Version Changes
Revised. Amendments from Version 1
We have added additional detail and clarification to the revised version of the article in response to comments raised by the reviewers. We have made clearer the definition and concept of social incentive which underpinned the intervention cover letter and the difference in this terminology from social pressure or social reward. We have also made minor amendments to Table 2 to remove BMI and EQ-5D-5L scores which the reviewers felt were not relevant. We added details about the PROMETHEUS programme and edited the description of the meta-analysis to address reviewer comments on the reason for the meta-analysis and the other studies that were included in this. We have also updated the discussion to also reflect the point that is mentioned in the methods that all participants were given a £5 monetary reward. Finally, we have updated two figures in the meta-analysis, due to a recently published new version (version 2) of one of the studies included in the meta-analysis ('Mitchell A, Cook L, Dean A, et al.: Using pens as an incentive for questionnaire return in an orthopaedic trial: an embedded randomised controlled retention trial') being published on F1000. This version made amendments to their results in light of a duplicate randomisation that was found in the host trial, these changes were negligible and did not affect the interpretation of the results. However this means that for accuracy the meta analysis figure of this paper has been updated to include these new figures.
Abstract
Background: Postal questionnaires are frequently used in randomised controlled trials to collect outcome data on participants; however, poor response can introduce bias, affect generalisability and validity, and reduce statistical power. The objective of this study was to assess whether a pen and/or social incentive text cover letter sent with a postal follow-up questionnaire increased response rates in a trial.
Method: A two-by-two factorial randomised controlled trial was embedded within the OTIS host trial. Participants due their 12-month (final) follow-up questionnaire were randomised to be sent: a pen; a social incentive text cover letter; both; or neither. The primary outcome measure was the proportion of participants in each group who returned the questionnaire. Secondary outcomes were: time to return, completeness of the questionnaire, necessity of a reminder letter, and the cost effectiveness.
Results: The overall 12-month questionnaire response rate was 721 out of 755 (95.5%). Neither the pen nor social incentive cover letter had a statistically significant effect on response rate: pen 95.2% vs. no pen 95.8%, adjusted OR 0.90 (95% CI 0.45 to 1.80; p=0.77); social incentive cover letter 95.2% vs. no social incentive cover letter 95.8%, adjusted OR 0.84 (95% CI 0.42 to 1.69, p=0.63). No statistically significant differences were observed between either of the intervention groups on time to response, need for a reminder or completeness. Therefore, neither intervention was cost-effective.
Conclusions: We found no evidence of a difference in response rates associated with the inclusion of a pen and/or social incentive cover letter with the final follow-up postal questionnaire of the host trial. However, when these results are combined with previous SWATs, the meta-analysis evidence remains that including a pen increases response rates. The social incentive cover letter warrants further investigation to determine effectiveness.
Trial registration: ISRCTN22202133 (21st June 2020).
Keywords: Retention, pen, social incentive, cover letter, randomised controlled trial, embedded trial, SWAT, postal questionnaire, response rate
Introduction
Randomised controlled trials (RCTs) are the gold standard to assess effectiveness of treatment options and to inform care decisions 1 , yet only a few hundred studies exist to assess the effectiveness of different methods to improve retention or recruitment into RCTs 2 .
Trial methodologists and funders have highlighted the need to evaluate participant recruitment and retention strategies in order to provide evidence on which to base decisions around the design and conduct of RCTs 3 .
Postal questionnaires are frequently used in randomised controlled trials to collect outcome data on participants; however, poor response can introduce bias, affect generalisability and validity, and reduce statistical power. Several systematic reviews report on the topic of retention strategies, including improving response rates to questionnaires 4– 7 . However, there remains a lack of definitive evidence regarding some commonly adopted practices such as sending a pen or using a cover letter with a questionnaire to encourage the participant to return it 8– 10 . The results of a study within a trial (SWAT) evaluating these two strategies are reported here.
Methods
Design
A two-by-two factorial RCT was embedded within the OTIS trial of occupational therapist-led home assessment and modification for the prevention of falls ( ISRCTN22202133) 11 . OTIS recruited participants over the age of 65 years who were at risk of falling. Participants were randomised to receive an occupational therapist delivered visit or usual care. They were followed up for 12 months for falls data and were sent postal questionnaires at four, eight and 12 months. This SWAT was embedded at the 12-month time point. Ethical approval for this SWAT was received from the NHS West of Scotland Research Ethics Committee 3 (16/WS/0154) and Health Research Authority and Research Ethics approval in July 2018. Approvals were obtained from the University of York, Department of Health Sciences Research Governance Committee. Participants provided informed consent to be enrolled into the OTIS trial and to be sent study related information by post. Consent for the SWAT was therefore waived by the above-named ethics committee.
Participants
A total of 779 participants due to receive their 12-month questionnaire between 16 th October 2018 and 2 nd August 2019 were randomised into the SWAT in a single tranche in September 2018. Participants who had withdrawn from the OTIS study prior to this were excluded from randomisation.
The allocation sequence was generated by the OTIS statistician, who was not involved with the sending of the questionnaires, using STATA v15 12 . The identification numbers of OTIS participants to be involved in the SWAT were randomised 1:1:1:1 in a single block. Because there were no descriptive details of the participants attached to the identification numbers this meant the randomisation was concealed.
Interventions
Table 1 details the combination of interventions sent in the post with the 12-month questionnaire. We included an unconditional £5 note with the questionnaire for all participants.
The non-standard cover letter offered a mild level of social incentive, in the form of a personalised table that indicated whether or not a questionnaire had been received from the participant at the earlier (4 and 8-month) time points. The concept of social incentive that underpinned the intervention for this study was that a social incentive is something that persuades people to behave in a certain way by the promise that their actions will be noticed or made public 10 . Therefore, the cover letter was intended to highlight to the participant that their questionnaire responses are noted and valued 10 .
Table 1. Intervention groups.
|
Pen
York Trials Unit branded pen, standard cover letter (Supplementary File 1) * |
Control Group
No pen, standard cover letter (Supplementary File 4). |
|
Pen and Social Incentive Cover Letter
York Trials Unit branded pen, social incentive cover letter (Supplementary File 3). |
Social Incentive cover letter
Social incentive cover letter ( Supplementary File 2), no pen. |
*Supplementary Files are available as Extended data 13 .
Blinding and quality assurance
Participants were blind to their participation. Research administrators and research team members posting the questionnaire packs were not blind to the intervention; however, administrators who recorded the outcome data were blind to allocation.
Primary objective
To assess whether a pen and/or social incentive text cover letter sent with the 12-month questionnaire increased postal questionnaire response rates for participants in the OTIS trial.
Primary outcome
The primary outcome was response rate, defined as the proportion of participants in each group who returned the 12-month questionnaire.
Secondary outcomes
Time to return 12-month questionnaire
The completeness of the 12-month questionnaire
The requirement for a reminder letter to be sent
Cost effectiveness
Statistical analysis
The data were analysed in SPSS v25 14 using two-sided tests at the 5% significance level on an intention-to-treat basis. Participants who withdrew or died before the 12-month questionnaire was sent were excluded from the analysis. The primary outcome was compared using a logistic regression model adjusting for age (retention is generally higher in participants ˂75 years and older adults may respond differently to incentives 15 ), gender (to control for potential differences in anticipation of social incentives between males and females 16 ) and host trial treatment allocation. The presence of an interaction between the two interventions was tested by introducing the interaction term into the logisit model. Time to questionnaire return (calculated as days from questionnaire sent to return) was analysed using Cox Proportional Hazards regression, adjusting for the same covariates as in the primary analysis. The proportional hazards assumption was assessed using Schoenfeld residuals 17 . Completeness of response (defined as number of items completed) was analysed by linear regression model and adjusted as for the primary analysis.
Cost effectiveness was calculated for each group using the total cost of the pen/letter/postage/stationary and staff time.
Due to SWATs typically being under-powered to show small effects, it is essential that the results are seen within the context of the wider literature. A fixed effect meta-analysis using the Mantel-Haenszel method was conducted using review manager v5.3 18 to pool the results of this study for enclosing a pen with the 12-month questionnaire with other RCT evidence. These were located utilising the Cochrane systematic review 7 search strategy (Supplementary file 14) in MEDLINE and EMBASE, along with hand searching of previous systematic reviews references, published retention research reference lists, conference papers and co-author personal knowledge of studies. The results of this study were pooled with four previous SWATs 8, 9, 19, 20 investigating the same intervention, with the same dichotomous outcome of response to the questionnaire or not. Pooled odds ratios and corresponding 95% CIs were calculated. Heterogeneity between trials was assessed using the Chi-squared and I 2 statistics. The meta-analysis was facilitated by the PROMoting THE USE of SWATs (PROMETHEUS) programme, which supports host trial teams to conducted SWATs and for data obtained to be collated and meta-analysed.
A meta-analysis of the results of the social incentive intervention was not undertaken as the only previous study using this was conducted within a cohort study rather than an RCT 10 .
Results
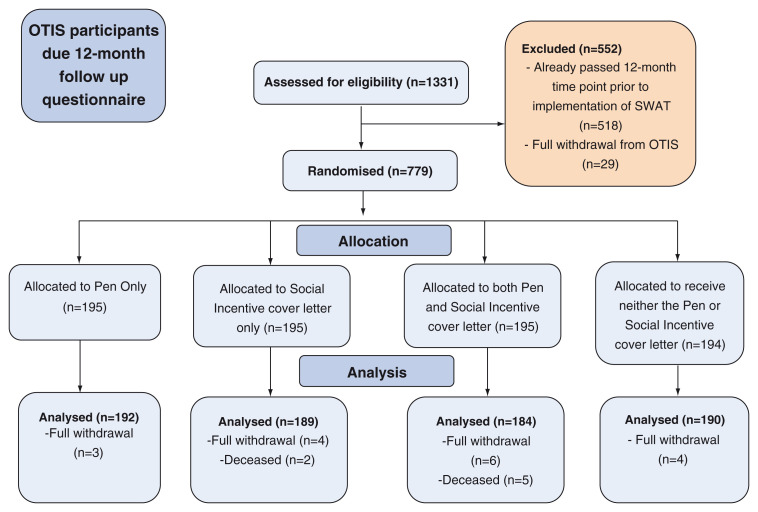
Figure 1 depicts the recruitment and retention of participants in the embedded trial. Table 2 presents summary statistics for the baseline characteristics of the SWAT participants.
Figure 1. Flow diagram depicting the recruitment and retention of participants in this embedded trial.
Table 2. Baseline characteristics of the SWAT participants.
| Pen only
(n=192) |
Pen and social
incentive cover letter (n=184) |
Social incentive
cover letter only (n=189) |
Standard 12-
month cover letter (control) (n=190) |
|
|---|---|---|---|---|
| Age | ||||
| n | 192 | 184 | 189 | 190 |
| Mean (SD) | 80 (6.3) | 80 (6.1) | 79 (6.2) | 80 (6.2) |
| Min, Max | 67, 98 | 66, 98 | 65, 98 | 69, 94 |
| Gender | ||||
| n | 192 | 184 | 189 | 190 |
| Male | 73 (38.0%) | 56 (30.4%) | 59 (31.2%) | 69 (36.3%) |
| Female | 119 (62.0%) | 128 (69.6%) | 130 (68.8) | 121 (63.7%) |
| Host trial randomisation | ||||
| n | 192 | 184 | 189 | 190 |
| OT visit (intervention) | 61 (31.8%) | 49 (26.6%) | 59 (31.2%) | 65 (34.2%) |
| GP standard care | 131 (68.2%) | 135 (73.4%) | 130 (68.8%) | 125 (65.8%) |
| Number of falls in 12 months prior to randomisation | ||||
| n | 145 | 139 | 149 | 135 |
| Mean | 2.2 (3.0) | 1.8 (1.4) | 2.0 (1.7) | 2.2 (2.1) |
| Min, Max | 1, 21 | 1, 11 | 1, 10 | 1, 15 |
#= How good or bad your health is today rated from 0 worst, 100 best.
Primary outcome
Between randomisation into the SWAT and being sent their 12-month questionnaire, 24 randomised participants either died or withdrew from the host trial and so were not sent the questionnaire. A total of 721/755 (95.5%) returned the 12-month questionnaire. The response rate was identical in the pen only group (184/192, 95.8%), social incentive cover letter only group (181/189, 95.8%) and control group (182/190, 95.8%). However, it was marginally lower in the pen and social incentive cover letter group (174/184, 94.6%).
No evidence of a difference in response rates was found between participants with or without pens (pen: 358/376 [95.2%]; no pen: 363/379 [95.8%]; adjusted OR 0.90, 95% CI 0.45 to 1.80, p=0.77) nor with or without the social incentive cover letter (cover letter: 355/373 [95.2%]; no cover letter: 366/382 [95.8%]; adjusted OR 0.84, 95% CI 0.42 to 1.69, p=0.63) ( Table 3).
Table 3. Primary outcome results.
| Primary
outcome |
Group | Hazard ratio (HR)/
Odds ratio (OR)/Mean difference (MD) |
95%
Confidence Interval |
p-value | Other |
|---|---|---|---|---|---|
|
Response
rate |
Pen received vs. not received | OR = 0.90 | 0.45, 1.80 | 0.77 | Total of 721/755 (95.5%)
returned tde 12-month questionnaire |
| Social incentive cover letter
received vs. not received |
OR = 0.84 | 0.42, 1.69 | 0.29 | ||
| Host trial allocation
(intervention vs. control) |
OR = 1.40 | 0.64, 3.23 | 0.38 | ||
| Age (per year) | OR = 0.96 | 0.91, 1.01 | 0.11 | ||
| Gender (male vs. female) | OR = 0.71 | 0.35, 1.44 | 0.35 |
The interaction between the interventions was found to be non-significant (interaction effect size estimate OR 0.79 95% CI 0.20, 3.15 p = 0.74).
Secondary outcomes
Time to return . Median time to return the questionnaire was nine days, with a mean of 12.2 days. No statistically significant difference between the groups was found ( Table 4).
Table 4. Secondary outcome results.
| Secondary
outcome |
Group | Hazard ratio (HR)/
Odds ratio (OR)/Mean difference (MD) |
95%
Confidence Interval |
p-value | Other |
|---|---|---|---|---|---|
| Time to return | Pen received vs. not
received |
HR = 1.08 | 0.93, 1.25 | 0.30 | Mean time for all participants to
return questionnaire = 12.2 days. Median time for all participants to return questionnaire = 9 days. |
| Social incentive cover
letter received vs. not received |
HR =1.101 | 0.87, 1.17 | 0.92 | ||
| Host trial allocation
(intervention vs. control) |
HR = 0.85 | 0.73, 1.00 | 0.05 | ||
| Age (per year) | HR = 0.99 | 0.97, 1.00 | 0.02 | ||
| Gender (male vs. female) | HR = 1.80 | 0.92, 1.26 | 0.35 | ||
|
Reminders
sent |
Pen received vs. not
received |
OR = 0.89 | 0.56, 1.42 | 0.63 | 83/755 (11.0%) required a reminder
p value associated with the Kruskal- Wallis test statistic p=0.190 |
| Social incentive cover
letter received vs. not received |
OR = 0.92 | 0.58, 1.47 | 0.74 | ||
| Host trial allocation
(intervention vs. control) |
OR = 1.611 | 1.00, 2.59 | 0.05 | ||
| Age (per year) | OR = 1.04 | 1.00, 1.08 | 0.03 | ||
| Gender (male vs. female) | OR = 0.87 | 0.53, 1.42 | 0.57 | ||
|
Completeness
of response |
Pen received vs. not
received |
MD = 0.14 | -0.46, 0.74 | 0.65 | Overall average completeness of the
questionnaires was 27.8/31 questions (89.6% complete) |
| Social incentive cover
letter received vs. not received |
MD = 0.09 | -0.69, 0.51 | 0.78 | ||
| Host trial allocation
(intervention vs. control) |
MD = -0.10 | -0.55, 0.75 | 0.77 | ||
| Age (per year) | MD = -0.10 | -0.46, 0.74 | 0.65 | ||
| Gender (male vs. female) | MD = -1.06 | -1.69, -0.42 | ˂0.001 |
Reminders sent . In total, 83/755 (11.0%) participants required a reminder letter. The pen and social incentive cover letter group required the least reminders (19/184 10.3%) and the control group required the most reminders (24/190 12.6%). No statistically significant evidence was found of a difference of participants requiring a reminder between the groups ( Table 4).
Completeness of response . Overall average completeness of the questionnaires was 27.8/31 questions (89.6% complete) with no evidence of a difference in completeness of the questionnaire between pen received or not ( Table 4).
Cost effectiveness . Due to the non-statistically significant effect of the interventions on response rates calculating overall associated costs provides evidence of potential cost savings not to send the social incentive cover letter and/or pen ( Extended data: Supplementary File 9 13 ).
Meta-analysis
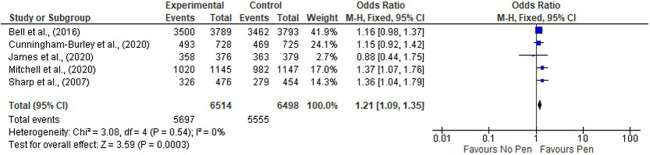
A fixed effect meta-analysis of enclosing a pen with a follow-up postal questionnaire on response rate was conducted ( Figure 2). This included five studies 8, 9, 21, 22 (n=13012 participants) and gave a statistically significant pooled OR favouring the intervention (1.21, 95% CI 1.09 to 1.35 p = 0.0004). Negligible heterogeneity was observed (chi-squared = 2.88 I 2= 0%). The risk of bias was low, as indicated by the Cochrane’s risk of bias tool assessment undertaken 23 ( Extended data: Supplementary File 10 13 ).
Figure 2. Meta-analysis of enclosing a pen with a questionnaire to increase response rate to a postal questionnaire.
Discussion
This SWAT found no evidence that sending a pen and/or a social incentive cover letter with a postal, trial follow-up questionnaire improved response rate, time to return, requirement for a reminder, or questionnaire completeness.
A limitation was the average age of the participants (79.9 years) giving a narrow age demographic thus restricting generalisability of results. Further investigation of the pen and social incentive cover letter in RCTs are required across more diverse populations.
The OTIS trial hosted three other methodological SWATs; therefore, there was a potential for contamination or interaction. It is preferable to plan all SWATs that will be undertaken in the early design stages 19 , to ensure they are planned accordingly to reduce the potential of this.
The overall response rate of the 12-month postal questionnaire for all SWAT participants was 95.7%, which may have been helped by the inclusion of £5 to all participants as standard. This high response rate is therefore difficult to improve upon, furthermore the incentives may not have been as effective with participants who are very committed to the behaviour 10 . The incentive required for committed participants may be different 10, 20 . A learning point being that future SWATS testing these interventions should avoid doing so in trials with already high response rates.
Conclusion
Whilst neither the pen nor the social incentive cover letter showed an effect on response rate, the meta-analysis evidence remains that including a pen increases response rates. This reinforces that for interventions where small effects are likely, it is important to undertake a number of trials and combine these to be confident of an intervention’s effectiveness. Further investigation of the social incentive cover letter in RCTs is required to determine effectiveness.
Data availability
Underlying data
Open Science Framework: Pen and Social Incentive Cover Letter Retention SWAT, https://doi.org/10.17605/OSF.IO/7TDRB 13 .
Extended data
Open Science Framework: Pen and Social Incentive Cover Letter Retention SWAT, https://doi.org/10.17605/OSF.IO/7TDRB 13 .
This project contains the following extended data:
Full study protocol
Supplementary File 1- Cover letter for the Pen only group.
Supplementary File 2 - Cover letter for the Social incentive cover letter only group.
Supplementary File 3 - Cover letter for the Pen and social incentive cover letter group.
Supplementary File 4 - Cover letter for the control group.
Supplementary File 5 - Results table by intervention group
Supplementary File 6 - Graph Survival curve of pen vs no pen and time taken to return 12-month questionnaire.
Supplementary File 7 - Graph Survival curve of Social incentive cover letter vs no social incentive cover letter and time taken to return 12-month questionnaire.
Supplementary File 8- Survival curve of host trial allocation and time taken to return 12-month questionnaire.
Supplementary File 9 – Costings table
Supplementary File 10 – Cochrane Risk of bias tool assessments for Bell et al., (2016) 8 , Sharp et al., (2006) 9 , Cunningham-Burley et al., (2020) 21 , Mitchell et al., (2020) 22 and James et al., (2020).
Supplementary File 11 – Summary of all SWATs undertaken in the OTIS study
Supplementary File 12 – Copy of the OTIS reminder letter
Supplementary file 13 – Summary of studies included in the meta-analysis
Supplementary File 14 – Copy of the search strategies of Brueton et al., (2014)
Reporting guidelines
Open Science Framework: CONSORT checklist for ‘Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled Study within a Trial’, https://doi.org/10.17605/OSF.IO/TYJDP 13 .
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
The authors would like to thank the embedded trial participants who returned 12-month questionnaires.
This manuscript has been written by the authors on behalf of the OTIS Study Team. Sophie Boyes (York Teaching Hospital NHS Foundation Trust); Belen Corbacho (University of York); Shelley Crossland (Leicestershire Partnership NHS Trust); Avril Drummond (University of Nottingham); Simon Gilbody (University of York); Catherine Hewitt (University of York); Sarah E Lamb (University of Oxford); Katie Whiteside (University of York); Jennifer McCaffery (University of York); Alison Pighills (Mackay Base Hospital; Mackay Australia and James Cook University); Clare Relton (University of Sheffield).
The results from this project will contribute to the evidence towards trial methodology for improving retention of participants. This will study will be linked with a national research programme PROMETHEUS led by York Trials Unit ( https://www.york.ac.uk/healthsciences/research/trials/research/swats/prometheus/) and findings will be combined with other studies in meta-analyses to detect small but cost effective differences. This will help future trials to be designed with effective interventions in place to maximise retention and avoid introduction of bias and reduced study power.
Funding Statement
The OTIS study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Programme grant number: 14/49/149). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This SWAT was funded by PROMETHEUS and the results from this project will contribute to the evidence towards trial methodology for improving retention of participants. This will study will be linked with a national research programme PROMETHEUS led by York Trials Unit (https://www.york.ac.uk/healthsciences/research/trials/research/swats/prometheus/) and findings will be combined with other studies in meta-analyses to detect small but cost effective differences. This will help future trials to be designed with effective interventions in place to maximise retention and avoid introduction of bias and reduced study power. The University of York is the study sponsor and has legal responsibility for the initiation and management of the trial (sponsor representative: Dr Michael Barber, Research and Enterprise Directorate, University of York, Ron Cooke Hub, Heslington, York, UK, YO10 5GE).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Eble A, Boone P, Elbourne D: On minimizing the risk of bias in randomized controlled trials in economics. The World Bank.2016. Reference Source [Google Scholar]
- 2. Clark L, Ronaldson S, Dyson L, et al. : Electronic prompts significantly increase response rates to postal questionnaires: a randomized trial within a randomized trial and meta-analysis. J Clin Epidemiol. 2015;68(12):1446–50. 10.1016/j.jclinepi.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 3. Edwards P, Roberts I, Clarke M, et al. : Increasing response rates to postal questionnaires: systematic review. BMJ. 2002;324(7347):1183. 10.1136/bmj.324.7347.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakash RA, Hutton JL, JØrstad-Stein EC, et al. : Maximising response to postal questionnaires - A systematic review of randomised trials in health research. BMC Med Res Methodol. 2006;6:5. 10.1186/1471-2288-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards PJ, Roberts IG, Clarke MJ, et al. : Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev. 2007; (2): MR000008. 10.1002/14651858.MR000008.pub3 [DOI] [PubMed] [Google Scholar]
- 6. Edwards PJ, Roberts I, Clarke MJ, et al. : Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009; (3): MR000008. 10.1002/14651858.MR000008.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brueton VC, Tierney JF, Stenning S, et al. : Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4(2):e003821. 10.1136/bmjopen-2013-003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bell K, Clark L, Fairhurst C, et al. : Enclosing a pen reduced time to response to questionnaire mailings. J Clin Epidemiol. 2016;74:144–150. 10.1016/j.jclinepi.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 9. Sharp L, Cochran C, Cotton SC, et al. : Enclosing a pen with a postal questionnaire can significantly increase the response rate. J Clin Epidemiol. 2006;59(7):747–54. 10.1016/j.jclinepi.2005.10.014 [DOI] [PubMed] [Google Scholar]
- 10. Cotterill S, Howells K, Rhodes S, et al. : The effect of using social pressure in cover letters to improve retention in a longitudinal health study: an embedded randomised controlled retention trial. Trials. 2017;18(1):341. 10.1186/s13063-017-2090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cockayne S, Pighills A, Adamson J: Can occupational therapist-led home environmental assessment prevent falls in older people? A modified cohort randomised controlled trial protocol. BMJ Open. 2018;8(9):e022488. 10.1136/bmjopen-2018-022488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. StataCorp: Stata Statistical Software: Release 15. College Station, T.S.L.2017. Reference Source [Google Scholar]
- 13. James S: Pen and Social Incentive Cover Letter Retention SWAT.2020. 10.17605/OSF.IO/7TDRB [DOI] [Google Scholar]
- 14. Corp I: IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. Released (2017). Reference Source [Google Scholar]
- 15. Chhatre S, Jefferson A, Cook R, et al. : Patient-centered recruitment and retention for a randomized controlled study. Trials. 2018;19(1):205. 10.1186/s13063-018-2578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spreckelmeyer KN, Krach S, Kohls G, et al. : Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4(2):158–65. 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoenfeld D: Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 18. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, T.C.C,2014. [Google Scholar]
- 19. Bower P, Brueton V, Gamble C, et al. : Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials. 2014;15:399. 10.1186/1745-6215-15-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Keefe DJ: Persuasion: Theory and Research. SAGE publishers,2002. Reference Source [Google Scholar]
- 21. Cunningham-Burley R, Roche J, Fairhurst C, et al. : Enclosing a pen to improve response rate to postal questionnaire: an embedded randomised controlled trial [version 1; peer review: awaiting peer review]. F1000Res. 2020;9:577. 10.12688/f1000research.23651.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell A, Cook L, Dean A, et al. : Using pens as an incentive for questionnaire return in an orthopaedic trial: an embedded randomised controlled retention tria. F1000Res. 2021;9:321. [Google Scholar]
- 23. Higgins JPT, Altman DG, Gøtzsche PC, et al. : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]