Abstract
Objective
To assess the dose-related effects of radial extracorporeal shock wave therapy on pain alleviation in knee osteoarthritis.
Methods
With the use of a 2 × 2 factorial randomized controlled design, 89 patients diagnosed with knee osteoarthritis were assigned to 1 of 4 treatment groups, which varied in terms of shock intensity (0.12 mJ/mm2, lower density, or 0.24 mJ/mm2, higher density) and shock number (2,000 impulses or 4,000 impulses), or to a placebo control. Each group received 4 sessions of radial extracorporeal shock wave therapy, one week apart. The primary outcome was pain intensity measured on a visual analogue scale, and the secondary outcome was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. Assessments were performed at baseline, after each session, and at 4-week follow-up.
Results
Two-way repeated-measures analysis of variance revealed a significant effect on the Pain score for intensity (p < 0.001), with no effect for number (p = 0.467) or the intensity–number interaction (p = 0.536). Similar results were obtained for the WOMAC scores, except for an association between number and WOMAC score (p = 0.036). At the 4-week follow-up, all treatment groups showed greater reductions in the Pain and WOMAC scores than the control group. In addition, scores decreased more at higher densities of shock intensity than at lower densities, while there was no significant difference between the 2,000- and 4,000-shock conditions.
Conclusion
Moderate-intensity radial extracorporeal shock wave therapy was effective, and a higher density might be more efficacious in alleviating pain in knee osteoarthritis.
LAY ABSTRACT
Extracorporeal shock wave therapy may be a viable treatment for knee osteoarthritis with local pain and dysfunction; however, there are no standards on how to choose the treatment parameters to obtain the best outcome. This study compared 5 different levels of amount of treatment in 89 patients with knee osteoarthritis, and found that a medium intensity of therapy was effective. In addition, a higher intensity of extracorporeal shock wave therapy, rather than higher shock numbers, might result in a higher level of alleviation of symptoms in these patients.
Key words: dose-response relationship, radial extracorporeal shock wave therapy, knee osteoarthritis
Knee osteoarthritis (KOA) is the most common degenerative joint lesion; it has a high prevalence and a negative impact on the quality of life of affected individuals (1). It has been reported in a case–control study carried out in North Staffordshire that 9.6% of men and 18% of women have symptomatic KOA at the age of 60 years and over (2). In addition, approximately 25% of the population older than 55 years reports at least one episode of knee pain each year in the United Kingdom.(3). The management of early- medium stage KOA is crucial; the main aims during this stage of conservative therapy for KOA are to relieve pain and enhance joint mobility (4, 5). Treatments for KOA include oral medication, exercise therapy, intra-articular drug injection, and physiotherapy. Among these treatments, radial extracorporeal shock wave therapy (rESWT) has recently attracted increasing attention (6). rESWT is widely used for pain relief and the treatment of musculoskeletal disorders, and has proven beneficial at specific stages of KOA (7).
An extracorporeal shock wave is a transient sequence of acoustic pulses with a high peak pressure of 100 MPa, followed by a negative pressure of approximately 5–10 MPa, with an energy density between 0.003 and 0.89 mJ/mm2 (8). Radial shock wave devices generate the maximum energy at the probe tip and then distribute it radially into the tissue, providing effective treatment (9). rESWT may be a more acceptable treatment for some patients because of its non-invasiveness, low complication rate, lack of required hospitalization and low cost compared with other approaches. However, rESWT has not met established efficacy standards, and the optimal dose is unknown. A meta-analysis indicated that the effects of rESWT are superior to those of a placebo and physical therapy for pain relief in KOA (10). Nevertheless, an RCT that applied a relatively small dose of radial extracorporeal shock waves did not demonstrate any statistically significant difference from a placebo treatment in terms of pain control in patients with severe KOA (11). Another systematic review identified that the positive energy flux density (EFD) should be as high as possible; however, the dose was based on the subjective feeling of the individual patient (12).
It is important to quantify the amount of rESWT needed to reduce symptoms of KOA and establish the efficacy of rESWT. Thus, the primary aim of this prospective randomized placebo-controlled study was to test whether there was a dose-response relationship between the treatment doses and reduction in pain and dysfunction. The secondary aim was to examine whether the mean change in pain and function scores was greater for the active rESWT conditions than for the placebo control 4 weeks after the final treatment.
MATERIAL AND METHODS
Design and patients
This study employed a randomized, placebo-controlled, doubleblind, 2×2 factorial design and was registered in the Chinese Clinical Trial Registry (number ChiCTR2,000030371). The 2 therapeutic factors were energy flux density (0.12 mJ/mm2, lower density (LD) or 0.24 mJ/mm2, higher density (HD)) and shock number (2,000 impulses or 4,000 impulses). Thus, the 4 active rESWT groups were LD/2,000, LD/4,000, HD/2,000 and HD/4,000. After the baseline assessments, subjects were randomized to the LD/2,000, LD/4,000, HD/2,000, HD/4,000 or placebo control groups.
The study was conducted at the outpatient rehabilitation medicine department of the Aerospace Center Hospital, Beijing, China, from June 2019 to February 2020 and approved by the medical ethics committee of Aerospace Center Hospital (number 20190528-JT-09).
Patients were diagnosed by 2 expert physicians according to the American College of Rheumatology criteria (13). Inclusion criteria were: age over 45 years; presence of unilateral knee joint pain unresponsive to conventional treatments for at least 3 months; Kellgren-Lawrence (K-L) grade II (small osteophytes, possible narrowing of the joint) or III (multiple, moderately sized osteophytes, definite joint space narrowing, some sclerotic areas, possible deformation of bone ends) seen on X-ray of the knee joint (14); and written informed consent form to participate in the study. Exclusion criteria were: bilateral knee joint symptoms; patients who had received ESWT in the past; patients who had undergone surgery in the involved knee joint or received an intra-articular injection in the preceding 6 months; secondary osteoarthritis of the knee joint (inflammatory or metabolic); contraindication for ESWT (metal implants, infection or tumour near the treatment area, blood-clotting disorders, and pregnancy); and severe primary cardiovascular disease, lung disease or other serious diseases that affect survival.
Interventions
The enrolled subjects received rESWT with the radial extracorporeal shock wave device, Swiss Dolor Clast (EMS Electro Medical Systems, Nyon, Switzerland), with the standard radial (blue) handpiece, and a metal applicator with a diameter of 10 mm for the treatment groups and 15 mm for the control group. rESWT was administered by one physician who was not involved in the enrolment and assessment of the patients. Before the treatment, subjects were placed in supine and prone positions successively, with the affected knee joint flexed at different angles to expose the pain points. Meanwhile, the physician located the pain points by palpating the anatomical marks around the knee joint (i.e. the peripatellar area, the medial and lateral condyles, and the popliteal fossa area, avoiding critical nerves and blood vessels), wiped an aqueous gel on the probe of a radial handpiece, and oriented the probe perpendicularly on the targeted area. There was no application of local anaesthesia or analgesic drugs during the sessions.
rESWT groups
Subjects received 4 sessions of rESWT, one week apart, with a shock frequency of 8 Hz per session. The treatment protocols for the 4 rESWT groups were as follows: LD/2,000, with a positive EFD of 0.12 mJ/mm2 and 2,000 impulses per session; LD/4,000, with a positive EFD of 0.12 mJ/mm2 and 4,000 impulses per session; HD/2,000, with a positive EFD of 0.24 mJ/mm2 and 2,000 impulses per session; and HD/4,000, with a positive EFD of 0.24 mJ/mm2 and 4,000 impulses per session.
Control group
The placebo control group also received 4 sessions of rESWT, one week apart, with a shock frequency of 8 Hz per session, but was treated with the minimum positive EFD 0.02 mJ/mm2 and 1,000 impulses per session (15).
All subjects were prevented from receiving any additional treatments, such as physical therapy, oral or parenteral steroid medications, anti-inflammatory drugs, stretching, acupuncture, orthotics, etc., throughout the treatment sessions.
Outcome measurements
The primary outcome measure was the pain intensity measured by visual analogue scale (VAS) (16) score, where 0 indicated no pain and 10 indicated maximum pain. VAS-pain was selected because it measures the severity of pain on movement and is widely used in efficacy studies of KOA treatments. The secondary outcome was physical function on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). The WOMAC assesses the symptoms of KOA and is a validated, disease-specific, self-reporting questionnaire. The index consists of 5 questions on the severity of knee pain, 2 on stiffness, and 17 on limitations in physical function. All items were rated on a Likert scale of 0 (no symptoms) to 4 (extreme symptoms), with a total range of 0–96 and higher scores indicating worse symptoms (17, 18).
Evaluations were performed at baseline, immediately after each of the 4 rESWT sessions, and at follow-up 4 weeks after the final rESWT session. The adverse effects and patients’ complaints during treatment were documented. The same physiatrist who was blinded to the participant’s treatment assignment conducted all the outcome measurements.
Sample size
A priori power analysis was performed (G*Power, v.3.1.9.2, Franz Faul, Germany). Based on the preliminary data, a desired statistical power of 80% was assumed to detect a 2-point difference with a standard deviation (SD) of 1.5 points in the VAS-pain score, comparing active treatments with the control, based on a 2-tailed t-test with a Bonferroni significance level of α = 0.05/4. Interaction effects between treatments and time were tested based on a 2-way analysis of variance (ANOVA) model at a significance level of α = 0.05 with a statistical power of 80%. A drop-out rate of 10% was allowed for in all 5 treatment conditions. With these parameters, 19 participants were needed for each of the 4 rESWT treatment conditions, and 13 participants were needed for the control condition. Allowing for drop-outs, 99 randomized participants were required in total.
Randomization and blinding
Patients were randomized to the LD/2,000, LD/4,000, HD/2,000, HD/4,000 or placebo control group after providing written informed consent. Randomization was performed by a person who was not involved in the study, and a computergenerated list of random numbers was used. The randomization numbers were concealed from the physiatrist who measured the outcomes in sequentially numbered, opaque, sealed and stapled envelopes. Thus, both the patients and physiatrist who measured the outcomes were blinded to the allocation. To maintain blinding, the interim statistical analyses were conducted by independent statisticians and the results were not shared with the patients or physiatrist before the end of the study.
Statistical analyses
Data were analysed using IBM SPSS statistics version 22 (IBM, Armonk, NY, USA). Interim analyses were specified to be performed considering both the intervention efficacy and potential safety concerns, when 50% and 90% estimated sample size (50 and 89 patients, respectively) were randomized and completed. Early stopping for efficacy was predetermined at a p-value < 0.001 for rejection of the null hypothesis to declare that the active treatments were superior to the control (19, 20). The trial was also planned to be ended if potential harmful side-effects were observed.
All analyses were on an intention-to-treat (ITT) basis with all randomized patients included in the analyses. Missing data from patients who withdrew after the initial visit were imputed by means of the “last observation carried forward” technique. The normality of distributions was verified using the Kolmogorov-Smirnov and Shapiro-Wilk tests for VASpain scores and WOMAC scores across different time-points and interventions. Descriptive analysis was used to analyse the demographic data in each group. Crude means and SDs of the VAS-pain and WOMAC scores were calculated for all groups.
To account for the correlation at different time-points among each individual, a 2-way repeated-measures ANOVA was performed. Data collected at baseline, after each of the 4 treatment sessions, and 4 weeks after the final treatment session were included as levels of the within-subject factor (time), and 2 types of interventions (energy flux density and shock number) were set as the between-subject factors. If significant treatment by time interactions for the VAS-pain and WOMAC scores were observed, simple effects tests were performed, followed by Bonferroni’s post hoc tests in a secondary analysis to determine the within- and between- treatment differences. In the post hoc analysis, a Bonferroni adjustment was used to account for multiple comparisons with the statistical p-value set at 0.05/k, where k indicated the number of comparisons.
RESULTS
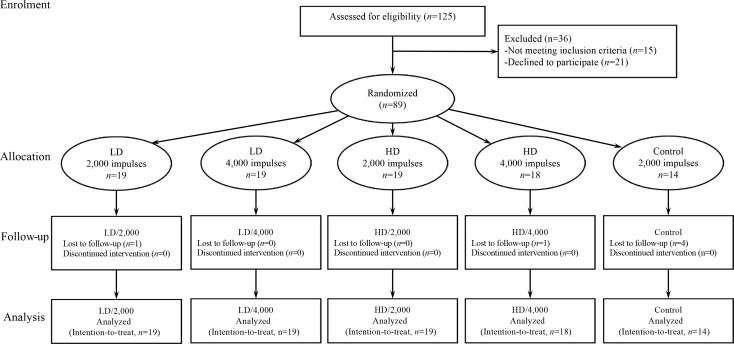
A total of 125 patients diagnosed with primary KOA were assessed for eligibility. Of these, 36 were excluded for not meeting the inclusion criteria (n = 15) or declining to participate (n = 21). Treatment allocation is shown in Fig. 1. Patients were randomized to 4 active treatment groups: LD/2,000 (n = 19), LD/4,000 (n = 19), HD/2,000 (n = 19), HD/4,000 (n = 18), or a placebo control group (n = 14). The trial was stopped for efficacy at the pre-planned interim analysis after enrolment of 89 patients, and all patients completed the 4-session experiment. Four patients were lost to follow-up due to unknown reasons, and 2 accepted other treatments during the follow-up period.
Fig. 1.
CONsolidated Standards of Reporting Trials (CONSORT) 2010 flow diagram. HD: higher density; LD: lower density.
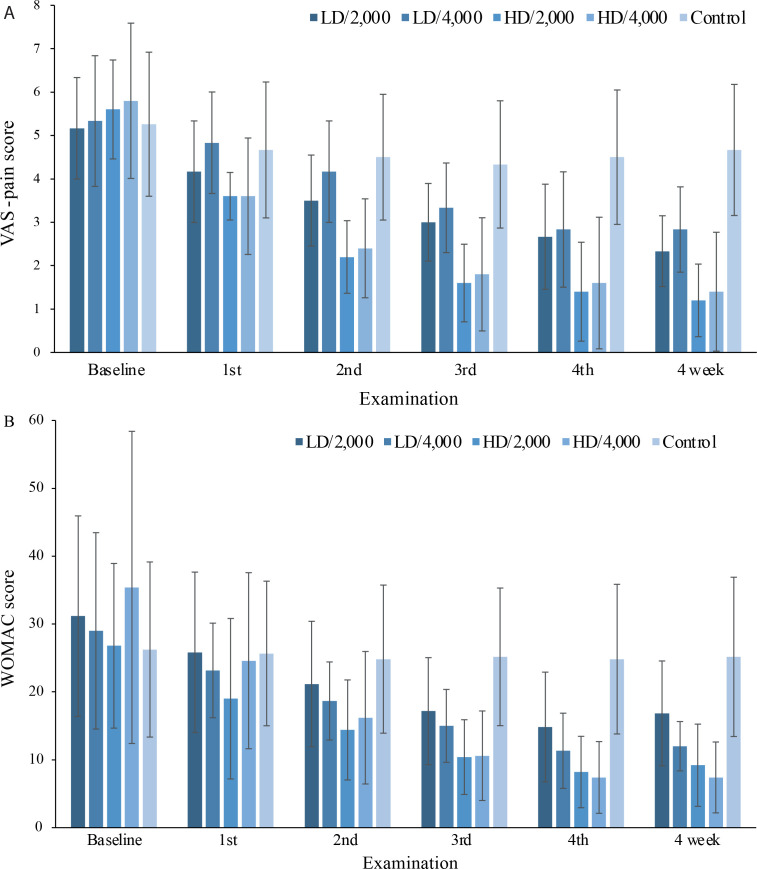
The baseline demographics and clinical characteristics of the patients are summarized in Table I. Changes in the crude means of the VAS-pain and WOMAC scores across the 5 groups from baseline to the 4-week follow-up are summarized in Figs 2A and 2B, respectively, both of which showed a decreasing trend.
Table I.
Patients’ baseline demographic and clinical characteristics
| Characteristics | LD/2,000 (n = 19) | LD/4,000 (n = 19) | HD/2,000 (n = 19) | HD/4,000 (n = 18) | Control (n = 14) |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 60.84 (8.36) | 62.70 (7.50) | 58.21 (9.47) | 63.65 (6.94) | 61.50 (5.43) |
| Female, n (%) | 11 (57.89) | 12 (63.16) | 10 (52.63) | 12 (66.67) | 8 (57.14) |
| BMI, kg/m², mean (SD) | 24.83 (1.73) | 24.35 (1.36) | 23.91 (1.56) | 25.96 (2.11) | 24.98 (1.32) |
| Duration of KOA, months, mean (SD) | 17.15 (5.36) | 19.92 (6.85) | 18.56 (7.48) | 16.67 (4.72) | 15.73 (8.37) |
| VAS-pain score, mean (SD) | 5.17 (1.17) | 5.33 (1.51) | 5.60 (1.14) | 5.80 (1.79) | 5.26 (1.66) |
| WOMAC score, mean (SD) | 31.17 (14.77) | 29.00 (14.59) | 26.80 (12.13) | 35.40 (24.50) | 26.25 (12.91) |
HD: higher density; LD: lower density; 4,000: 4,000 impulses; 2,000: 2,000 impulses; SD: standard deviation; BMI: body mass index; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Fig. 2.
Changes from baseline to the 4-week follow-up in the visual analogue scale (VAS)-pain scores and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores across all groups (mean (standard deviation (SD)). (A) The mean VAS-pain score of all groups at baseline, after the 1st, 2nd, 3rd, and 4th sessions of treatment, and at the 4-week followup. (B) Variations in the mean WOMAC score across all groups. Group: LD/2,000, n = 19; LD/4,000, n = 19; HD/2,000, n = 19; HD/4,000, n = 18; Control group, n = 14. VAS: visual analogue scale; HD: higher density; LD: lower density; 4,000: 4,000 impulses; 2,000: 2,000 impulses.
All further significance was calculated by 2-way repeated measures ANOVA. As shown in Table II, the significance of the treatment effect was p < 0.001 for intensity and p = 0.467 for number for the VAS-pain scores, p = 0.536 for the intensity-number interaction for the VAS-pain scores, p < 0.001 for intensity, and p = 0.036 for number for the WOMAC scores, and p = 0.552 for the intensity-number interaction for the WOMAC scores.
Table II.
Statistical analysis of visual analogue scale (VAS)-pain and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores for all treatment factors
| Treatment factors | VAS-pain scores |
WOMAC scores |
||
|---|---|---|---|---|
| F | *p-value | F | *p-value | |
| Intensity | 35.72 | < 0.001 | 17.11 | < 0.001 |
| Number | 0.55 | 0.467 | 5.01 | 0.036 |
| Intensity × number interaction | 0.40 | 0.536 | 0.37 | 0.552 |
| Intensity × time interaction | 11.84 | < 0.001 | 9.07 | 0.001 |
| Number × time interaction | 0.35 | 0.774 | 5.07 | 0.017 |
| Intensity × number × time interaction | 0.53 | 0.654 | 0.66 | 0.492 |
p-values relate to tests by 2-way repeated-measures analysis of variance (ANOVA).
The intensity × time interaction was significant for the VAS-pain and WOMAC scores. While the number× time interaction was not significant for the VASpain scores, it was significant for the WOMAC scores. The intensity × number × time interaction was significant for neither the VAS-pain nor the WOMAC scores. As significant treatment-by-time interactions for the VASpain and WOMAC scores were detected, simple effects tests were performed in the secondary analysis.
Within-treatment comparison between the 4-week follow-up and baseline
There were significant decreases (p < 0.001) over time in the VAS-pain and WOMAC scores, with the greatest change at the 4-week follow-up for all active treatments. For the combined HD condition, the reduction at the 4-week follow-up in the mean (95% confidence interval; 95% CI) VAS-pain score was 4.27 (3.47, 5.08) (p < 0.001) from baseline; for the LD condition, it was 2.73 (1.99, 3.45) (p < 0.001) from baseline; for the 4,000-impulse condition, it was 3.38 (2.62, 4.14) (p < 0.001) from baseline; and for the 2,000-impulse condition, it was 3.62 (2.86, 4.38) (p < 0.001) from baseline. Similarly, for the mean WOMAC score, for the HD condition, the reduction at the 4-week follow-up was 21.94 (17.82, 26.05) (p < 0.001) from baseline; for the LD condition, it was 15.82 (12.08, 19.57) (p < 0.001) from baseline; for the 4,000-shock condition, it was 21.40 (17.43, 25.38) (p < 0.001) from baseline; and for the 2,000-shock condition, it was 16.36 (12.43, 20.29) (p < 0.001) from baseline. For the control, there was no significant difference for either the mean VAS-pain score 0.69 (–0.34, 1.72) (p > 0.99) or the mean WOMAC score 4.42 (–0.94, 9.78) (p > 0.99) from baseline.
Comparisons among treatments at the 4-week follow-up
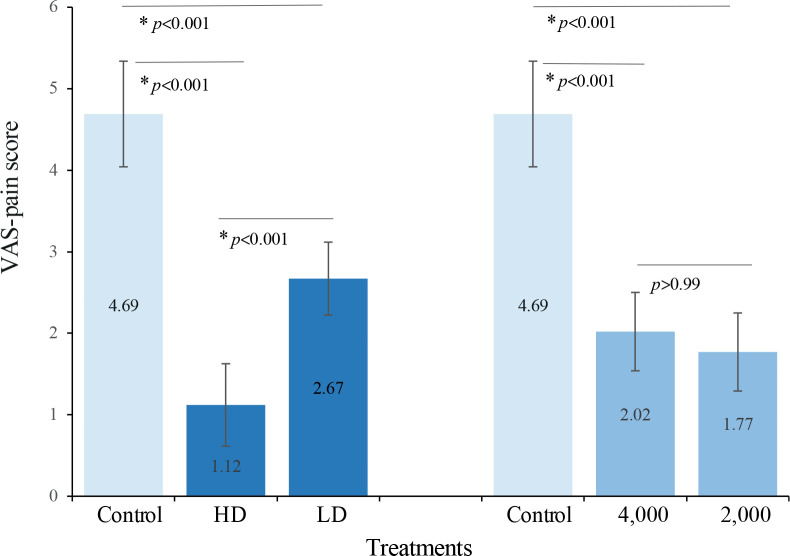
The differences among treatments were significant (p < 0.001) at the 4-week follow-up. As shown in Fig. 3, the HD condition was significantly more effective than the LD and control conditions in reducing the VAS-pain scores, and the mean differences between HD and LD and between HD and control (95% CI) were 1.55 (0.69, 2.40) (p < 0.001) and 3.58 (2.55, 4.61) (p < 0.001), respectively. The LD condition also differed from the control condition, and the mean difference (95% CI) was 2.03 (1.04, 3.02) (p < 0.001). For the 4,000- and 2,000–impulse conditions, which were not significantly different from each other, the mean difference was 0.24 (–0.61, 1.09) (p > 0.99), and both differed from the control condition, by 2.68 (1.67, 3.70) (p < 0.001) and 2.93 (1.92, 3.93) (p < 0.001), respectively.
Fig. 3.
Comparisons of the visual analogue scale (VAS)-pain scores among treatments at the 4-week follow-up. Values are estimated marginal means (95% confidence interval; 95% CI) from 2-way repeated measures analysis of variance (ANOVA). *p < 0.05/3, post hoc Bonferroni-adjusted tests for multiple comparisons (k=3). HD: higher density; LD: lower density; 4,000: 4,000 impulses; 2,000: 2,000 impulses.
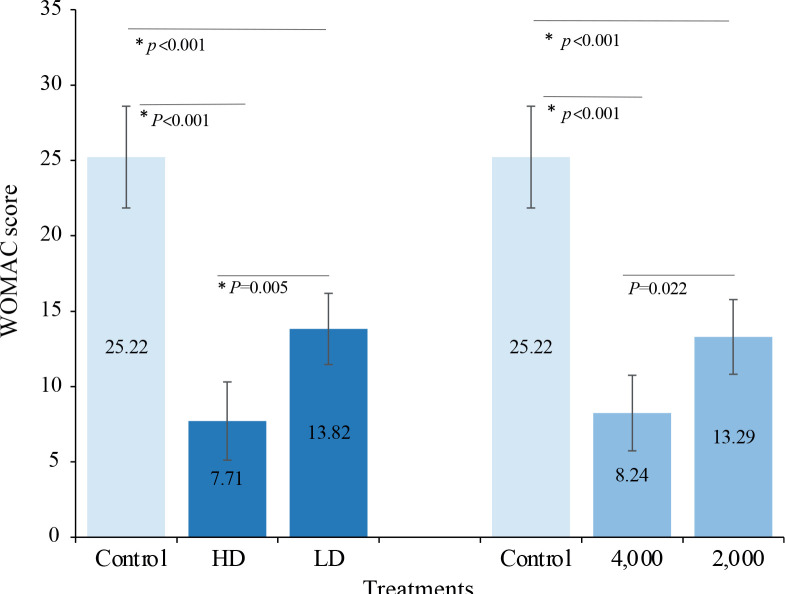
Similar results were obtained for the WOMAC scores (Fig. 4), which indicated a significant difference between the HD and LD conditions, with a mean difference (95% CI) of 6.11 (1.74, 10.49) (p = 0.005), between the HD and control conditions of 17.51 (12.16, 22.86) (p < 0.001), and between the LD and control conditions of 11.40 (6.24, 16.55) (p < 0.001). The results for the 4,000- and 2,000-impulse conditions indicated that both conditions were significantly different from the control, and the mean differences (95% CI) were 16.97 (11.66, 22.29) (p < 0.001) and 11.93 (6.71, 17.15) (p < 0.001), respectively, while there was no significant difference between the 2 conditions after the Bonferroni adjustment, 5.04 (0.63, 9.46) (p = 0.022).
Fig. 4.
Comparisons of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores among treatments at the 4-week follow-up. Values are estimated marginal means (95% confidence interval; 95% CI) from 2-way repeated measures analysis of variance (ANOVA). *p < 0.05/3, post hoc Bonferroni-adjusted tests for multiple comparisons (k = 3). HD: higher density; LD: lower density; 4,000: 4,000 impulses; 2,000: 2,000 impulses.
There were no adverse effects or complications after application of rESWT in any of the groups during the study period, and all patients were able to complete their treatments without any anaesthesia. Two patients reported minor skin reddening for a brief period following treatment.
DISCUSSION
The major finding of this study was that rESWT shock intensity has an independent effect on pain relief and functional improvement in patients with knee osteoarthritis, and the higher density condition led to greater improvements in the VAS-pain scores and WOMAC scores than the lower density or the control condition at the 4-week follow-up. This study further indicates that the shock number may decrease the WOMAC scores; nevertheless, no significant differences were detected between the 2,000- and 4,000-shock conditions at the 4-week follow-up.
Related research has found that there is a dose–effect relationship for rESWT. One study found that when endothelial progenitor cells of rats were treated with a low-energy (0.04–0.13 mJ/mm2) shock wave, the expression of endothelial nitric oxide synthase increased; when cells were treated with a high-energy (0.16 mJ/mm2) shock wave, most of the expression of cytokines declined, leading the cells to apoptosis. Finally, it was concluded that shock intensities, ranging from 0.10 to 0.13 mJ/mm2 and shock numbers ranging from 200 to 300 impulses were the optimal parameters for rESWT to treat cells in vitro (21).
Similar issues regarding the dose–response relationship have arisen in the clinical application of rESWT. Prior experience indicates that therapy parameters, such as the EFD per session, the interval between the sessions and the number of the sessions, will have an impact on the treatment effect. An RCT conducted by Ke et al. indicated that multiple-session rESWT has a clinically cumulative effect compared with single-session rESWT for patients with carpal tunnel syndrome (22). This study also proved the beneficial effects of multiple-session rESWT indirectly; however, the relevant content was not presented in the main results. A persistent decrease in outcome values was found across the 4 treatment sessions, until there was no significant difference between the final treatment and the 4-week follow-up, demonstrating that the treatment effects could last for at least 4 weeks after the final session.
However, few RCTs have investigated the dose– effect relationship between EFD and pain alleviation in patients with KOA. With respect to EFD, several studies have found “more is better”. One rESWT study of plantar fasciitis with positive results considered that the EFD was recommended to be maximum tolerable (23). In addition, a systematic review (12) demonstrated that the application of insufficient energy might adversely affect the outcome of rESWT. The mean EFD applied in all RCTs during rESWT for calcifying tendonitis of the shoulder with a positive outcome in the physiotherapy evidence database(Physiotherapy Evidence Database (PEDro; www.pedro.org.au [Accessed 2015 Oct 23 2015]) was 0.28±0.04 mJ/mm2, which was approximately 2.6 times more than the EFD applied in a negative RCT on rESWT (EFD = 0.11 mJ/mm2) (24). A similar situation was found for treating plantar fasciopathy and Achilles tendinopathy (25, 26).
Therefore, a key issue in studying rESWT interventions is quantification of the positive EFDs per treatment, i.e. the product of shock intensity by shock number. The EFD applied in KOA in previous research ranges from 0.04 to 0.25 mJ/mm2, and the shock number ranges from 1,000 to 4,000 impulses per treatment. Imamura et al. (11) showed a negative outcome with 3 sessions of rESWT, one week apart, on pain control in patients with severe KOA, where the positive EFD was 0.10–0.16 mJ/mm2 and the shock number was 2,000 impulses. Imamura et al. conjectured that higher total positive EFD might be required to achieve treatment success. Zhao et al. (27) used rESWT with total positive EFDs (with 4 sessions of treatment, one week apart, an EFD of 0.25 mJ/mm2, with 4,000 impulses each session) more than 4 times higher than the former and then demonstrated a significant superiority of rESWT over a placebo.
The current study quantified the EFD of each session. The shock intensity and shock number applied here are consistent with the recommendations of Chinese guidelines for extracorporeal shock wave therapy for musculoskeletal diseases (28), which advise rESWT of low-moderate intensity and 2,000–4,000 impulses per session be applied in KOA therapy. Our earlier study (2020, unpublished observations) found that the higher the shock intensity, the more likely patients are to experience local pain, swelling, and numbness caused by the shock waves, but no serious adverse events were reported within the therapeutic dose. The EFD for a moderate intensity could be well accepted by patients; therefore, a moderate intensity from 0.12 to 0.25 mJ/mm2 was applied. Doses of 0.12 and 0.24 mJ/mm2 were selected for the sake of the analysis.
These results provide implications for the selection of treatment parameters for rESWT in KOA: higher total EFDs can be achieved by a higher density of impulses rather than by more impulses when the frequency and number of sessions are fixed. A moderate intensity of EFD is recommended in the clinical management of KOA, ranging from 0.12 to 0.25 mJ/mm2; furthermore, a higher density is preferred if it can be tolerated by the individual patient without the application of local anaesthesia. In addition, the current study found a potential correlation between the shock number and functional improvement; nevertheless, it is not effective enough to detect significant differences between the 2,000- and 4,000-impulse conditions at all time-points, including the 4-week follow-up when the maximal change was obtained. Thus, the shock number could be applied with 2,000 or 4,000 impulses in the treatment of KOA, which might achieve similar results.
Study limitations
This study has a number of limitations. First, it offered substantial evidence to address dose–response questions with respect to treatment doses and pain alleviation in patients with KOA, but the maximal threshold is still not clear; thus, further research is needed to explore the therapeutic range of rESWT for KOA. Secondly, it was difficult for the placebo group to maintain compliance. To minimize this drop-out, the present study screened patients who had never been treated with ESWT and specified that the physician administering the treatment avoid communicating with participants about the treatment parameters. In addition, rESWT with a minimum intensity and 1,000 impulses was adopted to make the patients feel they were being treated. Finally, the sample is relatively small. The clinical trial was stopped early, based on the prespecified interim analysis, and significant differences were found between the HD and LD groups, indicating that the strength was sufficient; however, further multi-centre clinical trials are warranted to validate these findings. In addition, a potential association between shock number and functional improvement might exist, and this possibility deserves to be elucidated further in larger trials.
CONCLUSION
rESWT with an EFD of moderate intensity was found to be effective for pain alleviation and functional improvement therapy in patients with KOA. There was a dose–response relationship between the amount of EFD and alleviation of symptoms, independent of the shock number adopted per treatment; that is, the higher density compared with the lower density might be a better option for the treatment of patients with KOA. This study further indicates that a higher number of shocks might improve physical functioning in KOA; this possibility should be studied further in larger trials.
ACKNOWLEDGEMENT
This study was supported by a grant from the Medical and Health Research Project of Aerospace Science and Industry Corporation of China (grant no. 2019-LCYL-009).
REFERENCES
- 1.Grazina R, Andrade R, Bastos R, Costa D, Pereira R, Marinhas J, et al. Clinical management in early OA. Adv Exp Med Biol 2018; 1059: 111–135. [DOI] [PubMed] [Google Scholar]
- 2.Bedson J, Jordan K, Croft P. The prevalence and history of knee osteoarthritis in general practice: a case-control study. Fam Pract 2015; 22: 103–108. [DOI] [PubMed] [Google Scholar]
- 3.Peat G, McCarney RP, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001; 60: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kon E, Filardo G, Drobnic M, Madry H, Jelic M, Dijk N, et al. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2012; 20: 436–449. [DOI] [PubMed] [Google Scholar]
- 5.Gerald Gremion, David Gaillard, Pierre-Francois Leyvraz, Brigitte M Jolles. Effect of biomagnetic therapy versus physiotherapy for treatment of knee osteoarthritis: a randomized controlled trial. J Rehabil Med 2009; 41: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Pan Y, Yang Q, Guo ZG, Yue Q, Meng QG. Extracorporeal shockwave therapy for the treatment of knee osteoarthritis: a retrospective study. Medicine 2018; 97: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon SR, Kim JH. Effect of extracorporeal shock wave therapy on knee osteoarthritis. Ann Phys Rehabil Med 2014; 57: 37–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden JA, Toth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res 2001; 387: 8–17. [DOI] [PubMed] [Google Scholar]
- 9.Foldager CB, Kearney C, Spector M. Clinical application of extracorporeal shock wave therapy in orthopedics: focused versus unfocused shock waves. Ultrasound Med Biol 2012; 38: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Ma J, Zhao T, Gao F, Sun W. Application and efficacy of extracorporeal shockwave treatment for knee osteoarthritis: a systematic review and meta-analysis. Exp Ther Med 2019; 18: 2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura M, Alamino S, Hsing WT, Alfieri FM, Schmitz C, Battistella LR. Radial extracorporeal shock wave therapy for disabling pain due to severe primary knee osteoarthritis. J Rehabil Med 2017; 49: 54–62. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz C, Csaszar NB, Milz S, Schieker M, Maffulli N, Rompe JD, et al. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull 2015; 116: 115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheum 1986; 29: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957; 16: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA 2002; 288: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983; 16: 87–101. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Campbell J, Stevens J, Pilch L, Stewart C, Mahmood Z. Validation study of a computerized version of the Western Ontario and McMaster Universities VA3.0 Osteoarthritis Index. J Rheumatol 1997; 24: 2413–2415. [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15: 1833–1840. [PubMed] [Google Scholar]
- 19.Viele K, McGlothlin A, Broglio K. Interpretation of clinical trials that stopped early. JAMA 2016; 315: 1646–1647. [DOI] [PubMed] [Google Scholar]
- 20.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2016; 315: 2435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Yan X, Wang C, Tang T, Chai Y. The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J Surg Res 2014; 186: 484–492. [DOI] [PubMed] [Google Scholar]
- 22.Ke MJ, Chen LC, Chou YC, Li TY, Chu HY, Tsai CK, et al. The dose-dependent efficiency of radial shock wave therapy for patients with carpal tunnel syndrome: a prospective, randomized, single-blind, placebo-controlled trial. Sci Rep 2016; 6: 38244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow IHW, Cheing GLY. Comparison of different energy densities of extracorporeal shock wave therapy (ESWT) for the management of chronic heel pain. Clin Rehabil 2007; 21: 131–141. [DOI] [PubMed] [Google Scholar]
- 24.Kolk A, Yang KG, Tamminga R, van der Hoeven H. Radial extracorporeal shock-wave therapy in patients with chronic rotator cuff tendinitis: a prospective randomized doubleblind placebo-controlled multicentre trial. Bone Joint J 2013; 95-B: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 25.Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracorporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med 2005; 15: 119–124. [DOI] [PubMed] [Google Scholar]
- 26.Notarnicola A, Maccagnano G, Tafuri S, Forcignano MI, Panella A, Moretti B. CHELT therapy in the treatment of chronic insertional Achilles tendinopathy. Lasers Med Sci 2014; 29: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Z, Jing R, Shi Z, Zhao B, Ai Q, Xing G. Efficacy of extracorporeal shockwave therapy for knee osteoarthritis: a randomized controlled trial. J Surg Res 2013; 185: 661–666. [DOI] [PubMed] [Google Scholar]
- 28.[Academic congress of shockwave medical professional committee. Chinese guidelines for extracorporeal shock wave therapy for musculoskeletal diseases.] Chin J Frontier of Med Sci 2019; 11: 1–10 (in Chinese). [Google Scholar]