Abstract
A 45-year-old woman presented with a left-sided neck swelling following treatment a year prior for cervical spine chordoma. She had initially been managed surgically with a cervical vertebrectomy and a course of proton beam therapy. Although there had been a degree of residual tissue, her disease remained stable radiologically and clinically. Repeat MRI demonstrated an increasing left paravertebral mass and a head of pancreas metastasis, which shared pathological characteristics with chordoma. Given the advanced metastatic nature of her disease, imatinib was offered with a palliative intent. While waiting for treatment she developed a spinal cord compression, managed with radiotherapy. She commenced imatinib and her disease remained stable for 9 months before progressing clinically and radiologically. This case demonstrates an unusual pattern of metastatic chordoma and provides further rationale for imatinib in such patients.
Keywords: cancer intervention, malignant disease and immunosuppression, oncology, pharmacology and therapeutics, tyrosine kinase inhibitor
Background
Chordomas are rare tumours arising from embryological remnants of the notochord and totalling 1%–4% of skeletal malignancies. They are most commonly found in the sacrum, skull base and vertebrae and predominantly affect men. Chordomas are rarely identified in those below the age of 40, with a peak incidence between 50–60 years old. Median survival is 6 years. Although low grade, chordomas are locally invasive and have an insidious course. Therefore patients often have a delayed presentation with 5% having metastatic disease.1 Common sites for metastases include skin, bone, lungs and brain.2 Clinical features of chordoma vary widely depending on site affected and can be non-specific, ranging from cranial nerve palsies, radiculopathies and bone pain to loss of bladder and bowel function.
Histologically, chordomas can be classified as classical, chondroid or dedifferentiated, with the former being associated with an improved prognosis. They are classically described as having a characteristic physaliferous appearance with immunopositivity for epithelial markers, such as keratin, and S-100.3 Chondroid appearance has been associated with improved outcomes in contrast to dedifferentiated chordomas, which have a clinically more aggressive nature.4
Their clinically indolent presentation, in addition to anatomical location and need to preserve healthy neurological tissue, can present challenges to surgical resection. Proton-beam therapy is often used in conjunction with surgical excision. However, chordomas are relatively chemoresistant and radioresistant, meaning treatment options are limited in patients presenting with recurrence (>20%).
There are few current treatment protocols for recurrence. However, imatinib mesylate, a tyrosine kinase inhibitor (TKI), has been demonstrated to have some efficacy as a targeted therapy, with research demonstrating a period of disease stabilisation.5 6
This case demonstrates an extremely rare pattern of metastases in a patient with recurrent chordoma and disease stabilisation with imatinib.
Case presentation
A 45-year-old woman initially presented with severe neck pain (December 2015) and a left-sided neck lump (March 2016) in Cyprus, where she held dual residence. She had a background of mild asthma and anaphylaxis to penicillin and took no regular medication. She was a non-smoker and drank alcohol within limits. Her father died of liver cancer and her mother died in February 2019 (we are not clear on cause of death). She had two healthy brothers.
Following further imaging and multidisciplinary team (MDT) discussion, and appearances were in keeping with chordoma. She underwent a C3–C5 vertebrectomy, tumour debulking and insertion of iliac bone graft in July 2016. Pathology was consistent with chordoma, with expression of CAM 5.2, MNF and S100 demonstrated. Samples were also positive for brachyury. Postoperative MRI imaging demonstrated significant tumour bulk with residual left paraspinal soft tissue. She completed a course of proton beam therapy in Florida, USA, and repeat imaging between December 2016 and June 2017 showed reduction in the size of the tumour.
In June 2018, she noted a small swelling above the wound site and later attended for review with her neurosurgical team. No neurological deficit was noted, and a local recurrence was diagnosed.
Investigations
Initial CT and MRI imaging at presentation demonstrated a heterogeneously enhancing 62×39 mm mass in the prevertebral region of the lower neck, invading posteriorly into the C3–C5 vertebral bodies (figure 1).
Figure 1.
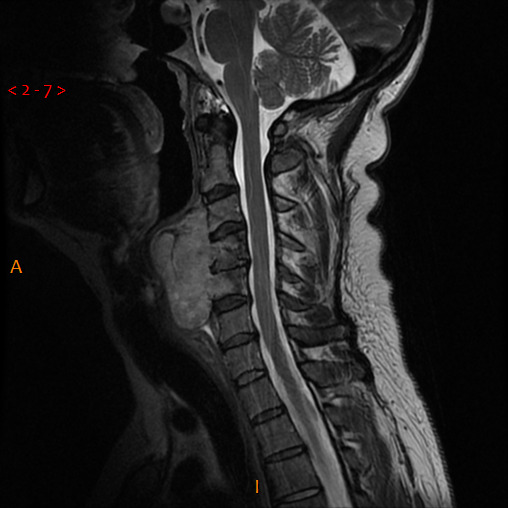
Sagittal view of T2-weighted MRI demonstrating initial cervical lesion.
Following her re-presentation in November 2018, MRI demonstrated a significant increase in the size of the left paravertebral soft tissue mass with components identified from C3 to T1 (figure 2). There was associated deviation of surrounding structures with deposits adjacent to the left sternocleidomastoid. She underwent excision of deposits adjacent to the previous incision and the larger deposit in the lower neck. Pathology confirmed deposits of chordoma in the former (positivity for cytokeratin AE1/3 and brachyury, patchy positivity S100, CK7 negative) but not the latter. No epidermal growth factor receptor mutation was detected; platelet‐derived growth factor receptor (PDGFR) was not analysed. A CT of the chest, abdomen and pelvis revealed bone involvement in the anterior left iliac crest and a 22×20 mm pancreatic neck lesion (figure 3); following discussion at both national sarcoma and pancreatic MDTs, a biopsy was attempted to help guide management. Due to difficult pharyngeal anatomy, multiple endoscopic ultrasound attempts were unsuccessful. A CT-guided biopsy of the head of pancreas mass revealed metastatic chordoma (positive for AE1/3, MJF, S100 negative). All serum tumour markers to date have been negative.
Figure 2.
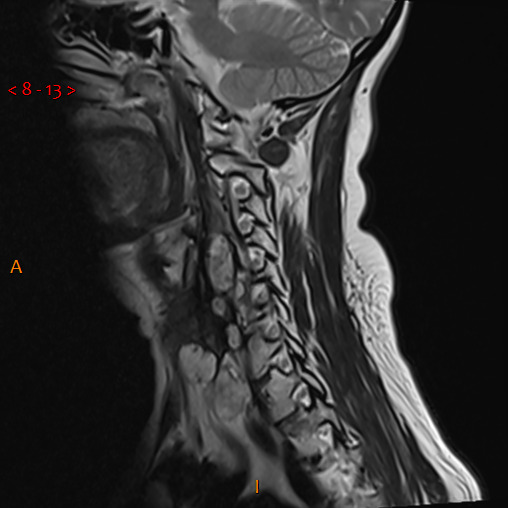
Sagittal view of T2-weighted MRI neck demonstrating recurrence of cervical disease.
Figure 3.
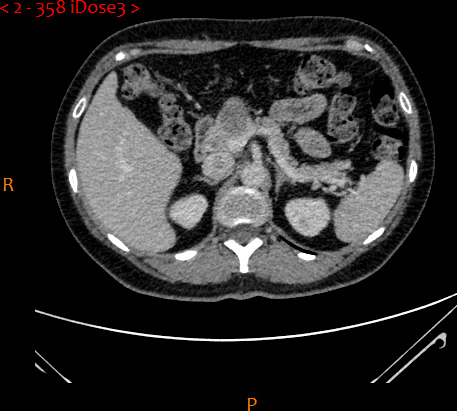
Axial view of CT of the abdomen demonstrating pancreatic head lesion.
Differential diagnosis
It was crucial to obtain a biopsy from the pancreatic mass to characterise the nature of it. Metastatic chordoma with pancreatic metastasis is extremely rare and a secondary synchronous pancreatic tumour had to be excluded.
Treatment
Given the metastatic nature of the patient’s chordoma, further surgery or radiotherapy were felt to be inappropriate. Funding for imatinib was sought and granted, with palliative intent to limit tumour growth, as guided by the local oncology team.
Prior to starting imatinib, the patient had an emergency admission to her local District General Hospital with a collapse. She was found to have impending malignant spinal cord compression, with increase in size of both the primary chordoma and the pancreatic metastasis. She was transferred to the regional oncology centre where she completed palliative radiotherapy (30 Gy in 10 fractions over 2 weeks) and started imatinib in May 2019.
Outcome and follow-up
Imatinib therapy was tolerated well with little toxicity for the duration of treatment. In August 2019, CT imaging demonstrated response of both the cervical and pancreatic lesion. However, further radiological investigation in February and May 2020 demonstrated increasingly rapid progression of both the left paratracheal and pancreatic lesions. Imatinib was discontinued in August 2020 to allow the patient to explore experimental therapies given the speed and degree of growth. Unfortunately her disease continued to progress and she died 9 months later.
Discussion
Metastases in chordoma affect 40% of patients, most commonly in the skin, bone and lungs.4 The identification of a pancreatic metastasis in our patient is extremely rare, with few cases reported in the literature.7 It is not uncommon for patients with chordoma to have symptoms of spinal cord involvement, given the primary tumour site and predilection for local invasion.8 However, the combination of cord compression and pancreatic disease demonstrates an extremely unusual pattern of recurrent metastatic chordoma.
Imatinib is a TKI which is already licensed in several malignancies, including gastro-intestinal stromal tumours (GIST) and chronic myeloid leukaemia. It inhibits multiple receptors including PDGFR‐β (PDGFRB) and KIT, both implicated in oncogenesis.9 10 A number of small scale studies have demonstrated efficacy in advanced chordoma.5 11 In a Phase II trial by Stacchiotti et al 64% of a cohort with advanced PDGFRB-positive chordoma had a clinical benefit (complete response + partial response + stable disease ≥6 months as defined by the Response Evaluation Criteria in Solid Tumours (RECIST)).6 Our case report correlates with the clinical response demonstrated in this paper and provides rationale for further, larger scale research into the efficacy of imatinib in this orphan malignancy. Other compounds have demonstrated promising results, including erlotinib and multikinase inhibitors such as sorafenib, with the latter having progression-free rates of 57.4% at 9 months, compared with 41% for imatinib.12 However, cohort sizes remain small and there have not yet been randomised trials to demonstrate best standard of care in chordoma.
In our patient, imatinib was well tolerated, with minimal toxicity. While adverse events have been reported in patients with chordoma managed with imatinib, such as oedema and fatigue, these are generally in line with those reported in other setting where imatinib is commonly used, such as GIST, and managed with dose reduction.6 13 In comparison, a cohort of patients on sorafenib experienced grade 3 and grade 4 toxicity in 77.8% and 14.8% of patients, respectively.12 Given the lack of systemic therapy available for chordoma and the relatively minimal toxicities of imatinib, these should not present a barrier to further investigation of this compound in advanced chordoma.
There is no current UK or international guideline for clinical management of recurrent metastatic chordoma, and no suitable clinical trials for such patients.14 Given the clinical benefit of imatinib to our patient and minimal toxicities experienced, there is reasonable rationale for further investigation into the utility of imatinib in this cohort, and compassionate use in the intervening period.
Learning points.
This is evidence of unusual pattern of disease with pancreatic involvement; consider unusual sites of metastases in patients with advanced chordoma.
Imatinib should be considered as a systemic immunotherapy in advanced metastatic chordoma, particularly where local therapy is not an option given its potential efficacy and minimal toxicity.
There are many promising targeted compounds in addition to imatinib, such as sorafenib; further randomised trials are needed into precision therapy for chordoma to establish best standard of care.
Acknowledgments
We would like to thank Dr Khalid Ali and Dr Thomas Elswood for their assistance in selecting radiological images for this case.
Footnotes
Contributors: IN proposed the case report and supervised the project. EVK wrote the report with contributions from KH. IN and EVK finalised the manuscript. TRE assisted in sourcing appropriate imaging.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol 2012;13:e69–76. 10.1016/S1470-2045(11)70337-0 [DOI] [PubMed] [Google Scholar]
- 2.Chambers PW, Schwinn CP. Chordoma. A clinicopathologic study of metastasis. Am J Clin Pathol 1979;72:765–76. 10.1093/ajcp/72.5.765 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell A, Scheithauer BW, Unni KK, et al. Chordoma and chondroid neoplasms of the spheno-occiput. An immunohistochemical study of 41 cases with prognostic and nosologic implications. Cancer 1993;72:2943–9. [DOI] [PubMed] [Google Scholar]
- 4.Chugh R, Tawbi H, Lucas DR, et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist 2007;12:1344–50. 10.1634/theoncologist.12-11-1344 [DOI] [PubMed] [Google Scholar]
- 5.Casali PG, Messina A, Stacchiotti S, et al. Imatinib mesylate in chordoma. Cancer 2004;101:2086–97. 10.1002/cncr.20618 [DOI] [PubMed] [Google Scholar]
- 6.Stacchiotti S, Longhi A, Ferraresi V, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol 2012;30:914–20. 10.1200/JCO.2011.35.3656 [DOI] [PubMed] [Google Scholar]
- 7.Morris AA, RABINOVITCH R. Malignant chordoma of the lumbar region; report of a case with autopsy; Comment on unusual metastases to the brain, lungs, pancreas, sacrum and axillary and iliac lymph nodes. Arch Neurol Psychiatry 1947;57:547. [PubMed] [Google Scholar]
- 8.Maccarty CS, David C. Chordoma.A study of fifty-nine cases. Cancer 1952;5:1170–8. [DOI] [PubMed] [Google Scholar]
- 9.Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N Engl J Med 2002;346:683–93. 10.1056/NEJMra013339 [DOI] [PubMed] [Google Scholar]
- 10.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006;24:4764–74. 10.1200/JCO.2006.06.2265 [DOI] [PubMed] [Google Scholar]
- 11.Hindi N, Casali PG, Morosi C, et al. Imatinib in advanced chordoma: a retrospective case series analysis. Eur J Cancer 2015;51:2609–14. 10.1016/j.ejca.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 12.Bompas E, Le Cesne A, Tresch-Bruneel E, et al. Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French sarcoma group (GSF/GETO). Ann Oncol 2015;26:2168–73. 10.1093/annonc/mdv300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–32. 10.1200/JCO.2007.13.4452 [DOI] [PubMed] [Google Scholar]
- 14.Stacchiotti S, Gronchi A, Fossati P, et al. Best practices for the management of local-regional recurrent chordoma: a position paper by the chordoma global consensus group. Ann Oncol 2017;28:1230–42. 10.1093/annonc/mdx054 [DOI] [PMC free article] [PubMed] [Google Scholar]


