Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, catheter ablation, cryoballoon, incidence, phrenic nerve injury
Abstract
Background:
Cryoballoon-based pulmonary vein isolation (PVI) has emerged as an effective treatment for atrial fibrillation. The most frequent complication during cryoballoon-based PVI is phrenic nerve injury (PNI). However, data on PNI are scarce.
Methods:
The YETI registry is a retrospective, multicenter, and multinational registry evaluating the incidence, characteristics, prognostic factors for PNI recovery and follow-up data of patients with PNI during cryoballoon-based PVI. Experienced electrophysiological centers were invited to participate. All patients with PNI during CB2 or third (CB3) and fourth-generation cryoballoon (CB4)-based PVI were eligible.
Results:
A total of 17 356 patients underwent cryoballoon-based PVI in 33 centers from 10 countries. A total of 731 (4.2%) patients experienced PNI. The mean time to PNI was 127.7±50.4 seconds, and the mean temperature at the time of PNI was −49±8°C. At the end of the procedure, PNI recovered in 394/731 patients (53.9%). Recovery of PNI at 12 months of follow-up was found in 97.0% of patients (682/703, with 28 patients lost to follow-up). A total of 16/703 (2.3%) reported symptomatic PNI. Only 0.06% of the overall population showed symptomatic and permanent PNI. Prognostic factors improving PNI recovery are immediate stop at PNI by double-stop technique and utilization of a bonus-freeze protocol. Age, cryoballoon temperature at PNI, and compound motor action potential amplitude loss >30% were identified as factors decreasing PNI recovery. Based on these parameters, a score was calculated. The YETI score has a numerical value that will directly represent the probability of a specific patient of recovering from PNI within 12 months.
Conclusions:
The incidence of PNI during cryoballoon-based PVI was 4.2%. Overall 97% of PNI recovered within 12 months. Symptomatic and permanent PNI is exceedingly rare in patients after cryoballoon-based PVI. The YETI score estimates the prognosis after iatrogenic cryoballoon-derived PNI.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03645577.
Graphic Abstract:
A graphic abstract is available for this article.
What Is Known?
Pulmonary vein isolation by radiofrequency or cryoballoon is an established treatment option for atrial fibrillation treatment.
Cryoballoon treatment is associated with a significantly higher risk of phrenic nerve injury.
Only single-center data with a limited number of patients are available for phrenic nerve injury and follow-up data are scarce.
What the Study Adds
The YETI registry is the largest survey analyzing the incidence, characteristics, prognostic factors, and recovery of phrenic nerve injury (PNI) during CB-based pulmonary vein isolation.
The incidence of periprocedural PNI was 4.2%. With a mean time to PNI of 127.7±50.4 s and a mean temperature at PNI of −49±8°C, PNI occurred relatively late during the energy application at relatively low temperatures and 82.5% of PVs were already isolated at the time of PNI.
At 12 months, PNI recovered in 97.0% of the patients.
The numerical value of the YETI score directly represent the probability of PNI recovery within 12 months.
Pulmonary vein isolation (PVI) is the cornerstone of invasive treatment of atrial fibrillation. Recently, cryoballoon-based PVI has emerged as a widespread and worldwide accepted ablation strategy.1 The second-generation cryoballoon (CB2, Arctic Front Advance, Medtronic, Inc, Minneapolis, MN) has demonstrated high procedural success rates, relatively short procedure times, high durability of PVI, and convincing long-term maintenance of sinus rhythm.2–7 In head-to-head comparisons such as the “Fire and Ice” trial and the “Freeze” cohort study, cryoballoon ablation was noninferior to RF ablation in terms of safety and clinical efficacy in patients with paroxysmal atrial fibrillation.8,9 However, cryoballoon ablation is associated with a significantly higher risk of phrenic nerve (PN) injury (PNI) compared with RF ablation particularly during ablation of the right-sided pulmonary veins (PVs).8,9 The reported incidence of PNI varies from 1.1% to 6.2% of patients.8–12 The predictors and outcome of periprocedural cryoballoon-related PNI have been earlier described.10,11,13,14 However, only single-center data with a limited number of patients are available, and follow-up data are scarce. The YETI registry (Worldwide survey on outcome after iatrogenic phrenic nerve injury during cryoballoon based pulmonary vein isolation) sought to assess the incidence, characteristics, prognostic factors of PNI recovery, and follow-up of periprocedural PNI during cryoballoon-based PVI in a large-scale worldwide population.
Methods
Study Design
For data privacy issues and regulations the data, methods used in the analysis, and materials used to conduct the research will not be available to other researchers for purposes of reproducing the results or replicating the procedures. In this retrospective, multicenter and multinational registry, we evaluated the incidence, characteristics, prognostic factors for PNI recovery and follow-up data of patients with PNI during cryoballoon-based PVI. Experienced electrophysiological centers from all over the world were invited to participate. All patients with PNI during CB2 or third (CB3) and fourth-generation cryoballoon (CB4) based PVI were eligible for the YETI registry. Patients treated with the first-generation cryoballoon were excluded. All patients were treated by cryoballoon-based PVI between May 2012 and June 2019. Additionally, the total number of patients treated with CB2, CB3, or CB4 was assessed for each individual center. The registry was approved by the local ethical review board of the University of Luebeck, Germany (AZ: 18-151A). Each participating center was responsible for its ethics approval by the local ethics committee. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All patient information was anonymized. The YETI registry was registered on www.clinicialtrials.gov (NCT03645577). Each participating center provided patients baseline characteristics, periprocedural characteristics, and follow-up data according to a standardized and uniform database. The primary end point was PNI at 12 months of follow-up. Secondary end points were the incidence of PNI, periprocedural characteristics, and prognostic factors.
Intraprocedural Management
Intraprocedural management of cryoballoon-based PVI has been described previously and was performed according to the individual centers protocols.15–17 In all patients, a 12F steerable sheath (Flexcath Advance, Medtronic) was utilized. The cryoballoon was advanced into the left atrium and directed toward the target PV via a spiral mapping catheter (Achieve, or Achieve Advance, Medtronic). The acute procedural end point was defined as persistent PVI verified by the spiral mapping catheter recordings. Safety algorithm for PNI prevention were utilized according to the individual centers´ protocols. Use of an esophageal temperature probe (CIRCA S-CATH, Circa Scientific, Englewood, CO or SensiTherm; St Jude Medical, St. Paul, MN) to monitor esophageal temperatures and to prevent esophageal injury was performed according to each individual centers protocols. Four different ablation protocols were utilized according to each centers´ preferences. A bonus freeze protocol (protocol No. 1, group 1) comprised a fixed freeze-cycle duration of 240 seconds, followed by an additional bonus freeze of 240 seconds after successful PVI.1 A no bonus freeze (240 seconds) protocol (protocol No. 2, group 2), applied a fixed freeze-cycle of 240 seconds without a bonus freeze after documentation of PVI.16,18 A no bonus freeze (180 seconds) protocol (protocol no. 3, group 3), applied a fixed freeze-cycle of 180 seconds without a bonus freeze after documentation of PVI. A time-to-effect (TTE) protocol (protocol No. 4, group 4) used a TTE-guided strategy based on continuous real-time recordings from the spiral mapping catheter according to each centers´ preferences.19,20 Postprocedural care was taken according to the latest recommendations and guidelines.1,15,21
Periprocedural Monitoring of Phrenic Nerve Function
Detailed techniques for monitoring the PN function and prevention of PNI have been previously described and were performed according to current standards and recommendations and each individual centers´ protocols.12,13,15,22
Generally, continuous PN pacing at maximum output and pulse width at a cycle length of 1000 to 1200 ms were performed during energy delivery to the septal PVs, using a diagnostic catheter positioned in the superior vena cava.23 Phrenic nerve capture was monitored using intermittent fluoroscopy and tactile feedback of diaphragmatic contraction by the operator’s hand positioned on the patient’s abdomen. In addition, the compound motor action potential (CMAP) was monitored as previously described.22 If a weakening or loss of diaphragmatic movement, and/or a 30% reduction of CMAP amplitude was noted either by palpation or by fluoroscopic imaging, the stability of the pacing catheter was reconfirmed. After reconfirming the stability of the pacing catheter, PNI was diagnosed if (1) a transient decline in the diaphragmatic movement or (2) a >30% decrease of the CMAP amplitude was observed. Refrigerant delivery was stopped by the single or double-stop technique.13 If PNI occurred, no additional freeze-cycle was applied to the septal PVs. Transient PNI was defined as complete recovery of PN function during hospitalization (1–3 days after PVI as individual institutional standard). All patients with PNI were scheduled for clinical and fluoroscopy or X-ray follow-up at 3 and 6 months and, thereafter, at 6-month intervals after the procedure if PNI did not recover. Persistent PNI was defined as the persistence of an elevated hemidiaphragm noted by fluoroscopy or chest X-ray if PNI recovered until 12 months. If PNI did not recover until 12 months, the PNI was defined as permanent. Symptomatic PNI was defined as proven PNI with dyspnea (light, moderate, or severe).
Statistical Analysis
All analyses were performed using STATA software version 14.0 (STATA Corp, Lake Drive Way, TX). Distributions of continuous variables were tested for normality using the Shapiro-Wilk test. Continuous variables are expressed as mean±SD for normally distributed, or as median (interquartile range), for non-normally distributed otherwise. Categorical variables are reported as counts (percentage).
Comparisons of continuous variables were performed using the Student t test for 2 groups or ANOVAs in case of multiple groups, or the corresponding nonparametric test, if not normally distributed. Comparisons of categorical variables were performed using Chi-squared or Fisher exact test, as appropriate.
Follow-up duration was calculated from the date of the procedure to the date of PNI recovery or censoring, which was defined as the most recent follow-up visit at which the end point could be ascertained. The overall cumulative rate of PNI recovery was estimated using the Kaplan-Meier method.
After testing for proportional hazard assumption, the association between prespecified predictors and PNI recovery at 12 months was assessed using Cox regression.
A multivariate-sparing Cox regression model was fit to control for confounders, including univariate predictors reaching a P of <0.1. The final model was fitted using a stepwise backward selection based on the Akaike’s information criterion.
Strength of association was reported using hazard ratios (HRs) for univariate analysis and adjusted HR for multivariate analysis. The discriminative performance of the model was reported through Harrell’s C-statistic.
For an individual patient, the probability of recovery from PNI at 12 months follow-up was derived using the following equation: P (PNI recovery at 1 year)=1−S0(12)exp(YETI Score)
with S0(12) representing the baseline recovery probability at 12 months and the YETI score representing the sum of the products of the predictors and associated coefficients from the final model for a given patient. All P reported are 2-sided, and a P<0.05 was considered statistically significant.
Results
Patients Population
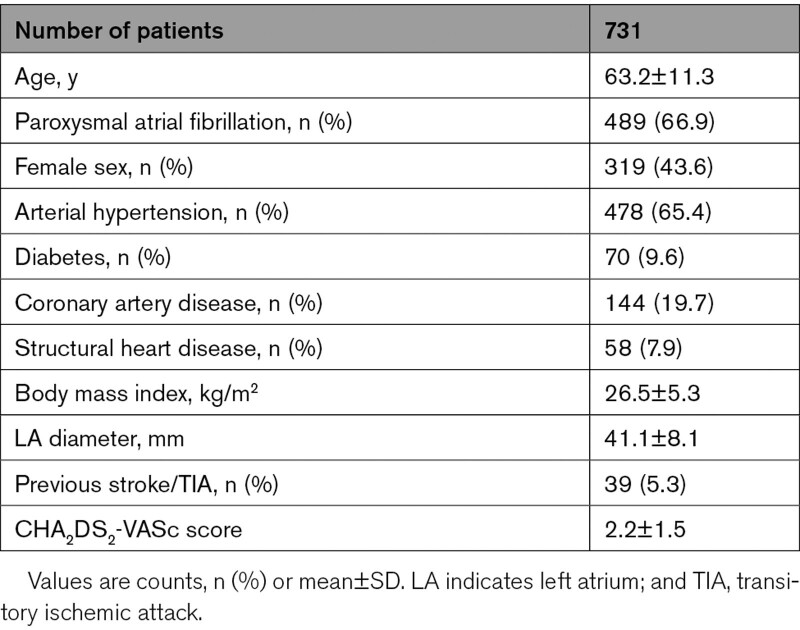
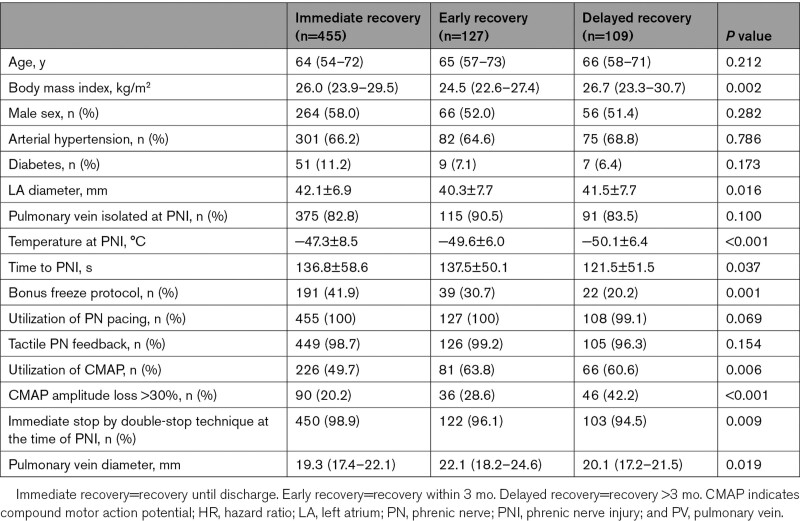
A total of 98 experienced electrophysiological centers from all over the world (17 countries, 5 continents) were invited to participate in the YETI registry. Data were obtained from 33/98 (34%) electrophysiological centers in 10 countries (Germany: n=15, Turkey: n=4, Japan: n=4, United States of America: n=3, China: n=1, Austria: n=2, Poland: n=1, Switzerland: n=1, Georgia: n=1, Egypt: n=1). A total of 17 356 patients underwent CB2-, CB3-, or CB4-based PVI, and a total of 731 patients (4.2%) experienced periprocedural PNI. PNI appeared in 2012 in 59 patients (8.1%), 2013: 80 patients (10.9%), 2014: 81 patients (11.1%), 2015: 139 patients (19.0%), 2016: 122 patients (16.7%), 2017: 152 patients (20.8%), 2018: 91 patients (12.4%) and 2019: 7 patients (1%). The patients’ baseline characteristics of patients with PNI are shown in Table 1.
Table 1.
Baseline Characteristics
Periprocedural Data
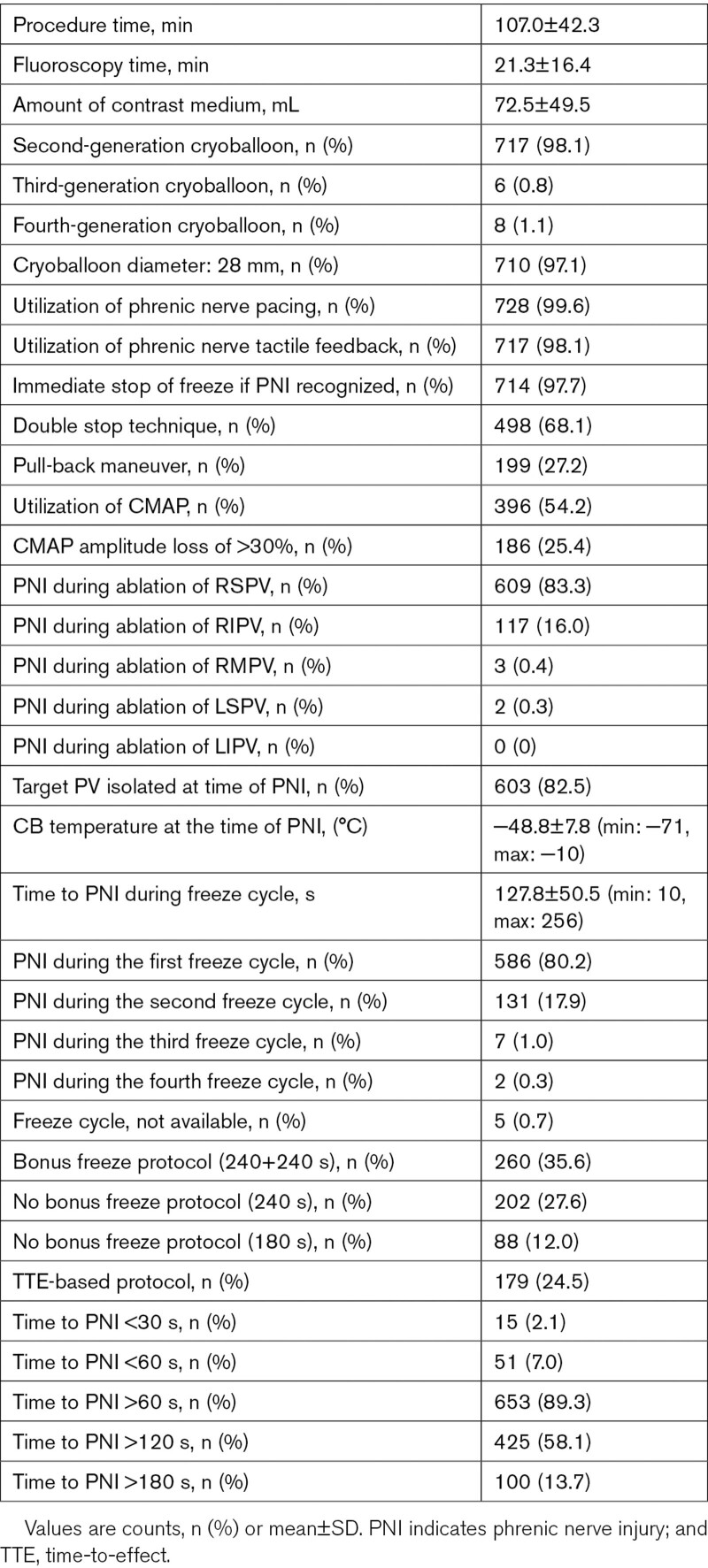
Periprocedural data are presented in Table 2. The data quality and integrity were relatively high. The time to PNI was available in 96.5% of patients, and the temperature at PNI was available in 97.2% of patients. Utilization of CMAP, CMAP amplitude loss >30%, and data concerning immediate stop by double-stop technique as well as baseline data were available in >99% of patients. A total of 717/731 (97.6%) patients were treated by the CB2, 6/731 (0.8%) by the CB3, and 8/731 (1.1%) by the CB4. In 710/731 (97.1%), the 28 mm cryoballoon and in 21/731 (2.9%) the 23 mm cryoballoon has been used. The PNI occurred during energy application along the right superior pulmonary vein (83.3%), right inferior pulmonary vein (16.0%), right middle pulmonary vein (0.4%), and left superior pulmonary vein (0.3%), whereas no PNI was observed during ablation at the left inferior pulmonary vein. The mean diameter of the target PV where PNI occurred was 20.1±3.9 mm. At the time of PNI and termination of energy application, the target PV was isolated in 82.5% of patients. The time to PNI after starting the cryoballoon application (time to PNI) was 127.7±50.4 seconds, with the shortest time to PNI of 10 seconds and the longest time to PNI of 256 seconds. The temperature measured at the time of PNI was −48.8±7.8 (minimum temperature: −71°C, maximum temperature −10°C). The PNI occurred during the first cryoballoon application in 80.2%, during the second cryoballoon application in 17.9%, and during the third cryoballoon application in 1.0% of patients.
Table 2.
Periprocedural Data
Monitoring and Prevention of Phrenic Nerve Injury
Utilization of PN pacing was performed in 99.6% and tactile feedback via the operators hand on the patients abdomen during PN pacing was performed in 98.1% of patients. Periprocedural observation of PN function via the CMAP was performed in 54.2% and a loss of ≥30% of CMAP amplitude before PNI was reported in 47.0% of patients. Immediate stop of energy delivery at the time of PNI via double-stop technique was reported in 68.1% of patients.
Recovery of Phrenic Nerve Injury
Complete recovery of PN function until the end of the procedure and at discharge 1 to 3 days after the procedure was observed in 53.9% (394/731) and 62.2% (455/731) of patients, respectively. Symptomatic PNI at discharge was observed in 16.4% (120/731) of patients.
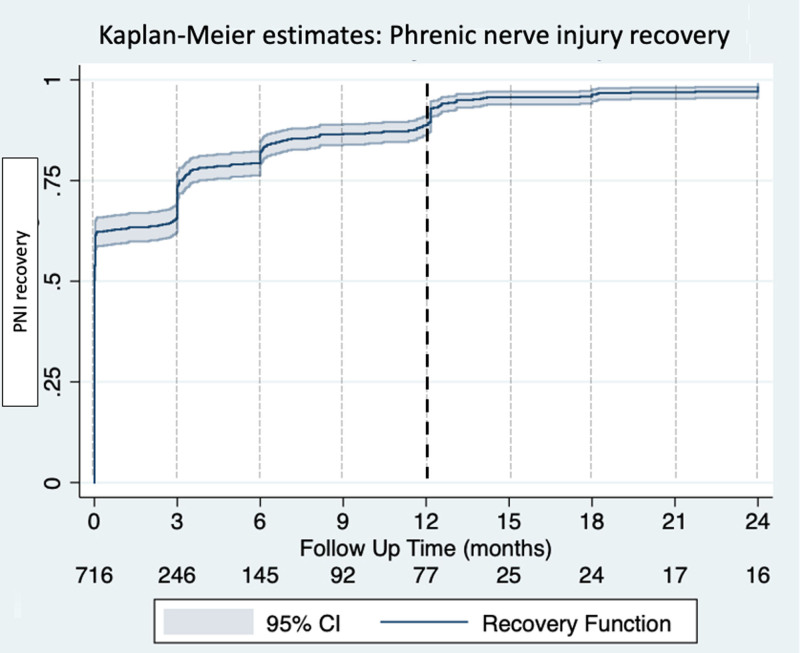
Recovery of PNI at 3 and 6 months of follow-up was found in 80.6% (577/716, with 15 patients lost to follow-up) and 89.6% (629/702, with 29 patients lost to follow-up), respectively. Symptomatic PNI at 3 and 6 months of follow-up was observed in 12.4% (89/716) and 4.8% (34/702), respectively. Recovery of PNI at 12 months of follow-up was found in 97.0% (682/703, with 28 patients lost to follow-up) and 16/703 (2.3%) reported symptomatic PNI. After a follow-up period of 24 months, PNI recovered in 98.7% (691/700, with 31 patients lost to follow-up) and only 2/700 patients (0.3%) reported symptomatic PNI. (-) The Kaplan-Meier estimates is showing the recovery for PNI within follow-up (Figure 1).
Figure 1.
Time course of phrenic nerve injury (PNI) recovery. The Kaplan-Meier estimates demonstrate the relative proportion of patients recovered after periprocedural PNI.
Prognostic Factors of PNI and YETI Score Derivation
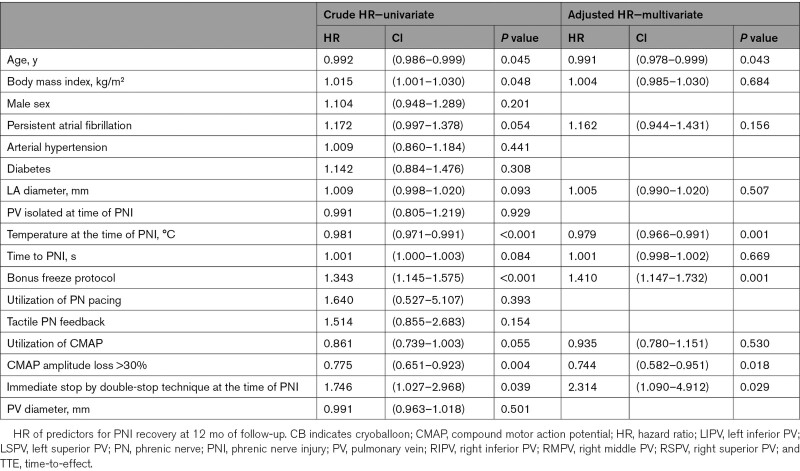
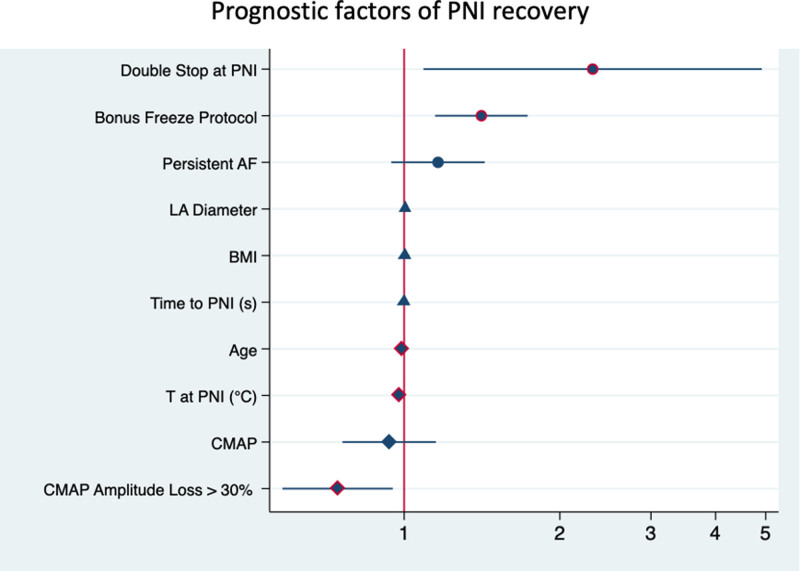
Table 3 shows the univariate and multivariate analysis of prognostic factors. After analyzing the data in the univariate Cox model, a multivariate analysis was performed on suitable parameters. Age was the single baseline and demographic variable that was associated with PNI recovery (HR, 0.991 [CI, 0.978–0.999], P=0.043). Detected procedural parameters for improved PNI recovery were utilization of a bonus freeze protocol (HR, 1.410 [95% CI, 1.147–1.732], P=0.001) and immediate stop by double-stop technique at the time of PNI (HR, 2.314 [95% CI, 1.090–4.912], P=0.029). Detected parameter for reduced PNI recovery were the following: temperature at PNI (HR, 0.979 [95% CI, 0.966–0.991], P<0.0001) and CMAP amplitude loss >30% (HR, 0.744 [95% CI, 0.582–0.951], P=0.018; Figure 2).
Table 3.
Predictors of PNI Recovery
Figure 2.
Prognostic factors for phrenic nerve injury (PNI) recovery. Multivariate analysis on prognostic factors of 12-mo PNI recovery. Hazard rates have been reported on a logarithmic scale. AF indicates atrial fibrillation; BMI, body mass index; CMAP, compound motor action potential; and LA, left atrium.
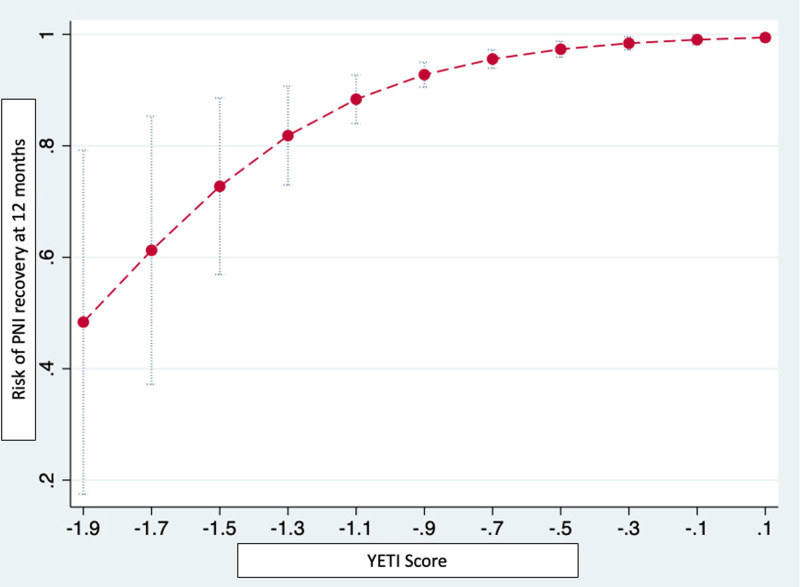
From the regression model, the YETI score was derived using the 5 predictors remaining significant at the multivariate model (age, nadir temperature at PNI, CMAP amplitude loss >30%, immediate stop by double-stop technique at the time of PNI, and bonus freeze protocol). Baseline survival function S0 was found to be 0.0161. The Harrell’s C statistic of the whole model resulted as 0.701 (95% CI, 0.629–0.773). The YETI score has a numerical value that will directly represent the probability of a specific patient of recovering from PNI within 12 months (Figure 3). An appropriate YETI score calculator will be available in the Supplemental Material.
Figure 3.
YETI score: prediction of phrenic nerve injury (PNI) recovery. The YETI score has a numerical value that will directly represent the probability of a specific patient of recovering from PNI within 12 mo. The YETI Score is calculated as −0.0073833×age−0.0187482×temperature+0.2339744 if bonus freeze protocol was utilized, +0.6743827 if immediate stop by double-stop technique at PNI was performed, −0.2743813 if CMAP amplitude loss >30% was detected. An appropriate YETI score calculator will be available online.
Impact of Parameter on the PNI Recovery Time
In addition to prognostic factors for PNI recovery, predictors on PNI recovery in different time intervals were tested (Table 4). A higher BMI, increased left atrium diameter, utilization of a bonus freeze protocol, smaller PV diameter, and immediate stop by double-stop technique at the time of PNI were more common in patients experiencing an immediate recovery (defined as recovery until discharge). Delayed recovery (defined as recovery or no recovery until 12 months) was detected for patients with the lower temperature at the time of PNI, shorter time to PNI, utilization of CMAP, and a CMAP amplitude loss >30%.
Table 4.
Recovery Time Prediction Analysis
Discussion
The YETI registry is the largest survey analyzing the incidence, characteristics, prognostic factors, and recovery of PNI during cryoballoon-based PVI. The major findings are (1) the incidence of periprocedural PNI was 4.2%. (2) With a mean time to PNI of 127.7±50.4 seconds and a mean temperature at PNI of −49±8°C, PNI occurred relatively late during the energy application at relatively low temperatures and 82.5% of PVs were already isolated at the time of PNI. (3) Recovery of PNI occurred in 53.9% of patients until the end of the procedure. At 12 months, PNI recovered in 97.0% of patients. Only 0.1% of the overall population (n=17 356) showed permanent and 0.06% demonstrated symptomatic and permanent PNI. (4). Prognostic factors for PNI recovery were age, cryoballoon temperature at PNI, utilization of a bonus-freeze protocol, immediate stop by double-stop technique at PNI and CMAP amplitude loss >30%. (5) The numerical value of the YETI score directly represent the probability of PNI recovery within 12 months.
Cryoballoon-based treatment of atrial fibrillation has shown excellent efficacy and safety for PVI and is increasingly performed worldwide. Periprocedural PNI represents the typical complication of balloon-based ablation procedures.24 The implementation of various techniques for early detection and prevention of PNI has reduced the rate of periprocedural PNI. Still, the reported incidence of PNI varies between 1.1% and 6.2%.8–12 With 4.2% of patients, the YETI registry might represent a reliable incidence and is reflecting the observations of a large number of cryoballoon users.
The recovery rate from PNI was relatively high. In accordance with other studies with smaller patient populations, 53.9% of PNI recovered till the end of the procedure, and 97% of PNI recovered within 12 months of follow-up.8,25 After 24 months follow-up, 98.7% of PNI recovered. With only 0.1% occurrence of permanent PNI and 0.06% of permanent and symptomatic PNI for the overall population of 17 356 patients, clinically relevant periprocedural PNI seems to be a rare phenomenon in cryoballoon-based PVI procedures.
An optimal PV occlusion is essential for durable lesions and maneuvers to push the cryoballoon into the PV are frequently performed. These maneuvers are reducing the anatomic distance between the cryoballoon and the PN, and thus explaining the higher rate of periprocedural PNI compared with RF-based PVI. To increase the distance between the cryoballoon and PN, pulling back the cryoballoon after initializing the ablation is a common strategy.24 It is known that neural tissue is gradually loses its function when exposed to low temperatures and is able to recover after rewarming, which may explain the observed high recovery rate after terminating the freezing process.26 In cryoballoon ablation, the lesion formation is relatively homogenous and is continuously penetrates the cardiac tissue from the endothelium. This is the most likely mechanism for the observed relatively late appearance of PNI during the freezing process after > 2 minutes observed at relatively low temperatures of almost −50°C as in previous reports.24 It has been shown that TTE-based protocols applying fewer and shorter freeze cycles compared with protocols with fixed freeze cycle duration.19,20,25,27 Furthermore, a lower incidence of the overall complication rate especially for PNI was observed when utilizing TTE-based protocols most likely driven by the lower number and shorter length of the applied freeze-cycles.25 Recently, the CIRCA-DOSE trial found similar 12-month outcome for PVI utilizing a 2- or 4-minute freeze cycle duration, which is underlying again the positive influence of a slenderer ablation strategies compared with long-lasting and bonus freeze protocols.28 However, the optimal freezing cycle strategy is required to be investigated. Non-TTE based protocols might be a predictor of PNI occurrences. Although the aim of YETI registry was not to detect predictors for PNI appearance, dosing seems to play an important role in the occurrence, and therefore prevention, of PNI. Interestingly, utilizing a bonus freeze protocol was associated with a higher PNI recovery rate (HR 1.410) compared with other common protocols. This observation might be explained by the fact that the PN function survived the first freeze undamaged, increasing the possibility to also survive the bonus freeze and at least get not injured as much as if the PNI function is already disabled during the first freeze. Anatomic factors likely play a critical role in this observation is likely, but this could not be proven by our clinical analysis, since no systematic preprocedural imaging by computer tomography has been performed.10
We found that the temperature at the time of PNI was a strong predictor of PNI recovery. The lower the temperature reached at the time of PNI, the lower was the probability of recovery within 12 months. Even though the time to PNI was not a PNI recovery predictor, yet the temperature at PNI is a parameter already containing the time to PNI because of the typical course of the energy delivery over time. As expected, we found that immediate stop by double-stop technique at the time of PNI was associated with a higher recovery from PNI and a CMAP amplitude loss >30% was associated with a lower rate of recovery from PNI. With a HR of 2.314 when immediately stopping the freeze cycle by double-stop technique, this factor was found to be the strongest prognostic factor for PNI recovery. Therefore, we strongly recommend utilizing of the double-stop technique in the case of PNI as well as a generally use of CMAP or another additional monitoring modality (eg, intra cardiac echocardiography) during all cryoballoon procedures.22 The uni- and multivariate analyses found that except of age PNI recovery cannot be predicted by baseline characteristics and depends almost exclusively on procedural characteristics. The multivariate Cox regression showed that PNI recovery over time is basically associated with peri-procedural characteristics and fairly independent from baseline demographics. To develop a score for prediction of PNI recovery at 12 months, the variables that resulted significant at the cox regression analysis were utilized. The numerical value of the YETI score will directly represent the probability of a specific patient of recovering from PNI within 12 months.
Limitations
The YETI registry has several limitations. First, the presented findings are based on a retrospective analysis. Nevertheless, the data were derived worldwide by a large number of centers, and the population represents the largest database on cryoballoon-driven PNI up to date.
Second, since only patients with periprocedural PNI were evaluated, the population represents only a subgroup of patients with cryoballoon-based PVI. Therefore, no predictors for the occurrence of periprocedural PNI could be evaluated, and the registry was focused on prognostic factors for PNI recovery. Third, not all patients met the end point of 12-month follow-up and were lost to follow-up. Fourth, the YETI score has not been prospectively validated in an independent cohort. This fact is limiting its validity.
Conclusions
With 4.2%, the periprocedural incidence of PNI during CB2-based PVI is relatively low. About 53.9% of PNI recovered until the end of the procedure. Overall, 97% of PNI recovered within 12 months. Symptomatic and permanent PNI is exceedingly rare in patients after cryoballoon-based PVI. As prognostic factors for PNI recovery age, cryoballoon nadir temperature at PNI, utilization of a bonus freeze protocol, immediate stop at PNI by double-stop technique, and CMAP amplitude loss >30% were identified. The YETI score might be a useful tool to predict recovery after iatrogenic cryoballoon-related PNI.
Article Information
Acknowledgments
We thank all the local investigators and assistant personnel for their great work. Furthermore, we thank all the YETI collaborators: Andreas Rillig, Ellen Hoffmann, Robert Pantlik, Feifan Ouyang, Makoto Sano, Emrie Tomaiko, Ugur Canpolat, and Sorin Popescu. Dr Heeger performed concept/design, data collection, data analysis and interpretation, drafting the article, critical revision, and approval. Dr Richard Tilz performed concept/design, critical revision, and approval. Drs Gasperetti and Proietti performed data analysis and interpretation. All other authors performed data ecollection, critical revision, and approval.
Sources of Funding
None.
Disclosures
Dr Heeger received travel grants and research grants from Medtronic, Boston Scientific, Biosense Webster and Cardiofocus and is a proctor and lecturer of Medtronic, Boston Scientific, Cardiofocus and Biosense Webster. Dr Kuck received travel grants and research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, Cryocath, and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis. He received speaker’s honoraria from Medtronic. Dr Tilz received travel grants from St. Jude Medical, Biosense Webster, Daiichi Sankyo, SentreHeart and Speaker’s Bureau Honoraria from Biosense Webster, Biotronik, Boston scientific, Pfizer, Topera, Bristol-Myers Squibb, Bayer, Sanofi Aventis, and research grants by Cardiofocus, Boston Scientific and Lifetech. Dr Aytemir is a proctor and lecturer for Medtronic, Biosense Webster, and Abbott. Dr Metzner received speaker’s honoraria and travel grants from Medtronic, Biosense Webster, and Cardiofocus. Dr Straube received honoraria for lectures from Medtronic, and Bristol-Myers-Squibb, outside the submitted work; and educational support from Pfizer. Dr Cay received travel grants and speaker’s honoraria from Medtronic, Biosense Webster, and Abbott and is a proctor of Medtronic. Dr Jędrzejczyk-Patej received consultant fees from Medtronic, Biotronik, Abbott, Boston Scientific. Dr Wissner is a consultant to Medtronic. The other authors report no conflicts.
Supplemental Material
YETI score calculator
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- CMAP
- compound motor action potential
- LA
- left atrium
- PN
- phrenic nerve
- PNI
- phrenic nerve injury
- PVI
- pulmonary vein isolation
- PV
- pulmonary vein
- RSPV
- right superior pulmonary vein
- TTE
- time-to-effect
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.121.010516.
For Sources of Funding and Disclosures, see page 31.
Contributor Information
Christian Sohns, Email: csohns@hdz-nrw.de.
Alexander Pott, Email: alexander.pott@uniklinik-ulm.de.
Andreas Metzner, Email: andreasmetzner1@web.de.
Osamu Inaba, Email: oinbcvm@tmd.ac.jp.
Florian Straube, Email: florian.straube@web.de.
Malte Kuniss, Email: m.kuniss@kerckhoff-klinik.de.
Arash Aryana, Email: a_aryana@outlook.com.
Shinsuke Miyazaki, Email: mmshinsuke@gmail.com.
Serkan Cay, Email: cayserkan@yahoo.com.
Joachim R. Ehrlich, Email: joachimehrlich@t-online.de.
Ibrahim El-Battrawy, Email: ibrahim.elbattrawy2006@gmail.com.
Martin Martinek, Email: martin.martinek@ordensklinikum.at.
Ardan M. Saguner, Email: ardansaguner@yahoo.de.
Verena Tscholl, Email: verena.tscholl@charite.de.
Kivanc Yalin, Email: yalinkivanc@gmail.com.
Evgeny Lyan, Email: lyanevgeny@gmail.com.
Wilber Su, Email: emrietomaiko@gmail.com.
Giorgi Papiashvili, Email: giorgi.papiashvili@gmail.com.
Maichel Sobhy Naguib Botros, Email: michael_sobhi81@yahoo.com.
Alessio Gasperetti, Email: alessio.gasperetti93@gmail.com.
Riccardo Proietti, Email: riccardoproietti6@gmail.com.
Erik Wissner, Email: erikwissner@gmail.com.
Daniel Scherr, Email: daniel.steven@me.com.
Masashi Kamioka, Email: kmasashi@fmu.ac.jp.
Hisaki Makimoto, Email: klein2004att@gmail.com.
Tsuyoshi Urushida, Email: uru@hama-med.ac.jp.
Tolga Aksu, Email: aksutolga@gmail.com.
Julian K.R. Chun, Email: j.chun@ccb.de.
Kudret Aytemir, Email: aytemir@hacettepe.edu.tr.
Ewa Jędrzejczyk-Patej, Email: ewajczyk@op.pl.
Karl-Heinz Kuck, Email: kuckkh@aol.com.
Tillman Dahme, Email: tillman.dahme@gmx.de.
Daniel Steven, Email: daniel.steven@me.com.
Philipp Sommer, Email: psommer@hdz-nrw.de.
References
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS)The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020:ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Heeger CH, Subin B, Wissner E, Fink T, Mathew S, Maurer T, Lemes C, Rillig A, Wohlmuth P, Reissmann B, et al. Second-generation cryoballoon-based pulmonary vein isolation: Lessons from a five-year follow-up. Int J Cardiol. 2020;312:73–80. doi: 10.1016/j.ijcard.2020.03.062 [DOI] [PubMed] [Google Scholar]
- 3.Aryana A, Kowalski M, O’Neill PG, Koo CH, Lim HW, Khan A, Hokanson RB, Bowers MR, Kenigsberg DN, Ellenbogen KA; Cryo-DOSING Investigators. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: Short- and long-term results of a multicenter study. Heart Rhythm. 2016;13:2306–2313. doi: 10.1016/j.hrthm.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 4.Akkaya E, Berkowitsch A, Zaltsberg S, Greiss H, Hamm CW, Sperzel J, Kuniss M, Neumann T. Five-year outcome and predictors of success after second-generation cryoballoon ablation for treatment of symptomatic atrial fibrillation. Int J Cardiol. 2018;266:106–111. doi: 10.1016/j.ijcard.2018.03.069 [DOI] [PubMed] [Google Scholar]
- 5.Reddy VY, Sediva L, Petru J, Skoda J, Chovanec M, Chitovova Z, Di Stefano P, Rubin E, Dukkipati S, Neuzil P. Durability of Pulmonary Vein Isolation with Cryoballoon Ablation: Results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) Study. J Cardiovasc Electrophysiol. 2015;26:493–500. doi: 10.1111/jce.12626 [DOI] [PubMed] [Google Scholar]
- 6.Bordignon S, Fürnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak B, Schulte-Hahn B, Schmidt B, Chun KR. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace. 2015;17:725–731. doi: 10.1093/europace/euu331 [DOI] [PubMed] [Google Scholar]
- 7.Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A, Wohlmuth P, Reissmann B, Tilz RR, Ouyang F, et al. Once Isolated, Always Isolated? Incidence and Characteristics of Pulmonary Vein Reconduction After Second-Generation Cryoballoon-Based Pulmonary Vein Isolation. Circ Arrhythm Electrophysiol. 2015;8:1088–1094. doi: 10.1161/CIRCEP.115.003007 [DOI] [PubMed] [Google Scholar]
- 8.Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, et al. ; FIRE AND ICE Investigators. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQ, Tebbenjohanns J, Noelker G, Tilz RR, Chun JKR, et al. ; FREEZE Cohort Study Investigators. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21:1313–1324. doi: 10.1093/europace/euz155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abugattas JP, de Asmundis C, Iacopino S, Salghetti F, Takarada K, Coutiño HE, Ströker E, De Regibus V, de Greef Y, Brugada P, et al. Phrenic nerve injury during right inferior pulmonary vein ablation with the second-generation cryoballoon: clinical, procedural, and anatomical characteristics. Europace. 2018;20:e156–e163. doi: 10.1093/europace/eux337 [DOI] [PubMed] [Google Scholar]
- 11.Fürnkranz A, Bordignon S, Schmidt B, Perrotta L, Dugo D, De Lazzari M, Schulte-Hahn B, Nowak B, Chun JK. Incidence and characteristics of phrenic nerve palsy following pulmonary vein isolation with the second-generation as compared with the first-generation cryoballoon in 360 consecutive patients. Europace. 2015;17:574–578. doi: 10.1093/europace/euu320 [DOI] [PubMed] [Google Scholar]
- 12.Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz R, Rillig A, Mathew S, Deiss S, Kamioka M, et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol. 2014;25:466–470. doi: 10.1111/jce.12358 [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki S, Kajiyama T, Watanabe T, Hada M, Yamao K, Kusa S, Igarashi M, Nakamura H, Hachiya H, Tada H, et al. Characteristics of Phrenic Nerve Injury During Pulmonary Vein Isolation Using a 28-mm Second-Generation Cryoballoon and Short Freeze Strategy. J Am Heart Assoc. 2018;7:e008249. doi: 10.1161/JAHA.117.008249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casado-Arroyo R, Chierchia GB, Conte G, Levinstein M, Sieira J, Rodriguez-Mañero M, di Giovanni G, Baltogiannis Y, Wauters K, de Asmundis C, et al. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm. 2013;10:1318–1324. doi: 10.1016/j.hrthm.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Su W, Kowal R, Kowalski M, Metzner A, Svinarich JT, Wheelan K, Wang P. Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm. 2015;12:1658–1666. doi: 10.1016/j.hrthm.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 16.Heeger CH, Wissner E, Wohlmuth P, Mathew S, Hayashi K, Sohns C, Reißmann B, Lemes C, Maurer T, Saguner AM, et al. Bonus-freeze: benefit or risk? Two-year outcome and procedural comparison of a “bonus-freeze” and “no bonus-freeze” protocol using the second-generation cryoballoon for pulmonary vein isolation. Clin Res Cardiol. 2016;105:774–782. doi: 10.1007/s00392-016-0987-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzner A, Reissmann B, Rausch P, Mathew S, Wohlmuth P, Tilz R, Rillig A, Lemes C, Deiss S, Heeger C, et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2014;7:288–292. doi: 10.1161/CIRCEP.114.001473 [DOI] [PubMed] [Google Scholar]
- 18.Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, Saitoh Y, Baltogiannis G, Irfan G, Coutiño-Moreno HE, et al. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12:673–680. doi: 10.1016/j.hrthm.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 19.Heeger CH, Wissner E, Mathew S, Hayashi K, Sohns C, Reißmann B, Lemes C, Maurer T, Fink T, Saguner AM, et al. Short tip-big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clin Res Cardiol. 2016;105:482–488. doi: 10.1007/s00392-015-0944-y [DOI] [PubMed] [Google Scholar]
- 20.Chun KR, Stich M, Fürnkranz A, Bordignon S, Perrotta L, Dugo D, Bologna F, Schmidt B. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm. 2017;14:495–500. doi: 10.1016/j.hrthm.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 21.Su W, Aryana A, Passman R, Singh G, Hokanson R, Kowalski M, Andrade J, Wang P. Cryoballoon Best Practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15:1348–1355. doi: 10.1016/j.hrthm.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 22.Franceschi F, Koutbi L, Gitenay E, Hourdain J, Maille B, Trévisan L, Deharo JC. Electromyographic monitoring for prevention of phrenic nerve palsy in second-generation cryoballoon procedures. Circ Arrhythm Electrophysiol. 2015;8:303–307. doi: 10.1161/CIRCEP.115.002734 [DOI] [PubMed] [Google Scholar]
- 23.Ghosh J, Singarayar S, Kabunga P, McGuire MA. Subclavian vein pacing and venous pressure waveform measurement for phrenic nerve monitoring during cryoballoon ablation of atrial fibrillation. Europace. 2015;17:884–890. doi: 10.1093/europace/euu341 [DOI] [PubMed] [Google Scholar]
- 24.Tohoku S, Chen S, Last J, Bordignon S, Bologna F, Trolese L, Zanchi S, Bianchini L, Schmidt B, Chun KRJ. Phrenic nerve injury in atrial fibrillation ablation using balloon catheters: Incidence, characteristics, and clinical recovery course. J Cardiovasc Electrophysiol. 2020;31:1932–1941. doi: 10.1111/jce.14567 [DOI] [PubMed] [Google Scholar]
- 25.Rottner L, Fink T, Heeger CH, Schlüter M, Goldmann B, Lemes C, Maurer T, Reißmann B, Rexha E, Riedl J, et al. Is less more? Impact of different ablation protocols on periprocedural complications in second-generation cryoballoon based pulmonary vein isolation. Europace. 2018;20:1459–1467. doi: 10.1093/europace/eux219 [DOI] [PubMed] [Google Scholar]
- 26.Andrade JG, Dubuc M, Ferreira J, Guerra PG, Landry E, Coulombe N, Rivard L, Macle L, Thibault B, Talajic M, et al. Histopathology of cryoballoon ablation-induced phrenic nerve injury J Cardiovasc Electr. 2014;25:187–194. doi: 10.1111/jce.12296 [DOI] [PubMed] [Google Scholar]
- 27.Heeger CH, Schuette C, Seitelberger V, Wissner E, Rillig A, Mathew S, Reissmann B, Lemes C, Maurer T, Fink T, et al. Time-to-effect guided pulmonary vein isolation utilizing the third-generation versus second generation cryoballoon: one year clinical success. Cardiol J. 2019;26:368–374. doi: 10.5603/CJ.a2018.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, et al. ; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.