Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, catheter ablation, electroporation, follow-up studies
Abstract
Background:
Pulsed field ablation (PFA) is a novel form of ablation using electrical fields to ablate cardiac tissue. There are only limited data assessing the feasibility and safety of this type of ablation in humans.
Methods:
PULSED AF (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF; https://www.clinicaltrials.gov; unique identifier: NCT04198701) is a nonrandomized, prospective, multicenter, global, premarket clinical study. The first-in-human pilot phase evaluated the feasibility and efficacy of pulmonary vein isolation using a novel PFA system delivering bipolar, biphasic electrical fields through a circular multielectrode array catheter (PulseSelect; Medtronic, Inc). Thirty-eight patients with paroxysmal or persistent atrial fibrillation were treated in 6 centers in Australia, Canada, the United States, and the Netherlands. The primary outcomes were ability to achieve acute pulmonary vein isolation intraprocedurally and safety at 30 days.
Results:
Acute electrical isolation was achieved in 100% of pulmonary veins (n=152) in the 38 patients. Skin-to-skin procedure time was 160±91 minutes, left atrial dwell time was 82±35 minutes, and fluoroscopy time was 28±9 minutes. No serious adverse events related to the PFA system occurred in the 30-day follow-up including phrenic nerve injury, esophageal injury, stroke, or death.
Conclusions:
In this first-in-human clinical study, 100% pulmonary vein isolation was achieved using only PFA with no PFA system–related serious adverse events.
Graphic Abstract:
A graphic abstract is available for this article.
What Is Known?
Pulmonary vein isolation by catheter ablation is recommended for patients with symptomatic atrial fibrillation who have failed antiarrhythmic drug therapy.
Pulsed field ablation delivered to cardiac tissue may offer safety and efficacy advantages due to the minimal thermal energy imparted to target tissue, the higher susceptibility of myocardial cells to electrical fields, and the ability to create transmural, contiguous lesions through irreversible electroporation.
The PULSED AF study (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF) is a prospective, interventional, single-arm, premarket global clinical trial designed to evaluate the safety and efficacy of pulsed field ablation using a pulmonary vein isolation–only catheter ablation strategy in patients with drug-refractory symptomatic paroxysmal and persistent atrial fibrillation.
What the Study Adds
A novel pulsed field ablation system used in a first-in-human pilot study achieved successful intraprocedural pulmonary vein isolation in 100% of patients. There were no serious adverse events attributed to the pulsed field ablation system during the 30-day follow-up.
The global prevalence of atrial fibrillation (AF) is growing.1,2 Catheter ablation for AF by pulmonary vein isolation (PVI) is recommended for symptomatic AF patients who have failed antiarrhythmic drug therapy.3 While effective, ablation can be associated with complications including pulmonary vein (PV) stenosis, phrenic nerve injury, cerebrovascular injury, and atrioesophageal fistula.4 Achieving a reduction in AF recurrence while minimizing safety complications remains a challenge for catheter ablation technologies.
Irreversible electroporation (IRE) is a mechanism of inducing cell death based on the application of electrical fields to tissue.5,6 When a threshold of electrical field strength and application duration is surpassed, the cell membrane becomes hyperpermeable and induces cell death with limited damage to the extracellular matrix. Pulsed field ablation (PFA) delivered to cardiac tissue may offer safety and efficacy advantages due to the minimal thermal energy imparted to target tissue, the higher susceptibility of myocardial cells to electrical fields, and the ability to create transmural, contiguous lesions through IRE.
The PULSED AF clinical trial (Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF; NCT04198701) is designed to determine the safety and efficacy of IRE in patients with a documented history of paroxysmal or persistent AF. We report the first-in-human pilot study phase describing the acute procedural efficacy and safety of a novel PFA system.
Methods
The authors declare that all supporting data are available within the article (and its Supplemental Material).
Trial Design
PULSED AF is a prospective, interventional, single-arm, premarket global clinical trial designed to evaluate the safety and efficacy of PFA using a PVI-only catheter ablation strategy. The PFA system being investigated (PulseSelect PFA System; Medtronic, Minneapolis, MN) is investigational and not approved in any geography. Patients were enrolled at 6 sites in 4 countries. Procedures were performed by one operator at each site in Canada (n=7), Australia (n=5), the United States (n=22 across 3 sites), and the Netherlands (n=4). The study protocol was approved by the ethics committee of each participating site.
PULSED AF is sponsored by Medtronic, Inc, and the study began enrollment in December 2019. The Steering Committee members are responsible for the scientific and clinical oversight with regard to trial execution. An independent Clinical Events Committee adjudicated adverse events for relatedness to the PFA system.
Participants
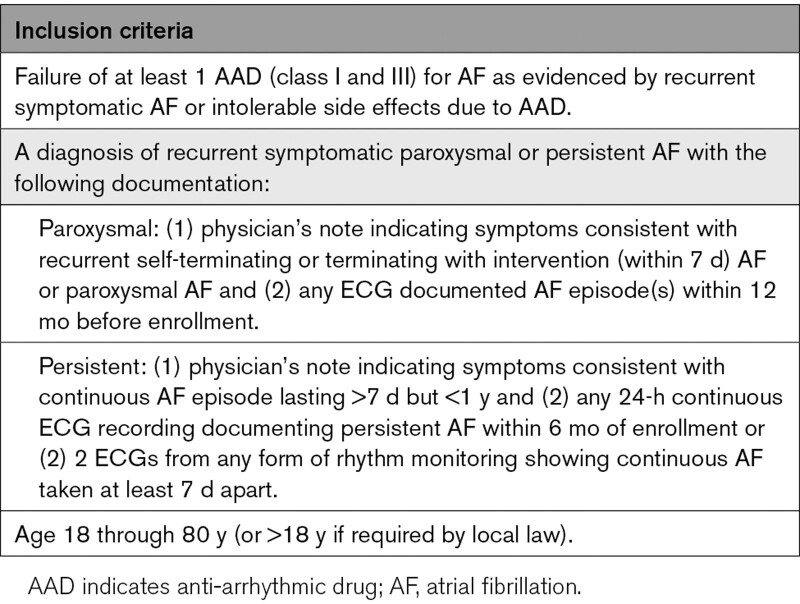
The study population includes patients 18 to 80 years of age undergoing first-time catheter ablation of paroxysmal or persistent AF that failed at least one antiarrhythmic drug (class I or III), which is in accordance with the current European and US guidelines for the management of AF.3,7,8 At least one episode of AF had to be documented by ECG within 1 year before enrollment in the trial for patients with paroxysmal AF. A subject with paroxysmal AF was defined as having AF that terminates spontaneously or with intervention within 7 days of onset. A subject with persistent AF was defined as having a continuous AF episode lasting longer than 7 days but <1 year. Within 6 months before enrollment in the trial, persistent AF patients provided documentation demonstrating either (1) 24-hour continuous ECG recording of continuous AF or (2) 2 electrocardiograms taken 7 days apart showing continuous AF. Detailed inclusion and exclusion criteria are available in Table 1 and Table S1. All patients provided written informed consent before study procedures in accordance with country-specific applicable data privacy acts, the Declaration of Helsinki, and the applicable laws for research using medical devices.
Table 1.
Inclusion Criteria
Before the procedure, a full baseline evaluation was performed, including medical history, physical examination, arrhythmia symptom review, medication review, 12-lead ECG, transthoracic echocardiogram, and transesophageal echocardiogram. The echocardiogram was performed to ensure a left ventricular ejection fraction of ≥35% and no gross enlargement of the left atrium (LA) >5.0 cm. Additionally, patients completed a chest computed tomography or magnetic resonance imaging scan at baseline to allow future characterization of any potential occurrences of PV stenosis. Patients were not excluded based on PV anatomy or size. Oral anticoagulation therapy was required for at least 3 weeks before the procedure and was not stopped before the procedure. No heparin bridging was allowed.
PFA System
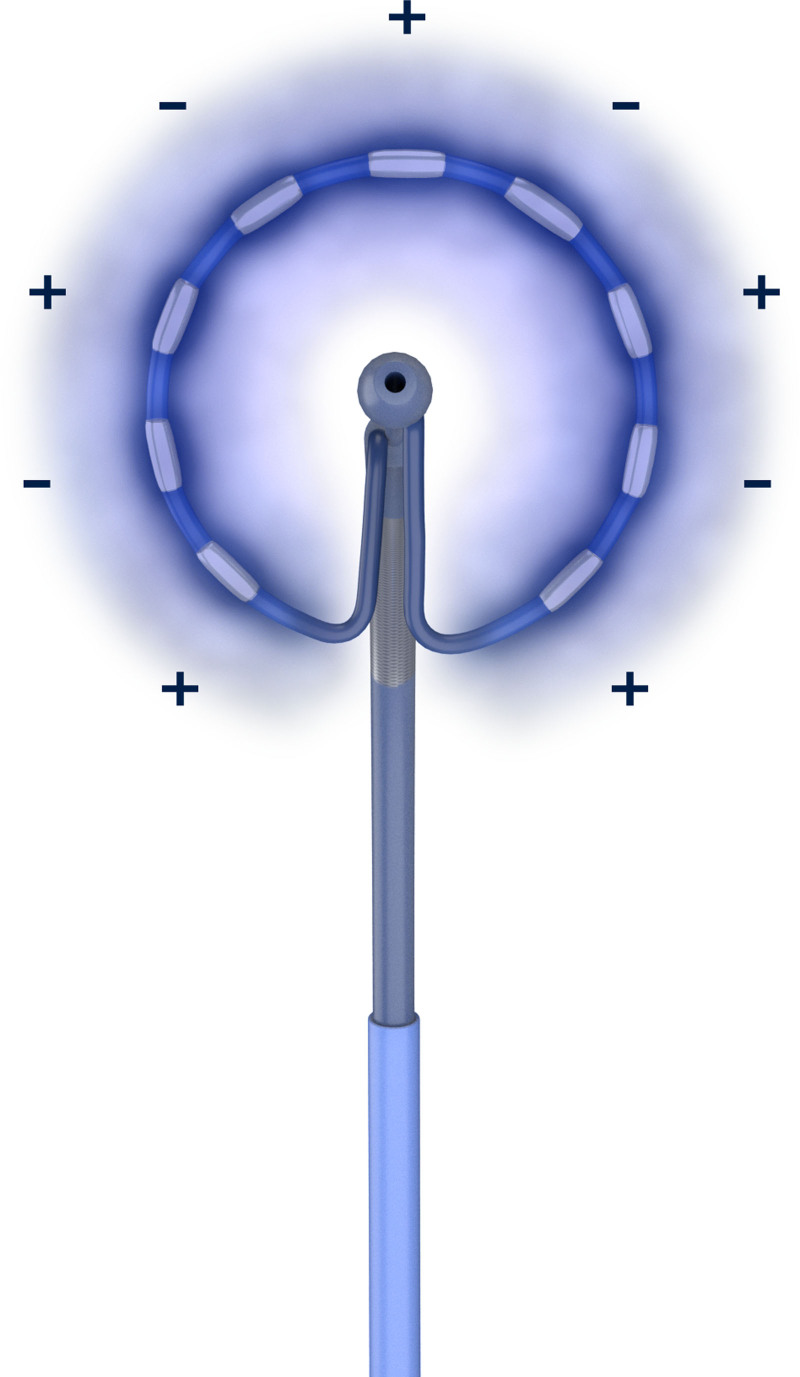
The Medtronic PulseSelect PFA System delivers a controlled biphasic, bipolar waveform to user-selectable electrodes through an over-the-wire, circular array with 9 gold electrodes (electrode length, 3 mm; 20° forward tilted array with a diameter of 25 mm; 9F shaft; Figure 1). The circular catheter is also capable of recording PV and atrial potentials, and it can also perform pacing. The system allows a variety of power profiles to be delivered. Each profile creates a different electrical field surrounding the array with voltages applied to the electrodes ranging from 500 to 1500 volts. The waveforms were delivered on the order of milliseconds in trains of pulses. No external grounding patch was used due to the bipolar nature of the delivery. Investigators used medical discretion regarding the appropriate profile level to isolate each PV. In each position, 4 deliveries of the specified energy profile were given for the lesions to reach their full extent. All deliveries were automatically synchronized to the cardiac cycle to occur during the ventricular refractory period, and as such, simultaneous atrial/ventricular pacing was not required. Each electrode can measure any temperature change from baseline to approximately 1 second post-delivery.
Figure 1.
Pulsed field ablation delivered to a 9-gold circular electrode array (electrode length, 3 mm; 20° forward tilted array with diameter of 25 mm; 9F shaft) in a biphasic, bipolar configuration generates an electric field confined to the area immediately surrounding the array.
PFA Procedure
Patients were sedated with general anesthesia or conscious sedation, and paralytics were not recommended. Intravenous heparin was administered before (or immediately following) transseptal puncture, and a target activated clotting time >350 s was required throughout the procedure and before the first energy delivery.
After femoral venous access, a 10F sheath was used for transseptal puncture and catheter introduction. The circular PFA catheter was introduced into the LA via a guidewire, and the PFA catheter’s circular array positioning at the PV was assessed by fluoroscopy or intracardiac echocardiography imaging. During a subset of cases, the catheter electrode array position was tracked in the electroanatomical mapping system. Before ablation, the guidewire was positioned inside the PV, and the circular array was navigated proximal to the PV ostium. Before PFA applications, a low-voltage electrical pulse was delivered from all 9 electrodes to test for phrenic nerve capture. The purpose of this low-voltage test pulse was to evaluate proximity of the catheter to the phrenic nerve and allow investigators to reposition the catheter as needed to reduce potential phrenic nerve injury. To perform the ablation, the physician operator then selected the therapy profile and monitored for electrogram amplitude reduction on the catheter. During each therapy delivery, an electrical artifact appeared lasting ≈100 to 200 milliseconds (Figure 2). The electrical artifact induced by PFA was not a function of the specific recording system used, and the PFA system was decoupled from the recording system during energy deliveries to isolate the patient from high-voltage circuitry. After a series of 4 deliveries, the catheter was rotated to a new position to achieve full circumferential isolation since the electric field has a gap between electrodes 9 and 1 (Figure 1). In general, this electrode distribution requires at least 4 catheter positions around each PV to achieve full isolation of the PV. Investigators aimed for a level of isolation that was near the level of the PV carina before expanding the lesions more antrally. In some patients, the catheter positionings were overlaid on the preablation voltage map and on the postablation voltage map. Deliveries were continued around each PV until the end point of PVI.
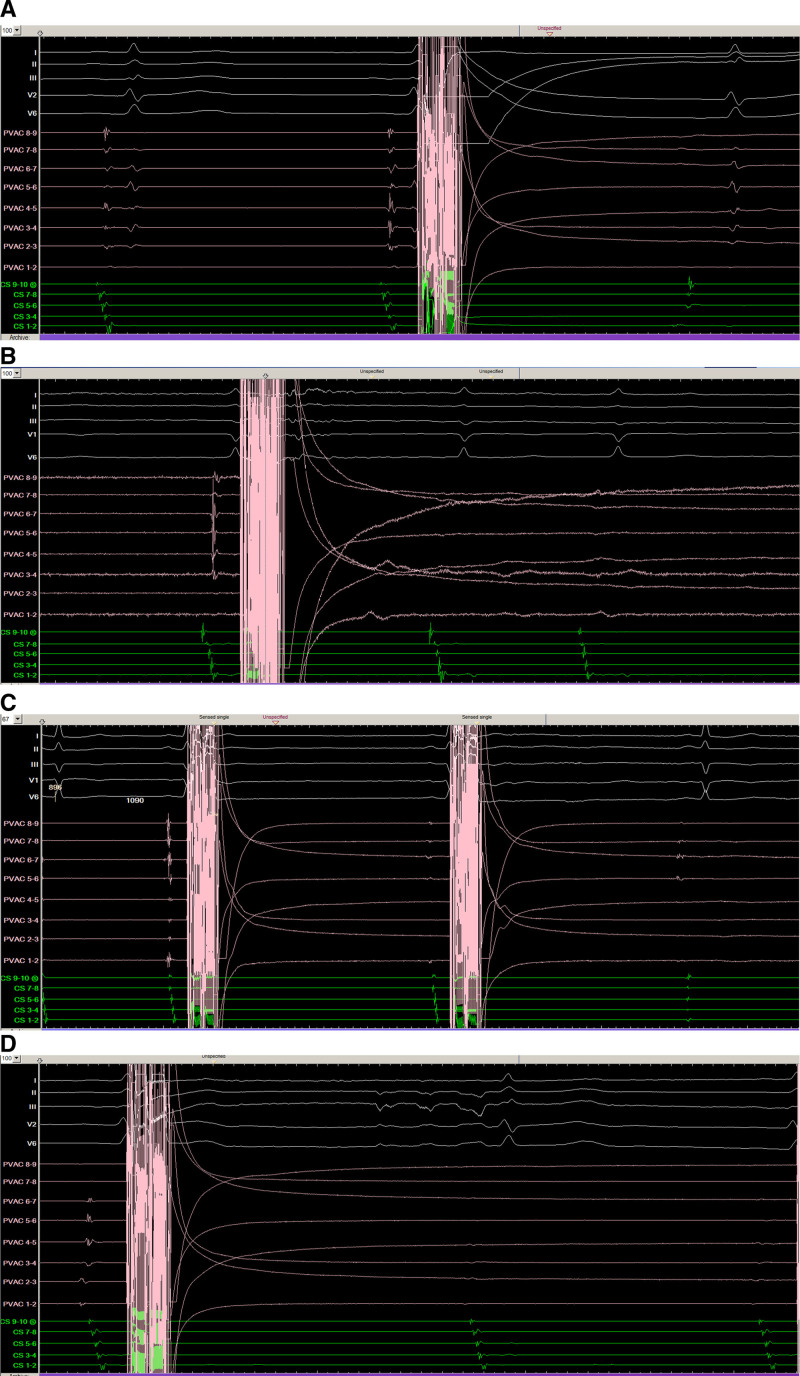
Figure 2.
Isolation of pulmonary veins with pulsed field ablation. Pulmonary vein potentials recorded from bipolar electrograms from the nine-gold electrode array are shown immediately before and after PFA delivery in the left inferior (A), left superior (B), right inferior (C), and the right superior (D) pulmonary veins demonstrating efficient electrical PV isolation.
Confirmation of intraprocedural isolation of each PV was assessed at least 20 minutes after the last PFA application within a specific PV. To prove isolation, demonstration of entrance block was required. If any residual PV connection was found, additional PFA therapy deliveries were applied until full entrance and exit block was achieved. Postablation voltage maps were used in a subset of patients at the operator’s discretion to further prove acute PVI before the end of the procedure. Adenosine or isoproterenol were used at the operator’s discretion but were not mandated by the protocol. Use of an ablation catheter other than the PFA circular array for PVI was considered an acute procedural failure.
Additional LA ablations outside of the PV antra were not allowed by protocol. Ablation of the cavotricuspid isthmus for treatment of atrial flutter was allowed if clinically necessary but was performed using a standard commercial radiofrequency ablation catheter. Pre- and postprocedure phrenic nerve pacing was performed in all patients. Luminal esophageal temperature monitoring was performed at operator discretion, and the position of the esophageal temperature probe was periodically adjusted to maintain close proximity to the electrode array of the ablation catheter.
Follow-Up
Upon hospital discharge, all patients underwent a medication review, physical exam, 12-lead ECG, and a National Institute for Health Stroke Scale assessment. A study visit was scheduled 30 days following the procedure, during which patients underwent a physical exam. A 12-lead ECG, medication review, and arrhythmia symptom review were also completed at this time point. Patients did not receive a follow-up chest computed tomography or magnetic resonance imaging scan within the 30-day follow-up period but received a cardiopulmonary exam during the 30-day study visit.
Study Outcomes
The efficacy objective of the first-in-human pilot study was to assess the achievement of intraprocedural PVI with the PFA system, where acute procedural failure was defined as (1) the inability to isolate all targeted PVs (assessed for entrance and, where assessable, exit block) during the index ablation procedure or (2) ablation using a nonstudy device to isolate any PV.
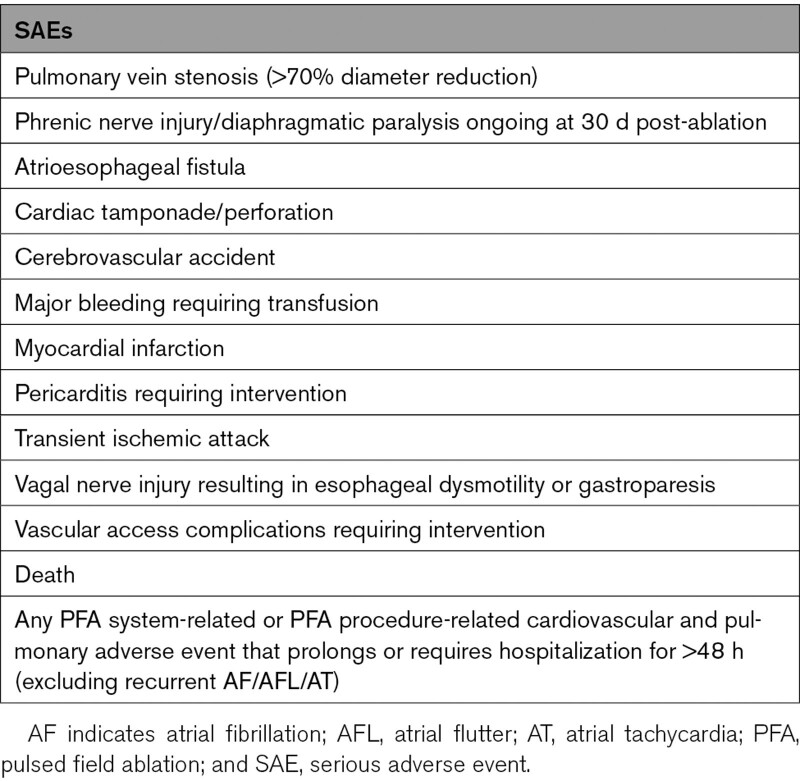
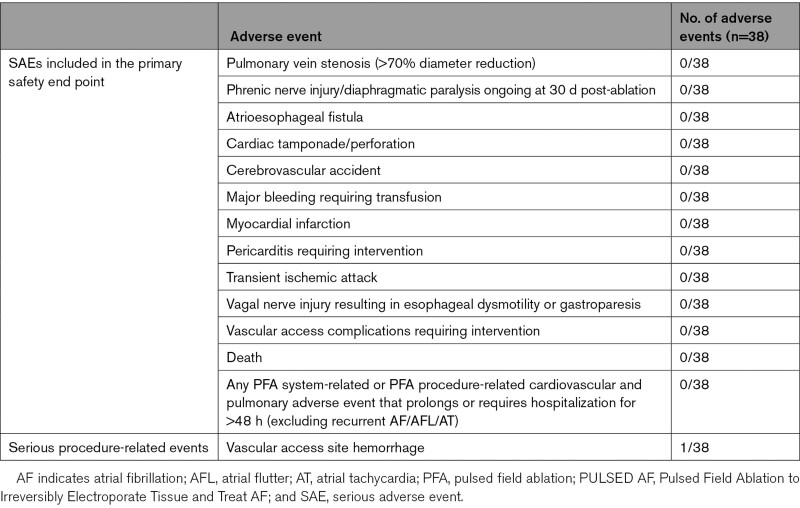
The safety objective of the pilot study was to assess the incidence of PFA system-related and PFA procedure-related serious adverse events (SAEs) within 30 days postablation. A list of SAEs included in this end point is provided in Table 2. All adverse events were collected in the study.
Table 2.
SAEs Included in the Primary Safety End Point
Statistical Analysis
The sample size of the first-in-human pilot study was not determined by a formal sample size calculation. Results are presented as a mean±SD for continuous variables, unless otherwise noted. Categorical variables are presented as a count and percentage. Statistical analyses were performed using the SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Study Patients
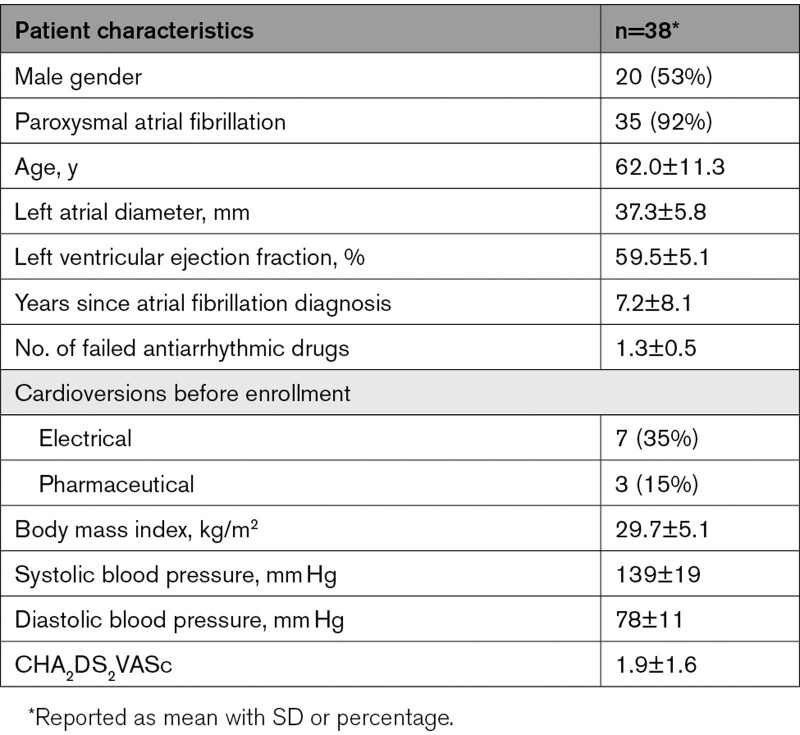
A total of 38 patients, 47% female, 92% paroxysmal AF, with an average age of 62.0±11.3 years underwent endocardial PFA of their PVs. The baseline data are available in Table 3.
Table 3.
Baseline Patient Characteristics
Procedural Characteristics
A subset of patients (9/38, 24%) were treated using conscious sedation, and the remainder received general anesthesia. Phrenic nerve stimulation was observed in 42% of PFA therapy deliveries in right-sided veins, and it was observed in 3% of PFA therapy deliveries in left-sided veins. Additionally, phrenic nerve stimulation in response to the low-voltage test pulse was only observed one time when the catheter was positioned deeper into the PV. The catheter was repositioned before any therapeutic deliveries. Although phrenic nerve capture was observed in response to PFA therapy deliveries, neither phrenic nerve injury nor transient stunning was observed in any patients intraprocedurally or during the follow-up period.
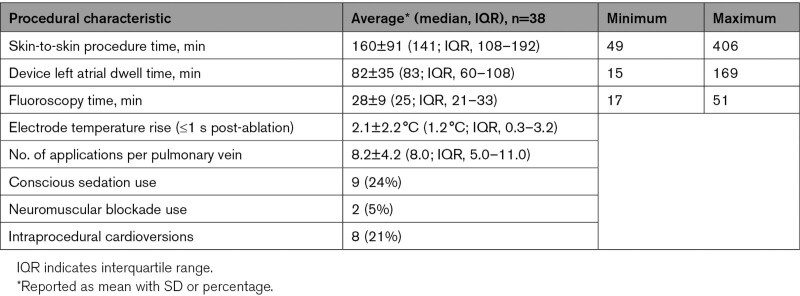
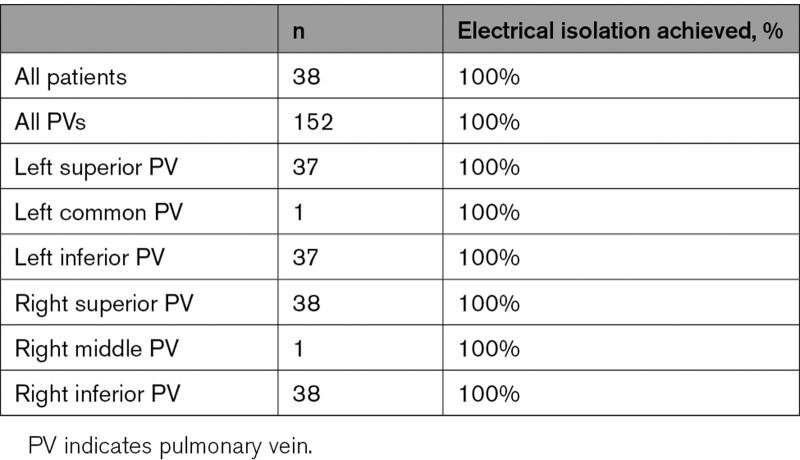
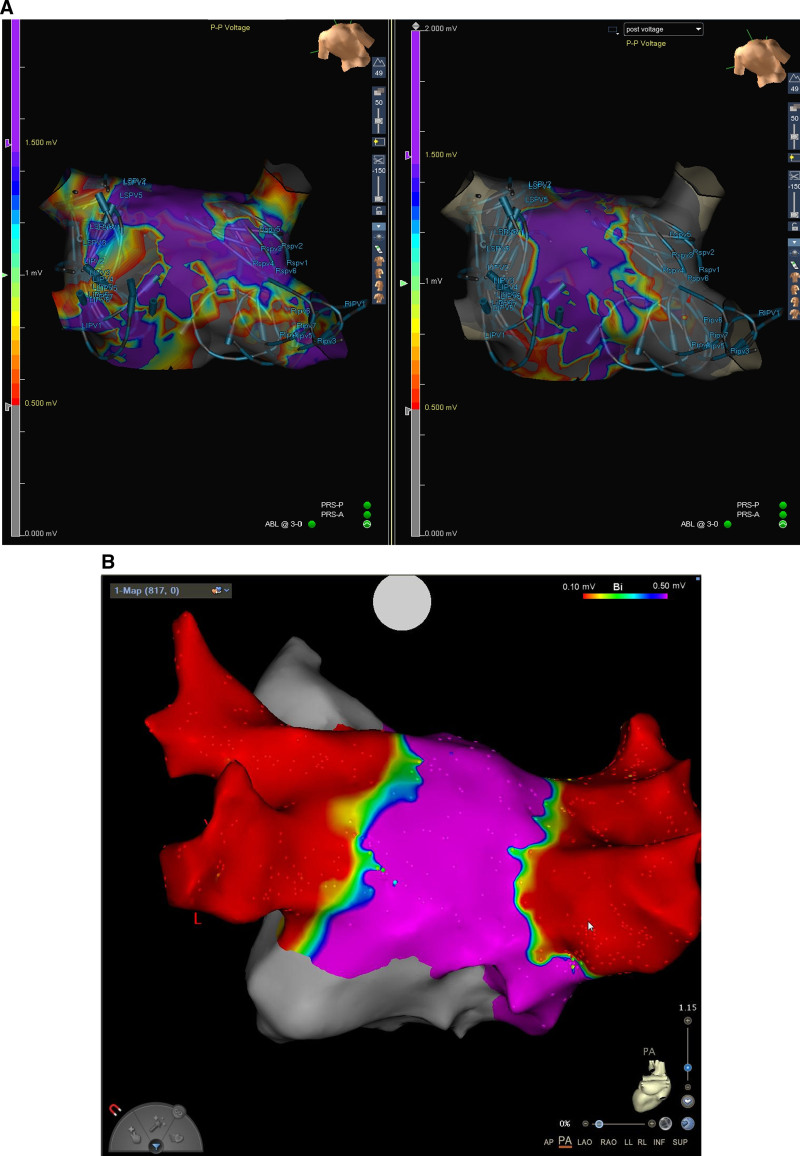
An average of 8.2±4.2 (median, 8.0; interquartile range [IQR], 5.0–11.0) energy applications were delivered around each of the PVs. The average temperature rise recorded directly from the electrodes during all ablations within 1-second postablation was 2.1±2.2°C (median, 1.2°C; IQR, 0.3–3.2°C; Table 4). No steam pops were observed during PFA delivery, and no char was observed on the catheter post-ablation. By procedural end, complete PVI was achieved in all 152 PVs attempted (Table 5). Samples of reduced PV potential amplitude and electrical isolation observed after PFA applications in each PV are provided in Figures 2 and 3, respectively. A sample video of PFA application is provided in the Supplemental Material, and typical voltage maps demonstrating ostial and antral PVI are shown in Figure 4.
Table 4.
Characteristics of Pulsed Field Ablation Procedures
Table 5.
Electrical Isolation of PVs
Figure 3.
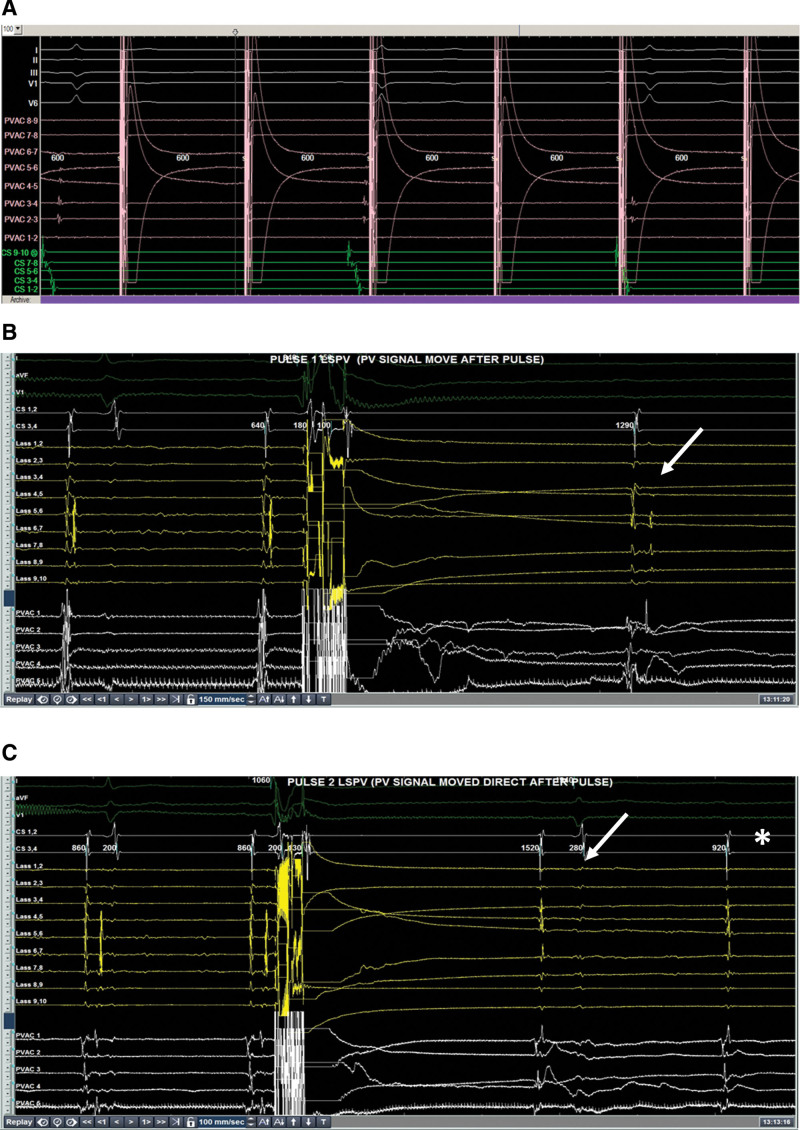
Evidence of pulmonary vein block recorded from bipolar electrograms from the 9-gold electrode array. In (A), we see exit block demonstrated by pacing within the left superior pulmonary vein with no conduction to the rest of the atrium (green coronary sinus signals). In (B), after one pulsed field application, we see delay in the pulmonary vein potential compared with preablation (white arrow). In (C), we see further delay in the pulmonary vein potential after one more application of pulsed field ablation (white arrow) and then pulmonary vein block (asterix).
Figure 4.
Postablation voltage maps of patients treated with pulsed field ablation. A demonstrates how the catheter electrode array position was tracked on a subset of cases using an electroanatomical mapping system. The catheter positionings were overlaid on the preablation voltage map (A, left) and on the postablation voltage map (A, right). At least 4 catheter positions were required around each pulmonary vein (PV) to achieve full ostial and antral PV isolation, as demonstrated in another patient (B).
Further procedural details are shown in Table 4. The skin-to-skin procedure time (from first sheath inserted to last sheath removed) was an average of 160±91 minutes (median, 141 minutes; IQR, 108–192). The average LA dwell time of the PFA catheter was 82±35 minutes (median, 83 minutes; IQR, 60–108), which included the 20-minute protocol required wait time after PVI. The average fluoroscopy time was 28±9 minutes (median, 25; IQR, 21–33). There were 8 (21%) intraprocedural cardioversions.
Safety
Luminal esophageal temperature monitoring was performed in a subset of patients at operator discretion (n=7). The maximum esophageal temperature recorded was 36.14±0.34°C, with a mean change of 0.06°C from baseline. No patients demonstrated any symptoms of esophageal injury during follow-up. No SAEs, strokes, or deaths related to the PFA system occurred in any patient out to 30 days post-procedure. Details are provided in Table 6.
Table 6.
Adverse Event Incidence in PULSED AF
Discussion
Main Findings
A novel PFA system used in a first-in-human pilot study achieved successful intraprocedural PVI in 100% of patients included in the study. There were no SAEs attributed to the PFA system during the 30-day follow-up. One serious procedure-related event was reported related to vascular access.
PFA Safety
PFA creates an electric field delivered within seconds that ablates cardiac tissue based on the mechanism of IRE.9 Cell membrane destabilization and subsequent cell death occurs in the cardiac tissue directly exposed to the electric field surrounding the array, ideally enabling ablation that is largely independent of thermal damage.5 Preclinical work has shown that biphasic, bipolar PFA can be achieved with minimal temperature rise.10 Furthermore, cardiac myocytes appear to have a lower threshold for IRE enabling cardiac ablation while minimizing the potential for collateral damage of other tissues. Animal models have shown preservation of phrenic nerve and esophageal tissue despite exposure to clinical PFA levels.11–16 PFA also seems to reduce the risk of PV stenosis.17,18 Therefore, one of the greatest potential advantages of PFA is offering a safety profile that may be superior to traditional radiofrequency or cryoablation. Worldwide surveys and large clinical trials suggest rates of complications ranging from 0.02% to 0.11% for atrioesophageal fistula, 0.1% to 2.2% for phrenic nerve injury, and 0.1% to 0.5% for PV stenosis.7 These are serious complications, and if their incidence can be reduced to almost zero, PFA will represent a significant step forward for cardiac catheter ablation of AF.
Our first-in-human study seems to support the largely nonthermal mechanism of PFA for lesion creation. Despite direct PFA delivery over the esophagus, there was almost no change in luminal temperature in the subset of patients where temperature monitoring was performed. Furthermore, the median change in electrode temperature detected by the generator within 1 second of PFA delivery was only about 1°C. Furthermore, we did not observe any occurrences of phrenic nerve injury or symptoms consistent with esophageal injury. Phrenic nerve function was routinely checked pre- and post-ablation in all patients. Postprocedural esophageal endoscopy was not performed in any patients. Steam pops or catheter char were not observed. The one serious procedure-related event that was observed was related to vascular access, unrelated to PFA energy delivery.
An average of 8.2±4.2 PFA applications was delivered to achieve PVI, which is aligned with prior studies with the same catheter and alternate radiofrequency energy source. Placement of the loop-shaped catheter to achieve both ostial and antral isolation drove the number of placements. Ablations were delivered without monitoring of contact force, indicating that PFA can achieve PVI without contact force–based monitoring and without thermal-related safety events.
PFA Efficiency
Because each of the 4 pulse trains delivered for PFA is delivered within a single cardiac cycle, the duration of energy application is very short compared with traditional thermal catheter ablation, which requires a sustained application to achieve tissue temperatures that irreparably damage cardiac tissue. Even with 4 applications at each ablation site, the duration of energy application is short compared with traditional thermal catheter ablation, which requires a sustained application to achieve tissue temperatures that irreparably damage cardiac tissue. Consequently, improved efficiency would be another potential key benefit for PFA.
Taking into account the first-in-human use of this PFA system and a study mandated 20-minute waiting period after the final PV was isolated; the LA dwell time for the procedure was only about 80 minutes. The total procedure time of ≈160 minutes represented the total time from sheath insertion to sheath removal (including transfer from the electrophysiology laboratory to the recovery room). It also included the time for any cavotricuspid isthmus ablation and voltage map creation. Examined within the context of a regulated clinical study, even these preliminary results suggest the possibility of a significant efficiency improvement for PFA over thermal ablation.
Limitations
This present analysis is limited by the single-arm clinical study design and small sample size. Further research with a larger clinical population and longer duration outcomes is warranted and will be initiated with the pivotal PULSED AF trial. Additional future clinical studies are needed to fully understand the impact of PFA energy settings on PVI, learning curve characteristics, incidence of silent cerebral emboli and silent cerebral lesions, and the impact of anesthesia on this novel procedure. Also, we did not perform systematic evaluation of the esophagus with endoscopy or magnetic resonance imaging to look for more subtle forms of esophageal injury. Finally, the purpose of this first-in-human pilot was to observe acute procedural end points, and longer term results will be reported in a larger subset of patients during the pivotal study.
Conclusions
In this first-in-human pilot trial, PFA using a novel circular array system achieved 100% acute PVI with no SAEs attributed to the PFA technology or catheter within the first 30 days post-procedure. A PULSED AF pivotal trial will evaluate the safety and efficacy of PFA in achieving PVI in a large group of patients with longer term follow-up.
Article Information
Acknowledgments
We would like to acknowledge Mark Stewart for his contributions to the development of this technology and support of the first-in-human clinical procedures. We would like to thank Dr Damijan Miklavčič and Dr Bor Kos for their guidance in optimizing the delivery of pulsed field ablation therapy. We would like to thank Dr Lars Mattison for his contribution to data interpretation and manuscript development. We would like to thank Josh Treadway and Jennifer Diouf for their commitment to execution and success of the clinical study. Also, we would like to thank Rachel Siciliano and Kevin Ruben for their knowledge and support of clinical procedures.
Sources of Funding
This study was fully funded by Medtronic, Inc.
Disclosures
Dr Verma, Dr Boersma, Dr Calkins, Dr Haines, Dr Hindricks, Dr Kuck, Dr Marchlinski, Dr Natale, Dr Packer, and P. Sanders receive consultation funds from Medtronic, Inc. Dr Onal and Dr Rosen are employees of Medtronic, Inc. Dr Marchlinski receives funds from Medtronic (consultant/advisory board), Biosense Webster (consultant/advisory board), Boston Scientific, and Biotronik (consultant/Director Fellow’s Educational Court).
Supplemental Material
Table S1
Video S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- IQR
- interquartile range
- IRE
- irreversible electroporation
- LA
- left atrium
- PFA
- pulsed field ablation
- PULSED AF
- Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF
- PV
- pulmonary vein
- PVI
- pulmonary vein isolation
- SAE
- serious adverse event
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.121.010168.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Lucas Boersma, Email: l.boersma@antoniusziekenhuis.nl.
David E. Haines, Email: dhaines@beaumont.edu.
Andrea Natale, Email: dr.natale@gmail.com.
Francis E. Marchlinski, Email: francis.marchlinski@uphs.upenn.edu.
Prashanthan Sanders, Email: prash.sanders@adelaide.edu.au.
Hugh Calkins, Email: hcalkins@jhmi.edu.
Douglas L. Packer, Email: Crowson.Jacqueline@mayo.edu.
John Hummel, Email: john.hummel@osumc.edu.
Birce Onal, Email: birce.onal@medtronic.com.
Sofi Rosen, Email: sofi.rosen@medtronic.com.
Karl-Heinz Kuck, Email: kuckkh@aol.com.
Gerhard Hindricks, Email: gerhard.hindricks@leipzig-ep.de.
Bradley Wilsmore, Email: bwilsmore@gmail.com.
References
- 1.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S, Zei PC, Epstein LM, Tedrow UB, Michaud GF, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:854–860. doi: 10.1111/jce.13484 [DOI] [PubMed] [Google Scholar]
- 5.Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48:63–91. doi: 10.1146/annurev-biophys-052118-115451 [DOI] [PubMed] [Google Scholar]
- 6.Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015;62:4–20. doi: 10.1109/TBME.2014.2367543 [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66–e93 [DOI] [PubMed] [Google Scholar]
- 9.Sugrue A, Vaidya V, Witt C, DeSimone CV, Yasin O, Maor E, Killu AM, Kapa S, McLeod CJ, Miklavčič D, et al. Irreversible electroporation for catheter-based cardiac ablation: a systematic review of the preclinical experience. J Interv Card Electrophysiol. 2019;55:251–265. doi: 10.1007/s10840-019-00574-3 [DOI] [PubMed] [Google Scholar]
- 10.Stewart MT, Haines DE, Miklavčič D, Kos B, Kirchhof N, Barka N, Mattison L, Martien M, Onal B, Howard B, et al. Safety and chronic lesion characterization of pulsed field ablation in a Porcine model. J Cardiovasc Electrophysiol. 2021;32:958–969. doi: 10.1111/jce.14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, Howard B. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. doi: 10.1016/j.hrthm.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 12.Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink A, Doevendans P, Wittkampf F. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol. 2017;10:e004672. doi: 10.1161/CIRCEP.116.004672 [DOI] [PubMed] [Google Scholar]
- 13.Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck ED, Donskoy E, Neuzil P, Dukkipati SR, Reddy VY. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol. 2020;13:e008303. doi: 10.1161/CIRCEP.119.008303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koruth JS, Kuroki K, Kawamura I, Stoffregen WC, Dukkipati SR, Neuzil P, Reddy VY. Focal pulsed field ablation for pulmonary vein isolation and linear atrial lesions: a preclinical assessment of safety and durability. Circ Arrhythm Electrophysiol. 2020;13:e008716. doi: 10.1161/CIRCEP.120.008716 [DOI] [PubMed] [Google Scholar]
- 15.Yavin H, Brem E, Zilberman I, Shapira-Daniels A, Datta K, Govari A, Altmann A, Anic A, Wazni O, Anter E. Circular multielectrode pulsed field ablation catheter lasso pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021;14:e009229. doi: 10.1161/CIRCEP.120.009229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–1844. doi: 10.1016/j.hrthm.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 17.Howard B, Haines DE, Verma A, Packer D, Kirchhof N, Barka N, Onal B, Fraasch S, Miklavčič D, Stewart MT. Reduction in pulmonary vein stenosis and collateral damage with pulsed field ablation compared with radiofrequency ablation in a canine model. Circ Arrhythm Electrophysiol. 2020;13:e008337. doi: 10.1161/CIRCEP.120.008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Driel VJ, Neven KG, van Wessel H, du Pré BC, Vink A, Doevendans PA, Wittkampf FH. Pulmonary vein stenosis after catheter ablation: electroporation versus radiofrequency. Circ Arrhythm Electrophysiol. 2014;7:734–738. doi: 10.1161/CIRCEP.113.001111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.