Abstract
Background
Ibrutinib therapy is associated with an increased risk of atrial fibrillation (AF) in chronic lymphocytic leukemia (CLL). Risk assessment tools and outcomes of AF in these patients are not well described.
Methods
We performed a retrospective review of patients with CLL treated with ibrutinib at Mayo Clinic between October 2012 and November 2018.
Results
Two hundred ninety-eight patients were identified with a median time on ibrutinib of 19 months (range 0.23–69.7 months). Fifty-one patients developed treatment-emergent AF; the risk of treatment-emergent AF at 6 months, 1 year, and 2 years was 9%, 12%, and 16%, respectively. The following were associated with an increased risk of treatment-emergent AF on multivariable analyses: past history of AF (hazard ratio [HR] 3.5, p = 0.0072) and heart failure (HR 3.4, p = 0.0028). Most patients are able to continue ibrutinib therapy (dose reduced in 43%). Development of treatment-emergent AF was associated with shorter event-free survival (EFS; HR 2.0, p = 0.02) and shorter overall survival (OS; HR 3.2, p = 0.001), after adjusting for age, prior treatment status, TP53 disruption, heart failure, valvular disease, and past history of AF.
Conclusions
Patient comorbidities, rather than CLL-related factors, predict risk of treatment-emergent AF in patients treated with ibrutinib. Although the vast majority of patients with treatment-emergent AF are able to continue ibrutinib (with dose reduction in 43%), treatment-emergent AF appears to be associated with worse outcomes, independent of other adverse prognostic factors.
Keywords: Chronic lymphocytic leukemia, Small lymphocytic lymphoma, Ibrutinib, Atrial fibrillation
Background
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is the most prevalent lymphoid malignancy in the USA, with approximately 140,000 people living with the disease [1]. Therapy with ibrutinib, a Bruton tyrosine kinase inhibitor (BTK), has revolutionized the management of CLL, with unprecedented progression-free survival (PFS) and overall survival (OS), even among patients with high-risk disease features such as unmutated immunoglobulin heavy chain (IGHV) genes and del17p/TP53 mutation [2–4]. Despite these impressive outcomes, few patients achieve deep remission status (i.e., complete response or negative minimal residual disease) on single-agent ibrutinib, necessitating continuous therapy. Long-term treatment with ibrutinib is associated with an increased risk of developing cardiovascular complications, particularly atrial fibrillation (AF) [5]. In the pivotal phase 3 trials that led to approval of ibrutinib, approximately 5–9% of patients developed AF [6] compared with 0.5–2.4% in the comparator arms [7–9]. Additionally, the incidence of treatment-emergent AF among patients treated with ibrutinib increases over time, with up to 10% of patients treated developing the condition in longer-term follow-up [10–12].
Although a number of studies have proposed risk models [6, 13–15] to predict for AF occurring in CLL patients who receive ibrutinib, these risk prediction models have not been compared with each other nor has one emerged as a gold standard. Additionally, the optimal management of CLL patients who develop AF on ibrutinib therapy remains unclear, particularly with respect to continuation of ibrutinib, with some groups recommending to discontinue ibrutinib, whereas others suggest continuing ibrutinib [16–21]. Finally, the risk of bleeding with concomitant use of anticoagulation therapy and ongoing ibrutinib use remains a significant concern, since there is limited data on the latter combination in CLL patients [22].
In the present study, we studied in detail the risk factors for developing AF in a clinical cohort of CLL patients treated with ibrutinib at Mayo Clinic and determine the optimal risk profiling for these patients prior to initiating ibrutinib treatment by comparing available risk prediction models to each other. Additionally, we describe treatment decisions related to ibrutinib and outcomes of patients who developed AF and their subsequent clinical outcomes with a particular focus on concomitant anticoagulation or antiplatelet therapy and the risk of bleeding.
Methods
Study subjects
The Mayo Clinic CLL Database includes patients with CLL seen at Mayo Clinic since 1995 who provide written consent for their medical records to be used for research [23–25]. We identified all patients with CLL treated with ibrutinib between October 30, 2012, and November 1, 2018. This study was conducted with approval by the Mayo Clinic Institutional Review Board. Sociodemographic and clinical data extracted from the medical record and the CLL Database included age, race, sex, prior CLL therapy, Rai stage, IGHV mutation status, chromosomal aberrations detected by CLL fluorescence in situ hybridization (FISH), TP53 mutation status, and CD49d and CD38 expression. Treatment data, including start and end date of ibrutinib therapy, and dosing of ibrutinib, was extracted by manual review of the electronic health record. Patients who had an electrocardiogram (EKG) and/or an echocardiogram within 36 months of ibrutinib start were also identified through chart review, and their P-R interval and left atrial size was recorded from their most recent EKG and echocardiogram prior to ibrutinib start, respectively. Heart failure was defined as a clinical diagnosis of heart failure recorded by the patient’s primary physician or cardiologist. Valvular heart disease was defined as either abnormal echocardiography findings, past history of valve replacement, or clinical diagnosis of valvular heart disease in the setting of abnormal findings on physical examination.
We identified all patients in the study who developed AF using the electronic medical record. Patients with AF were stratified into three different groups: (a) Persistent AF: patients who were in AF at the commencement of ibrutinib therapy (these patients were not included in the risk factor models); (b) recurrent AF: patients with history of AF but in sinus rhythm at ibrutinib start and who developed AF while on ibrutinib; and (c) incident AF: patients who developed AF on ibrutinib therapy with no previous history of AF. Treatment-emergent AF was defined as all patients who had incident AF and/or recurrent AF. Risk stratification for AF was calculated for all patients using three different methods: (a) Framingham Heart Study (FHS) AF score [23]; (b) Mayo CLL AF risk score [14]; and (c) Italian AF risk score [15]; details on these methods and scores are included in Table 4. The data required to compute these risk estimator tools was obtained by review of the patient’s medical records at the time of ibrutinib start. For patients who did not have EKG data available, the P-R interval was assumed to be 160 milliseconds, the mid-point of the normal range, for the FHS risk score calculator.
Table 4.
Comparison of treatment-emergent AF risk prediction models (n = 290)
| Model | N (%) | Hazard ratio (95% CI) | p value |
|---|---|---|---|
| Framingham risk* | |||
| < 10% | 163 (56) | Reference | |
| 10–20% | 75 (26) | 2.2 (1.1–4.3) | 0.02 |
| > 20% | 52 (18) | 3.4 (1.7–6.5) | 0.0003 |
| Mayo CLL AF Score** | |||
| 0–1 | 86 (30) | Reference | |
| 2–3 | 90 (31) | 1.9 (0.7–5.1) | 0.20 |
| 4 | 64 (22) | 2.9 (1.1–7.7) | 0.03 |
| 5–7 | 50 (17) | 6.4 (2.6–16.0) | < 0.0001 |
| Italian AF risk score*** | |||
| 0 | 18 (6) | Reference | |
| 1–2 | 176 (61) | 1.7 (0.2–13.0) | 0.64 |
| 3–4 | 59 (20) | 5.2 (0.7–41.0) | 0.12 |
| > = 5 | 37 (13) | 10.8 (1.4–85.3) | 0.02 |
Italicized table entries indicate P-value < 0.05
Predictive components are age, sex, BMI, systolic blood pressure, treatment for hypertension, PR interval, significant murmur, and prevalent heart failure
Calculated as age > 75.3 points, > 65.2 points, male sex 1 point, HTN 1 point, valve disease 2 points. The scores are then categorized into four risk groups: 0–1; 2–3; 4; 5–7
Calculated as age > 65.1 point, male gender 1 point, valve disease 2 points, cardiomyopathy 3 points, hyperthyroidism 1 point, chronic lung disease 1 point, DM 1 point, severe infections 1 point
For each occurrence of AF, the severity of the AF event was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v 5.0 [26]; the date of diagnosis of AF and treatments (including modification to the ibrutinib treatment regimen) were abstracted. CHA2DS2-VASc score, a stratification for the risk of stroke in patients with AF, was computed from information in the medical record [27] at the time of diagnosis of AF. Anticoagulation and antiplatelet therapy data was abstracted, including the type of anticoagulation and/or antiplatelet therapy used and duration of anticoagulant/antiplatelet therapy. Bleeding complications were classified according to the International Society on Thrombosis and Haemostasis (ISTH) criteria. Major bleeding was defined as fatal bleeding, symptomatic bleeding in a critical organ (e.g., intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular), or bleeding that resulted in a hemoglobin drop of at least 2 g/dL or that required at least 2 units of blood [28]. Clinically relevant non-major bleeding was defined as bleeding that did not meet the major bleeding definition above but required [1] medical intervention by a health care professional, [2] hospitalization, or [3] face-to-face evaluation [29].
Statistical analysis
Treatment-emergent AF was defined as new or recurrent AF after initiation of ibrutinib treatment. AF at any time was defined as a time-dependent variable indicating presence of AF at any time, including prior to initiation of ibrutinib treatment. Chi-square and Fisher’s exact tests were used to compare discrete variables. The Kruskal-Wallis test was used to compare continuous variables. Event-free survival (EFS) was defined as the time from initiation of ibrutinib therapy to disease progression, initiation of next line of therapy, or death due to any cause. Overall survival (OS) was defined as the time interval between start of ibrutinib therapy and the date of death (regardless of cause of death). The Fine-Gray method was used to evaluate competing risks, specifically competing risk of death in the time to treatment-emergent AF models. Kaplan-Meier plots were generated to display EFS and OS. Multivariable Cox proportional hazards regression analysis was used to determine factors which predicted development of treatment-emergent AF, both overall and also in the subset without persistent AF, and to determine factors that predicted shorter EFS and OS. AF at any time was considered as a time-dependent variable for the EFS and OS analyses. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R 3.4.2.
Results
Patient cohort
A total of 298 patients contributed 565 years of ibrutinib exposure with a median time on ibrutinib of 19 months (range 0.2–69.7). The median age at ibrutinib initiation was 68 years (range 35–93), 210 (70.5%) were males, and 208 (69.7%) received ibrutinib for relapsed/refractory CLL (median number of prior lines of therapy = 1, range 0–15). Table 1 shows the baseline characteristics of all patients at the time of the initiation of ibrutinib. Eight (2.7%) patients were in persistent AF at the start of ibrutinib therapy. Of the remaining 290 patients, 21 (7%) patients had a prior history of AF (but were in normal sinus rhythm at the onset of ibrutinib therapy).
Table 1.
Baseline demographics of all patients at the start of ibrutinib therapy
| No AF at ibrutinib start (N = 290) | Persistent AF at ibrutinib start (N = 8) | Total (N = 298) | |
|---|---|---|---|
| Age | |||
| Median (range) | 68 (35–93) | 76 (66–84) | 68 (35–93) |
| Males | 203 (70.0%) | 7 (87.5%) | 210 (70.4%) |
| Rai stage | |||
| Missing | 28 | 0 | 28 |
| Rai 0 | 37 (14.1%) | 0 (0.0%) | 37 (13.7%) |
| Rai 1 or 2 | 91 (34.7%) | 1 (12.5%) | 92 (34.1%) |
| Rai 3 or 4 | 134 (51.1%) | 7 (87.5%) | 141 (52.2%) |
| FISH | |||
| Missing | 9 | 0 | 9 |
| Negative | 44 (15.7%) | 1 (12.5%) | 45 (15.6%) |
| Other* | 3 (1.1%) | 1 (12.5%) | 4 (1.4%) |
| 13q- | 86 (30.6%) | 1 (12.5%) | 87 (30.1%) |
| trisomy 12 | 45 (16.0%) | 2 (25.0%) | 47 (16.3%) |
| 11q- | 59 (21.0%) | 2 (25.0%) | 61 (21.1%) |
| 17p- | 44 (15.7%) | 1 (12.5%) | 45 (15.6%) |
| IGHV mutation | |||
| Missing | 38 | 2 | 40 |
| Unmutated | 193 (76.6%) | 2 (33.3%) | 195 (75.6%) |
| Prior therapies | |||
| Median (range) | 1 (0–15) | 2 (0–4) | 1 (0–15) |
| BMIa | |||
| Median (range) | 26.6 (17.5–50.8) | 27.2 (21.2–38.5) | 26.6 (17.5–50.8) |
| Prior history of AF | 21 (7.2%) | 8 (100%) | 29 (9.7%) |
Body mass index
Other FISH results include del17p centromere, IGH/BCL3 translocation, and del6q-
Overall, 51 patients developed treatment-emergent AF (39 incident AF and 12 recurrent AF). The estimated cumulative incidence of treatment-emergent AF at 6 months, 1 year, and 2 years was 9% (95% CI 6–13), 12% (95% CI 9–17), and 16% (95% CI 12–21), respectively (Fig. 1). The estimated cumulative incidence between time to AF was notably different between the incident AF and recurrent AF groups with 1-year, 2-year, and 3-year estimated cumulative incidence of 9% (95% CI 6–14), 13% (95% CI 10–19), and 16% (95% CI 12–22) for the incident AF group compared with 43% (95% CI 26–71), 48% (95% CI 30–76), and 56% (95% CI 36–86) for the recurrent AF group. Of note, 9/21 (42.9%) patients with a history of AF did not have a recurrence of AF while on ibrutinib therapy (median time on ibrutinib = 23.3 months [range 9.2–66.6 months]).
Fig. 1.
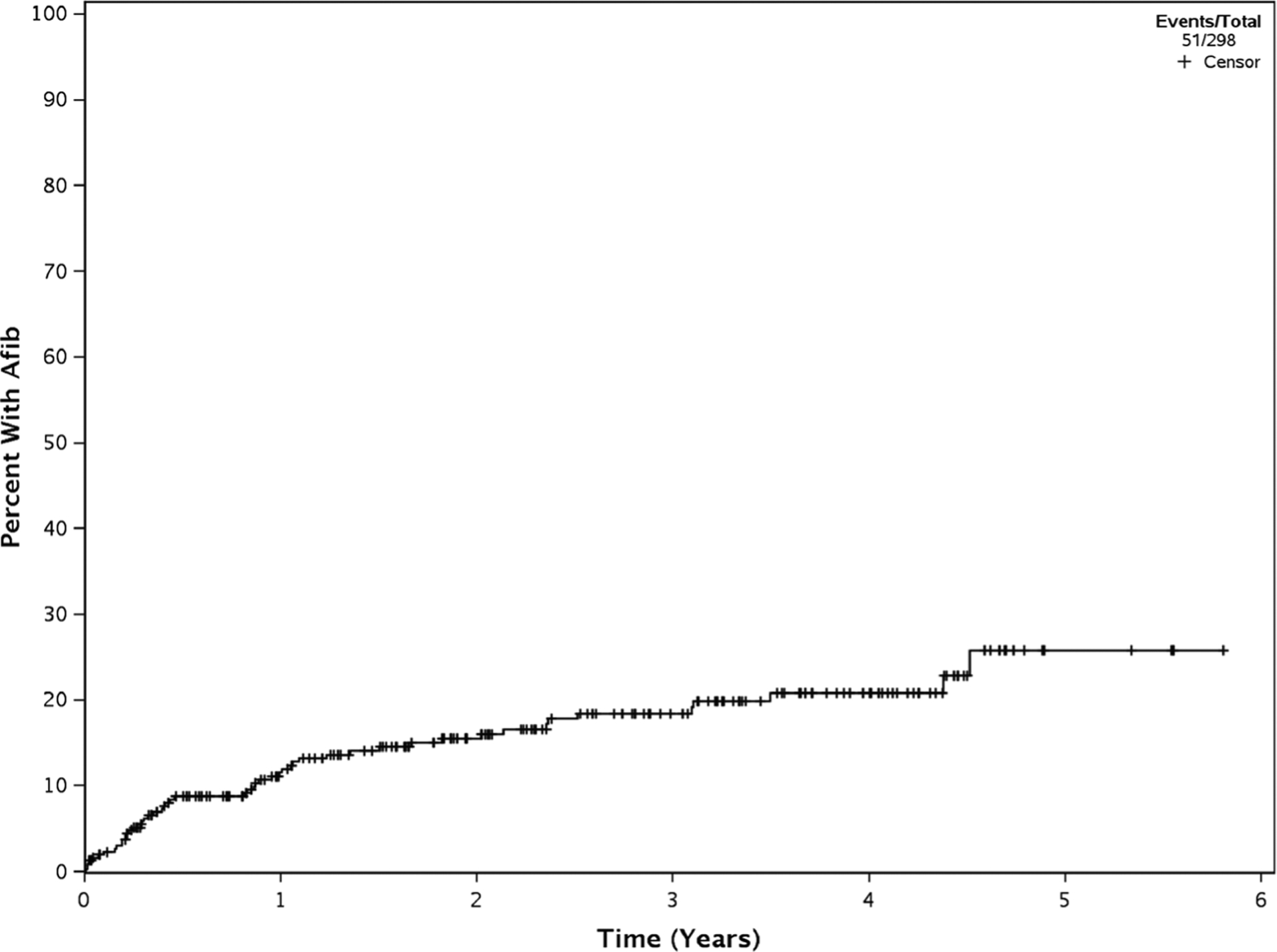
Cumulative incidence of treatment-emergent atrial fibrillation
Risk factors for developing AF
Table 2 presents risk factors for the development of treatment-emergent AF. On univariable analysis, the following risk factors were statistically significant for developing AF: history of AF (HR 4.8, 95% CI 2.5–9.4, p < 0.0001), heart failure (HR 4.8, 95% CI 2.6–8.9, p < 0.0001), valvular heart disease (HR 3.4, 95% CI 2.0–5.9, p < 0.0001), age ≥ 65 years (HR 2.5, 95% CI 1.3–5.1, p = 0.008), sleep apnea (HR 2.1, 95% CI 1.02–4.4, p = 0.04), and hypertension (HR 1.8, 95% CI 1.003–3.1, p = 0.049). Echocardiography was performed within 3 years of ibrutinib start in 53 (18.3%) patients. Left atrial volume index and ejection fraction were not associated with the development of AF on univariable analysis. Electrocardiography was performed within 3 years of ibrutinib start in 69 (23.8%) patients. P-R interval was not associated with the development of AF on univariable analysis. None of the CLL disease-related characteristics was found to be associated with the development of AF. Multivariable analysis identified prior history of AF (3.5, 95% CI 1.4–8.7, p = 0.0072) and heart failure (HR 3.4 95% CI 1.5–7.7, p = 0.0028) as independent risk predictors of developing treatment-emergent AF (Table 3). After excluding 21 patients with a prior history of AF from analysis, age ≥ 65 years, heart failure, and valvular heart disease approached statistical significance in their ability to predict treatment-emergent AF (Table 3).
Table 2.
Univariable analyses of risk factors for treatment-emergent AF among patients who did not have AF at ibrutinib start (n = 290)
| Patients (N = 290) | HR (95% CI) | p value | |
|---|---|---|---|
| Age | |||
| ≥ 65 | 180 (62.0%) | 2.5 (1.3–5.1) | 0.008 |
| Age as continuous variable (for each year older) | 1.06 (1.03–1.09) | < 0.0001 | |
| Sex | |||
| Male | 203 (70.0%) | 1.3 (0.7–2.4) | 0.49 |
| Rai | |||
| Rai 0 | 37 (14.1%) | ||
| Rai 1 or 2 | 91 (34.7%) | 0.8 (0.3–2.0) | 0.69 |
| Rai 3 or 4 | 134 (51.1%) | 0.8 (0.3–1.8) | 0.59 |
| FISH | |||
| Normal/13q/12+ | 175 (60.3%) | ||
| 11q/17p | 103 (35.5%) | 0.7 (0.4–1.3) | 0.28 |
| Missing | 12 (4.1%) | ||
| IGHV mutation | |||
| Mutated | 59 (23.4%) | 1.2 (0.6–2.5) | 0.62 |
| Prior therapies | |||
| No prior | 89 (30.7%) | ||
| One prior | 66 (22.8%) | 1.2 (0.5–2.5) | 0.71 |
| Two or more prior | 135 (46.6%) | 0.9 (0.5–1.8) | 0.84 |
| BMI | |||
| ≥ 30 | 78 (26.9%) | 1.3 (0.7–2.3) | 0.39 |
| TP53 disruption | |||
| Normal | 227 (80.5%) | ||
| 17p- or abnormal | 55 (19.5%) | 0.9 (0.4–1.8) | 0.76 |
| Hypertension | |||
| Yes | 134 (46.2%) | 1.8 (1.003–3.1) | 0.049 |
| Diabetes mellitus | |||
| Yes | 41 (14.1%) | 1.2 (0.5–2.6) | 0.73 |
| Myocardial infarction | |||
| Yes | 32 (11.0%) | 1.3 (0.6–2.8) | 0.56 |
| Coronary artery disease | |||
| Yes | 64 (22.1%) | 1.4 (0.8–2.6) | 0.24 |
| Heart failure | |||
| Yes | 29 (10.0%) | 4.8 (2.6–8.9) | < 0.0001 |
| Obstructive sleep apnea | |||
| Yes | 31 (10.7%) | 2.1 (1.02–4.4) | 0.04 |
| Valvular heart disease | |||
| Yes | 46 (15.9%) | 3.4 (2.0–5.9) | < 0.0001 |
| Cardiac murmur | |||
| Yes | 47 (16.2%) | 3.0 (1.7–5.3) | 0.0001 |
| Coronary artery bypass graft | |||
| Yes | 11 (3.8%) | 1.01 (0.2–4.1) | 0.99 |
| TIAc or stroke | |||
| Yes | 11 (3.8%) | 1.1 (0.2–4.6) | 0.94 |
| History of AF | |||
| Yes | 21 (7.2%) | 4.8 (2.5–9.4) | < 0.0001 |
| Chronic lung disease | |||
| Yes | 37 (12.8%) | 1.5 (0.7–3.0) | 0.29 |
| Severe infections | |||
| Yesa | 26 (9.0%) | 1.8 (0.8–3.8) | 0.15 |
| Baseline systolic BP (mmHg) | |||
| N | 287 | ||
| Median (range) | 123 (89182) | 0.99 (0.971.01) | 0.20 |
| Baseline PR interval (ms) | |||
| Nd | 69 | ||
| Median (range) | 160 (112–236) | 1.004 (0.99–1.02) | 0.53 |
| Enlarged atrial size (≥ 29 LA volume index) on echo | |||
| Nd | 53 | ||
| Yes | 36 (67.9%) | 1.0 (0.4–2.8) | 0.94 |
Italicized table entries indicate P-value < 0.05
Severe infections were classified as infections before ibrutinib therapy began which required hospitalization or parenteral antibiotics
There were two patients with hyperthyroidism. 1 patient with hyperthyroidism developed AF
Transient ischemic attack
Not available for all patients
Table 3.
Multivariable analyses of time to AF in patients with no AF at ibrutinib start (n = 290) and in the subset with no prior history of AF (n = 269)
| Parameter | At risk for AF (n = 290) | No prior history of AF (n = 269) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age > = 65 | 1.79 (0.84–3.83) | 0.13 | 2.20 (0.90–5.39) | 0.08 |
| Hypertension | 1.04 (0.53–2.03) | 0.92 | 1.15 (0.49–2.70) | 0.74 |
| Heart failure | 3.42 (1.53–7.66) | 0.003 | 2.51 (0.98–6.42) | 0.06 |
| Valvular heart disease | 1.36 (0.54–3.40) | 0.51 | 2.48 (0.95–6.46) | 0.06 |
| History of AF | 3.48 (1.42–8.66) | 0.007 | ||
Italicized table entries indicate P-value < 0.05
Comparison of models to predict risk of treatment-emergent AF
Table 4 presents a comparison of the AF risk prediction tools and the distribution of risk groups in our cohort, respectively. Figures 2 a, b, and c show the time to treatment-emergent AF according to the Framingham, Mayo CLL, and Italian AF risk scores, respectively. Based on lower Akaike information criteria (AIC), the Italian score (AIC = 513) was best able to predict risk of treatment-emergent AF versus the Mayo CLL risk score (AIC = 524) and the Framingham risk score (AIC = 530). However, each prediction tool performed well in our cohort of patients as there were clear separations in time to AF by each risk group.
Fig. 2.
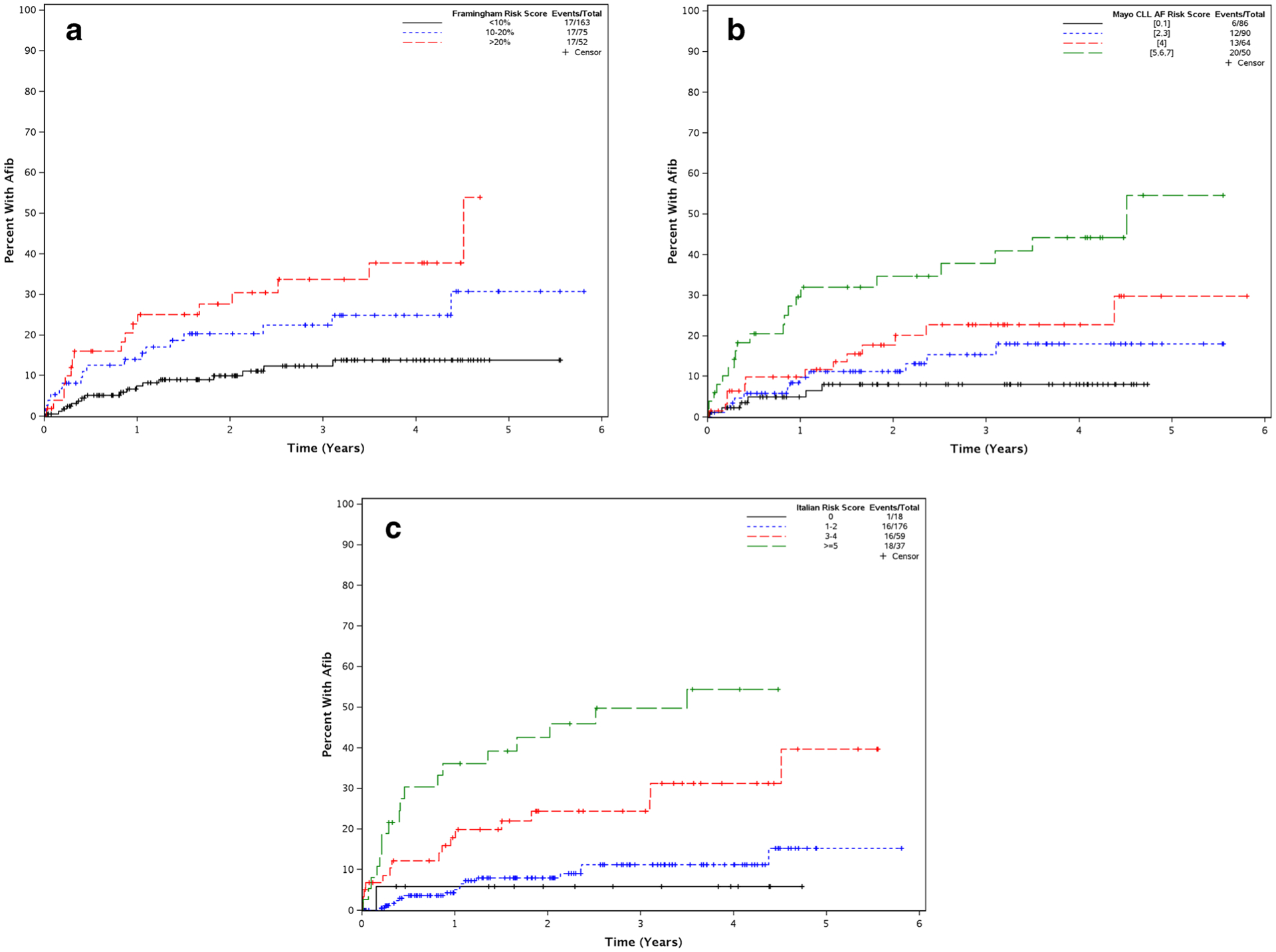
a Time to atrial fibrillation Framingham risk score. b Time to atrial fibrillation Mayo CLL AF risk score. c Time to atrial fibrillation Italian AF risk score
Management of treatment-emergent AF (n = 51)
Figure 3 depicts the management of patients with AF. Treatment-emergent AF was grade ≥ 3 in 13 (25%) patients. Rate control measures (predominantly using beta-blockers) were used for the management of treatment-emergent AF in 27 (53%) patients, rhythm control (using digoxin) in 2 (4%) patients, and one patient (2%) had both. Sixteen (31%) patients underwent additional interventions including atrioventricular nodal ablation (n = 3), cardioversion (n = 11), and placement of a permanent pacemaker (n = 2). Five (10%) patients did not receive any specific therapy for their treatment-emergent AF. At the time of AF onset, 12 (24%) patients held ibrutinib temporarily and subsequently resumed the original dose, 22 (43%) patients reduced the dose of ibrutinib without a hold, and 11 (22%) patients continued their ibrutinib dose without interruption from the prior dose prior to the onset of AF. Six (12%) patients permanently discontinued ibrutinib due to treatment-emergent AF.
Fig. 3.
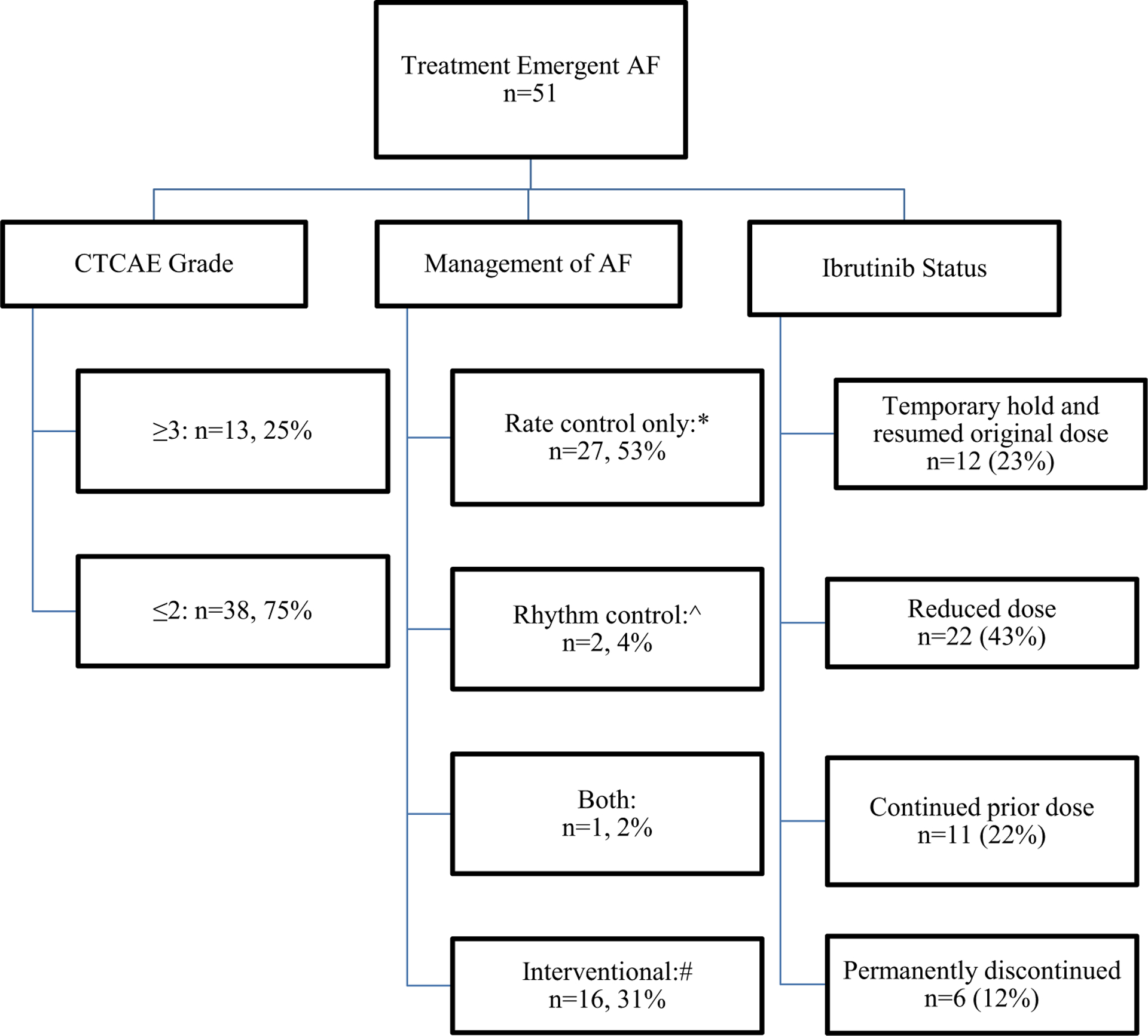
Treatment-emergent atrial fibrillation
*Labetalol, metoprolol, carvedilol, atenolol
^digoxin
#AV node ablation (n=3); cardioversion (n=11); permanent pacemaker (n=2)
Stroke prevention in patients with treatment-emergent AF
Figure 4 presents the anticoagulation and/or antiplatelet strategy for stroke prevention stratified by CHA2DS2-VASc score. Supplemental Table 1 provides the anticoagulation and anti-platelet drug therapies used. Of 51 patients with treatment-emergent AF, 42 (82%) had a CHA2DS2-VASc score of ≥ 2. Of these 42 patients, 17 (40%) received anticoagulation alone, 6 (14%) received antiplatelet agents, 4 (10%) patients received both, and 15 (36%) received neither. Of 9 patients with CHA2DS2-VASc score ≤ 1, 4 (44%) received anticoagulation alone, 2 (22%) received antiplatelet agents alone, and 3 (34%) received neither. During a median observation time of 9.9 months (range 0–45.2 months) after AF was identified, no thrombotic strokes occurred in these 51 patients with treatment-emergent AF.
Fig. 4.
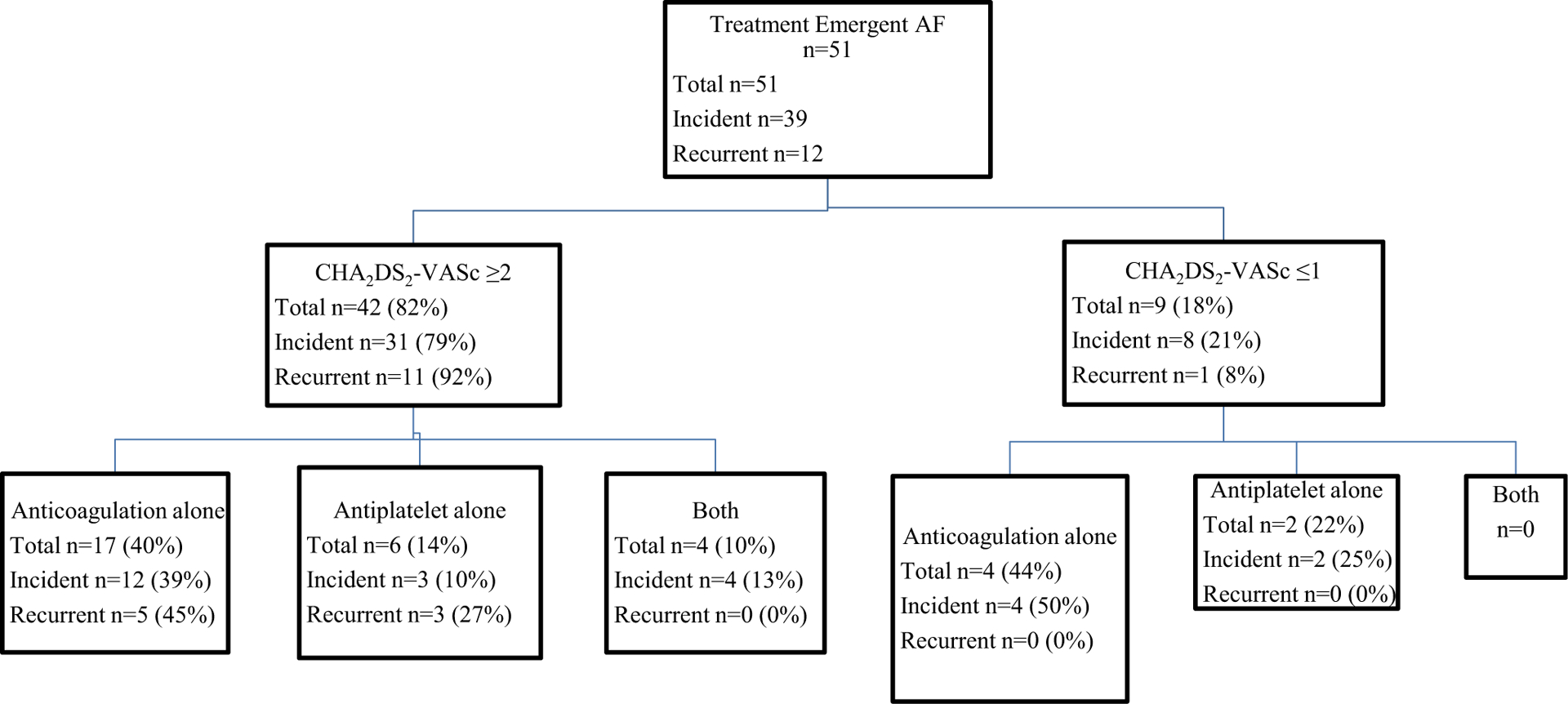
Stroke prevention in patients with treatment-emergent AF
Anticoagulation agents: apixaban n=15 (29%), rivaroxaban n=5 (10%), enoxaparin n=3 (6%), dabigatran n=1 (2%), warfarin n=1 (2%)
Antiplatelet agents: aspirin 81 mg n=9 (18%), aspirin 325 mg n=2 (4%), dual antiplatelet therapy n=1 (2%)
Two major bleeds occurred after the identification of treatment-emergent AF. The first patient developed an intracranial bleed requiring burr hole evacuation while on ibrutinib therapy. He was on antiplatelet therapy with aspirin and anticoagulation with rivaroxaban. Ibrutinib was permanently discontinued in this patient. The second patient with a major bleed (gastrointestinal) was not on any antiplatelet or anticoagulation therapy. Ibrutinib was temporarily held for this patient and resumed at the original dose. Clinically relevant non-major bleeding occurred in 12 patients. The breakdown of these events is presented in Supplemental Table 2. Ibrutinib was continued at the same dose in ten patients, and concomitant anticoagulation was discontinued in four patients.
Patients with persistent AF
Prior to beginning ibrutinib therapy, eight patients were in persistent AF. All 8 patients had a CHA2DS2-VASc score of ≥ 2 at the start of ibrutinib therapy. Two patients received both anticoagulation and antiplatelet therapy, three patients anticoagulation alone, one patient antiplatelet therapy alone, and two patients received no stroke prophylaxis. Five patients were started on 140 mg daily ibrutinib, one patient at 140 mg every other day, and one patient at 280 mg daily due to concomitant anticoagulation therapy and concern for bleeding. One patient began full-dose ibrutinib at 420 mg daily. The median time on ibrutinib therapy was 21.3 months (range 14.4–53.3 months), and none required modification of their ibrutinib therapy due to worsening of AF. One patient experienced a clinically relevant non-major bleed (bruising).
Outcomes
The median EFS of the entire cohort was 3.9 years, and median OS was 5.3 years. On univariable analysis, the development of treatment-emergent AF was associated with shorter EFS (HR 2.7, 95% CI 1.6–4.5, p < 0.001) and shorter OS (HR 4.1, 95% CI 2.3–7.3, p < 0.001, Table 5). In multivariable analyses, treatment-emergent AF remained a significant predictor of EFS after adjusting for prior CLL treatment status, and TP53 disruption, heart disease, valvular heart disease, and past history of AF (HR 2.0, 95% CI 1.1–3.8, p = 0.02). Treatment-emergent AF also remained a significant predictor of shorter OS after adjusting for age, prior CLL treatment status, TP53 disruption, heart failure, valvular heart disease, and past history of AF (HR 3.2, 95% CI 1.6–6.3, p = 0.001).
Table 5.
Univariable and multivariable analyses of factors predicting EFS and OS
| EFS | |||||
|---|---|---|---|---|---|
| Variable | N | Univariable | Multivariable* | ||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | |||||
| <65 (ref) | 110 | ||||
| ≥ 65 | 180 | 1.2 (0.8–1.8) | 0.52 | 1.0 (0.6–1.6) | 0.94 |
| Prior treatment (relapse/refractory) | |||||
| None (ref) | 89 | ||||
| Prior treatment | 201 | 2.8 (1.6–5.0) | 0.0005 | 2.5 (1.4–4.6) | 0.002 |
| TP53 disruption | |||||
| No (ref) | 227 | ||||
| Yes | 55 | 2.0 (1.3–3.0) | 0.003 | 2.1 (1.3–3.3) | 0.002 |
| Treatment-emergent AF** | |||||
| No AF (ref) | 246 | ||||
| AF | 44 | 2.7 (1.6–4.5) | 0.0002 | 2.0 (1.1–3.8) | 0.02 |
| Heart failure | |||||
| No (ref) | 261 | ||||
| Yes | 29 | 1.5 (0.8–2.6) | 0.21 | 0.9 (0.4–1.7) | 0.66 |
| Valvular heart disease | |||||
| No (ref) | 244 | ||||
| Yes | 46 | 1.7 (1.04–2.7) | 0.03 | 1.8 (1.1–3.2) | 0.03 |
| History of AF | |||||
| No (ref) | 269 | ||||
| Yes | 21 | 1.9 (1.0–3.6) | 0.04 | 1.1 (0.502.2) | 0.89 |
| Sex | |||||
| Female (ref) | 87 | ||||
| Male | 203 | 0.7 (0.5–1.1) | 0.11 | ||
| Rai | |||||
| Rai 0 (ref) | 37 | ||||
| Rai 1 or 2 | 91 | 0.6 (0.3–1.2) | 0.15 | ||
| Rai 3 or 4 | 134 | 0.6 (0.3–1.2) | 0.14 | ||
| FISH | |||||
| Normal/13q/12+ (ref) | 175 | ||||
| 11q/17p | 103 | 1.3 (0.8–1.9) | 0.30 | ||
| IGHV mutation | |||||
| Mutated (ref) | 59 | ||||
| Unmutated | 193 | 1.4 (0.8–2.4) | 0.24 | ||
| OS | |||||
| Variable | N | Univariable | Multivariable* | ||
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | |||||
| < 65 (ref) | 110 | ||||
| ≥ 65 | 180 | 1.9 (1.03–3.4) | 0.04 | 1.9 (1.0–3.8) | 0.06 |
| Prior treatment (relapse/refractory) | |||||
| None (ref) | 89 | ||||
| Prior treatment | 201 | 5.3 (1.9–14.6) | 0.001 | 5.0 (1.8–14.0) | 0.002 |
| TP53 disruption | 282 | ||||
| Normal (ref) | 227 | ||||
| Yes | 55 | 2.9 (1.7–4.9) | 0.0001 | 3.8 (2.1–6.9) | < 0.0001 |
| Treatment-emergent AF** | |||||
| No AF (ref) | 237 | ||||
| AF | 53 | 4.1 (2.3–7.3) | < 0.0001 | 3.2 (1.6–6.3) | 0.001 |
| Heart failure | |||||
| No (ref) | 261 | ||||
| Yes | 29 | 2.2 (1.1–4.2) | 0.02 | 1.1 (0.5–2.3) | 0.86 |
| Valvular heart disease | |||||
| No (ref) | 244 | ||||
| Yes | 46 | 2.0 (1.1–3.5) | 0.02 | 2.1 (1.1–4.2) | 0.03 |
| History of AF | |||||
| No (ref) | 269 | ||||
| Yes | 21 | 1.5 (0.7–3.2) | 0.35 | 0.5 (0.2–1.3) | 0.14 |
| Sex | |||||
| Female (ref) | 87 | ||||
| Male | 203 | 0.7 (0.4–1.1) | 0.11 | ||
| Rai | |||||
| Rai 0 (ref) | 37 | ||||
| Rai 1 or 2 | 91 | 0.7 (0.3–1.7) | 0.44 | ||
| Rai 3 or 4 | 134 | 1.1 (0.5–2.5) | 0.79 | ||
| FISH | |||||
| Normal/13q/12+ (ref) | 175 | ||||
| 11q/17p | 103 | 1.5 (0.9–2.6) | 0.11 | ||
| Mutation | |||||
| Mutated (ref) | 59 | ||||
| Unmutated | 193 | 0.97 (0.5–1.9) | 0.92 | ||
Italicized table entries indicate P-value < 0.05
Multivariable analysis was run on 282 patients without permanent AF who also had data to assess TP53 disruption
Run as a time-dependent variable
Discussion
In this large retrospective analysis of CLL patients treated with ibrutinib, the risk of treatment-emergent AF at 2 years was ~ 16%, which is significantly higher than that reported in ibrutinib registration trials [11, 12]. As would be expected, comorbidities of patients (particularly past history of atrial fibrillation and heart failure) and not CLL characteristics (such as Rai stage, CLL FISH profile or IGHV mutation status) predicted the development of treatment-emergent AF. Most patients with treatment-emergent AF were able to continue ibrutinib therapy; however, the dose of ibrutinib was reduced in ~ 50% patients. Approximately 80% patients with treatment-emergent AF had a high CHADS2-VASC score (≥2), and despite inconsistent use of anticoagulation due to the increased risk of bleeding, no patient developed a thrombotic stroke after a median observation of ~ 10 months. Finally, the development of treatment-emergent AF was associated with a shorter EFS and OS, independent of other adverse characteristics such as age, prior therapy, and TP53 disruption, suggesting that the occurrence of AF is associated with poor outcomes in CLL patients who receive ibrutinib.
In a pooled analysis of 756 patients who received ibrutinib (either as a single agent or in combination with chemotherapy) in 4 clinical trials, the risk of treatment-emergent AF was 6.5% after a median exposure time of 13 months [12]. In other analyses of cohorts among ibrutinib-treated patients outside the context of a clinical trial, the risk of atrial fibrillation has ranged between 5 and 10%, depending on the patient population, length of follow-up, and the way by which AF was ascertained [4, 30]. Our results suggest that the risk of treatment-emergent AF may be significantly higher (median age in our cohort was 68 years) and is likely influenced by age and comorbidity of the cohort treated. Although data from clinical trials indicate that the risk of treatment-emergent AF is highest in the first few months after the start of therapy, results from our study indicate that the risk increases with longer follow-up, such that it was 9% at 6 months, 12% at 1 year, and ~ 16% at 2 years following ibrutinib therapy. This finding has important clinical implications in general practice, since the vast majority of patients are likely to be on ibrutinib therapy for an extended period of time.
There are several models proposed to predict the risk of atrial fibrillation, both in the general population as well as in CLL patients who have received non-ibrutinib-based and ibrutinib-based therapy. We evaluated the ability of the Framingham risk score, Mayo CLL AF risk score, and the Italian AF risk score to predict the risk of treatment-emergent AF in our cohort of patients. All models predicted the risk of treatment-emergent AF reasonably well. The risk factor with the highest hazard ratio for the occurrence of treatment-emergent AF was a past history of AF at the time of ibrutinib initiation. With the recent approval of venetoclax in both relapsed/refractory CLL and frontline CLL (in combination with obinutuzumab), these findings suggest that ibrutinib may be a less optimal choice in patients with prior history of atrial fibrillation. On subset analysis, excluding patients with prior history of AF from the multivariable model, we found that hypertension, heart failure, and age ≥ 65 years were factors associated with a higher risk of treatment-emergent AF (although the p value did not reach < 0.05, likely due to the small number of events). Other studies have suggested that a baseline EKG (demonstrating a long PR interval) and echocardiogram can predict the risk of treatment-emergent AF—and should be routinely obtained in all patients initiating ibrutinib therapy [31]. Given the small numbers of patients who had these tests done in our study, we are unable to confirm these findings which is likely due to our limited ability to detect a difference. However, given that patient comorbidities can predict risk of treatment-emergent AF, we believe it could be prudent to rely on clinical characteristics alone (such as those provided by the Italian and Mayo CLL AF scores) to risk stratify CLL patients who are about to start ibrutinib-based therapy.
AF is associated with an increased risk of stroke and thromboembolism, which can lead to significant morbidity and mortality [32]. Preventing complications of AF relies upon therapeutic anticoagulation with a variety of agents, including the vitamin K antagonist warfarin [33] and direct oral anticoagulants acting upon Factor Xa [30, 34] and thrombin [31]. However, treatment with ibrutinib causes platelet dysfunction [35–37], increasing the risk of bleeding [38, 39]. This presents a conundrum for the treating physician where the combined risk of anticoagulation therapy with the platelet dysfunction of ibrutinib and underlying bleeding risk of CLL may present an unacceptably high risk of bleeding for a patient with a cardiovascular complication ordinarily requiring anticoagulation therapy. There is limited data on the use of other anticoagulants such as oral direct thrombin inhibitors or low molecular weight heparin in conjunction with ibrutinib. Of the 51 patients with treatment-emergent AF in our study, 25 (50%) received anticoagulation in conjunction with ibrutinib. The most commonly used anticoagulant was apixaban, followed by rivaroxaban, enoxaparin, and dabigatran. Unfortunately, one of these patients (who was on concomitant rivaroxaban and aspirin) experienced a major bleed. Approximately 50% of patients with clinically relevant non-major bleeding were on concomitant anticoagulation with ibrutinib. With the availability of venetoclax for both frontline and relapsed CLL, the threshold for discontinuing ibrutinib in the context of treatment-emergent AF has become lower; expert opinion has recommended discontinuing ibrutinib in such situations [22]. Although acalabrutinib may be used in an off-label setting in such a situation (it is approved by the Food and Drug Administration for relapsed mantle cell lymphoma in the USA), there are no data comparing the risk of atrial fibrillation or bleeding between ibrutinib and acalabrutinib in a randomized study. Our results suggest that with appropriate dose reduction of ibrutinib and close monitoring, ibrutinib may safely be combined with oral direct factor Xa inhibitors in CLL patients who develop treatment-emergent AF, although an individualized decision needs to be made for each patient after a careful discussion of risks and benefits of such an approach, given the lack of prospective data.
Patients with persistent AF prior to beginning ibrutinib therapy are an understudied population. Our study demonstrated that ibrutinib did not cause worsening of AF, and no strokes occurred in this group of patients. Although venetoclax may be a better initial choice in this population [40], many such patients will ultimately progress after venetoclax and ultimately need to consider BTKi therapy, particularly those with high-risk molecular features such as del 17p/TP53 mutation. Our limited data shows that ibrutinib may be considered in these patients where access to venetoclax may be an issue. There is limited data describing the impact of treatment-emergent AF on progression-free survival (PFS) and OS in CLL patients treated with ibrutinib. In the retrospective analysis of 756 CLL patients treated with ibrutinib on clinical trials [21], there was no difference in PFS among patients who developed AF relative to those who did not. In contrast to these findings, our study suggests that patients with treatment-emergent AF experience a shorter OS, independent of other adverse prognostic factors such as age, prior CLL therapy, and TP53 disruption. These findings have important implications in the management of CLL patients, since depending on age and comorbidity, up to 1 in 6 patients treated with ibrutinib may experience treatment-emergent AF during the course of therapy.
Our study has several important limitations. First, the study focuses on patients treated at an academic center, which could have introduced selection bias in the patient population evaluated and the treatments rendered. Given the retrospective nature of the study, no uniform guidelines were available to practicing clinicians about how to respond among patients who experienced AF with respect to dose modifications, ibrutinib discontinuation, or use of concomitant antiplatelet or anticoagulation in patients. Thus, our results represent the clinical practices that have been utilized at our institution, which may vary at other institutions. Additionally, the understanding of ibrutinib-related toxicities and other aspects of management of patients on ibrutinib, including those developing AF, evolved during the study interval (2012–2018). Finally, given the small numbers of patients with treatment-emergent AF, we are unable to exclude the possibility that dose reduction of ibrutinib contributed to the shorter EFS and OS.
The results of our study, obtained from careful follow-up of patients treated at our center, provide important information to practicing clinicians about the prediction, incidence, and management of AF in patients with CLL treated with ibrutinib. Patient comorbidities including history of AF and heart failure predicted risk of treatment-emergent AF in patients treated with ibrutinib. Most patients who develop AF are able to continue ibrutinib therapy but the occurrence of AF appears to be associated with adverse clinical outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank the participating patients treated at Mayo Clinic. The results of this study were presented as a poster at the Annual American Society of Clinical Oncology Meeting in Chicago, IL, in June 2019. The conduct of this research was supported in part by the Henry J Predolin Foundation. Sameer A. Parikh also acknowledges support from the Mayo Clinic K2R Career Development Program.
Competing interest
SAP: Research funding has been provided to the institution from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, AbbVie, TG Therapeutics, and Ascentage Pharma for clinical studies in which Sameer A. Parikh is a principal investigator. Sameer A. Parikh has also participated in Advisory Board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, Verastem Oncology and AbbVie (he was not personally compensated for his participation). NEK: Research funding has been provided to the institution from Pharmacyclics, Acerta, Tolero & MEI Pharma for which Neil E. Kay is a principal investigator. Dr. Kay has also participated as a member of the Data and Safety Monitoring Board for Cytomx Therapeutics, Infinity Pharm, Agios Pharm, Celgene, and MorphoSys. He has also participated in Advisory Board meetings of Pharmacyclics and Janssen. WD: Research funding has been provided to the institution from Merck for which Wei Ding is a principal investigator. SSK: Sponsored Research Funding provided to the laboratory from Novartis, Kite, Actinium, MorphoSys, Lentigen, Humanigen, and Tolero. SSK is inventor on patents licensed to Novartis and Humanigen. SSK is a co-founder of Leah Labs and SensImmune and he has participated as a consultant to Leah Labs and Kiniksa. TDS: Research funding has been provided to the institution from Pharmacyclics, Janssen, Genentech, GlaxoSmithKline, Celgene, Cephalon, and Hospira for which Tait D. Shanafelt is a principal investigator. All other authors have no conflict of interest to report.
Abbreviations
- AF
atrial fibrillation
- AIC
Akaike information criteria
- BTKi
Bruton tyrosine kinase inhibitor
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- CTCAE
Common Terminology Criteria for Adverse Events
- EFS
event-free survival
- EKG
electrocardiogram
- FISH
fluorescence in situ hybridization
- ISTH
International Society on Thrombosis and Haemostasis
- HR
hazard ratio
- IGHV
immunoglobulin heavy chain
- OS
overall survival
- PFS
progression-free survival
- SLL
small lymphocytic lymphoma)
Footnotes
Compliance with ethical standards
The Mayo Clinic Institutional Review Board approved this study and all patients consented to the use of their medical records for research purposes.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00277-020-04094-3) contains supplementary material, which is available to authorized users.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute. [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O’Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ, RESONATE-2 Investigators (2015. Dec 17) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 373(25):2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, Jelinek DF, Braggio E, Leis JF, Zhang CC, Coutre SE, Barr PM, Cashen AF, Mato AR, Singh AK, Mullane MP, Little RF, Erba H, Stone RM, Litzow M, Tallman M (2019. Aug 1) Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381(5):432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, Parikh SA, Coutre S, Hurria A, Brown JR, Lozanski G, Blachly JS, Ozer HG, Major-Elechi B, Fruth B, Nattam S, Larson RA, Erba H, Litzow M, Owen C, Kuzma C, Abramson JS, Little RF, Smith SE, Stone RM, Mandrekar SJ, Byrd JC (2018) Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 379(26):2517–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS (2014. Dec 11) Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 124(25):3829–3830 [DOI] [PubMed] [Google Scholar]
- 6.Thompson PA, Levy V, Tam CS, Al Nawakil C, Goudot FX, Quinquenel A et al. (2016) Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol 175(3):462–466 [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM et al. (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 371(3):213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy MS, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M, HELIOS investigators (2016) Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 17(2):200–211 [DOI] [PubMed] [Google Scholar]
- 9.Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, Siegal D (2016) The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 128(1):138–140 [DOI] [PubMed] [Google Scholar]
- 10.Ahn IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S, Lotter J, Housel S, Stetler-Stevenson M, Yuan CM, Maric I, Calvo KR, Nierman P, Hughes TE, Saba NS, Marti GE, Pittaluga S, Herman SEM, Niemann CU, Pedersen LB, Geisler CH, Childs R, Aue G, Wiestner A (2018) Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 131(21):2357–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiczer TE, Levine LB, Brumbaugh J, Coggins J, Zhao Q, Ruppert AS, Rogers K, McCoy A, Mousa L, Guha A, Heerema NA, Maddocks K, Christian B, Andritsos LA, Jaglowski S, Devine S, Baiocchi R, Woyach J, Jones J, Grever M, Blum KA, Byrd JC, Awan FT (2017) Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv 1(20):1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A et al. (2018) Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 18(10):648–657 e15 [DOI] [PubMed] [Google Scholar]
- 13.Lentz R, Feinglass J, Ma S, Akhter N (2019) Risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk Lymphoma 60(6):1447–1453 [DOI] [PubMed] [Google Scholar]
- 14.Shanafelt TD, Parikh SA, Noseworthy PA, Goede V, Chaffee KG, Bahlo J, Call TG, Schwager SM, Ding W, Eichhorst B, Fischer K, Leis JF, Chanan-Khan AA, Hallek M, Slager SL, Kay NE (2017) Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma 58(7):1630–1639 [DOI] [PubMed] [Google Scholar]
- 15.Visentin A, Deodato M, Mauro FR, Autore F, Reda G, Vitale C, Molica S, Rigolin GM, Imbergamo S, Scomazzon E, Pravato S, Frezzato F, Facco M, Piazza F, Cesini L, Tedeschi A, Laurenti L, Cortelezzi A, Coscia M, Cuneo A, Foà R, Semenzato G, Trentin LA (2018) Scoring system to predict the risk of atrial fibrillation in chronic lymphocytic leukemia and its validation in a cohort of ibrutinib-treated patients. Blood 132(Suppl 1):3118 [Google Scholar]
- 16.Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG et al. (2018) Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol 4(12):1491–1500 [DOI] [PubMed] [Google Scholar]
- 17.Lentz R, Feinglass J, Ma S, Akhter N (2019) Risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk Lymphoma 7:1–7 [DOI] [PubMed] [Google Scholar]
- 18.Khalid S, Yasar S, Khalid A, Spiro TP, Haddad A, Daw H (2018) Management of atrial fibrillation in patients on ibrutinib: a Cleveland Clinic experience. Cureus. 10(5):e2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reda G, Fattizzo B, Cassin R, Mattiello V, Tonella T, Giannarelli D, Massari F, Cortelezzi A (2018) Predictors of atrial fibrillation in ibrutinib-treated CLL patients: a prospective study. J Hematol Oncol 11(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boriani G, Corradini P, Cuneo A, Falanga A, Foà R, Gaidano G, Ghia PP, Martelli M, Marasca R, Massaia M, Mauro FR, Minotti G, Molica S, Montillo M, Pinto A, Tedeschi A, Vitolo U, Zinzani PL (2018) Practical management of ibrutinib in the real life: focus on atrial fibrillation and bleeding. Hematol Oncol 36(4):624–632 [DOI] [PubMed] [Google Scholar]
- 21.Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, Yang T, Reddy NM, Funck-Brentano C, Brown JR, Roden DM, Moslehi JJ (2019) Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol 74(13):1667–1678 [DOI] [PubMed] [Google Scholar]
- 22.Stephens DM, Byrd JC (2019) How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 133(12):1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang H, Reichard KK, Rabe KG, Hanson CA, Call TG, Ding W, Kenderian SS, Muchtar E, Schwager SM, Leis JF, Chanan-Khan AA, Slager SL, Braggio E, Smoley SA, Kay NE, Shanafelt TD, van Dyke DL, Parikh SA (2019) IGH translocations in chronic lymphocytic leukemia: clinicopathologic features and clinical outcomes. Am J Hematol 94(3):338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel PJ, Larson MC, Kabat B, Call TG, Ding W, Kenderian SS, Bowen D, Boysen J, Schwager SM, Leis JF, Chanan-Khan AA, Muchtar E, Hanson CA, Slager SL, Kay NE, Chaffee KG, Shanafelt TD, Parikh SA (2018) Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br J Haematol 183(3):421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh SA, Rabe KG, Call TG, Zent CS, Habermann TM, Ding W, Leis JF, Schwager SM, Hanson CA, Macon WR, Kay NE, Slager SL, Shanafelt TD (2013) Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol 162(6):774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ugurel S, Rohmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A et al. (2017) Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer 83:247–257 [DOI] [PubMed] [Google Scholar]
- 27.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 137(2):263–272 [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Kearon C (2005) Subcommittee on control of anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694 [DOI] [PubMed] [Google Scholar]
- 29.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S (2015) Subcommittee on Control of A. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13(11):2119–2126 [DOI] [PubMed] [Google Scholar]
- 30.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365(11):981–992 [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151 [DOI] [PubMed] [Google Scholar]
- 32.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr et al. (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 130(23):e199–e267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friberg L, Rosenqvist M, Lip GY (2012) Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 125(19): 2298–2307 [DOI] [PubMed] [Google Scholar]
- 34.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM, the ROCKET AF Steering Committee (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365(10):883–891 [DOI] [PubMed] [Google Scholar]
- 35.Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, Cole-Sinclair M, Reynolds J, Filshie R, Schischka S, Khot A, Sandhu S, Keating MJ, Nandurkar H, Tam CS (2015) Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 29(4):783–787 [DOI] [PubMed] [Google Scholar]
- 36.Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, Bordet JC, Tam C, Sié P, Ysebaert L, Payrastre B (2014) Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 124(26):3991–3995 [DOI] [PubMed] [Google Scholar]
- 37.Lipsky AH, Farooqui MZ, Tian X, Martyr S, Cullinane AM, Nghiem K et al. (2015) Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 100(12):1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones JA, Hillmen P, Coutre S, Tam C, Furman RR, Barr PM, Schuster SJ, Kipps TJ, Flinn IW, Jaeger U, Burger JA, Cheng M, Ninomoto J, James DF, Byrd JC, O’Brien SM (2017) Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol 178(2):286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JR, Moslehi J, O’Brien S, Ghia P, Hillmen P, Cymbalista F et al. (2017) Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 102(10):1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, de la Serna J, Jaeger U, Cartron G, Montillo M, Humerickhouse R, Punnoose EA, Li Y, Boyer M, Humphrey K, Mobasher M, Kater AP (2018) Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 378(12):1107–1120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


