Abstract
Introduction
Awareness-raising campaigns play a central role in efforts to combat drug resistance. These campaigns assume that knowledge deficits drive poor practices that increase resistance. Therefore, increasing awareness will promote prudent practices and reduce resistance. However, most awareness campaigns have been developed and evaluated in high-income and public health settings. Consequently, it is not clear whether these campaigns are effective in low-income and middle-income countries and/or within animal health settings.
Methods
Focus group discussions and in-depth interviews were used to collect narratives of veterinary drug use among Maasai pastoralists (n=70), animal health professionals (n=10) and veterinary drug sellers (n=5). Thematic analysis was used to identify recurring themes across narratives and groups.
Results
Narratives of Maasai and animal health professionals indicated that Maasai treated their livestock with limited input from the professional sector and that non-prudent treatment practices were observed (eg, using antimicrobials as ‘energizers’). Professionals linked these practices to knowledge and attitudinal deficits among the Maasai, while Maasai narratives highlighted the importance of climatic uncertainties and cultural beliefs surrounding veterinary care.
Conclusion
Narratives of veterinary drug use from animal health professionals are consistent with the knowledge deficit assumption guiding awareness-raising efforts. In contrast, Maasai narratives highlight how animal health practices are patterned by cultural norms interacting with factors largely outside of Maasai control, including a constrained professional veterinary sector. If these cultural and structural contexts remain unconsidered in awareness-raising strategies, current campaigns are unlikely to motivate practices necessary to limit drug resistance, especially within low-income and middle-income settings.
Keywords: qualitative study, prevention strategies, public health, health education and promotion
Key questions.
What is already known?
-
Drug resistance, especially antimicrobial resistance, is a worldwide threat to public and animal health, food safety and economic security.
Awareness creation among users, prescribers and sellers of drugs has been a dominant strategy to address drug resistance because the filling in of ‘knowledge deficits’ is assumed to promote better practices (eg, prudent use of antibiotics) that will limit the emergence and spread of resistance.
What are the new findings?
Narratives of Maasai pastoralists emphasise that drivers of use and misuse of veterinary drugs partially stem from sociocultural and structural factors, including reductions in veterinary services and increasing impacts of climate change.
Narratives of veterinary use among animal health professionals and sellers of veterinary drugs emphasise knowledge deficits among the Maasai and a reticence to accept new knowledge results in the misuse of veterinary drugs, thereby driving resistance.
What do the new findings imply?
Awareness-raising strategies founded upon understandings of drug resistance in high-income countries are unlikely to produce sustained impact on drug use practices and drug resistance in low-income and middle-income countries.
Awareness campaigns meant to address drug resistance in low-income settings should be tailored to the sociocultural factors and structural inequalities informing animal health-seeking practices in livestock communities.
Introduction
Awareness-raising campaigns feature prominently in strategies to address the health and economic threats posed by increasing levels of drug resistance in public and animal health. Indeed, improving awareness of drug resistance is the first stated target of the action plans of the WHO,1 the Food and Agriculture Organization of the United Nations (FAO)2 and the World Organisation for Animal Health (OIE).3 These awareness creation efforts are exemplified by the World Antimicrobial Awareness Week (WAAW), which occurs every November and was initially launched at the World Health Assembly in 2015 with support from the FAO, OIE and WHO member states. The primary objective of the week is to promote, through awareness-raising, prudent use of antimicrobial drugs in public and animal health to limit the emergence and transmission of antimicrobial resistance. In 2021, for example, the specific theme was ‘Spread Awareness, Stop Resistance’. The overarching theme of every WAAW is ‘Antimicrobials: Handle with Care’—a message advocated through key messages of using antimicrobials only when prescribed by a certified professional (health or veterinary), not sharing antimicrobials and always completing a full course of treatment. Emphasis on prudent drug use is also common in campaigns at regional and national levels. A recent review of 60 awareness campaigns across 46 countries found that the most common message—adopted by 78% of the campaigns—was that ‘misuse and overuse of antibiotics causes resistance’, while the second most frequent—adopted by 72% of the campaigns—was ‘if we use antibiotics incorrectly we will lose them/they will become ineffective’.4
The prominence placed on prudent drug use in awareness campaigns is due to the widely held belief that misuse of drugs is a major force driving drug resistance.5 6 The link between use and resistance is supported by numerous empirical studies that find antimicrobial use correlates with resistance levels in hospitals7 8 and on farms.9 10 In addition, use and resistance are positively correlated when examined at national/regional levels.11–13 Drug stewardship programmes, of which awareness-raising is one aspect, provide evidence that reductions in drug use can decrease resistance levels in agriculture14 and public health.15 16 Critically, however, evaluation of stewardship and awareness campaigns has largely been confined to the public health sectors of high-income countries (HICs). For example, a recent review of stewardship interventions targeted at reducing antibiotic-prescribing practices in low-income and middle-income countries (LMICs) found no studies that evaluated changes in the animal health sector.17
Several reasons exist that question whether awareness campaigns founded upon understandings of drug resistance in HICs, and within the public health sector, would be effective at limiting resistance in LMICs and particularly in animal health. First, the common targets of awareness campaigns in HICs are prescribers of drugs (ie, doctors and veterinarians).15 Within LMICs, the widespread availability of over-the-counter drugs, including antimicrobials and anthelmintics, means that prescribers are not the sole custodians of these drugs and many farmers administer veterinary drugs to livestock without consultation from trained professionals.18 19 Access to professionals is further limited by distance and cost in LMICs.20 21 These constraints make it impractical for most farmers in LMICs to heed awareness messages that stress veterinary drugs should only be used when prescribed by a health professional. Second, the theoretical premise underlying prudent use messaging in HICs—that overuse or misuse is the main driver of resistance patterns—is not consistent with studies conducted within LMICs. Often, these studies do not show an association between drug use and resistance levels in agriculture,22 people23 24 or at national levels.25 Instead, these studies suggest that poor hygiene and sanitation—by encouraging the transmission of resistant micro-organisms—overwhelms selection from drug use.22 23 26 As such, even if awareness campaigns promote prudent practices, their impact on drug resistance may be minimal without corresponding improvements in hygiene and sanitation infrastructures. Moreover, the term ‘prudent’ is highly contextual: what is deemed prudent use from a scientific and HIC standpoint could be considered imprudent in the LMIC context, when a lack of infrastructure and prevention services means the only economically viable option for a livestock farmer is ‘overusing’ antimicrobials.27 28
Acknowledging the contextual differences outlined above, calls have been made to develop more targeted drug stewardship strategies, including awareness campaigns, within LMICs.4 29 30 To date, these calls have largely focused on public health with less emphasis on animal health.31 To help inform awareness-raising strategies within the animal health sector in LMICs, we collected narratives of veterinary drug use among Tanzanian Maasai pastoralists along with narratives from animal health professionals who provide the Maasai animal health services and the individuals who sell them veterinary drugs (ie, agrovets). Thematic analysis of these narratives was then used to identify and theorise the factors underlying treatment patterns in Maasai communities. Finally, we critically appraise these factors to assess the suitability of current awareness-raising strategies and suggest how stewardship strategies can be improved to limit the development and spread of drug resistance in LMICs.
Methods
Study population
Maasai pastoralists
The majority of Maasai pastoralists reside in rural areas of the Maasai Steppe in northern Tanzania and southern Kenya. Most live in extended family compounds called bomas, which are organised along patrilineal lines, and many Maasai continue to practise polygyny. Livestock, primarily cattle, sheep and goat, continue to provide significant nutritional and economic inputs, although many Maasai households now grow some food crops, mostly maize and beans. Livestock care, including healthcare, begins at a young age, with Maasai children tending calves and kids as early as 5–6 years. Boys progress to herding adult goats, sheep and cattle as they age and become moran (warriors), while girls and women, although still tending for animals (eg, milking), will begin to focus more on household chores and childcare.32–36 Older Maasai men usually make the final decisions regarding livestock treatment. However, it is common for men to be gone for long periods, especially to find work in mines or wage labour jobs in urban areas (eg, security guards).35 37 38 Consequently, animal health decisions can also be made by wives and older children.36
Animal health professionals and agrovets
Maasai communities are served by several types of animal health providers with varying expertise in animal health. Providers with the most training are termed livestock field officers (LFOs), who are employed by the Tanzanian Ministry of Livestock and Fisheries (MoLF) and at minimum must have a 2-year diploma in animal health. LFOs are assigned by the MoLF to villages or wards depending on perceived needs and not based on an LFO’s ethnic background or place of origin. For example, of the 12 LFOs in the current study area, only 3 were Maasai. A recurring issue throughout Tanzania is that the number of LFOs working in a district or ward is well below the estimated necessary number. In Longido District, for example, there are a total of 12 LFOs, while the total need estimated by the Tanzanian government is almost two times that number (ie, 23).
Beginning in the 1990s, the increasing gaps in veterinary services referenced above encouraged the training of community members on animal health.39 Originally, these members were referred to as ‘Community Animal Health Workers’, but the Tanzanian government no longer recognises this as a category of animal health service provider. As such, we refer to them as they are referenced within communities as ‘Wahudumu wa afya ya Mifugo ngazi ya Jamii’ (veterinary health workers) or WAMIJA. In this study, all interviewed WAMIJAs were Maasai who resided within the study area. These individuals, most within the last 4 years, had received short trainings (around a month) on animal health, most from a programme called Maisha Bora funded by the Belgium government. As originally conceived during the 1990s, trained community members were meant to provide ancillary animal health support, especially in areas where LFOs had not been positioned. However, our experiences in Longido suggested that WAMIJAs rarely collaborate or communicate with LFOs in the provision of animal healthcare.
Animal health services are also provided by privately owned shops called agrovet shops, which sell veterinary medicines and other health-related products (eg, disinfectants). In the current study location, agrovet shops, whose employees we refer to as ‘agrovets’, can be grouped into two types. One group of agrovets were LFOs who operated agrovet shops as side businesses and none of these individuals were Maasai. The second group were individuals, of which two were Maasai, who owned agrovet shops but did not possess specialised training in animal health. An important development that occurred during the study period was the MoLF instituting a policy requiring every agrovet shop to be managed by an animal professional with at least a certificate in animal health from a government-recognised institution. Although agrovet shops need to be managed by these professionals, our observations suggested that family members (eg, especially wives and older children) or employees who may not have animal health training often assist customers.
Study design and sampling
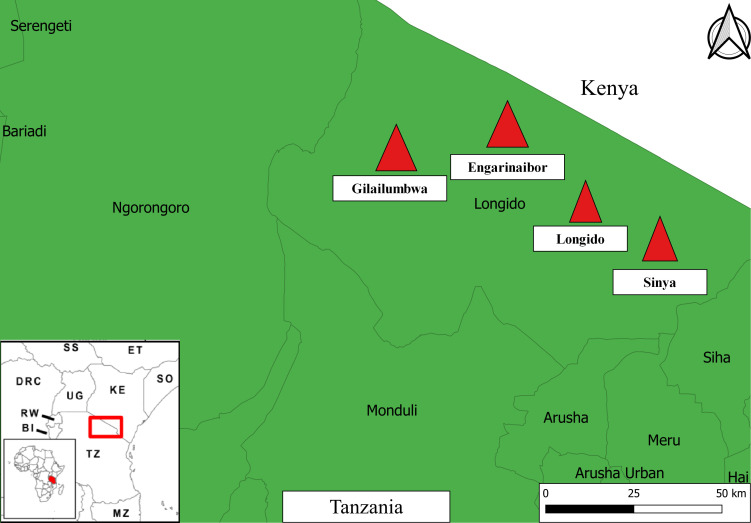
Data analysed in the current study were collected during a larger mixed-methods study of veterinary drug use and drug resistance in Maasai communities (for a general overview of this project, see Caudell et al).40 Data were collected in Longido District, Arusha Region in northern Tanzania between 14 November and 14 December 2018 (see figure 1). Four wards within Longido District were targeted for sampling, namely (1) Longido, (2) Sinya, (3) Gilailumbwa and (4) Engarinaibor. These locations were purposefully selected as communities in these wards represent a spectrum of Maasai livelihoods, from continued reliance on pastoralism (Sinya and Gilailumbwa) to greater reliance on crops or agropastoralism (Longido and Engarinaibor). The study communities are also geographically dispersed, with some (Sinya and Gilailumbwa) far away from animal health offices and agrovet shops (see online supplemental material 1.0 for more background on study locations).
Figure 1.
Map of study locations. Approximate study locations are indicated by red triangles. For inset map, SS is South Sudan, ET is Ethiopia, DRC is Democratic Republic of Congo, UG is Uganda, KE is Kenya, SO is Somalia, RW is Rwanda, BI is Burundi, TZ is Tanzania, MZ is Mozambique. Base layers for the map were downloaded from OpenStreetMap contributors (http://www.vdsgeo.com/osm-data.aspx) and licensed under Creative Commons Attribution-ShareAlike 2.0. The map was created using QGIS V.3.14.
bmjgh-2021-006958supp001.pdf (178.7KB, pdf)
Six focus group discussions (FGDs) were conducted with livestock keepers, with group size ranging from 6 to 12 persons (n=55 total persons). FGD participants were mostly heads of households and were conveniently selected. Additional data were collected through in-depth interviews (IDIs) of ‘influential persons/opinion shapers’ (n=10), who were all Maasai and included traditional elders considered as experts on livestock matters within the community. IDIs were also conducted with LFOs (n=5), WAMIJAs (n=5) and agrovets (n=5) (see online supplemental tables S1 and S2 for interview guides for Maasai livestock keepers and animal health service professionals, respectively).
Analysis of qualitative data was guided by a content thematic analysis approach.41 Audio recordings from FGDs and IDIs were transcribed into text format from Kiswahili and Maa and translated to English. The data were read and reread, noting down initial and diverse ideas in relation to the themes of discussion and interviews. Initial data analysis was conducted by coding data and applying a deductive framework approach to the predesigned categorical themes.41 For example, ‘animal diseases’ had different probes and leading themes, such as ‘knowledge’ and ‘identification’ and ‘treatment seeking’. Each theme was probed by asking the ‘how’, ‘why’ and ‘who’ of the process (eg, who seeks out treatment and how?). Subthemes were identified within the predesigned themes and lower-level codes were assigned to these themes. Examples of subthemes and associated lower-level codes are presented in online supplemental table S3. Coding was performed by PEM and supported by ERM.
Prior to the interviews, an information sheet containing study details and the risk/benefits of participation was provided to potential participants who could read and was read out to those who could not. Written informed consent was sought from study participants who could write and thumbprints were requested from those who could not write.
Patient and public involvement
The public were involved in conducting the study. Specifically, Maasai community leaders and local animal health professionals were consulted to assist in the recruitment of persons for FGDs and IDIs (see online supplemental materials for more information on participant selection).
Results
Demographic data from FGDs and IDIs
Maasai
A total of 55 Maasai livestock keepers participated in the six FGDs across the four wards, 33 (60%) of whom were male and 22 (40%) were female. Only 3 had secondary education, while 25 (45%) had primary education and 27 (50%) had no education at all. A slight majority (53%) fell in the 41–70 age group, with the remaining falling in the 20–40 age group (see online supplemental table S4 for the number of FGDs in each ward and online supplemental table S5 for more detailed demographic information on FGD participants). Opinion shapers selected for IDIs were all Maasai men aged between 41–80 years old, most having some level of primary education. Opinion shapers included village and hamlet chairmen, traditional leaders, and village and ward executive officers.
Animal health professionals and agrovets
Interviewed LFOs were all men between the ages of 20 and 40, with four having a work experience of between 6 and 12 years and one with 1–5 years of experience. Three LFOs had a diploma in veterinary science and two had a bachelor’s degree in veterinary science. WAMIJAs were all between 40 and 80 years of age and included three men and one woman. All had certificates in animal disease management, with three working as WAMIJAs between 6 and 12 years and one less than 5 years. For interviewed agrovets, three out of the five were men, majority of whom were between 20 and 40 years of age. All agrovets have between 1 and 5 years of experience, with three having no animal health training and two having a certificate or diploma in an animal health field (see online supplemental table S6 for the number of IDIs in each ward and online supplemental table S7 for more demographic information on IDI participants).
Qualitative results
In the following sections, we highlight the major themes emerging from the qualitative data analysis, supporting each theme with quotes from our FGDs and IDIs.
Maasai narratives of veterinary drug use
Theme 1: veterinary drugs as ‘energizers’ and ‘health promoters’
Maasai respondents spoke of using veterinary drugs to perform several functions beyond disease treatment. These functions can be grouped into interconnected roles of (1) promoting health (afya in Kiswahili), (2) energising and (3) fattening. Some drugs, particularly anthelmintic drugs, were believed to have the capacity to fulfil all roles, but were especially effective as energisers. As one respondent noted, with agreement from the group:
During the dry season, I notice my goats and cattle have emaciated, because of the dry-sunny condition they do not get enough pasture, that is why they become so weak. I go to the agrovet I tell him/her “doc, because of the weather condition, my livestock conditions have deteriorated, they are weak, what drugs should I give them? Together with their weak condition, what can you give me that can help remove diseases and give them strength so that they can remain as such [strong] until the rains come, where we will have enough pastures, so that they can regain strength?” —R4-FGD-MALE-FU-LONG-01
As with ‘energizing drugs’, drugs that Maasai respondents recalled when discussing ‘fattening’ (kunenepesha in Kiswahili) were mostly anthelmintic. Some Maasai believed the drugs themselves fattened the animal, while others believed the drugs changed animal behaviour (eg, grazing behaviours), which allowed the animal to gain weight. Regarding the former, one respondent said:
These fattening drugs we also use during the rainy season. You can first give them de-wormers, which you inject in the skin, that we call Enginyois [‘worm’ in Maa], or use the drug you inject in its body [i.e., intramuscular], we call it Nalisan [a trade name], so that they can eat pasture normally and become healthy. That is when we say it fattens. —R5-FGD-02-MALE-FOLLOW UP-SINYA
A constant across these differing narratives of ‘energizing drugs’ was the importance of combining different drugs. This process was described as follows by an elder:
First, it (ie, Ivermectin) kills the small parasites inside the animals’ stomach, also…It can be…that which fattens the livestock. …Dewormize Plus- we give goats and sheep to fatten them especially after giving the Ivermectin. Besides fattening them, the drug helps the animal to get rid of small worms passing them through their faeces. —IDI-FU-0017
Theme 2: different drugs for different seasons
Discussions surrounding veterinary drugs as ‘fatteners’ and ‘energizers’ highlighted the important role of seasonality in treatment practices. More specifically, certain drugs were preferred during the dry season, while others were perceived as functioning well during the rainy season. For example, the drug levamisole was preferred during the rainy season, while Nalisan, a commercial albendazole, was a ‘dry season drug’. As an influential elder commented:
You give them deworming drugs after every 3 months…also there are drugs you give only during dry season, drugs that if you give them during the rainy season they won’t work. There is also a drug we give them during the rainy season hence it does not function when you give them during the dry season. —IDI-OS-LONG3-0004
While there was some disagreement among FGD participants on the appropriate drugs for seasons, the underlying logic was framed around ecological and epidemiological differences across the seasons. The Maasai believe that it is during the long rainy season when the disease burden is highest—a belief consistent with epidemiological research in East Africa livestock populations39—which means that most drugs used during the rainy season are perceived as ‘treatment drugs’. It is the time when most veterinary drugs are bought and used, and others hoarded, for the next season. As a Maasai woman said:
We decide any treatment for our livestock by preparing for the upcoming season. This plan is best implemented in April (rainy season) when there is enough grass and water. That is when I allocate one of my big bulls for sale in order to purchase enough drugs for the livestock. The best time to make decisions is April when the pastures are plenty. —R3-FGD Women-FGD-LONG4-0003
While disease pressures are perceived to be lower in the dry season, animals are anticipated to be more susceptible to diseases during the dry season as they are weaker due to insufficient pastures and water. To overcome these periods of harshness, some Maasai said they injected drugs both for energising and fattening, which they said was to successfully manoeuvre the dry season and ensure a good market price. Summing up this seasonal strategy, one Maasai respondent said:
I have a small addition to make, (before giving drugs) we look at the season, such as dry season for instance. During this time the livestock are very weak and emaciated. You give them the drugs that are meant to give the cattle strength, because to make the journey to where water is can take a long time. When they return you can decide to give them drugs to wear off tiredness from the journey. —R3-FGD-01-MALE-FUP-LONGIDO
Theme 3: veterinary drugs as vaccine replacements
Across our interviews, Maasai respondents kept employing the terms kinga (prevention), chanjo (vaccination) and tiba (treatment), interchangeably when discussing veterinary drugs, making it clear that these drugs, like vaccines, were believed to have some abilities to prevent disease. This prevention ability is clear in how Maasai narrate the drug administration process. During one of our visits to bomas to observe livestock treatment, the head of the boma, with help from his 10-year old son, was injecting the anthelmintic ivermectin into his goats and explained, “I usually make sure I vaccinate my animals after every three months,” he told us “…it helps a lot and it makes the animals look good.” Throughout his description, he continually used the Kiswahili word for vaccine (chanjo). Requesting him to clarify the vaccination process, he told us that the drug prevents them (sheep and goats) from getting diseases during the period of 3 months. This belief—that drugs perform similar roles as vaccines—was echoed in our FGDs. However, drugs were thought to act more as ‘delayers’ of diseases, while vaccines were perceived more as ‘long-term fixes’. As one respondent told us:
I cannot talk much about vaccination drugs…We are not very familiar with vaccination drugs but [we are aware] about drugs for prevention. For example, there is this disease called orkipei (CCPP), if you do not have almasi (brand name Alamycin) drug, which is the exact drug for CCPP, you can inject terramycin instead. Now, that gives you time to make arrangements to seek for the drug that treats [Alamycin], now that is prevention. —IDI-INFL-LONG4-0007
Maasai beliefs regarding the equivalence between vaccines and veterinary drugs also meant vaccines could be used for treatment. As with drugs, some Maasai respondents believed that vaccines could be applied when the animal is diseased. To explain this activity, Maasai respondents used the phrase chanjo ya kutibu, which literally translates to ‘vaccines for treating’. These beliefs impact livestock treatment patterns, as one animal health professional told us:
I was on a vaccination mission recently, to prevent LSD [lumpy skin disease], an outbreak, which has vehemently attacked our district. One farmer had cattle that were clearly already infected, as the symptoms were obvious. He too wanted his animal vaccinated. I explained to him that we cannot vaccinate the animal because it was already infected, and the vaccine only works on those that are not yet infected. He was adamant and refused to leave. So, I told him to wait until I finish the rest. To satisfy his demands, I have 5 cc left, I decided to inject in order to please him. He was very happy after that and left. —R6-IDI-Follow-Up
Theme 4: preference for broad-spectrum drugs and drug experimentation
Discussions with livestock keepers highlighted a preference for broad-spectrum veterinary drugs and particularly broad-spectrum antibiotics, which act on two major bacterial groups, Gram-positive and Gram-negative. Popular broad-spectrum drugs included tylosin, terramycin, and amoxicillin. To the Maasai, the preference for these drugs was borne out of necessity to protect the livestock from present diseases as well as other unknown/yet to be known potential threats. Failure of one broad-spectrum drug led to the application of others, which often continued until an improvement was achieved or the animal died. As a Maasai respondent described the treatment of Contagious Caprine Pleuropneumonia:
Currently, terramycin is like an extra drug now, which you can use whenever you see the animal is not well, and it can be better after using it. However, when it does not get well you use tylosin and when the animal did not get well again you can use almasin…. —R7-FGD-MEN-LONG4-0004
Maasai livestock keepers would often shift from one broad-spectrum drug to another, usually based not on knowledge of the ingredients or advice of animal health professionals, but from past experiences and discussions with extended family and friends. When a preferred drug did not work, the obvious option was to make a change to another drug, preferably a broad-spectrum drug.
Descriptions of how the Maasai use broad-spectrum drugs also emphasised the role of experimentation in the treatment process. Describing the application of a drug named Ofomen, a Maasai participant said:
Ofomen, you just inject it in your animal if they have emaciated too much, you inject one cc after every now and then and observe because these drugs are not for diseases, they are just for health/energizing. —R6-IDI-FUP (emphasis ours)
Different versions of ‘administer every now and then and observe’ were found across different drug types, often in association with not knowing the disease that was afflicting the animal(s). A Maasai man elaborated on this process when describing the steps taken after cattle and goats come back weak from the temporary bomas (ie, where livestock are taken in the dry season):
First, they do not have good health but it is impossible to know why, none of them are ill because none shows any symptoms of illness. They consume pasture and drink water as usual. That is when you take such drug called Enginyois [“worm” in Maa]. You use Enginyois after seeing that the livestock is weak but there is no symptom of illness whatsoever, only that they are weak. You take that drug and inject all of them. After that, that is when you observe their development. —R8-FGD-01-MALE-FUP-LONGIDO
Animal health professional and agrovet narratives of antimicrobial use
Theme 1: lack of knowledge among the Maasai drives drug use and misuse
In our IDIs with animal health professionals and agrovets, the most common reason given for veterinary drug use patterns among the Maasai, and particularly drug misuse, was their lack of knowledge/awareness about the importance of prudent use and drug resistance. As one animal health professional described livestock treatment among the Maasai:
It is only him who observes the animals’ symptom and hurriedly travels to Arusha [a major city in northern Tanzania] without any advice from his livestock veterinary officer to give him a proper diagnosis and prescription for appropriate drugs for the illness. After arriving to Arusha he buys drug of his liking, comes home, decides to estimate his own dose for the animal, that’s arbitrary drug use, and…that is a contribution to the increase of drug resistance. —IDI-LFO-LONG1-001
Another professional provided more details on how this lack of knowledge impacted drug administration:
All the treatments are done by the farmers themselves. That is why I admit that because it is them who treat, the amount of drug and where to inject is a big challenge for them. They go against the drug instruction…Farmers memorize all cattle with the same weight simply because they are cattle and inject the same CC and then the drugs fail. Drug resistance is caused by this community injecting habits, not the drug. I blame the farmer for injecting while he or she is not trained to do so. They avoid paying the vet officer to do it and at the end they tarnish the name of the drug as being resistant, it is not resistant, they underdose. —IDI-LFO-LONG1-002
As alluded to in the quote above, animal health professionals also claimed the Maasai tend to reject new information due to misconceptions about the capabilities of veterinary drugs. A widely held belief among animal health professionals was that pastoralists in the area did not observe recommended treatment guidelines. For example, it was believed that a common practice among the Maasai was to inject the sick animal once and if the animal regained strength and appeared better no more injections would be given. Animal health professionals also claimed that if they prescribed a drug with a treatment period of more than 1 day Maasai customers would perceive the drug as weak. There were also suggestions that some agrovets will not explain the full dose in fear of the customer perceiving the drug as ‘weak’. Finally, animal health workers told us ‘new ideas’, such as the importance of vaccination, were not always positively received. A local field officer reported that he has tried to educate Maasai to vaccinate but only a handful tended to accept his advice. As he further explained:
I have tried to advocate for vaccination but the response has been so little….most wait until their livestock have started dying that is when they demand for vaccine, but when the livestock is alive and healthy if you tell them to vaccinate he tells you, “is the animal sick you are telling me to vaccinate, for what?” —IDI-LFO-LONG4-0005
Theme 2: agrovets as promoters of use and misuse
Trained animal health professionals also tended to blame agrovets for the use and misuse of veterinary drugs. In the case of vaccinations, for example, it was suggested that some agrovets were against vaccination as this may impact drug sales. As one professional asserted:
You know these business people, if you vaccinate their agrovet shops will not market any treatment drugs. If you vaccinate for CCPP no one will ever buy drugs. This farmer is not aware that there is a vaccine for it and he is not told. The agrovet owner does not want to create vaccine awareness because he will lose drug clients…. —R1-MALE-WAMIJA-001
This belief particularly applied to the owners of agrovet shops who were also government veterinary officers, which was common in our study area, and led to several conflicts of interest. First, these individuals were perceived as being in control of vaccinations, as vaccinations are often regulated by the Tanzanian government, and they had the power to limit supply of vaccinations and drive demand for veterinary drugs in their shops. Second, it was believed that some agrovet shop owners who were also government employees charged for both the vaccination and their service in administering the vaccine, the latter of which should be provided at no/subsidised cost.
When probing animal health workers about Maasai use of veterinary drugs as ‘energizers’ and ‘fatteners’, respondents confirmed this was a regular practice, while professionals again highlighted the role of agrovets in perpetuating these practices. In terms of purchasing patterns, our agrovet respondents said the Maasai would often ‘demand those drugs’ upon entering the shop. Trained animal health professionals claimed these issues were exacerbated as some agrovets were not ‘professionals in drugs’, so they were not in the position to probe why a Maasai needed a drug. Due to this lack of knowledge, animal health professionals believed that agrovets could become champions in promoting arbitrary drug use. As one professional told us:
They don’t know (about the correct drug) because many of them who are selling the drugs are not specialists, it is a business which is being operated haphazardly, anyone can do it…. —IDI-LFO-LONG4-0005
Discussion
Narratives of veterinary drug use in northern Tanzania reveal that the reasons for use and perceived misuse vary across those who keep livestock and those who provide animal health services. Here, we first examine how these narratives reflect the political, cultural and economic contexts that constrain interactions between Maasai communities and the animal health service sector and how these constraints may perpetuate the non-prudent treatment practices targeted by awareness-raising campaigns. We then discuss how an understanding of the contexts patterning veterinary care among the Maasai informs current and future strategies to use awareness-raising campaigns in addressing drug resistance.
Narratives of veterinary drug use from Maasai pastoralists and those working in the animal health sector highlight that the veterinary service sector remains largely unengaged by Maasai livestock keepers. LFOs and agrovets continually emphasised that Maasai treated their own livestock with little input. These accounts are consistent with results from an earlier quantitative survey among these same Maasai communities, which found that only 7% of Maasai reported ‘always going to a veterinarian when an animal is sick’. This lack of engagement with animal health professionals is not unique to the Maasai and has been documented in livestock communities, especially those in rural areas, across sub-Saharan Africa and Asia.36 42–45
Lack of engagement of Maasai with the professional veterinary sector is partially a consequence of the adoption of structural adjustment programmes (SAPs), which were implemented in Tanzania, and across LMICs, during the late 1980s and early 1990s and led to major structural gaps in veterinary services. Led by the World Bank and the International Monetary Fund (IMF), SAPs allowed favourable loan terms if governments accepted a set of conditions. These conditions included large-scale divestment in public services and the favouring of privatisation, for example through market liberalisation, which neoliberal economists at the World Bank and IMF argued would improve the quality, efficiency and coverage of services (health, telecommunications, education, etc).46 47 However, while substantial divestments in public services occurred across much of sub-Saharan Africa, the push towards privatisation has largely not compensated for service gaps created through divestment, especially in rural areas48 where most Maasai and other livestock-keeping communities tend to live. Global comparisons highlight the failure of SAPs to improve the coverage of animal health services. The ratio of veterinarians to livestock in African countries is about 20 times lower compared with HICs (ie, Denmark, France, Spain and USA). In Tanzania, which has the third highest livestock population in Africa, a 2015 report estimated that only 20% of livestock keepers have access to veterinary professionals.49 Privatisation of animal health services further restricted engagement with professionals by assigning or increasing costs to once-free or subsidised services, such as vaccinations.21
While structural changes in the veterinary sector have impacted Maasai engagement with animal health professionals, the narratives also suggest that, even when available, Maasai may not seek advice or treatment from animal health professionals. In accounting for these tendencies, our previous work has highlighted the role of cultural norms in patterning livestock healthcare among Maasai communities. Many Maasai livestock owners view the Maasai, and by extension themselves, as experts or ‘doctors’ in veterinary care. These norms may justify the self-treatment of sick animals and drive scepticism of non-Maasai animal health professionals. Even if advice or treatment is sought, some Maasai would not admit to self-treatment to the LFO as they were apprehensive of being lectured on their bad practice.36 Admissions of self-treatment failures, combined with lectures from non-Maasai veterinarians, challenge the norms of Maasai as ‘veterinary doctors’. Importantly, these sociocultural influences may continue to limit engagement even in the context of a properly resourced professional veterinary sector. Conversely, additional divestment in the animal health sector that impacts the quality of veterinary care may engender further mistrust of animal health professionals and validate Maasai self-perceptions as ‘veterinary doctors’.
Maasai narrations of veterinary drugs as ‘disease delayers’ suggest how sociocultural factors may interact with gaps in veterinary services in ways that give rise to non-prudent treatment practices. Beliefs that veterinary drugs could play a vaccination-like role may have emerged or been strengthened as divestment in the veterinary sector limited the provision of vaccination services of the Tanzanian government. Couched within the neoliberal logic of SAPs, vaccines became to be viewed more as the responsibility of an individual engaging the private sector and paying a cost. The post-SAP Animal Health Strategy in Tanzania, for example, emphasised that ‘farm level disease control is the responsibility of the livestock keeper and services such as drugs, vaccines and inputs should be sought from the private sector’ (emphasis added).50 Our observations in Longido, however, suggest that encouragement of the private sector has not yielded higher-quality services. For example, Maasai respondents complained that private vaccine providers were only interested in ‘big sales’ (eg, agrovets would only offer vaccination amounts for a herd of 1000 goats) and those with smaller herds were often discouraged from buying vaccinations. With access limited, and beliefs that veterinary drugs could function similar to vaccines, it seems probable that most Maasai would prefer veterinary drugs over vaccines as these drugs are easily accessed at local agrovet shops, they know how to use drugs and little additional technology is needed (eg, a refrigerator to keep vaccines cold).
Interactions between the animal health sector and sociocultural logics of veterinary care are further evident in the role of experimentation in livestock treatment. The narratives presented here, combined with our previous work on veterinary care in these communities,36 51 52 suggest that treatment episodes among the Maasai follow a similar pattern. When an animal becomes sick, owners will largely draw on their ethnoveterinary knowledge as well knowledge from family and friends, especially community members with a reputation for keeping livestock healthy. From this knowledge, they will rationalise and hypothesise the disease/condition afflicting their animal(s) and decide a ‘proper treatment’. If the proper treatment involves veterinary drugs, the owner will go to an agrovet shop to purchase the drug. After purchasing the drug, the owner will then administer the drug themselves, observe the animal, and then decide whether the remaining doses should be given, the dose should be altered or a new drug should be administered. This experimentation process, which would be considered non-prudent in the context of drug resistance, is indicative of a lack of diagnostic inputs, either clinical or laboratory-based, from the professional veterinary sector. Laboratory diagnosis, which is most likely to correctly identify the disease and so result in appropriate drug and treatment regimen being administered, is too costly for most Maasai in our study area, who now must pay for sample collection costs, transport (the nearest laboratory is ≈85 km away) and the diagnosis itself, except during outbreaks recognised by the government.50 This lack of diagnostic input may also have encouraged Maasai preferences for broad-spectrum drugs, a preference that has also been documented in self-treatment in public health.53 By killing a wider range of pathogens, broad-spectrum drugs likely decrease the number of ‘experimental trials’, thereby reducing the costs of treatment. However, broad-spectrum drugs produce greater selective pressures for drug resistance in contrast to narrow-spectrum drugs, which are more likely to be used if the disease is correctly diagnosed.54
Narratives of Maasai livestock owners further suggest that veterinary care may be altered by climate change in ways that promote non-prudent practices, particularly the use of drugs as ‘energizers’ and the seasonal hoarding of drugs. Climate change, which is already affecting the health and productivity of Maasai livestock (eg, milk production),55 is expected to impact the onset and duration of the dry and rainy seasons.56 These impacts are likely to affect veterinary care, as Maasai narratives commonly stressed the seasonal patterning of drug use. For example, some drugs were used as ‘energizers’ to manage the longer distances livestock had to travel to forage in the dry season. Therefore, if Maasai perceive and/or experience harsher or longer dry seasons, the use of drugs as ‘energizers’ may increase. Likewise, if the rains come more sporadically and are not confined to defined periods, as both recent trends and models predict,57–59 the Maasai may respond to the increased disease pressures associated with the rains by hoarding veterinary drugs across the entire year instead of prior to the traditional onset of the rainy seasons. Given the Maasai and their livestock are already experiencing the impact of climate change, more research is needed to understand how veterinary drug use and ethnoveterinary knowledge of the Maasai will be impacted by climatic change and the downstream effects on drug resistance.
Veterinary drug narratives from the animal health service sector, including animal health workers and agrovets, were consistent with the prevailing assumptions of awareness-raising strategies. First, gaps in knowledge and attitudes at the individual level result in non-prudent drug use practices.55 Second, and consistent with Western conceptions of drug resistance, it is non-prudent drug use practices that represent the major drivers of drug resistance.5 6 Animal health professionals in Longido, consistent with professionals from other LMIC settings60 and HICs,56 claimed it was a lack of ‘correct knowledge’ among the Maasai and a mindset that prevented the acceptance of new knowledge that drove non-prudent practices (eg, administering the same drug dosage regardless of animal weight). Underlying the beliefs of animal health professionals in Longido, as with those crafting awareness-raising strategies in the organisations tasked with addressing antimicrobial resistance (eg, WHO), is the logic of biomedical individualism. Biomedical individualism represents disease as an expression of an individual’s choices. As an expression of individual choice, reductions in disease risk can be achieved by increasing awareness, thereby allowing individuals to make better, more informed choices.57 58
Consequences for awareness campaigns and drug stewardship
The underlying causes of veterinary care narratives in Longido, Tanzania, including the structure of veterinary services, sociocultural norms of veterinary care, and the influence of ‘Western’ understandings of drug resistance and the logic of biomedical individualism, provide insight into the effectiveness of conventional awareness-raising strategies. While animal health service providers draw on the logic of biomedical individualism to stress the need for increased awareness among the Maasai, evidence strongly suggests that awareness-raising alone is not sufficient to produce more prudent drug use practices. Surveys conducted within LMICs, including among Tanzanian Maasai, have found that an individual’s knowledge of and attitudes towards drug use and resistance do not correlate with their reported drug use practices, indicating a disconnect between awareness and practices.30 40 51 59 Although awareness-raising can produce behavioural changes, these impacts tend to occur in high-income communities and less so among those communities burdened by structural inequalities in wealth, health and environmental quality,58 61 62 a burden shared by many communities across LMICs. Given the Maasai and other pastoralists are often, along with foragers, the most socially, politically and economically disadvantaged communities within LMICs,63 64 awareness-raising strategies are likely to be particularly ineffective in addressing drug resistance within these communities. Beyond being ineffective, current awareness-raising strategies may even hold negative consequences for Maasai livelihoods. Perceived as giving the ‘correct’ knowledge and attitudes, the deployment of these awareness campaigns may validate stereotypes of Maasai and other pastoralists as ‘primitive’ or ‘backward’65 66 and those who ‘need education on animal husbandry practices’ (cited in Hodgson).67 The spread of these stereotypes, supported by colonialist and postcolonialist policies (eg, recognising land rights for sedentary farmers but not nomadic pastoralists68), provides a broader cautionary tale on how the construction of awareness campaigns based on Western discourses on health and development may exacerbate existing inequalities in LMICs.
Considering the likely ineffectiveness and potential negative consequences of current awareness-raising strategies, what additional steps are needed to develop awareness strategies that produce reductions in drug resistance in the Maasai and other livestock-keeping communities in LMICs? Our analysis of drug use narratives highlights four important areas. First, the development of impactful stewardship strategies will require an understanding of the cultural and social logics of veterinary care that pattern treatment and animal health-seeking practices. Current efforts to understand the drivers of drug use practices in LMICs have largely relied on knowledge, attitudes and practices surveys.1 30 31 However, the questions comprising these surveys, consistent with the logic of biomedical individualism and the need to ‘fill-in’ knowledge/attitudinal deficits, are focused on assessing individual levels of knowledge, attitudes and practices and so usually extract an individual from the social23 and cultural factors that guide treatment practices.69 To uncover these factors will require social scientists, particularly anthropologists, using qualitative and observational methods to understand the sociocultural contexts of veterinary care and how these contexts pattern the social dynamics between livestock owners and animal health service providers.
Second, drug resistance narratives from animal health professionals in Longido reflect the dominant discourse on drug resistance within HICs that misuse of drugs is the primary driver of resistance. This perspective is not consistent with the growing evidence that patterns of drug resistance in LMICs are influenced more by practices and infrastructures related to hygiene, sanitation and biosecurity.23 25–27 In addition, these assumptions may lead to stricter regulations on veterinary drugs within LMICs, where a lack of access to these drugs continues to cause considerably higher mortality and morbidity than drug-resistant infections.70 Consequently, accurate portrayals of drug resistance in LMICs need to inform the development of awareness-raising campaigns, trainings and/or curriculums targeted at animal health service providers. The content of these campaigns will not only need to emphasise the diverse drivers of drug resistance in LMICs but also the structural inequalities and sociocultural contexts that pattern veterinary care. To properly contextualise these trainings will require input from local communities and ethnographic investigations on animal health and indigenous disease knowledge. To this end, anthropologists are well placed to assist in the design of awareness campaigns and trainings as they can operate as ‘interlocutors’ between varying knowledge sets, for example, the ethnoveterinary knowledge of the Maasai and the Western biomedical understandings of animal health.67
If awareness strategies that recognise the sociocultural and economic contexts of veterinary care are to produce sustained behavioural change, a necessary third step is to address the broader structural forces that drive drug resistance in LMICs. The need for a more structural approach to understand health-seeking and ultimately reduce health inequalities has long been recognised in public health71 72 and more recently in addressing One Health issues.73 74 The need for a structural approach to understand and address drug resistance is emerging, for example, in the work of Chandler,27 who argues that antimicrobial drugs have become engrained in our modern infrastructure, largely by filling in for structural deficiencies in biosecurity, hygiene and sanitation. Although structural deficiencies and inequalities caused by decades of divestment in the health services and infrastructure will require broad-scale policy changes (eg, new funding schemes for the veterinary sector), initial efforts can focus on developing awareness campaigns targeted at upstream actors. These upstream actors include policy makers within governments as well as actors within the organisations who both advise and help enact these policies, including those at the FAO, OIE and WHO.
Finally, most of the drugs our Maasai respondents reported to use as ‘energizers’ and ‘fatteners’ were anthelmintics. As anthelmintics target ‘macro-organisms’, resistance to these drugs would not fall within a strict interpretation of ‘antimicrobial resistance’, which is the most used term when discussing drug resistance. How drug resistance is described and defined within strategies and policies is vitally important in efforts to understand and limit resistance. Strict definitions of antimicrobial resistance may funnel research, funding and interventions towards antimicrobial drugs (eg, antibiotics, antivirals) at the expense of anthelmintic drugs. Such trends would be unfortunate as anthelmintic resistance, like resistance to antimicrobials, is increasing globally.75–77 Therefore, awareness campaigns meant to address drug resistance generally should make it clear that efforts to address ‘antimicrobial resistance’ are understood to include anthelmintic resistance as well.
Conclusion
Competing narratives of users, prescribers and sellers of veterinary drugs in northern Tanzania stress that awareness campaigns to address drug resistance in LMICs must acknowledge the sociocultural and structural contexts in which veterinary care occurs. Awareness campaigns that disregard these contexts and continue to focus on ‘filling in gaps’ in Western and biomedical understandings of animal health and drug resistance are unlikely to produce sustained impacts on treatment practices and consequently on drug resistance.
Acknowledgments
We would like to acknowledge the contributions of the Longido research team and those who provided us with narratives, including Maasai livestock keepers, animal health professionals and agrovets.
Footnotes
Handling editor: Seye Abimbola
Twitter: @drekabali2012, @cortney_price, @fofasina
MC and PEM contributed equally.
Contributors: MC, PEM, ERM, AD-G, EK and TK all contributed to designing the study. MC, PEM, ERM, MO and FOF conducted the study. MC, PEM, ERM, AD-G, EK, CP, MO, TK and FOF collaborated in reporting the results. MC is responsible for the overall content of the work.
Funding: The study was funded by the Fleming Fund of the UK (GCP/GLO/710/UK).
Disclaimer: The views expressed here are those of the authors and do not necessarily reflect the views or policies of the Food and Agriculture Organization of the United Nations.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Transcriptions of qualitative interviews are available upon reasonable request from the co-first authors.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Medical Research Coordinating Committee of the National Institute for Medical Research (NIMR) in Tanzania (certificate clearance number NIMR/HQ/R.8a/Vol.IX/2926). Participants gave informed consent to participate in the study before taking part.
References
- 1.WHO . Global action plan on antimicrobial resistance. Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 2.FAO . The FAO action plan on antimicrobial resistance 2021-202. Rome, Italy: Food and Agriculture Organization, 2021. http://www.fao.org/3/a-i5996e.pdf [Google Scholar]
- 3.OIE . The OIE strategy on antimicrobial resistance and the Prudent use of antimicrobials. Paris, France: World Organisation for Animal Health, 2016. https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf [Google Scholar]
- 4.Huttner B, Saam M, Moja L, et al. How to improve antibiotic awareness campaigns: findings of a who global survey. BMJ Glob Health 2019;4:e001239. 10.1136/bmjgh-2018-001239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat 2000;3:303–11. 10.1054/drup.2000.0167 [DOI] [PubMed] [Google Scholar]
- 6.Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 7.Westh H, Zinn CS, Rosdahl VT. An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microb Drug Resist 2004;10:169–76. 10.1089/1076629041310019 [DOI] [PubMed] [Google Scholar]
- 8.Rogues AM, Dumartin C, Amadéo B, et al. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect Control Hosp Epidemiol 2007;28:1389–95. 10.1086/523280 [DOI] [PubMed] [Google Scholar]
- 9.Dorado-García A, Dohmen W, Bos MEH, et al. Dose-Response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg Infect Dis 2015;21:950–9. 10.3201/eid2106.140706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoneit C, Burow E, Tenhagen B-A, et al. Oral administration of antimicrobials increase antimicrobial resistance in E. coli from chicken--a systematic review. Prev Vet Med 2015;118:1–7. 10.1016/j.prevetmed.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 11.Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014;14:13. 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantziaras I, Boyen F, Callens B, et al. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother 2014;69:827–34. 10.1093/jac/dkt443 [DOI] [PubMed] [Google Scholar]
- 13.Dorado-García A, Mevius DJ, Jacobs JJH, et al. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in the Netherlands. J Antimicrob Chemother 2016;71:3607–19. 10.1093/jac/dkw308 [DOI] [PubMed] [Google Scholar]
- 14.Tang KL, Caffrey NP, Nóbrega DB, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health 2017;1:e316–27. 10.1016/S2542-5196(17)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017;17:990–1001. 10.1016/S1473-3099(17)30325-0 [DOI] [PubMed] [Google Scholar]
- 16.Schuts EC, Hulscher MEJL, Mouton JW, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016;16:847–56. 10.1016/S1473-3099(16)00065-7 [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson A, Ebata A, MacGregor H. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics 2019;8:2. 10.3390/antibiotics8010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan DJ, Okeke IN, Laxminarayan R, et al. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis 2011;11:692–701. 10.1016/S1473-3099(11)70054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auta A, Hadi MA, Oga E, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect 2019;78:8-18. 10.1016/j.jinf.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 20.Ahuja V, Morrenhof J, Sen A. The delivery of veterinary services to poorer communities: the case of rural Orissa, India. Rev Sci Tech 2003;22:931–48. 10.20506/rst.22.3.1452 [DOI] [PubMed] [Google Scholar]
- 21.Onono JO, Wieland B, Rushton J. Factors influencing choice of veterinary service provider by pastoralist in Kenya. Trop Anim Health Prod 2013;45:1439–45. 10.1007/s11250-013-0382-7 [DOI] [PubMed] [Google Scholar]
- 22.Subbiah M, Caudell MA, Mair C, et al. Antimicrobial resistant enteric bacteria are widely distributed amongst people, animals and the environment in Tanzania. Nat Commun 2020;11:228. 10.1038/s41467-019-13995-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caudell MA, Mair C, Subbiah M, et al. Identification of risk factors associated with carriage of resistant Escherichia coli in three culturally diverse ethnic groups in Tanzania: a biological and socioeconomic analysis. Lancet Planet Health 2018;2:e489–97. 10.1016/S2542-5196(18)30225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brander RL, Walson JL, John-Stewart GC, et al. Correlates of multi-drug non-susceptibility in enteric bacteria isolated from Kenyan children with acute diarrhea. PLoS Negl Trop Dis 2017;11:e0005974. 10.1371/journal.pntd.0005974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collignon P, Beggs JJ, Walsh TR, et al. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health 2018;2:e398–405. 10.1016/S2542-5196(18)30186-4 [DOI] [PubMed] [Google Scholar]
- 26.Ramay BM, Caudell MA, Cordón-Rosales C, et al. Antibiotic use and hygiene interact to influence the distribution of antimicrobial-resistant bacteria in low-income communities in Guatemala. Sci Rep 2020;10:13767. 10.1038/s41598-020-70741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandler CIR. Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun 2019;5:53. 10.1057/s41599-019-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denyer Willis L, Chandler C. Quick fix for care, productivity, hygiene and inequality: reframing the entrenched problem of antibiotic overuse. BMJ Glob Health 2019;4:e001590. 10.1136/bmjgh-2019-001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017;23:812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Haenssgen MJ, Xayavong T, Charoenboon N, et al. The consequences of AMR education and awareness raising: outputs, outcomes, and behavioural impacts of an antibiotic-related educational activity in Lao PDR. Antibiotics 2018;7:95. 10.3390/antibiotics7040095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardefeldt LY, Gilkerson JR, Billman-Jacobe H, et al. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J Vet Intern Med 2018;32:1092–9. 10.1111/jvim.15083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fratkin E. East African Pastoralism in transition: Maasai, Boran, and Rendille cases. Afr Stud Rev 2001;44:1–25. 10.2307/525591 [DOI] [Google Scholar]
- 33.Galaty J. Maasai expansion and the new East African pastoralism. In: Being Maasai: ethnicity and identity in East Africa. Athens, OH: Ohio University Press, 1993: 61–86. [Google Scholar]
- 34.Homewood K. Development, demarcation and ecological outcomes in Maasailand. Africa 1995;65:331–50. 10.2307/1161050 [DOI] [Google Scholar]
- 35.McCabe JT, Leslie PW, Deluca L. Adopting cultivation to remain pastoralists: the diversification of Maasai Livelihoods in northern Tanzania. Hum Ecol Interdiscip J 2010;38:321–34. 10.1007/s10745-010-9312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangesho PE, Caudell MA, Mwakapeje ER, et al. "We are doctors": Drivers of animal health practices among Maasai pastoralists and implications for antimicrobial use and antimicrobial resistance. Prev Vet Med 2021;188:105266. 10.1016/j.prevetmed.2021.105266 [DOI] [PubMed] [Google Scholar]
- 37.Baird TD, Gray CL. Livelihood diversification and shifting social networks of exchange: a social network transition? World Dev 2014;60:14–30. 10.1016/j.worlddev.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCabe JT. Cattle bring us to our enemies. Ann Arbor, MI: University of Michigan Press Ann Arbor, 2004. [Google Scholar]
- 39.Thuranira-McKeever C, Shaw A, Machila N, et al. Seasonal influences on livestock keeping in a sedentary crop-livestock system. Trop Anim Health Prod 2010;42:705–17. 10.1007/s11250-009-9478-5 [DOI] [PubMed] [Google Scholar]
- 40.Caudell MA, Dorado-Garcia A, Eckford S, et al. Towards a bottom-up understanding of antimicrobial use and resistance on the farm: a knowledge, attitudes, and practices survey across livestock systems in five African countries. PLoS One 2020;15:1–26. 10.1371/journal.pone.0220274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405. 10.1111/nhs.12048 [DOI] [PubMed] [Google Scholar]
- 42.Afakye K, Kiambi S, Koka E, et al. The impacts of animal health service providers on antimicrobial use attitudes and practices: an examination of poultry layer farmers in Ghana and Kenya. Antibiotics 2020;9:554. 10.3390/antibiotics9090554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oladele OI, Antwi MA, Kolawole AE. Factors influencing demand for animal health services and knowledge of biosecurity among livestock farmers along border villages of South Africa and Namibia. International Journal of Applied Research in Veterinary Medicine 2013;11:123–9 http://www.jarvm.com/articles/Vol11Iss2/Vol11%20Iss2VETOladele.pdf [Google Scholar]
- 44.Olaogun SC, Ifarajimi RO, Muhammad HA. Bovine dermatophilosis: awareness, knowledge, perception and drug usage practices amongst cattle herders in Oyo state, Nigeria. Bangladesh Journal of Veterinary Medicine 2020;18:37–46. 10.33109/bjvmjd2020rm2 [DOI] [Google Scholar]
- 45.Odeniran PO, Macleod ET, Ademola IO, et al. Practices of cattle keepers of Southwest Nigeria in relation to bovine trypanosomosis. Trop Anim Health Prod 2019;51:2117–26. 10.1007/s11250-018-1694-4 [DOI] [PubMed] [Google Scholar]
- 46.Gauthier J, Simeon M, De Haan C. The effect of structural adjustment programmes on the delivery of veterinary services in Africa. Citeseer 1999:133–56. [Google Scholar]
- 47.Sones KR, Catley A, African/Union/Interafrican Bureau for Animal Resources . Primary animal health care in the 21st century: shaping the rules, policies and institutions. Proceedings of an international conference held in Mombaa; 15-18 October 2002, Nairobi, Kenya, 2003. [Google Scholar]
- 48.Lamichhane DK, Shrestha S. Determinants of farmers' choice for veterinary service providers in Nepal Mountains. Trop Anim Health Prod 2012;44:1163–8. 10.1007/s11250-011-0053-5 [DOI] [PubMed] [Google Scholar]
- 49.Ministry of Livestock and Fisheries Development . Tanzania livestock Modernisation initiative. Dodoma, Tanzania, 2015. Available: https://livestocklivelihoodsandhealth.org/wp-content/uploads/2015/07/Tanzania_Livestock_Modernization_Initiative_July_2015.pdf [Accessed 15 Oct 2021].
- 50.Rutabanzibwa A. Veterinary legal reform in Tanzania. Mombasa, Kenya, 2002. [Google Scholar]
- 51.Mangesho PE, Caudell MA, Mwakapeje ER, et al. Knowing is not enough: a mixed-methods study of antimicrobial resistance knowledge, attitudes, and Practises among Maasai pastoralists. Front Vet Sci 2021;8:152. 10.3389/fvets.2021.645851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caudell MA, Quinlan MB, Quinlan RJ, et al. Medical pluralism and livestock health: ethnomedical and biomedical veterinary knowledge among East African agropastoralists. J Ethnobiol Ethnomed 2017;13:7–15. 10.1186/s13002-017-0135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Om C, Daily F, Vlieghe E, et al. "If it's a broad spectrum, it can shoot better": inappropriate antibiotic prescribing in Cambodia. Antimicrob Resist Infect Control 2016;5:58. 10.1186/s13756-016-0159-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen KCM, Schwensen SAF, Henriksen DP, et al. Antimicrobial resistance in the Bacteroides fragilis group in faecal samples from patients receiving broad-spectrum antibiotics. Anaerobe 2017;47:79–85. 10.1016/j.anaerobe.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization . World antibiotic awareness week. Available: https://www.who.int/news-room/campaigns/world-antibiotic-awareness-week [Accessed 1 Jun 2020].
- 56.Cattaneo AA, Wilson R, Doohan D, et al. Bovine veterinarians' knowledge, beliefs, and practices regarding antibiotic resistance on Ohio dairy farms. J Dairy Sci 2009;92:3494–502. 10.3168/jds.2008-1575 [DOI] [PubMed] [Google Scholar]
- 57.Fee E, Krieger N. Understanding AIDS: historical interpretations and the limits of biomedical individualism. Am J Public Health 1993;83:1477–86. 10.2105/ajph.83.10.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baum F, Fisher M. Why behavioural health promotion endures despite its failure to reduce health inequities. Sociol Health Illn 2014;36:213–25. 10.1111/1467-9566.12112 [DOI] [PubMed] [Google Scholar]
- 59.Haenssgen MJ, Charoenboon N, Zanello G, et al. Antibiotic knowledge, attitudes and practices: new insights from cross-sectional rural health behaviour surveys in low-income and middle-income south-east Asia. BMJ Open 2019;9:e028224. 10.1136/bmjopen-2018-028224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odetokun IA, Akpabio U, Alhaji NB, et al. Knowledge of antimicrobial resistance among veterinary students and their personal antibiotic use practices: a national cross-sectional survey. Antibiotics 2019;8:243. 10.3390/antibiotics8040243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frohlich KL, Potvin L. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health 2008;98:216–21. 10.2105/AJPH.2007.114777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Link BG, Phelan JC. Understanding sociodemographic differences in health--the role of fundamental social causes. Am J Public Health 1996;86:471–3. 10.2105/ajph.86.4.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawson DW, Borgerhoff Mulder M, Ghiselli ME, et al. Ethnicity and child health in northern Tanzania: Maasai pastoralists are disadvantaged compared to neighbouring ethnic groups. PLoS One 2014;9:e110447. 10.1371/journal.pone.0110447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hedges S, Borgerhoff Mulder M, James S, et al. Sending children to school: rural livelihoods and parental investment in education in northern Tanzania. Evolution and Human Behavior 2016;37:142–51. 10.1016/j.evolhumbehav.2015.10.001 [DOI] [Google Scholar]
- 65.Homewood K, Kristjanson P, Trench P. Staying Maasai?: Livelihoods, conservation and development in East African rangelands. In: Studies in human ecology and adaptation series. New York: Springer, 2009. [Google Scholar]
- 66.Blewett RA. Property rights as a cause of the tragedy of the commons: institutional change and the pastoral Maasai of Kenya. Eastern Economic Journal 1995;21:477–90. [Google Scholar]
- 67.Hodgson DL, Maasai B. Becoming indigenous: Postcolonial politics in a neoliberal world. Bloomington, Indiana: Indiana University Press, 2011. [Google Scholar]
- 68.Shettima AG, Tar UA. Farmer-pastoralist conflict in West Africa: exploring the causes and consequences. Information, society and justice journal 2008;1:163–84. [Google Scholar]
- 69.McKinn S, Trinh DH, Drabarek D, et al. Drivers of antibiotic use in Vietnam: implications for designing community interventions. BMJ Glob Health 2021;6:e005875. 10.1136/bmjgh-2021-005875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frost I, Craig J, Joshi J. Access barriers to antibiotics. Washington D.C.: Center For Disease Dynamics, Economics & Policy, 2019. https://cddep.org/publications/access-barriers-to-antibiotics/ [Google Scholar]
- 71.Farmer P. Infections and Inequalities: The Modern Plagues. Berkeley, CA: University of California Press, 2001. [Google Scholar]
- 72.Singer M, Bulled N, Ostrach B, et al. Syndemics and the biosocial conception of health. Lancet 2017;389:941–50. 10.1016/S0140-6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- 73.Wallace RG, Bergmann L, Kock R, et al. The dawn of structural one health: a new science tracking disease emergence along circuits of capital. Soc Sci Med 2015;129:68–77. 10.1016/j.socscimed.2014.09.047 [DOI] [PubMed] [Google Scholar]
- 74.Bardosh K. One health: politics and zoonotic disease in Africa. New York, NY: Routledge, 2016. [Google Scholar]
- 75.Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 2004;20:477–81. 10.1016/j.pt.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 76.Sangster NC, Cowling A, Woodgate RG. Ten events that defined anthelmintic resistance research. Trends Parasitol 2018;34:553–63. 10.1016/j.pt.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 77.Hodgkinson JE, Kaplan RM, Kenyon F, et al. Refugia and anthelmintic resistance: concepts and challenges. Int J Parasitol Drugs Drug Resist 2019;10:51–7. 10.1016/j.ijpddr.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-006958supp001.pdf (178.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Transcriptions of qualitative interviews are available upon reasonable request from the co-first authors.