. Am J Cardiol. 2008;101:1371–1372. [DOI] [PubMed] [Google Scholar]
- 15.Rickard J, Kumbhani DJ, Baranowski B, Martin DO, Tang WH, Wilkoff BL. Usefulness of cardiac resynchronization therapy in patients with adriamycin-induced cardiomyopathy. Am J Cardiol. 2010;105:522–526. [DOI] [PubMed] [Google Scholar]
- 16.Singh JP, Solomon SD, Fradley MG, et al. Association of cardiac resynchronization therapy with change in left ventricular ejection fraction in patients with chemotherapy-induced cardiomyopathy. JAMA. 2019;322:1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitzalis MV, Iacoviello M, Romito R, et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol. 2005;45:65–69. [DOI] [PubMed] [Google Scholar]
- 18.Cintron G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI17–23. [PubMed] [Google Scholar]
- 19.Chairs TF, Daubert J-C, Saxon L, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management: a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society; and in collaboration with the Heart Failure Society of America (HFSA), the American Society of Echocardiography (ASE), the American Heart Association (AHA), the European Association of Echocardiography (EAE) of the ESC and the Heart Failure Association of the ESC (HFA). Europace. 2012;14:1236–1286. [DOI] [PubMed] [Google Scholar]
- 20.Maass AH, Vernooy K, Wijers SC, et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace. 2018;20:e1. [DOI] [PubMed] [Google Scholar]
- 21.Mangiavacchi M, Gasparini M, Faletra F, et al. Clinical predictors of marked improvement in left ventricular performance after cardiac resynchronization therapy in patients with chronic heart failure. Am Heart J. 2006;151:477. e1–.e6. [DOI] [PubMed] [Google Scholar]
- 22.Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm. 2012;9:S3–S13. [DOI] [PubMed] [Google Scholar]
- 23.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 24.D’Andrea A, Caso P, Scarafile R, et al. Effects of global longitudinal strain and total scar burden on response to cardiac resynchronization therapy in patients with ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2009;11:58–67. [DOI] [PubMed] [Google Scholar]
- 25.Ghani A, Delnoy PPH, Adiyaman A, et al. Response to cardiac resynchronization therapy as assessed by time-based speckle tracking imaging. Pacing Clin Electrophysiol. 2015;38:455–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [FE], upon reasonable request.


