ABSTRACT
We report 13 genomic sequences of human bocavirus 1 isolated from pediatric inpatients in Fukushima, Japan, using an air-liquid interface culture of human bronchial tracheal epithelial cells. This work suggests the endemic circulation of a human bocavirus variant with a unique amino acid signature in Fukushima.
ANNOUNCEMENT
Human bocavirus (HBoV) belongs to the family Parvoviridae, with a genome consisting of approximately 5.5 kb of single-stranded DNA (ssDNA) and heterotelomeric DNA, and was first detected in Sweden in 2005 (1). HBoV has four subtypes (HBoV1 to HBoV4), and the genome contains three major open reading frames (ORFs), which encode nonstructural proteins (NSs), a nuclear phosphoprotein (NP1), and viral capsid proteins (VPs) (2). The NS ORF encodes several NSs (NS1, NS1-70, NS2, NS3, and NS4) (3). Three VPs (VP1, VP2, and VP3) are generated by the alternative splicing of VP mRNA by the NP1 protein (4). Some viruses contain putative up1 and ORFX genes (5). Recent studies showed HBoV1 can cause lower respiratory tract diseases as a monoinfection and not only in combination with other respiratory pathogens, suggesting the importance of HBoV as a pathogen of respiratory infection ranked second or third after respiratory syncytial virus (6–8). Nevertheless, few full-length genomes of HBoV1 have been registered in databases; in particular, the hairpin sequences at both ends, which are necessary for viral genome replication, are often lacking (9, 10).
In this study, 13 nearly complete genome sequences of HBoV1 isolates, including hairpin sequences, were determined (Table 1). Nasopharyngeal swab specimens were collected from pediatric inpatients in Fukushima, Japan, between 2018 and 2021, and those that were HBoV1 positive by multiplex real-time PCR assays for respiratory viruses (11, 12) were used for virus isolation using an air-liquid interface culture of human bronchial tracheal epithelial cells (HBTEC-ALI) prepared as described previously (13, 14). Nucleic acids were extracted from virus stock with a QIAamp viral RNA minikit (Qiagen, Hilden, Germany) (DNA was extracted simultaneously). The libraries for next-generation sequencing were prepared using a NEBNext Ultra II RNA library preparation kit for Illumina (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s instructions. Although HBoV is an ssDNA virus, indexed libraries were obtained using this kit. The indexed libraries were analyzed for 2 × 150 cycles on a DNBSEQ-G400 instrument at GENEWIZ (South Plainfield, NJ, USA). Reads were trimmed and then de novo assembled or mapped (based on the number of HBoV reads obtained) to the reference sequence (GenBank accession number JQ923422) using CLC Genomics Workbench v21.0.4 with default settings. The coverage of the assembled sequences was checked by mapping. The gene annotations were analyzed by VAPiD v1.6.6 (15).
TABLE 1.
Registered HBoV1 sequences
| Isolate name | Accession no. | Run data accession no. | Diagnosis | Sequence constitution method | Total no. of reads | Total no. of mapped reads | Avg coverage (×) | Length (bases) | GC content (%) | Infection typea |
|---|---|---|---|---|---|---|---|---|---|---|
| Fukushima_H181_2018 | LC651167 | DRR328227 | Acute bronchitis | Assembling | 14,227,470 | 1,009,293 | 26,081.54 | 5,371 | 42.17 | Monoinfection |
| Fukushima_H216_2018 | LC651168 | DRR328228 | Acute pneumonia | Assembling | 13,208,520 | 575,157 | 14,809.66 | 5,484 | 42.12 | Monoinfection |
| Fukushima_H254_2018 | LC651169 | DRR328229 | Acute pneumonia, febrile convulsion | Assembling | 13,259,719 | 781,149 | 20,516.24 | 5,561 | 42.37 | Monoinfection |
| Fukushima_H315_2018 | LC651170 | DRR328230 | Acute bronchitis | Assembling | 26,811,533 | 5,702,012 | 151,634.54 | 5,590 | 42.06 | Monoinfection |
| Fukushima_H565_2019 | LC651171 | DRR328231 | Acute bronchitis | Assembling | 8,726,152 | 272,385 | 7,244.30 | 5,596 | 42.61 | Monoinfection |
| Fukushima_O210_2018 | LC651172 | DRR328232 | Acute epiglottitis | Assembling | 30,670,275 | 689,693 | 18,204.93 | 5,450 | 42.46 | Monoinfection |
| Fukushima_O234_2018 | LC651173 | DRR328233 | Bronchopneumonia | Assembling | 27,303,129 | 45,973 | 1,211.76 | 5,291 | 42.55 | Coinfection with adenovirus 2 |
| Fukushima_O278_2018 | LC651174 | DRR328234 | Acute pneumonia | Assembling | 14,346,631 | 2,248,645 | 59,245.02 | 5,472 | 42.27 | Monoinfection |
| Fukushima_OR5_2020 | LC651176 | DRR328235 | Acute pharyngitis | Mapping | 9,533,747 | 3,719 | 98.18 | 5,539 | 42.5 | Monoinfection |
| Fukushima_OR59_2020 | LC651177 | DRR328236 | Bronchial asthma | Assembling | 62,190,703 | 454,271 | 12,043.63 | 5,501 | 42.46 | Monoinfection |
| Fukushima_OR65_2020 | LC651178 | DRR328237 | Pneumonia | Mapping | 13,282,899 | 26,465 | 677.54 | 5,421 | 42.44 | Coinfection with rhinovirus |
| Fukushima_OR72_2020 | LC651179 | DRR328238 | Febrile convulsion | Assembling | 10,367,690 | 834,422 | 22,050.27 | 5,490 | 42.69 | Monoinfection |
| Fukushima_OR189_2021 | LC651175 | DRR328239 | Intussusception | Assembling | 8,612,873 | 725,001 | 19,253.62 | 5,402 | 42.41 | Monoinfection |
Coinfection was determined by multiplex real-time PCR assays for respiratory viruses (12).
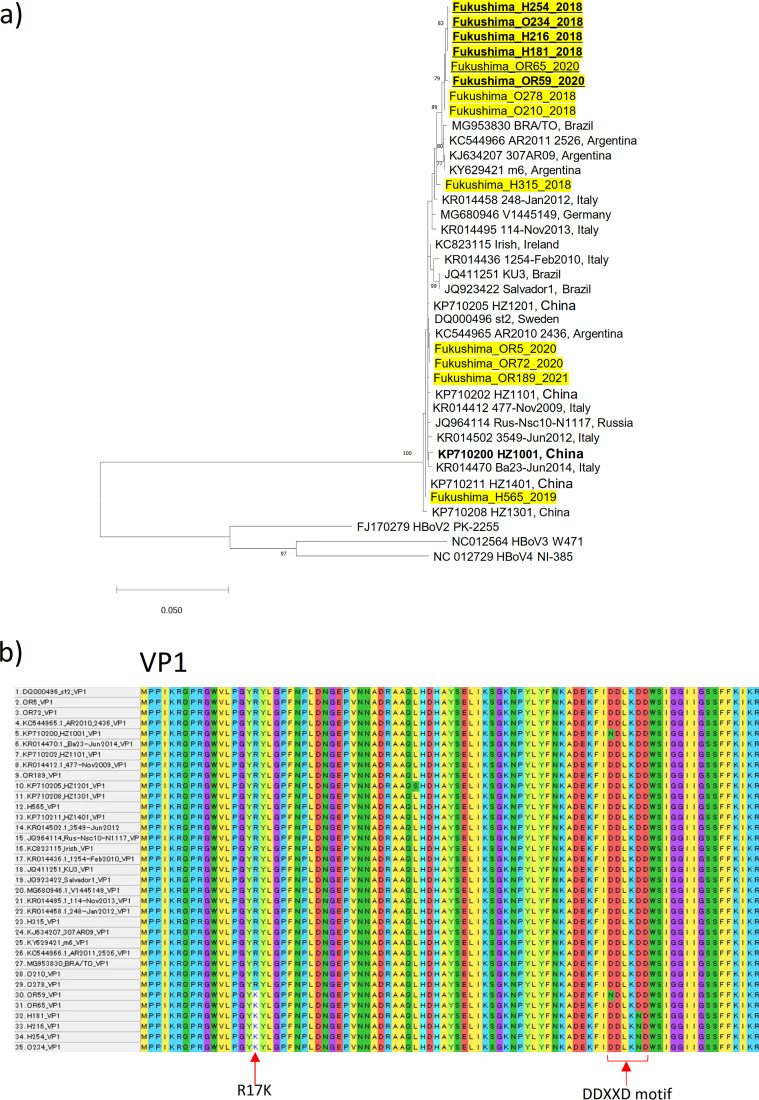
The phylogenetic analysis of the VP1 protein sequences showed that the Fukushima isolates clustered in distinct lineages, closely related to viruses described from around the globe (Fig. 1a). Characteristically, isolates OR59, OR65, H181, H216, H254, and O234 have a substitution at the 17th amino acid in the VP1 protein (R to K) and, except for OR65, also an amino acid substitution in the DDXXD motif (amino acid positions 68 to 72 in VP1), which disrupts this putative metal binding domain (16, 17) (Fig. 1b). Strains carrying both of these amino acid substitutions are not present in databases, suggesting that this unique variant was endemic in Fukushima from 2018 to 2021.
FIG 1.
(a) Phylogenetic analysis using the nucleotide sequences of VP1 was performed using MEGA-X software (v10.1.8). The maximum likelihood method was used to generate the phylogenetic tree. Five hundred bootstrap replicates were performed, and only values above 70 are shown. Reference HBoV1 sequences were obtained from GenBank. The new isolates are marked in yellow. The sequences carrying the disrupted DDXXD motif are shown in bold, and strains carrying the R17K amino acid substitution are underlined. The numbers show the bootstrap values. The scale bar shows the number of changes per position. (b) The alignment of VP1 amino acid sequences was prepared using MEGA-X software. Red arrows indicate the positions of amino acid substitutions.
Human subjects were enrolled after approval from the ethics committee of our institute (approval numbers 1001 and 1087).
Data availability.
The nearly complete genome sequences have been deposited in GenBank under accession numbers LC651167, LC651168, LC651169, LC651170, LC651171, LC651172, LC651173, LC651174, LC651175, LC651176, LC651177, LC651178, and LC651179 (Table 1). The raw reads were deposited under BioProject number PRJDB12572. Run data have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers DRR328227, DRR328228, DRR328229, DRR328230, DRR328231, DRR328232, DRR328233, DRR328234, DRR328235, DRR328236, DRR328237, DRR328238, and DRR328239.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Japan Agency for Medical Research and Development (grants 20fk0108119h0601 and 21fk0108119j0602).
Contributor Information
Kazuya Shirato, Email: shirato@niid.go.jp.
Jelle Matthijnssens, KU Leuven.
REFERENCES
- 1.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen AY, Cheng F, Lou S, Luo Y, Liu Z, Delwart E, Pintel D, Qiu J. 2010. Characterization of the gene expression profile of human bocavirus. Virology 403:145–154. doi: 10.1016/j.virol.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen W, Deng X, Zou W, Cheng F, Engelhardt JF, Yan Z, Qiu J. 2015. Identification and functional analysis of novel nonstructural proteins of human bocavirus 1. J Virol 89:10097–10109. doi: 10.1128/JVI.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W, Cheng F, Shen W, Engelhardt JF, Yan Z, Qiu J. 2016. Nonstructural protein NP1 of human bocavirus 1 plays a critical role in the expression of viral capsid proteins. J Virol 90:4658–4669. doi: 10.1128/JVI.02964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildgen O, Qiu J, Soderlund-Venermo M. 2012. Genomic features of the human bocaviruses. Future Virol 7:31–39. doi: 10.2217/fvl.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moesker FM, van Kampen JJ, van der Eijk AA, van Rossum AM, de Hoog M, Schutten M, Smits SL, Bodewes R, Osterhaus AD, Fraaij PL. 2015. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect 21:964.E1–964.E8. doi: 10.1016/j.cmi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang W, Yin F, Zhou W, Yan Y, Ji W. 2016. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep 6:20246. doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbeke V, Reynders M, Flore K, Vandewal W, Debulpaep S, Sauer K, Cardoen F, Padalko E. 2019. Human bocavirus infection in Belgian children with respiratory tract disease. Arch Virol 164:2919–2930. doi: 10.1007/s00705-019-04396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q, Deng X, Yan Z, Cheng F, Luo Y, Shen W, Lei-Butters DCM, Chen AY, Li Y, Tang L, Söderlund-Venermo M, Engelhardt JF, Qiu J. 2012. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog 8:e1002899. doi: 10.1371/journal.ppat.1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen W, Deng X, Zou W, Engelhardt JF, Yan Z, Qiu J. 2016. Analysis of cis and trans requirements for DNA replication at the right-end hairpin of the human bocavirus 1 genome. J Virol 90:7761–7777. doi: 10.1128/JVI.00708-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaida A, Kubo H, Takakura K, Iritani N. 2010. Detection and quantitative analysis of human bocavirus associated with respiratory tract infection in Osaka City, Japan. Microbiol Immunol 54:276–281. doi: 10.1111/j.1348-0421.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaida A, Kubo H, Takakura K-i, Sekiguchi J-i, Yamamoto SP, Kohdera U, Togawa M, Amo K, Shiomi M, Ohyama M, Goto K, Hase A, Kageyama T, Iritani N. 2014. Associations between co-detected respiratory viruses in children with acute respiratory infections. Jpn J Infect Dis 67:469–475. doi: 10.7883/yoken.67.469. [DOI] [PubMed] [Google Scholar]
- 13.Shirato K, Kawase M, Matsuyama S. 2018. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology 517:9–15. doi: 10.1016/j.virol.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkman R, Koekkoek SM, Molenkamp R, Schildgen O, van der Hoek L. 2009. Human bocavirus can be cultured in differentiated human airway epithelial cells. J Virol 83:7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shean RC, Makhsous N, Stoddard GD, Lin MJ, Greninger AL. 2019. VAPiD: a lightweight cross-platform viral annotation pipeline and identification tool to facilitate virus genome submissions to NCBI GenBank. BMC Bioinformatics 20:48. doi: 10.1186/s12859-019-2606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J, Rodionov D, De Schutter JW, Lin YS, Tsantrizos YS, Berghuis AM. 2017. Crystallographic and thermodynamic characterization of phenylaminopyridine bisphosphonates binding to human farnesyl pyrophosphate synthase. PLoS One 12:e0186447. doi: 10.1371/journal.pone.0186447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang X, Zhang R, Wu C, Guo Y, Mao X, Guo G, Zhang Y, Wang D-C, Li D, Zou Q. 2012. Modeling studies with Helicobacter pylori octaprenyl pyrophosphate synthase reveal the enzymatic mechanism of trans-prenyltransferases. Int J Biochem Cell Biol 44:2116–2123. doi: 10.1016/j.biocel.2012.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nearly complete genome sequences have been deposited in GenBank under accession numbers LC651167, LC651168, LC651169, LC651170, LC651171, LC651172, LC651173, LC651174, LC651175, LC651176, LC651177, LC651178, and LC651179 (Table 1). The raw reads were deposited under BioProject number PRJDB12572. Run data have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers DRR328227, DRR328228, DRR328229, DRR328230, DRR328231, DRR328232, DRR328233, DRR328234, DRR328235, DRR328236, DRR328237, DRR328238, and DRR328239.