Abstract
Objective
To determine whether the psychological benefits of intense, inpatient, multimodal rehabilitation for persons with Huntington’s disease (HD), as found in earlier studies, also apply in a shorter, day-care setting.
Design
Prospective, non-randomized cohort study.
Subjects
Twenty patients attending a group-based 8-week (3 days/week) rehabilitation programme aimed at persons in early stages of HD.
Methods
An explorative cohort study on register data from a specialized rehabilitation centre, including descriptive data, number of cancellations, a self-reported evaluation, and measures of psychiatric symptoms, health-related quality of life, sense of coherence and physical function at baseline and at the end of rehabilitation.
Results
Patients’ attendance rate was almost 90%. Patients were satisfied, and displayed significantly reduced anxiety and depression and improved health-related quality of life after rehabilitation. Baseline measures of sense of coherence showed significant negative correlation with the number of cancelled days of rehabilitation. Physical function improved, but did not correlate significantly with psychological outcome measures.
Conclusion
These results indicate that an 8-week multimodal day-care rehabilitation programme can be tolerable, reduce psychiatric symptoms, and improve health-related quality of life for people with HD. A higher sense of coherence seems to promote attendance rates. Further larger studies, including the impact of cognition and disease progression on the treatment effect, are warranted.
LAY ABSTRACT
Huntington’s disease (HD) is characterized by a triad of clinical features; motor, cognitive, and psychiatric. Physical training seems to have positive effects on motor symptoms. Earlier studies have shown that intense, inpatient, multimodal rehabilitation may also reduce psychiatric symptoms. The present study aimed to explore whether the psychological benefits are retained in a shorter, day-care setting.This study included 20 patients attending a group-based rehabilitation programme. The results show that participants had an almost 90 % attendance rate and were satisfied with the programme. After rehabilitation, they displayed significantly reduced anxiety and depression and improved quality of life. Sense of coherence showed a significant negative correlation with the number of cancelled days of rehabilitation. These results indicate that a shorter multimodal day-care rehabilitation programme can be easier to tolerate than longer programmes, and still reduce psychiatric symptoms and improve quality of life for people with HD. A higher sense of coherence appears to promote the attendance rate.
Key words: Huntington’s disease, multimodal treatment, depression, anxiety, health-related quality of life, sense of coherence
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by an expanded CAG repeat in the HTT gene (1). The clinical presentation of HD includes motor (chorea, dystonia, bradykinesia, akinesia, and eye movements), (2) cognitive (slowing in process speed, impaired executive functions, social cognition, attention, and working memory) (3, 4) and psychiatric symptoms (most notably depression, anxiety disorders, apathy, and irritability) (5, 6). Motor and cognitive symptoms are progressive, whereas most common psychiatric symptoms have a complex relationship with the disease process (7, 8).
Depression has a prevalence of 40–50% in HD (8). Anxiety often co-exists with depression and has a prevalence ranging from 13% to 71% (7). Depression and anxiety do not correlate with the duration of the illness (7, 8). Apathy, however, is highly correlated with disease progression (9), although it might be reduced with stimulating input and structure (3).
There is currently no cure for HD, but symptoms can be partly treated (10). There are indications that environmental factors and lifestyle may modulate the onset and progression of HD (11, 12), suggesting that rehabilitation could be a good option for symptomatic relief.
There is evidence to suggest that physiotherapy and exercise may be helpful for balance, motor function, flexibility, and gait speed (13–15). Studies investigating a combination of physiotherapy and occupational therapy in home-based and partly clinical settings mainly show positive results in motor symptoms (16, 17).
Over the past 2 decades, a few studies have investigated the effect of more intense multimodal rehabilitation for people with HD. The results are promising in terms of motor functions and physical quality of life (18–20). An early pilot-study shows some cognitive benefits (18), which have not been replicated in later studies (19–21). The rehabilitation programmes also seem to have a positive effect on psychiatric symptoms, with reduced depression and anxiety (19, 20). Psychological outcomes are highlighted in interviews with patients (22) in which the importance of being part of a group and mental and social outcomes was emphasized in addition to physical outcomes. The rehabilitation programmes evaluated are recurring inpatient rehabilitation, lasting 1–2 years. The programmes have a high dropout range (16.2–72.5%), which may be due to disease-related problems. As an extensive multimodal rehabilitation seems to affect not only physical symptoms, but also psychological factors, the question arises as to whether this also applies in a shorter day-care setting.
The aim of the present study was to evaluate the tolerability and effect of a 25-day, group-based, multimodal rehabilitation programme for people with HD on psychiatric symptoms, health-related quality of life (HRQoL) and psychological health factors. Thus, the programme was evaluated concerning tolerability, measured as dropout rate, cancellations and patient satisfaction. Furthermore, the effect of the programme on psychiatric symptoms (anxiety and depression), HRQoL, and sense of coherence, and the role of demographic, psychological or disease-related factors that influence the outcome of the programme were evaluated. As secondary aims, the physical effects of the programme, and the relationship between physical and psychological effects were evaluated.
METHODS
This explorative cohort study is based on register data from the rehabilitation centre’s registry, for the period 2014–2017.
Settings
A specialized neurological rehabilitation centre in Stockholm that manages outpatient rehabilitation for HD.
Subjects
The study includes all 20 patients who completed the programme in 2014–2017. The patients were over 18 years of age, diagnosed with HD, and able to take an active part in group activities. The programme was aimed at patients in the early stages of the disease.
Description of the rehabilitation programme
The programme being evaluated consisted of 25 days of rehabilitation (approximately 4 h/day), 3 days/week for 8 weeks. Each group comprised 2–5 patients.
An experienced team, consisting of a specialist in rehabilitation medicine, a neuropsychologist, an occupational therapist, a physical therapist, a speech therapist, a social worker, and rehabilitation assistants, conducted the rehabilitation.
Most of the treatment was given in a group setting, and consisted of information and education, group counselling, physical training focusing on strength, balance, and relaxation, speech therapy and creative activities (e.g. ceramics, leatherwork, gardening). Treatments that were offered individually included guidance on social support and insurance issues, assessment and recommendations concerning safe swallowing, and subscription for equipment (physical aids, cognitive aids and sleep aids). The needs of the family were addressed and, if possible, meetings with relatives were held in parallel with the rehabilitation.
There was some flexibility in the programme, with the possibility to modify the intensity and distribution between different parts of the rehabilitation for each group. The core elements, however, stayed the same over the evaluation period and are described in more detail in Appendix I.
As a multimodal rehabilitation, the knowledge from each profession permeates all parts of the treatment. Specific neuropsychological interventions regarding cognitive symptoms included education about brain functions and cognition, cognitive strategy training (internal and external strategies) in sessions where theory and practice were combined. Cognitive strategies were also noted and trained in creative activities and during physical training sessions.
Psychiatric symptoms were addressed, particularly in weekly counselling groups where mental health, work-life, social life and thoughts and feelings about the rehabilitation were discussed. The patients with more pronounced psychiatric symptoms were offered individual follow-up with the neuropsychologist during the programme. The focus on finding enjoyable activities to practice both cognition and exercise also had a psychological component, with the intention of breaking negative cycles of inactivity (partly due to disease-specific problems with initiative and apathy) and depression. During the rehabilitation, especially towards the end, the emphasis was on finding ways to continue with the positive activities that started during rehabilitation.
Data collection
Demographic data collected were: patients’ age, sex, years since diagnosis, information about living conditions (i.e. if the patient was living alone or had support at home from a spouse/parent/s) and earlier experience of HD-specific rehabilitation in the clinic (yes/no).
Outcome measures
Tolerability measures
To test the tolerability of the programme, in additions to dropouts (i.e. patients who started the programme but discontinued prematurely), the number of cancellations (i.e. days of rehabilitation in which the patient did not participate) was registered.
At the end of the rehabilitation programme, the patients completed a written evaluation, rating their overall impression of the rehabilitation, treatment by staff, the relevance of the content, increased knowledge about their difficulties and resources, and effect on daily life on a 5-grade scale.
Measures of psychological effects
For assessments regarding psychiatric symptoms (more specifically anxiety and depression), HRQoL and psychological health factors, self-rating questionnaires were distributed at the start of the rehabilitation programme, i.e. baseline (T1) and the end of the rehabilitation programme (T2). The patients completed the forms on their own, but part of the rehabilitation team was available for questions.
Psychiatric symptoms were measured with Hospital Anxiety and Depression Scale (HADS) (23), a questionnaire designed to measure anxiety and depression for patients in somatic care. The scale is divided into 2 subscales, 1 for anxiety and 1 for depression. Each subscale consists of 7 statements, which are answered on a 4-graded scale from 0 to 3. A total above 8 for each subscale indicates a possible anxiety or depression state with clinical significance (24). The mean scores for the general population in Sweden (25) are 4.55 (standard deviation (SD) = 3.73) for the anxiety subscale and 3.98 (SD = 3.46) for the depression subscale.
HRQoL was measured with EuroQol Visual Analogue Scale (EQ-VAS). EQ-VAS is part of the standardized instrument EuroQol five-dimensional questionnaire (EQ-5D) (26) that was developed to measure HRQoL. EQ-VAS records the patient’s self-rated health on a vertical visual analogue scale, from 0 (worst imaginable health state) to 100 (best imaginable health state). The total mean EQ-VAS for the general population in Sweden is 83.3 (27).
Psychological health factors were measured with the Sense of Coherence – 29 item scale (SOC-29) (28). The questionnaire was developed to measure sense of coherence, which consists of 3 interrelated components: comprehensibility (the sense that you can understand events and reasonably predict what will happen in the future), manageability (the belief that things are manageable and within your control), and meaningfulness (the feeling that things are meaningful and there is a good reason to care about what happens). Sense of coherence was originally presented as a global orientation that predicts how people manage stressful situations and stay well. Studies have shown that, even though the sense of coherence has a moderating effect on health (29), it is not as stable as initially assumed (30), and thus might be affected by rehabilitation.
Measures of physical functioning
During the period of evaluation, different measurements were used to evaluate physical improvement. When available, data at T1 and T2 for the Mini-Balance Evaluation Systems Test (Mini-BEST), Timed Up and Go test (TUG), and 6-Minute Walk Test (6MWT) were analysed. Mini-BEST is a performance measure designed to analyse several postural control systems that may contribute to poor functional balance in adults (31). TUG is an item in the Mini-BEST that requires both static and dynamic balance, using the time that a person takes to rise from a chair, walk 3 m, turn around, walk back to the chair and sit down. The 6MWT is a submaximal exercise test used to assess aerobic capacity and endurance (32), measuring the distance walked in 6 min. Physical measures were conducted by the treating physiotherapist.
Statistical methods and data management
To measure treatment efficacy, the change in mean value was compared between T1 and T2. For continuous, normally distributed data and normally distributed data at ordinal level comparisons were performed by paired Students t-test. The Wilcoxon signed-rank test was used for assessing skewed variables. Due to the small sample size, the effect size (Cohen’s d) was calculated when the p-value was < 0.2.
The data-set was controlled for outliers using box-plot diagrams. If outliers were found, calculations were made with and without outliers and discussed in further detail.
To test correlations between normally distributed variables the Pearson correlation coefficient was used, and Spearman’s rank correlation was used for skewed variables.
Data from the first enrollment was used for patients who attended the programme more than once during the period 2014–2017.
All analyses were performed using statistical software, IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. The statistical threshold was set at p < 0.05.
Ethical considerations
The intervention evaluated in this study is part of a clinical routine and posed no risk to the patients. As the study is a register study with no access to information that could reveal the identity of the patients, ethical approval was not required.
All patients were provided with oral and written information about the register study at the start of the rehabilitation period. Participation was voluntary, and the patient was entitled to cancel his or her participation at any time.
RESULTS
Demographic data
Of the 20 patients 80% (n = 16) were female. The mean age was 51.6 (SD 11.9), age range 23–74 years. The mean time since diagnosis was 3.9 (SD 4.3) years, with a range of 0–13 years. The percentage of patients living with a spouse or parent/s were 65 (n = 13), and 85% (n = 17) had not previously participated in HD-specific rehabilitation in the outpatient clinic.
There were no sex differences in any measurements at baseline, except for HRQoL (EQ-VAS) t(18) = –2.62, p < 0.05, where males had a significantly higher score.
Tolerability
During 2014–2017, a total of 22 patients were enrolled in the programme. Two patients (both male) did not complete the 8-week-period, giving a 9.09% dropout rate. Given the small sample and risk of identification of individuals, no subsequent analyses have been made on dropouts. The mean number of cancellations during the 25-day programme was 2.7 (SD 3.1, n = 20), giving an 89.2% attendance rate. The number of cancellations was not equally distributed, with a median value of 2 (range 0–12). The number of cancellations had a significant negative correlation with T1 overall SOC-29 score (r = –0.51, p = 0.021) and T1 SOC-29 Manageability (r z = –0.47, p = 0.034). No significant correlation was found between the number of cancellation and demographic factors, psychiatric symptoms or HRQoL at T1. No significant correlation was found between the number of cancellations and treatment effects on any variable.
Data from the written evaluation (self-reported outcome) are shown in Table I. The scores were generally high, indicating overall satisfaction with the programme. The total score was not significantly correlated with the number of cancellations or differences between T2 and T1 on any variable.
Table I.
Self-reported evaluation at the end of the rehabilitation programme (n = 20)
| Questions | Mean (SD) | Median (range) |
|---|---|---|
| What is your overall impression of the rehabilitation you have received? | 4.6 (0.6) | 5.0 (3–5) |
| How do you feel that you have been treated by the staff? | 4.8 (0.4) | 5.0 (4–5) |
| Has the content of the rehabilitation been relevant to you? | 4.3 (0.8) | 4.0 (2–5) |
| Has the rehabilitation given you increased knowledge about your difficulties and resources? | 4.2 (0.8) | 4.0 (2–5) |
| Do you feel that rehabilitation has affected your everyday life in any way? | 4.5 (0.6) | 4.5 (4–5) |
| Total score | 22.4 (2.7) | 23.0 (15–25) |
For each question, the minimum value was 1 (not good/not at all/worsened the situation) and the maximum value was 5 (very good/completely/improved the situation). Minimum value on the total score was 5, maximum 25. SD; standard deviation.
Psychological effects
Mean and median scores for the psychological outcome measures are shown in Table II. There was a significant treatment effect (the difference between T2 and T1) on both anxiety and depression, with small to medium effect sizes. A lower HADS score post-treatment indicates reduced anxiety and depression after the rehabilitation programme. The data for HADS was adjusted for outliers, as one patient’s results on HADS T2 differed greatly (2.8 SD) from the rest of the patient’s scores, which had a disproportionately high impact on the results. Results including the outlier are shown in Table II. A significant treatment effect, with medium effect size, was found regarding HRQoL. Increasing scores in EQ-VAS indicate improvement in HRQoL after the rehabilitation programme. No significant treatment effects were found for the sense of coherence.
Table II.
Psychological outcome measures as measured at baseline (T1) and the end of the rehabilitation programme (T2), mean/median difference and statistical results (n = 20)
| Variables | T1 | T2 | Diff T2–T1 | pvalue | Effect size |
|---|---|---|---|---|---|
| HADS-A (n = 19)*, mean (SD) | 8.3 (5.1) | 6.4 (4.4) | –1.9 | 0.03 | 0.4 |
| HADS-A, median (range) | 9.0 (0–16) | 6.5 (0–21) | –2.5 | 0.13 | 0.3 |
| HADS-D (n = 19)*, mean (SD) | 4.9 (3.5) | 4.1 (3.1) | –0.8 | 0.01 | 0.3 |
| HADS-D, median (range) | 4.5 (0–14) | 4.0 (0–16) | –0.5 | 0.13 | 0.1 |
| EQ-VAS, mean (SD) | 67.3 (20.7) | 77.3 (17.1) | 9.9 | 0.05 | 0.5 |
| SOC-29 CP, median (range) | 41.0 (27–58) | 42.5 (27–56) | 1.5 | 0.85 | |
| SOC-29 MA, median (range) | 46.5 (21–65) | 44.5 (27–63) | –2.0 | 0.99 | |
| SOC-29 ME, median (range) | 41.0 (21–52) | 36.5 (27–54) | –4.5 | 0.48 |
Effect size (Cohen’s d) was calculated when the p-value was < 0.2. Effect sizes < 0.2 are considered trivial, 0.2 represents a small effect size, 0.5 a medium effect size, and 0.8 a large effect size (34). p-value ≤ 0.05 in bold. HADS-A: Hospital Anxiety and Depression Scale, Anxiety; HADS-D: Hospital Anxiety and Depression Scale, Depression; EQ-VAS: EuroQol Visual Analogue Scale; SOC-29 CP: Sense of Coherence – 29 item scale, Comprehensibility; SOC-29 MA: Sense of Coherence – 29 item scale, Manageability; SOC-29 ME: Sense of Coherence – 29 item scale, Meaningfulness; Diff: difference; SD: standard deviation.
Outlier excluded.
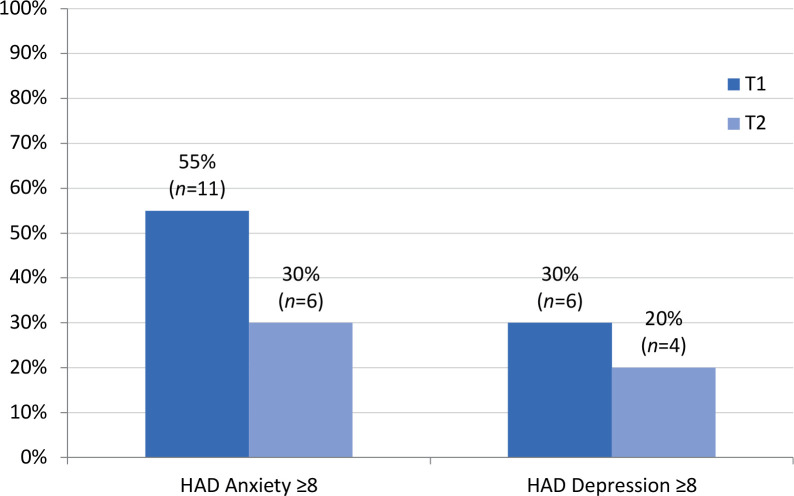
In addition to reduced mean scores for psychiatric symptoms, the number of patients with symptoms of clinical significance was lower after rehabilitation, as shown in Fig. 1.
Fig. 1.
Percentage of patients (n = 20) who had Hospital Anxiety and Depression Scale (HADS) subscale scores indicating a possible anxiety/depression state with clinical significance (≥ 8) (24) at baseline (T1) and the end of the rehabilitation programme (T2).
No significant sex difference was found regarding treatment on any of the effect measurements. Living conditions, age, or year since diagnoses did not correlate with treatment effects on any psychological outcome measure.
Physical functions
Mean and median scores for physical tests are shown in Table III. Measures of physical functions improved, but the difference for Mini-BEST was the only one that was statistically significant (with medium to large effect size), indicating a better balance. There was no significant correlation between any physical measures and the treatment effect of anxiety, depression, or HRQoL.
Table III.
Physical outcome measures as measured at baseline (T1) and the end of the rehabilitation programme (T2), mean/median difference and statistical results
| Variables | T1 | T2 | Diff T2–T1 | pvalue | Effect size |
|---|---|---|---|---|---|
| Mini-BEST (n = 17), median (range) | 22.0 (9–26) | 24.0 (15–27) | 2.0 | 0.01 | 0.7 |
| TUG (n = 15), mean (SD) | 10.2 (5.2) | 9.2 (3.3) | –1.0 | 0.11 | 0.2 |
| 6MWT (n = 13), mean (SD) | 488.2 (109.7) | 506.2 (112.8) | 18.0 | 0.12 | 0.2 |
Effect size (Cohen’s d) was calculated when the p-value was <0.2. Effect sizes <0.2 are considered trivial, 0.2 represents a small effect size, 0.5 a medium effect size and 0.8 a large effect size (34). A reduced number of patients is due to missing data. p-value ≤ 0.05 in bold.
Mini-BEST: Mini-Balance Evaluation Systems Test; TUG: Timed Up and Go test; 6MWT: 6-Minute Walk Test; Diff: difference; SD: standard deviation.
DISCUSSION
This study investigated whether an 8-week multimodal day-care rehabilitation programme was tolerable and could reduce psychiatric symptoms and improve HRQoL and sense of coherence for people with HD.
Although the study size was limited, with only 20 patients, it indicates that the rehabilitation programme was well tolerated, reduced symptoms of depression and anxiety, and had a positive effect on HRQoL for people affected by HD.
The dropout rate of 9.1% was lower than earlier studies of multimodal rehabilitation. Piira et al. (19, 20) reported a 16,2% dropout rate for a 1 year programme and a 40% dropout rate for a 2-year programme, and Zinzi et al. (18) reported a dropout rate of 72.5%. Difficulties fulfilling a programme are not surprising, given the cognitive impairment of executive functions (most important lack of initiative and awareness) and psychiatric symptoms, which are prominent in HD (3, 4). A reasonable assumption would be that participation in a day-care setting would result in a high number of cancellations rather than dropouts. However, the attendance rate of 89.2% was acceptable. No correlations were found between the number of cancellations and the effect on any outcome measures, which may indicate that the cancellation rate was not high enough to harm the outcome of the rehabilitation.
The relatively low dropout and high attendance rates show that the programme was well tolerated. The shorter course of rehabilitation may be an advantage, given the disease-related problems described earlier. A longer rehabilitation programme is more likely to be cancelled prematurely and may therefore not be as gratifying as a shorter rehabilitation that is fulfilled. The evaluation at the end of the rehabilitation confirmed that the programme was perceived as relevant, effective, and that the patients had a high overall satisfaction. It is of interest that the patients’ ability to attend the rehabilitation programme and find it meaningful, fits well with how the loss of motivation, initiative and spontaneity in HD might be reduced by stimulating input and structure (3). Care should be taken when interpreting the results from the evaluation form, however, as it was not validated.
Some factors could be thought to relate to the tolerability of the rehabilitation, e.g. if the patient was living alone (i.e. did not have daily support from family to attend the rehabilitation) or had high psychiatric symptoms. In the present study, however, no significant correlations were found between the number of cancellation and demographic factors, psychiatric symptoms or HRQoL at baseline. The only significant correlation for cancellations was with the sense of coherence (SOC-29 total score) and Manageability subscale at baseline. A negative correlation suggests that a high sense of coherence and, especially, a high sense of manageability, are related to a lower cancellation rate. This could be of interest, as it might indicate that efforts to make life for people with HD more predictable, have meaning and, most importantly, infuse a sense of control, could increase participation in rehabilitation and perhaps other types of medical treatment.
The significantly lower results on anxiety and depression after rehabilitation indicate that the rehabilitation programme had a positive effect on mental health, in line with earlier studies of multimodal rehabilitation (18–20). At the group level, the mean score for anxiety changed from clinically significant to under clinical significance. After rehabilitation, the mean score for anxiety was within the normal range for the population in Sweden (25). The number of patients who had a score indicating psychiatric symptoms of clinical significance, for both depression and anxiety, was also lower post-treatment. Most noteworthy, patients who had a score indicating problems with anxiety decreased from over half of the patients to less than one-third after rehabilitation. It is of note that the mean depression score at baseline was lower than expected, given the high prevalence of depression in HD found in earlier studies (8). The fact that the patients had few depression symptoms to begin with is probably a reason for the relatively small effect size of the intervention. However, the rehabilitation programme seemed to promote psychiatric well-being, at both clinical and sub-clinical levels.
There were significantly higher HRQoL scores after rehabilitation than at baseline. Medium effect size indicates notable real-life changes, and that the result should be considered of importance. Earlier studies (19, 20) have shown an increase in physical quality of life. For this study, a more global assessment was used, which did not differentiate between physical and mental quality of life. The fact that there was no significant correlation between improved physical measures and improved HRQoL indicated that the greater satisfaction with current health state was not solely due to physical improvements.
The rehabilitation programme had no significant effect on psychological health factors, as measured with SOC-29. This might, of course, be due to lack of increased comprehensibility, manageability and meaningfulness. One may speculate, however, that the measurement was not sensitive to change, given that it was originally designed to capture stable properties. It might be of more interest as a descriptive measurement, given the significant correlations with the number of cancellations described above.
One aim of this study was to determine if any demographic, psychological, or disease-related factors were influencing the outcome. In the present study, no factors were related to the effectiveness of the rehabilitation. The programme was aimed at patients in earlier stages of the disease, as they were assumed to benefit more from a rehabilitation programme with a focus on information, compensating strategies and preserving activity. In the current study, the measure of disease progression was years since diagnosis, which did not seem to be related to outcome effects. However, years since diagnosis are not an optimal measure, as the disease progression varies widely between individuals. The time of diagnosis is also dependent on when the patient has met the appropriate clinic. For someone who is a known carrier of the HD gene, this will, of course, be sooner than for someone with no known family history of HD. In other words, years since diagnosis should not be confused with years since disease onset.
Effects on motor symptoms were not of main interest in the current study. However, significant improvements were found on one measure of balance, and non-significant improvements on other measures of balance, aerobic capacity and endurance. As mentioned above, there was no significant correlation between physical improvements and changes in HRQoL or number of cancellations. The same was true with the decrease in depression and anxiety; the change seemed to be unrelated to physical improvements. Thus, the study implies that the psychiatric symptoms might have been due to the neuropsychological elements of the rehabilitation, or simply a result of the combined effect of multimodal rehabilitation.
As to the generalization of the results, the depression rating at the start of rehabilitation was noticeable low. More notably, there was a very unequal sex distribution. There are no sex differences in the prevalence of HD (3), yet 80% of the sample was female. This could be due to chance, but it could also represent a bias in the inclusion process of the programme (as described in Appendix I).
There are several other obvious limitations to the study. The sample size was small, and the absence of a control group hampers the ability to draw firm conclusions. Self-assessment questionnaires might also be a suboptimal choice, given the typical lack of awareness in HD. The data were collected as part of the rehabilitation, by the rehabilitation staff. Hence, the data were not anonymous, and the examiners were not blinded, which of course increased the risk of observer bias and expectancy effects. However, the SOC-29 score did not improve, which speaks against an overall expectancy effect.
CONCLUSION
The results of this study, albeit with limitations, indicate that an 8-week multimodal day-care rehabilitation programme can be tolerable, reduce psychiatric symptoms (anxiety and depression) and improve HRQoL for people with HD. A sense of coherence seems to be related to attendance rate, indicating that efforts to make life more understandable, manageable and meaningful for people with HD might increase participation in treatments.
It is of importance to note that HD is a slowly progressive disease. Many people with HD will live one-third of their life with active illness. A shorter rehabilitation period should be viewed as a complement to, and not a replacement for, long-term treatments. As a complementary programme, it appears to be a good way to boost not only physical functions but also psychological health and quality of life. As the daily cost of day-care rehabilitation is approximately 40% less than for inpatient rehabilitation (33), it might also be more applicable than the earlier evaluated rehabilitation programmes.
Replicating the current study with a larger cohort and/or randomized controls would give more robust and interpretable data, ideally with a follow-up. It would also be of interest to include measurements of cognitive function and/or a wider assessment of the clinical performance (e.g. Unified Huntington’s Disease Rating Scale; UHDRS) to clarify whether cognition and disease progression affect the treatment effect and if there is an optimal time during the disease for multimodal day-care rehabilitation.
ACKNOWLEDGEMENTS
The authors thank Olof Hjorth, PhD, at the Department of Psychology, Uppsala University, for his support and assistance during the statistical analyses. We thank the rehabilitation clinic for providing the data. Furthermore, we thank the professional staff involved in the programme for their dedicated work and valuable input. Finally, we want to express our gratitude to all the patients involved in this study and their families.
Appendix I. Description of the rehabilitation programme.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993; 72: 971–983. [DOI] [PubMed] [Google Scholar]
- 2.Schiefer J, Werner CJ, Reetz K. Clinical diagnosis and management in early Huntington’s disease: a review. Degen Neurolog Neuromusc Dis 2015; 5: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snowden JS. The neuropsychology of Huntington’s disease. Arch Clin Neuropsychol 2017; 32: 876–887. [DOI] [PubMed] [Google Scholar]
- 4.Dumas EM, van den Bogaard SJ, Middelkoop HA, Roos RA. A review of cognition in Huntington’s disease. Front Biosci (Schol Ed) 2013; 5: 1–18. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KE, Marder KS. An overview of psychiatric symptoms in Huntington’s disease. Curr Psychiatry Rep 2001; 3: 379–388. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblatt A. neuropsychiatry of Huntington’s disease. Dialog Clin Neurosci 2007; 9: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale M, van Duijn E. Anxiety in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2015; 27: 262–271. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, et al. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci 2005; 17: 496–502. [DOI] [PubMed] [Google Scholar]
- 9.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington disease. Neuropsychiat Neuropsychol Behav Neurol 2001; 14: 219–226. [PubMed] [Google Scholar]
- 10.Kieburtz K, Reilmann R, Olanow CW. Huntington’s disease: current and future therapeutic prospects. Mov Disord 2018; 33: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 11.Mo C, Hannan AJ, Renoir T. Environmental factors as modulators of neurodegeneration: insights from gene–environment interactions in Huntington’s disease. Neurosci Biobehav Rev 2015; 52: 178–192. [DOI] [PubMed] [Google Scholar]
- 12.Trembath MK, Horton ZA, Tippett L, Hogg V, Collins VR, Churchyard A, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord 2010; 25: 1444–1450. [DOI] [PubMed] [Google Scholar]
- 13.Bachoud-Levi AC, Ferreira J, Massart R, Youssov K, Rosser A, Busse M, et al. International guidelines for the treatment of Huntington’s disease. Front Neurol 2019; 10: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz NE, Rao AK, Kegelmeyer D, Kloos A, Busse M, Hartel L, et al. Physical therapy and exercise interventions in Huntington’s disease: a mixed methods systematic review. J Huntingtons Dis 2017; 6: 217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilney B, Morris ME, Perry A. Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: a systematic review. Neurorehabil Neural Repair 2003; 17: 12–24. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JA, Cruickshank TM, Penailillo LE, Lee JW, Newton RU, Barker RA, et al. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: a pilot study. Eur J Neurol 2013; 20: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 17.Cruickshank TM, Thompson JA, Dominguez DJ, Reyes AP, Bynevelt M, Georgiou-Karistianis N, et al. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: an exploratory study. Brain Behav 2015; 5: e00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinzi P, Salmaso D, De Grandis R, Graziani G, Maceroni S, Bentivoglio A, et al. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: a pilot study. Clin Rehabil 2007; 21: 603–613. [DOI] [PubMed] [Google Scholar]
- 19.Piira A, van Walsem MR, Mikalsen G, Nilsen KH, Knutsen S, Frich JC. Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: a prospective intervention study. PLoS Curr 2013; 5: ecurrents.hd.9504af71e0d1f87830c25c394be47027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piira A, van Walsem MR, Mikalsen G, Oie L, Frich JC, Knutsen S. Effects of a two-year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: a prospective intervention study. PLoS Curr 2014; 6: ecurrents.hd.2c56ceef7f9f8e239a59ecf2d94cddac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Walsem MR, Piira A, Mikalsen G, Fossmo HL, Howe EI, Knutsen SF, et al. Cognitive performance after a one-year multidisciplinary intensive rehabilitation program for Huntington’s disease: an observational study. J Huntingtons Dis 2018; 7: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frich JC, Rothing M, Berge AR. Participants’, caregivers’, and professionals’ experiences with a group-based rehabilitation program for Huntington’s disease: a qualitative study. BMC Health Serv Res 2014; 14: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 24.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 25.Lisspers J, Nygren A, Soderman E. Hospital Anxiety and Depression Scale (HAD): some psychometric data for a Swedish sample. Acta Psychiatr Scand 1997; 96: 281–286. [DOI] [PubMed] [Google Scholar]
- 26.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 27.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014, p. 19–30. [PubMed] [Google Scholar]
- 28.Antonovsky A. Unraveling the mystery of health. How people manage stress and stay well. San Francisco: Jossey-Bass; 1987, p. 218 [Google Scholar]
- 29.Eriksson M, Lindstrom B. Antonovsky’s sense of coherence scale and the relation with health: a systematic review. J Epidemiol Community Health 2006; 60: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson M, Lindstrom B. Validity of Antonovsky’s sense of coherence scale: a systematic review. J Epidemiol Community Health 2005; 59: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med 2010; 42: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 33.Vårdgivarguiden SLL. Neurologisk rehabilitering, planerad specialiserad [The caregiver guide SLL. Neurological rehabilitation, planned specialized.] [Updated 2019 May 22; cited 2020 Aug 24]. Available from: https://vardgivarguiden.se/administration/verksamhetsadministration/rapportera/rapporteringsanvisningar-a-o/neurologiskrehabilitering-planerad-specialiserad/ (in Swedish).
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]