Abstract
Knowledge of cardiovascular adaptations in athletes has predominantly focused on males, with limited data available on females who compromise a substantial percentage of all collegiate athletes. A multicenter retrospective cohort review of preparticipation cardiovascular screening data of 329 National Collegiate Athletic Association Division I female athletes was performed. This included physical exams, electrocardiograms, and echocardiograms. Female athletes in class IB sports had elevated systolic blood pressure (p = 0.01). For electrocardiograms, 7 (2%) had abnormal findings: 100% were white; 6 of 7 (86%) participated in IIC sports. Black athletes had longer PR intervals (p ≤ 0.001), whereas white athletes had longer QTc and QRS durations (p = 0.02 and 0.01, respectively). Athletes in IC and IIC sports had longer QTc intervals (p = 0.01). For echocardiographic parameters, no differences were noted based on race. However, significant differences were noted based on classification of sport: athletes in class IC sports had higher left-atrial volume indexes and E/A ratios. Athletes in class IB and IIC had increased left-ventricular wall thicknesses and aortic root dimensions. In conclusion, among one of the largest cohorts of collegiate female athlete preparticipation cardiac screening data to date, significant differences in various parameters based on classification of sport and race were observed. These categorizations should be considered when interpreting cardiovascular screening in female collegiate athletes to improve screening and guide future research.
Over the past several decades, increased emphasis has been placed on cardiovascular screening of collegiate athletes. This trend has led to the evolution of the preparticipation evaluation (PPE) to include screening electrocardiograms (ECGs) and transthoracic echocardiograms (TTEs). In 2017, the International Criteria for Electrocardiographic Interpretation in Athletes provided an updated consensus of normal versus abnormal ECG changes in athletes.1 Similarly, initial studies reviewing TTEs in male athletes have noted distinct differences in the athletic heart such as larger left ventricular cavities, septal sizes, and wall thickness with unclear clinical significance.2 Whereas such initial studies have aided our understanding of cardiac differences in athletes, the overwhelming majority have focused on men. Since the “Title IX Act” of 1972 there has been a dramatic increase in female participation in competitive sports.3 Additionally, mortality due to cardiovascular disease has continued to decrease overall in the United States, but the decline is almost nonexistent among younger women.4 As much of the existing literature has focused on cardiac changes in male athletes, a better understanding of the normal physiologic cardiac changes occurring in elite female athletes is important to provide a framework to assess for those at risk for adverse cardiovascular outcomes. Accordingly, we investigated physical and cardiac evaluations from the time of PPE in a cohort of National Collegiate Athletic Association Division I female athletes.
Methods
A retrospective cohort review of PPE data of 329 eligible female collegiate athletes cleared for participation from the University of Florida (2012 to 2019) and the University of Georgia (2010 to 2015) was performed. Female athletes were eligible for inclusion if they were enrolled in the University of Florida Athletic Association Cardiac Databank or presented for the institutional requirement PPE to participate in National Collegiate Athletic Association Division I athletics at the University of Georgia during the respective dates. Basketball, soccer, lacrosse, track and field, cross country, softball, gymnastics, swimming, and volleyball athletes were included. PPE included American Heart Association Preparticipation Cardiovascular Screening 14-element personal and family history, physical examination, a 12-lead ECG, and a TTE.5 All athletes received full clearance to play, and none were known to have been excluded during their subsequent career. Subgroup analyses were performed based on self-reported race and categorization of static and/or dynamic component of training based on the American Heart Association and/or American College of Cardiology Scientific Statement Task Force 1 (Table 1).6 Categories for race were “black” and “white.” Eight athletes reported “other” category for race and were excluded from race analyses due to small number.
Table 1.
Classification of sport based on static/dynamic component6
| A. (Low dynamic <50%) | B. (Moderate dynamic 50–75%) | C. High dynamic (>75%) | |
|---|---|---|---|
| III. High Static (>30%) | Gymnastics Field (throwing) |
N/A | N/A |
| II. Moderate Static (10–20%) | N/A | Field (jumping) Track (sprint) |
Swimming Track (mid-distance) Basketball Lacrosse |
| I. Low Static (<10%) | N/A | Softball Volleyball |
Soccer Track (distance)/XC |
N/A= No athlete(s) participating in this category included
Standard 12-lead ECGs were performed on all athletes at a speed of 25 mm/s using automatic measurements. Six physicians analyzed all of the ECGs in the study with 81% read by 1 cardiologist for clinical purposes. Baseline characteristics included heart rate, QRS duration, QTc duration, and PR interval calculated by automatic ECG machine measurements. ECG findings were subsequently classified as normal, borderline, or abnormal based on the international criteria1 at the time of data entry by trained clinical staff with physician supervision. Most studies were performed en masse by Athletic Heart7 during routine PPE at each institution. Eight physicians analyzed all TTEs in the study with 80% being read by 1 cardiologist. Athletes who missed initial screening days had TTEs performed on a GE Vivid E9 echocardiography machine with an M5 cardiac probe at each institution’s designated cardiology office. Measurements were based on the American Society of Echocardiography recommendations for chamber quantification in adults.8 Left atrial diameter, interventricular septum thickness, posterior wall thickness, left ventricular end diastolic diameter (LVEDD), left ventricular (LV) end systolic diameter, and aortic root diameter were measured from a parasternal long axis view. Left atrial volumes were calculated using bi-plane method of disks and indexed to body surface area left atrium volume index, LV diastolic function was assessed using pulsed wave Doppler at the tips of the mitral valve leaflets in diastole (E/A ratio), in addition to tissue Doppler imaging of the lateral and medial mitral valve annulus (e′). LV systolic function was calculated using biplane method of disks, or visually in athletes with suboptimal image quality. Valvular disease of ≥ moderate severity was assessed based on recommendations of the American Society of Echocardiography guidelines and standards for echocardiographic assessment of valve stenosis and native valvular regurgitation.9,10
Data analyses were performed using the IBM SPSS V.24 statistical package (IBM, Armonk, NY). Data were summarized using descriptive statistics. Median and interquartile ranges were reported in addition to means and standard deviations as many variables showed evidence of significant skew. Main effects of race and static and/or dynamic sport classification6 were assessed using parametric or non-parametric analyses depending on normality of the data (independent samples t test or Mann-Whitney U for race; analysis of variance or Kruskal-Wallis for static and/or dynamic sport classification). Statistical significance for main effects was defined a priori as unadjusted p <0.05. Significant main effects of static and/or dynamic sport classification were followed up with post hoc pairwise comparisons with Bonferroni adjustment for multiple comparisons. A p <0.05 was considered statistically significant.
Results
A total of 329 collegiate female athletes were included (Table 2). Black female athletes were taller, weighed more, and had higher body surface area than white athletes (all p <0.001). When classified by the static and/or dynamic component of their sport, most (56%) were in category IIC followed by IC (18%), IB (13%), IIB (7%), and IIIA (6%). There was a significant effect of static and/or dynamic component of sport on height, weight, body mass index, body surface area, and systolic blood pressure. Athletes in category IC had the greatest values for height, weight, body surface area, and systolic blood pressure.
Table 2.
Characteristics of study population
| Overall, mean (SD) [IQR] | Race (n = 322) | p value | Static/dynamic sport group (n = 329) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black, mean (SD) (n = 72) | White, mean (SD) (n = 250) | IB*, mean (SD) (n = 44) | IC†, mean (SD) (n = 60) | IIB‡, mean (SD) (n = 23) | IIC§, mean (SD) (n = 184) | IIIA¶, mean (SD) (n = 18) | ||||
| Age (y) | 18.68 (0.86) [18.2–18.8] | 18.9 (1.0) | 18.6 (0.79) | 0.2 | 18.5 (0.7) | 18.6 (0.8) | 19.4 (1.4) | 18.7 (0.8) | 18.5 (0.6) | 0.09 |
| Height (cm) | 171.0 (9.2) [165.1–175.6] | 174.6 (9.9) | 169.9 (8.8) | < 0.01 | 175.0 (10.6) | 167.0 (6.2) | 169.0 (7.2) | 172.5 (9.0) | 161.8 (8.2) | <0.01 |
| Weight (kg) | 66.8 (11.6) [59.0–73.4] | 71.90 (14.6) | 65.3 (10.2) | <0.01 | 72.2 (10.0) | 61.4 (9.9) | 59.8 (4.9) | 68.4 (11.7) | 64.5 (14.2) | <0.01 |
| BMI (Kg/m2) | 22.8 (2.9) [20.9–24.2] | 23.4 (3.5) | 22.6 (2.7) | 0.07 | 23.7 (3.5) | 22.0 (2.8) | 21.0 (1.7) | 22.9 (2.7) | 24.4 (3.0) | <0.01 |
| BSA (m2) | 1.78 (0.19) [1.65–1.88] | 1.86 (0.2) | 1.75 (0.17) | <0.01 | 1.9 (0.2) | 1.7 (0.2) | 1.7 (0.1) | 1.8 (0.2) | 1.7 (0.2) | <0.01 |
| Heart Rate (bpm) | 59 (10.1) [61–76] | 61 (9.9) | 59 (10.1) | 0.1 | 73.5 (11.5) | 66.5 (13.0) | 67.1 (8.5) | 68.5 (12.1) | 70.7 (15.6) | 0.07 |
| SBP (mm Hg) | 118.1 (10.1) [111–125] | 118.7 (10.2) | 118.1 (10.0) | 0.6 | 120.8 (10.8) | 115.3 (9.7) | 120.2 (9.0) | 118.6 (9.9) | 113.9 (9.9) | 0.01 |
| DBP (mm Hg) | 71.1 (7.5) [66–76] | 71.4 (7.0) | 71.1 (7.6) | 0.7 | 72.2 (7.8) | 70.5 (8.3) | 70.4 (6.0) | 71.3 (7.2) | 68.9 (8.8) | 0.5 |
bpm = beat per minute; IQR = interquartile range; SD = standard deviation; y = years.
n = 44; softball = 22, volleyball = 22.
n = 60; soccer = 42, track (distance)/cross country = 18.
n = 23; field (jumping) = 4 track (sprint) = 19.
n = 184; swimming = 83, track (mid-distance) = 4, basketball = 57, lacrosse = 40.
n = 18; gymnastics = 14, field (throwing) = 4.
Baseline ECG characteristics are summarized in Table 3. Values for QRS duration, QTc duration, and PR duration were within the normal range when compared with normal values for adults.11 Black female athletes had longer PR intervals (p <0.01) whereas white athletes had longer QRS and QTc durations (p = 0.01 and 0.02). A difference was noted in QTc duration between the static and/or dynamic category of sport. Women in highly dynamic and/or low-moderate static sports (category IC and IIC) had longer QTc durations when compared with women in low dynamic and/or high static sports (category IIIA) (p = 0.002 and 0.001). There were 7 athletes with abnormal ECG findings: all were white, 6 were in Group IIC, and 1 was in Group IC. The abnormal ECG characteristics included 3 with T-wave inversions, 1 with pathologic Q-wave, 2 with prolonged QT intervals, and 1 with premature ventricular contractions. ECG findings were classified as borderline in 4 athletes: 3 white and 1 black. When grouped by static and/or dynamic component of sport, borderline ECG characteristics were noted in 2 athletes in Group IC, 1 in Group IB, and 1 in Group IIC. The borderline findings included 2 left atrial enlargement and 2 right axis deviations.
Table 3.
Baseline electrocardiographic characteristics by race and static/dynamic component of sport
| Overall, mean (SD) [IQR] | Race (n = 322) | p value | Static/dynamic sport group (n = 329) | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black, mean (SD) (n = 72) | White, mean (SD) (n = 250) | IB, mean (SD) (n = 44) | IC, mean (SD) (n = 60) | IIB, mean (SD) (n = 23) | IIC, mean (SD) (n = 184) | IIIA, mean (SD) (n = 18) | ||||
| HR | 59.4 (10.1) (53–65) | 61.4 (9.9) | 58.9 (10.0) | 0.04 | 61.6 (11.1) | 59.0 (10.7) | 61.0 (10.5) | 58.8 (9.6) | 60.6 (9.7) | 0.57 |
| QRS duration (ms) | 90.4 (9.0) (85–96) | 87.6 (9.2) | 91.1 (8.8) | 0.01 | 92.6 (9.9) | 89.5 (9.3) | 86.8 (9.1) | 91.1 (8.3) | 88.4 (8.6) | 0.07 |
| QTc duration (ms) | 410.5 (20.8) (398–423) | 405.3 (21.0) | 411.7 (20.4) | 0.02 | 404.5 (17.4) | 414.2 (16.8) | 401.7 (21.3) | 413.5 (21.6) | 393.9 (18.2) | <0.01 |
| PR Interval (ms) | 151.2 (21.9) (136–163) | 159.6 (20.8) | 148.7 (21.7) | <0.01 | 148.1 (22.3) | 150.4 (20.6) | 156.1 (17.5) | 152.1 (22.4) | 146.4 (24.3) | 0.30 |
HR = heart rate; IQR = interquartile range; ms = millisecond; SD = standard deviation.
Cardiac dimensions, derived by echocardiography, are summarized in Table 4. All values were within the normal range defined by the American Society of Echocardiography guidelines except for the mitral valve E/A ratio. There was no significant effect of race on cardiac dimensions. However, when analyzed by the static/dynamic component to sport, a significant main effect was noted for left atrial dimension, LVEDD, LV end systolic diameter, posterior wall thickness, aortic root diameter, interventricular septum thickness, left atrial volume index, and mitral Valve E/A ratio. Women in class IIC sports had greater values for posterior wall thickness (p = 0.003), interventricular septum thickness (p = 0.01), and LVEDD (p = 0.025) compared with women in class IIB sports. With regard to left atrial dimension, women in class IIC sports had greater values compared with women in class IIIA sports (p = 0.03). Women in class IIC sports had greater values for LVEDD compared with women in class IIIA sports (p = 0.02). Women in class IC sports had higher E/A ratios compared with women in class IIC sports (p = 0.04).
Table 4.
Cardiac dimensions by race and static/dynamic component of sport
| Overall mean (SD) [IQR] | Race | p value | Static/dynamic sport group | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black, mean (SD) (n = 72) | White, mean (SD) (n = 250) | IB, mean (SD) (n = 44) | IC, mean (SD) (n = 60) | IIB, mean (SD) (n = 23) | IIC, mean (SD) (n = 184) | IIIA, mean (SD) (n = 18) | ||||
| LA Dimension (cm) (n=288) | 3.2 (0.4) [3.0–3.5] | 3.1 (0.5) | 3.2 (0.4) | 0.4 | 3.2 (0.4) | 3.2 (0.3) | 3.0 (0.4) | 3.2 (0.4) | 2.9 (0.3) | 0.014 |
| LVESD (cm) (n=312) | 3.1 (0.4) (2.9–3.3) | 3.1 (0.5) | 3.1 (0.4) | 0.3 | 3.1 (0.4) | 3.1 (0.3) | 2.9 (0.3) | 3.1 (0.4) | 2.9 (0.3) | 0.048 |
| LVEDD (cm) (n=325) | 4.7 (0.5) (4.4–5.0) | 4.5 (0.7) | 4.7 (0.5) | 0.1 | 4.7 (0.4) | 4.6 (0.5) | 4.5 (0.4) | 4.7 (0.6) | 4.5 (0.4) | 0.002 |
| PWT (cm) (n=328) | 0.9 (0.1) (0.8–1.0) | 0.9 (0.2) | 0.9 (0.1) | 0.9 | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.001 |
| IVS (cm) (n=329) | 0.9 (0.1) (0.8–1.0) | 0.9 (0.1) | 0.9 (0.1) | 0.5 | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.007 |
| ARD (cm) (n=302) | 2.6 (0.3) (2.4–2.8) | 2.6 (0.3) | 2.6 (0.3) | 0.4 | 2.6 (0.2) | 2.5 (0.3) | 2.4 (0.2) | 2.6 (0.3) | 2.5 (0.3) | 0.021 |
| LA IDX (ml/m2) (n=233) | 27.7 (8.2) (22–33) | 28.2 (9.3) | 27.6 (8.0) | 0.8 | 26.4 (9.4) | 30.4 (8.8) | 25.3 (7.1) | 27.9 (7.5) | 24.2 (6.2) | 0.022 |
| LV EF (n=323) | 59.8 (4.1) (57.5–62.5) | 59.3 (4.3) | 59.9 (4.1) | 0.06 | 58.8 (2.9) | 59.0 (2.9) | 59.5 (2.4) | 60.3 (4.9) | 60.1 (2.8) | 0.521 |
| MV E/A Ratio (n=252) | 2.6 (0.3) (1.8–2.6) | 2.1 (0.5) | 2.2 (0.6) | 0.1 | 2.3 (0.6) | 2.4 (0.6) | 2.1 (0.7) | 2.1 (0.5) | 2.3 (0.6) | 0.03 |
| e’ Lat (n=208) | 19.0 (3.5) (16.7–21.0) | 18.5 (3.7) | 19.1 (3.4) | 0.3 | 19.9 (3.9) | 18.8 (3.8) | 19.3 (2.8) | 18.6 (3.4) | 19.2 (2.4) | 0.347 |
| e’ Med (n=204) | 13.0 (2.2) (11.6–14.4) | 12.9 (2.4) | 13.0 (2.2) | 0.6 | 13.1 (2.2) | 12.8 (1.8) | 13.4 (2.2) | 13.0 (2.1) | 12.0 (3.4) | 0.899 |
| E/Lat e’ (n=205) | 5.3 (1.3) (4.5–6.0) | 5.2 (1.2) | 5.2 (1.3) | 0.8 | 5.1 (1.2) | 5.6 (1.2) | 4.8 (0.9) | 5.3 (1.4) | 5.1 (1.4) | 0.268 |
ARD = aortic root diameter; e’ Lat = lateral mitral annulus e prime; E/Lat e’ = mitral valve e/lateral e prime ratio; e’ Med = medial mitral annulus e prime; IDX = left atrium volume index; IQR = interquartile range; IVS = interventricular septum thickness; LA = left atrium; LV EF = left ventricular ejection fraction; LVEDD = left ventricular end diastolic diameter; LVESD = left ventricular end systolic diameter; MV E/A = mitral valve E/A ratio, PWT = posterior wall thickness; SD = standard deviation.
White athletes had the highest percentage of valvular insufficiency (49.6%). No athletes had any degree of valvular stenosis. Tricuspid valve insufficiency was the most common valvular insufficiency (Figure 1). When analyzed by static/dynamic component of sports, for valvular insufficiency of moderate or greater, athletes in class IIC had the highest prevalence (n = 4, moderate tricuspid regurgitation (TR) = 2, moderate PR = 1, moderate and/or severe TR = 1) followed by athletes in class IC (n = 1, moderate TR = 1). Overall, athletes in class IIB had the highest frequency of valvular insufficiency (62%). Only 1 (0.3%) had a bicuspid aortic valve. Left-ventricular hypertrophy was present in 33 (10%) of athletes, and 5 (1.5%) had an atrial septal defect whereas 1 (0.3%) had a ventricular septal defect. An aortic root diameter ≥3.7 cm was found in only 1 (0.3%) athlete and 6 (1.8%) had a dilated left ventricle with LVEDD >5.6 cm.
Figure 1.
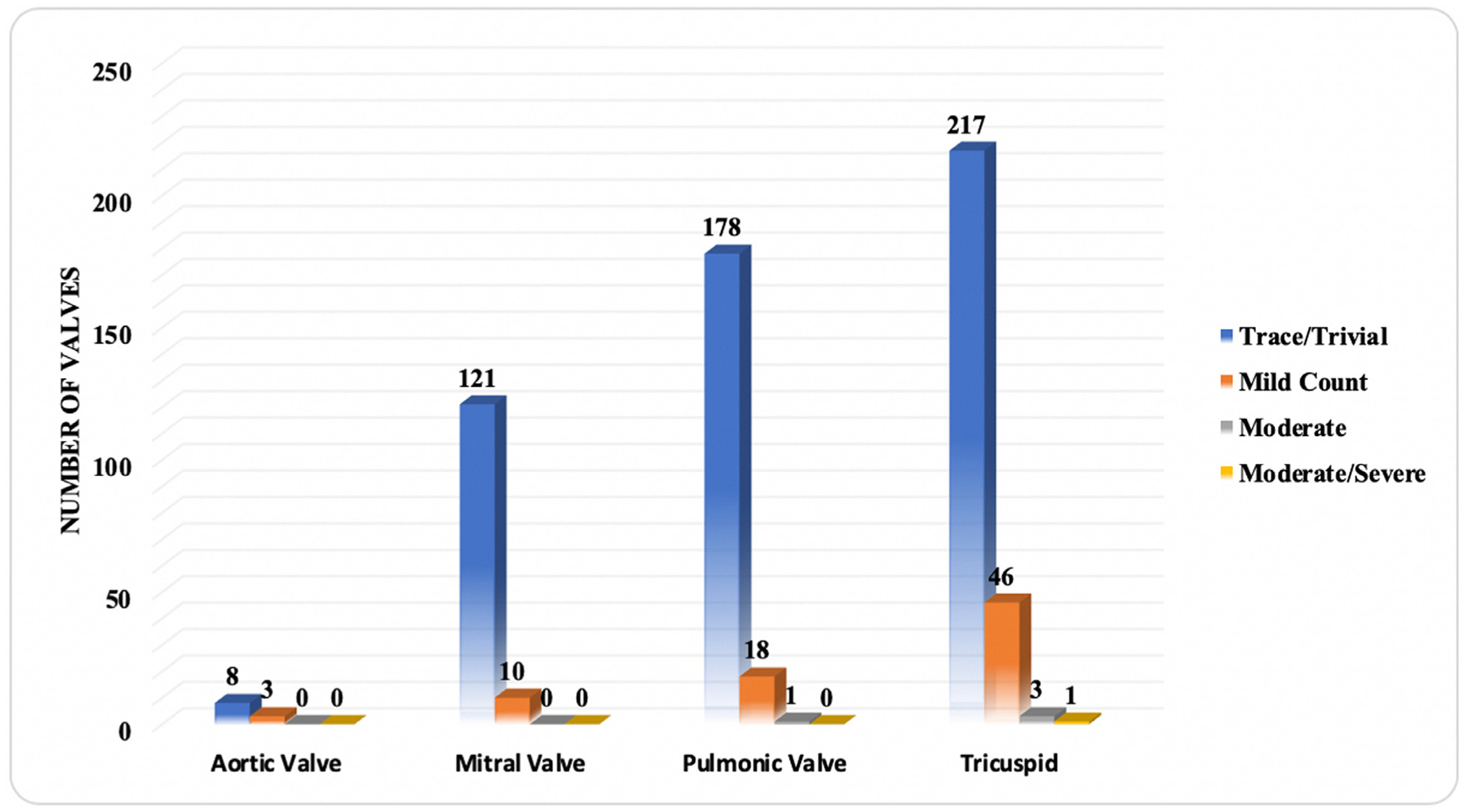
Valvular insufficiency in collegiate female athletes.
Discussion
Athletes can develop physiologic or pathologic cardiac remodeling due to intense physical training regimens and changes in body habitus. The majority of studies examining cardiac remodeling in collegiate athletes have focused on men. We sought to identify the PPE features of female athletes, including ECG and TTE data, to establish a reference for collegiate female athletes. In general, the majority of our findings were similar to published normative values.8 There was a significant difference noted in height, weight, and body surface area between white and black athletes, with black athletes being taller, weighing more, and having higher body surface area. When separated into static and/or dynamic aspect of sport, there were significant differences between height, weight, body surface area, body mass index, and systolic blood pressure. Athletes in low static and moderately dynamic sports (softball and volleyball) on average had the highest values for height, weight, body surface area, and systolic blood pressure.
The ECG findings for QRS, QTc, and PR duration were all within normal limits compared with standard normal values. Black athletes had longer PR intervals whereas white athletes had longer QRS durations. QTc duration was longer in athletes who participated in sports with a high dynamic component. Seven female athletes (2%) had abnormal ECG findings. No pathologic findings were identified, and they all had subsequent normal TTEs. The majority of athletes with abnormal ECG findings participated in moderately static and/or highly dynamic sports (IIC). Of note, this group did contain the largest number of athletes (IIC n = 184), so the incidence of abnormal ECG findings should be taken with caution.
TTE measurements were within the normal range when compared with the American Society of Echocardiography values for adults.8,10,12 There was a trend toward higher E/A ratio (>2) in both black and white athletes which is a common finding among trained athletes.13 There were no significant differences in TTE measurements by race; however, when athletes were grouped by static and/or dynamic level of sports, there were statistically significant differences in left atrial and LV dimension, LV wall thickness, aortic root diameter, and mitral valve E/A ratio. The results indicate the importance of the static and/or dynamic component of the sport with regard to cardiac remodeling and diastolic filling. Prior studies have revealed that endurance athletes (low-moderate static and/or highly dynamic) tend to have volume adaptations with more significant chamber dilation on TTE, including left atrial size, as well as early diastolic filling and an elevated E/A ratio, likely as a consequence of higher preload and cardiac output.14–16 LV dilation manifests as an increase in LVEDD, thus traditional threshold values for pathologic LVEDD should not be utilized among endurance athletes.15 In contrast, strength and power activities (high static and/or low-moderate dynamic) show adaptations based on a pressure challenge which can lead to mild concentric LV hypertrophy, sparing the atria and right ventricle.14
Care should be taken with extending data from studies in male athletes to female athletes as multiple studies have shown differences in trends in PPE, ECG, and echocardiogram data between male and female athletes. In a study of 80 collegiate male American style football players, no significant difference was noted in height, weight and body surface area based on race.16 This is in contrast to our data on female athletes, which noted significantly higher values for height, weight and body surface area in black athletes. Furthermore, there was a trend towards higher average blood pressure values among male collegiate athletes when compared with our female athletes (systolic blood pressure 126 ± 10 vs 118 ± 10). A similar trend of differences in PPE characteristics has also been reported in European studies. In a systematic investigation of 2,352 Olympic athletes participating in a variety of Olympic sports by Pelliccia et al, a significant difference was noted in systolic blood pressure between male and female athletes, with male athletes having higher blood pressures.17 Likewise, differences in ECG characteristics based on sex have been observed in athletes.18 In a study of 1,436 collegiate athletes participating in a variety of sports including soccer, running, strength training and fighting sports, male athletes were noted to have significantly higher PR intervals, QRS durations, incidence of sinus bradycardia, incomplete right bundle branch block, early repolarization and QRS voltage criteria for LV hypertrophy. Female athletes had higher QTc intervals.18 Similarly, in the International Criteria, important differences based on sex are noted, including females having a higher incidence of anterior T wave inversions and longer QT intervals.1
In addition to differences in PPE and ECG, distinct differences have also been noted on TTE evaluation of collegiate male and female athletes. Mean LVEDD in collegiate male American style football players was 53 ± 5 mm compared with 47 ± 5 mm among our athletes.19 Similarly, mean values for interventricular septum thickness, posterior wall thickness, left atrial dimension, aortic root diameter, and LV ejection fraction were all higher than those for female athletes in our study.19 Differences in TTE findings between elite male and female athletes have also been reported in European studies.17,20,21 In a systematic review of Olympic athletes by Pelliccia et al, male athletes had statistically significant greater septal wall thickness, LV posterior wall thickness, LV cavity dimension, LV ejection fraction, left atrial dimension, and aortic root diameter than female athletes. Reasons for differences in the adaption of the elite female athlete’s heart compared with the elite male athlete’s heart are uncertain. Nevertheless, these differences highlight the fact that data from the male athletic population should not simply be extrapolated to their female counterparts.
To the best of our knowledge this is the first summary of such data exclusive to Division I female athletes. This is very timely considering concerns about the lack of decline in adverse outcomes among younger women, which is seen in the remainder of the population. The training of female athletes is likely widely varied before beginning their collegiate careers, but if longitudinal follow-up yields significant findings in adverse outcomes, this is likely to foster training changes. Due to institutional privacy policies, and the fact that it was not possible to de-identify data from certain subgroups, over-reading was eliminated. In some instances, sample groups were sufficiently powered to detect main effect, however subgroup analysis was not sufficient to detect pairwise differences. Furthermore, due to the retrospective nature of the analysis, a Kappa index of agreement of the ECG measurements was unable to be performed.
In conclusion, for a cohort of NCAA division I female athletes, PPE, ECG, and TTE results were within established normative values aside from mitral valve E/A ratios which were elevated but still considered a normal finding in athletes.1,8,10,12,17 Significant differences were noted based on separation into static/dynamic component of sport in systolic blood pressure, abnormal ECG findings, left atrial dimension, LV wall thickness, aortic root diameter, and mitral valve E/A ratio. This reinforces that the classification of sport is an important factor in cardiovascular remodeling and interpretation of cardiovascular screening tests. We believe that these data will help to identify trends in cardiovascular adaptation among female athletes that will lead to improved screening and guide future research.
Acknowledgment
The authors wish to thank Monica Towns for her help with obtaining informed consent, data collection, and entry.
Funding:
This work was supported in part by the American Medical Society for Sports Medicine (AMSSM) Foundation Research Grant 2016 awarded to KE, Leawood, Kansas, and the University of Florida REDCap uses the NIH National Center for Advancing Translational Sciences (NCATS) grant UL1 TR001427, Bethesda, Maryland. CJ Pepine is supported by NIH/NHLBI Grants UM1 HL087366 and R01 HL146158-01, Bethesda, Maryland, and the US Department of Defense, DOD CDMRP PR161603, Arlington, Virginia, the Gatorade Foundation, Gainesville, FL, and the McJunkin Family Foundation Trust, Plantation, FL. All funders had no role in the study design; in collection, analysis, and interpretation of data; in writing the report; and in the decision to submit the manuscript for publication.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JM, Gerche A, Ackerman MJ, Borjesson M, Salerno JC, Asif IM, Owens DS, Chung EH, Emery MS, Froelicher VF, Heidbuchel H, Adamuz C, Asplund CA, Cohen G, Harmon KG, Marek JC, Molossi S, Niebauer J, Pelto HF, Perez MV, Riding NR, Saarel T, Schmied CM, Shipon DM, Stein R, Vetter VL, Pelliccia A, Corrado D. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017;51:704–731. [DOI] [PubMed] [Google Scholar]
- 2.Edenfield KM, Reifsteck F, Carek S, Harmon KG, Asken BM, Dillon MC, Street J, Clugston JR. Echocardiographic measurements of left ventricular end-diastolic diameter and interventricular septal diameter in collegiate football athletes at preparticipation evaluation referenced to body surface area. BMJ Open Sport Exerc Med 2019;5:e000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med 2000;19:163–173. vii. [DOI] [PubMed] [Google Scholar]
- 4.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, Okin PM, Saul JP, Salberg L, Van Hare GF, Soliman EZ, Chen J, Matherne GP, Bolling SF, Mitten MJ, Caplan A, Balady GJ, Thompson PD. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age): a Scientific Statement from the American Heart Association and the American College of Cardiology. Circulation 2014;130:1303–1334. [DOI] [PubMed] [Google Scholar]
- 6.Levine BD, Baggish AL, Kovacs RJ, Link MS, Maron MS, Mitchell JH. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 1: classification of sports: dynamic, static, and impact: a Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2015;132:e262–e266. [DOI] [PubMed] [Google Scholar]
- 7.Athletic Heart Metabolic & Cardiac Research Institute. Athletic heart: metabolic & cardiac evaluation. Available at: https://athletic-heart.com/. Accessed on July 10, 2020.
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28. 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23. quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 11.Kossmann CE. The normal electrocardiogram. Circulation 1953;8:920–936. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A, Caso P, Severino S, Galderisi M, Sarubbi B, Limongelli G, Cice G, D’Andrea L, Scherillo M, Mininni N, Calabro R. Effects of different training protocols on left ventricular myocardial function in competitive athletes: a Doppler tissue imaging study. Ital Heart J 2002;3:34–40. [PubMed] [Google Scholar]
- 14.Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the “grey zone” of clinical uncertainty. Can J Cardiol 2016;32:429–437. [DOI] [PubMed] [Google Scholar]
- 15.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 2002;40:1856–1863. [DOI] [PubMed] [Google Scholar]
- 16.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr 2015;28:236–244. [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia A, Adami PE, Quattrini F, Squeo MR, Caselli S, Verdile L, Maestrini V, Di Paolo F, Pisicchio C, Ciardo R, Spataro A. Are Olympic athletes free from cardiovascular diseases? Systematic investigation in 2352 participants from Athens 2004 to Sochi 2014. Br J Sports Med 2017;51:238–243. [DOI] [PubMed] [Google Scholar]
- 18.Bessem B, de Bruijn MC, Nieuwland W. Gender differences in the electrocardiogram screening of athletes. J Sci Med Sport 2017;20:213–217. [DOI] [PubMed] [Google Scholar]
- 19.Crouse SF, White S, Erwin JP, Meade TH, Martin SE, Oliver JM, Joubert DP, Lambert BS, Bramhall JP, Gill K, Weir D. Echocardiographic and blood pressure characteristics of first-year collegiate American-style football players. Am J Cardiol 2016;117:131–134. [DOI] [PubMed] [Google Scholar]
- 20.Pelliccia A, Kinoshita N, Pisicchio C, Quattrini F, Dipaolo FM, Ciardo R, Di Giacinto B, Guerra E, De Blasiis E, Casasco M, Culasso F, Maron BJ. Long-term clinical consequences of intense, uninterrupted endurance training in Olympic athletes. J Am Coll Cardiol 2010;55:1619–1625. [DOI] [PubMed] [Google Scholar]
- 21.Caselli S, Di Paolo FM, Pisicchio C, Di Pietro R, Quattrini FM, Di Giacinto B, Culasso F, Pelliccia A. Three-dimensional echocardiographic characterization of left ventricular remodeling in Olympic athletes. Am J Cardiol 2011;108:141–147. [DOI] [PubMed] [Google Scholar]


