Abstract
Background:
Knee osteoarthritis (OA) is associated with chronic inflammation in somatic structures, which alters sensory afferents and leads to plastic changes in the nervous system.
Methods:
A systematic literature review was carried out, without language restrictions, period, or status of publication. The database used were Medline, EMBASE, Cochrane Library and clinicaltrials.gov. Extra bibliographic references were extracted through the discussion with specialists, and through scientific researches in conference papers.
Results:
The electronic search found 938 articles. When excluding duplicates and applying the inclusion/exclusion criteria, 5 studies were considered: 2 using EEG and 3 using TMS. Significant reduction of EEG activity in the cingulate medium cortex, reduction of conditioned pain modulation (CPM) in studies with EEG, as well as the occurrence of an association between pain and motor response threshold/intracortical pain facilitation in studies with TMS were observed.
Conclusions:
The study contributes to a better understanding of the neurophysiological changes seen in the cingulate medium cortex, decrease in CPM and motor response threshold/intracortical pain facilitation. Advances in neuroplasticity studies may aid in the screening for early diagnosis of knee OA in the future. However, more studies are necessary.
Keywords: electroencephalography, knee, osteoarthritis, transcranial magnetic stimulation
Key Points
-
1.
Electroencephalography and magnetoencephalogram findings showed that neuroplastic changes reduce the inhibition effect of pain promoted by the inhibitory descending systems of pain.
-
2.
Studies involving transcranial magnetic stimulation found a lower inhibition of corticospinal system in chronic pain, generating dysfunctional state of modulatory pathways—the descending inhibitory pathway of pain.
1. Introduction
In the last few years, the aging process has become an important governmental issue in many developed and developing countries because of the significant increase in life expectancy.[1] As the human being ages, it's general health state suffers various transformations, including some dysfunctions and wears; amongst them, the diseases that affect cartilage function, for example knee osteoarthritis (OA).[2]
Knee OA is one of the main causes of incapacity in the adult and elderly population. It is a heterogeneous pathology characterized by a complex and multifactorial character[3–5] which causes changes in peripheral and central sensitization.[6–17]
The pain associated to the articular damage is highly variable[18] with few relations to specific radiological changes, and many times underestimated by physicians. The knee OA can lead to structural changes previously described in literature, segmental sensitization, and also promote central sensitization, with reduction of the pain threshold, hyperalgesia in and out previously sensitized areas, resulting in generalized pain.[19–26]
It is associated with chronic inflammation in somatic structures, which alters sensory afferent and leads to plastic changes in the nervous system.[27–30]
With the constant painful peripheral stimulus, there are changes in neuronal plasticity associated with less intracortical inhibition, which enhances the levels of pain and dysfunction.[31]
There are studies that indicate the change from inhibition state to maladaptive facilitation state by the descending modulating systems in chronic pain, with a decreased capacity to dissociate pain.[32,33]
These changes were associated with abnormal activity in the cingulate cortex, amygdala, insula, nucleus accumbens, and prefrontal areas.[32]
Knee OA may cause beyond the structural changes already described in literature: segmental and central sensitivity, reduction of pain threshold such as hyperalgesia inside and outside the already sensitized areas, which can lead to generalized pain.[34] It is associated with chronic inflammation in somatic structures, which alters sensory afferents and leads to plastic changes in the nervous system.[30]
Neuroplasticity is the name given to changes in function, structure, and organization of the nervous system that occurs continuously during a person's lifetime in response to internal factors related to the sensitive afferent visceral system and external factors, like motor learning and peripheral sensitive stimulation.[35] It is the way of the nervous system to mold itself to the individual needs and adapt itself to the environment where it lives. Effects of the neuroplasticity associated with musculoskeletal disorders like knee OA have been demonstrated in the peripheral nervous system, spinal cord, brainstem, sensory, and motor rotations of the brain plus mesolimbic and prefrontal cortex areas.[36]
The main changes in the nervous system due to neuroplasticity associated with knee OA occurred in the mesolimbic and prefrontal cortex areas and the best biomarkers to identify the effects of chronic pain in patients with OA involve the changes in the activity of these regions.[37]
The afore mentioned studies studied the morphology in the functional magnetic resonance imaging (fMRI). However, it is still necessary to carry out studies with functional assessment methods such as electroencephalogram (EEG), transcranial magnetic stimulation (TMS), and functional near-infrared saturation (fNIRS) of patients with pain due to knee OA in order to analyze functional changes.
Given the existence of neuroplastic mechanisms for pain modulation and other symptoms of knee OA, a systematic review of the literature is made necessary to identify important markers in the diagnosis and progress of this disease.
2. Objectives
The objective of this study is to realize a systematic study review which used neurophysiological assessment methods such as EEG, fNIRS, or TMS to verify neuronal plasticity effects in people with knee OA.
3. Methodology
This systematic review was realized according with the preferred reporting items for systematic review and meta-analysis protocols.[38] Ethical approval was not necessary because this research was carried out exclusively with scientific texts to review the scientific literature. Thus, approval by the Ethics Committee (CEP/CONEP System) is not required, as stated in Resolution 510/2016 in its Article 1. The study was registered in the PROSPERO database under the number CRD42018100688.
The elaboration of the scientific question was based on the PICO strategy,[39] considering: patients with knee OA(Patient/Problem); neurophysiological assessment methods such as EEG, fNIRS, or TMS (Intervention). There was not a standard intervention to be considered in this study (control/comparison). All the available outcomes in the literature were considered in the analysis (Outcomes).
3.1. Eligibility criteria
3.1.1. Types of study
The systematic review should screen and select the same methodology and research articles in order to provide an unbiased analysis. Nevertheless, most studies that correlate pain with neurophysiological changes are exploratory. Thus, it was decided to include all kinds of studies in order to carry out a complete analysis of the published studies and possible methodologies to be developed and explored in the future.
The following model studies were included: randomized controlled trials; non-randomized controlled trials; descriptive observational studies like case-report or case series; reviews. The studies were included according to their data relevancy and regardless of their publication status.
3.1.2. Types of participants
The participants in the studies were human adults, at least 40 years old, with knee OA of any duration, evaluated, or treated in any kind of institution. Patients must follow only clinical and radiological diagnosis of knee osteoarthritis.
Also, in order to clarify the neurophysiological analysis and to exclude other influences than osteoarthritis on pain processing, patients must not have any other cause that may enhance plastic changes such as: previous surgery in the affected area or diseases of the peripheral and central nervous system, infectious, inflammatory, rheumatic or neoplastic diseases, psychiatric disorders, stroke, neuropathy, and other causes of pain.[36]
3.1.3. Types of intervention
The interventions chosen in this study were assessment methods such as: EEG, fNIRS, or TMS applied in study participants.
3.1.4. Types of outcome measures
Primary outcome: cortex neurophysiological changes in people with knee OA.
3.1.5. Types of parameters analyzed
Authors, date, and location (country) of publication, number of patients used in the study, gender of patients, knee affected by OA (right lateral, left lateral, or bilateral) Kellgren–Lawrence (KL) knee OA scale, duration of pain resulting from OA, functional tests, visual analogue scale (VAS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were analyzed in this study.
Stimuli in tests were also analyzed: wave changes according to stimuli (relevant evoked potentials for pain); number of painful stimuli made in protocol; intensity of painful stimuli; time interval tested; use or not of analgesics during tests which should be the same in each study (as long as the use of analgesics can affect both peripheral and central function); VAS (pre-test and post-test).
3.1.6. EEG
Acoustic evoked potential components N1/N2, P1/P2; location of occurrence of peaks of N1/N2 and P1/P2; increase or decrease of N1/N2 and P1/P2. Changes in temporoparietal cortex and mid/anterior cingulate cortex; amplitude/speed variation of delta waves and theta waves.
3.1.7. fNIRS
Wavelength absorption of [HbO] and [Hb] (concomitant or paradoxical variation), location and temporal relationship with painful stimuli; optical density or attenuation; intensity of emitted light; duration of brightness peak; tissue oxygenation index, and tissue saturation.
3.1.8. TMS
Motor evoked potential (MEP): represents the activation of muscle fibers of motor units stimulated in the contralateral hemisphere.
Cortical silent period: it is reproduced in the application of transcranial stimulus during the voluntary contraction of the effector muscle and presents soon after the MEP.
Motor threshold: refers to the lowest stimulus intensity capable of generating a MEP.
Central motor conduction time: conduction time of the nerve impulse from the cortex to the target muscle (cortex-muscle).
Peripheral motor conduction time (PMCT): PMCT = MEP – Peripheral conduction time.
3.2. Exclusion criteria
The studies were excluded if: the interventions were made in animals or children; the methods used to neurophysiological assessment were not EEG, fNIRS, or TMS to verify neuronal plasticity.
Letters to editor, incomplete articles, incomplete study protocols, and not published studies were not considered in this revision.
3.3. Literature review
The research was realized from May 2018 until January 2019, without language or date restrictions in the following databases: Medline (via PubMed)—www.pubmed.com; EMBASE—www.embase.com; Cochrane Library—www.thecochranelibrary.com; National Health Institute database; ClinicalTrials.gov—www.clinicaltrials.gov.
Using the PubMed search tools, we selected the MeSH terms from the most relevant publications to realize a new research in order to obtain more articles that could potentially be included in this systematic review.
In addition, it was realized a manual search of theses, annals of congresses and meetings, references, study records, and contact with experts in the field.
3.3.1. Search strategies
The keywords were used equally in all databases, respecting their heterogeneities (e.g., terms “Emtree” and terms “MeSH” will be mapped in Embase and Medline, respectively).
The keywords were: “osteoarthritis,” “knee,” “brain mapping,” “gyrus cinguli,” “cerebral,” “cortex,” “sensorimotor cortex,” “motor cortex,” “theta rhythm,” “delta rhythm,” “evoked potentials,” “transcranial magnetic stimulation,” “electroencephalography,” “spectroscopy,” “near-infrared,” “cerebral cortex.”
The search strategy was: (Osteoarthritis, Knee) AND ((brain mapping) OR (Gyrus Cinguli) OR (Cerebral Cortex) OR (Sensorimotor Cortex) OR (Motor Cortex) OR (theta rhythm) OR (delta rhythm) OR (evoked potentials) OR (transcranial magnetic stimulation) OR (electroencephalography) OR (spectroscopy) OR (near-infrared) OR (cerebral cortex)).
3.3.2. Data extraction
Data from each study were extracted independently by 3 authors. Discordances were resolved by consensus. If no consensus was reached, the three authors would ask for the fourth author's specialist opinion to clarify the doubts and follow the decision of the fourth author.
All studies were analyzed according to their titles and abstracts, following the inclusion and exclusion criteria. If eligibility criteria were met, the full text would be extracted. All full-text studies evaluated were described in the “Results” section.
Missing data were elucidated by contacting the authors directly.
3.3.3. Data validation
Three authors performed the data validation through the discussion of the selected papers.
The risks of bias for the observational studies was assessed using the criteria of the Study Quality Assessment Tools | National Heart, Lung, and Blood Institute (NHLBI).[40] Intervention-type studies were analyzed through the guidelines of the Cochrane Back Review Group (CBRG).[41]
All studies were considered.
4. Results
4.1. Trial flow
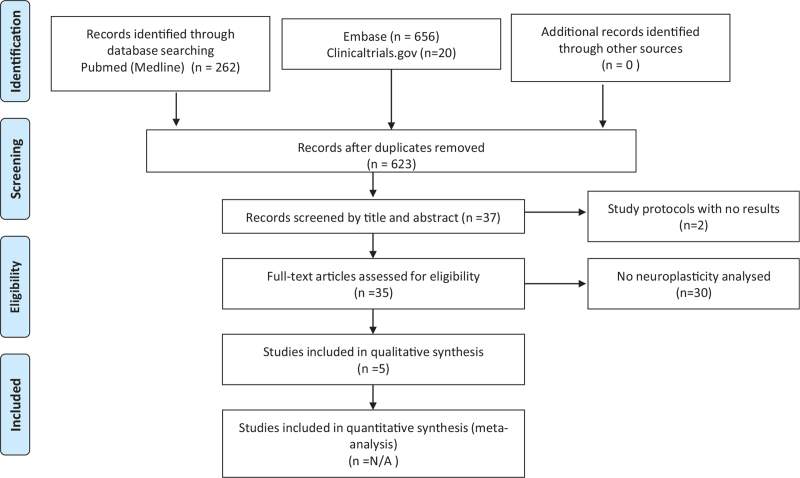
The electronic search in the PubMed database found 938 articles related to neurophysiological assessment methods in patients with knee OA. By excluding 123 duplicates and articles based on title and abstract, we considered 37 potentially eligible studies. Of those, 2 studies were incomplete protocol studies and 30 studies are not related to neurophysiological assessment methods, such as EEG, cerebral fNIRS or TMS, or studying neuroplasticity in patients with knee OA according to their relevance and data update (Fig. 1).
Figure 1.
PRISMA flowchart: neurophysiological assessment methods in patients with knee OA. OA = osteoarthritis, PRISMA = preferred reporting items for systematic review and meta-analysis protocols.
4.2. Quality of evidence
After reading the articles included in the systematic review, the following factors were analyzed to determine the level of evidence: study design, selection, detection, loss, reporting, and information (measurement) biases.
A total of 5 studies were included in the qualitative analysis (Table 1). Of the 5 articles, 3 studied neuroplasticity through TMS and 2 used EEG in their methodologies. No studies about fNIRS were found in this research. All studies had low risk of bias within their limitations. Study data by Quante et al[42] presented poor reports of selection, detection, and reporting bias criteria; Preece et al[43] presented flaws in relation to the detection bias criteria; Kittelson et al[44] failed in relation to selection and detection bias criteria due to lack of data and lack of complete information on the study methodology.
Table 1.
Bias risk analysis.
| Author, year, country | Quante et al (2008), Neustadt, Holstein, Germany[42] | da Graça-Tarragó M et al (2016), Porto Alegre, Brazil[33] | Preece et al (2016), Manchester, United Kingdom[43] | Kittelson et al (2014), Colorado, USA[44] | da Graça-Tarragó et al (2016), Porto Alegre, Brazil[45] |
| Study description | Case series without control group | Cross-sectional study | Prospective cohort study | Case series with control group | Randomized clinical trial |
| Selection bias | N/A | Low | Low | N/A | Low |
| Detection bias | N/A | Low | N/A | N/A | Low |
| Loss bias | Low | Low | Low | Low | Low |
| Report bias | N/A | Low | Low | Low | Low |
| Information bias | Low | Low | Low | Low | Low |
4.3. Studies’ characteristics
All included studies consist of published full studies, and doubts about the available data were complemented by contacting the authors. The studies present no conflicts of interest.
The demographic characteristics of studies are displayed in Table 2. All studies contained a total of 127 patients, of whom 84 (66.14%) presented knee OA. In the studies, the mean age of the patients was 62.79 years and the mean BMI was 28.764 kg/m2. Populations involved 16.54% of men and 83.46% of women. The WOMAC scores averaged 54.04 in the da Graça-Tarragó M et al[33,45] and Preece et al[43] studies. These averages were similar in the studies.
Table 2.
Demographic characteristics of the studies.
| Author, publication date and country | Number of patients (total = 127) | Mean age, yr | Sex | Mean body mass index, kg/m2 | Quantification of pain (pre-stimuli) and duration | Grade of knee osteoarthritis (Kellgren–Lawrence scale) and laterality of the analyzed knees |
| Quante et al (2008), Neustadt, Holstein Germany[42] | Total = 12OA = 12C = 0 | OA = 61.4 ± 10.5 | M = 3F = 9 | Mean weight 83.4 ± 12.4 kgMean height 167.2 ± 9.0 cmMean BMI = 29.83 | 5.83 ± 0.94>6 months | IVUnilateral knee osteoarthritis |
| da Graça-Tarragó M et al (2016), Porto Alegre, Brazil[33] | Total = 31OA = 21C = 10 | OA = 64.50 (SD = 7.72)C = 34.10 (SD = 11.64) | M = 0F = 31 | OA = 27.53(SD = 5.11)C = NA | WOMACc 57.92 (SD = 13.25)>6 months | III–IVN/A (not reported knee osteoarthritis laterality) |
| Kittelson et al (2014), Colorado, USA, Colorado, USA[44] | Total = 37OA = 17C = 20 | OA = 63.9 ± 1.8C = 58.3 ± 2.5 | M = 18 (C = 10; OA = 8)F = 19 (C = 10; OA = 9) | OA = 28.3 ± 1.0 C = 25.0 ± 2.5 | - | N/A (not reported knee osteoarthritis laterality) |
| Preece et al (2016), Manchester UK[43] | Total = 21OA = 21C = 0 | OA = 62C = 61 | M = 10F = 11 | OA = 29 (SD = 4)C = 27 (SD = 4) | WOMACs = 9.6WOMACc = 45 | Varying degrees OA (II–IV)Unilateral and bilateral OA |
| da Graça-Tarragó et al (2016), Porto Alegre, Brazil[45] | Total = 12OA = 13C = 13 | OA = 62.15 (SD = 7.44)C = 66.85 (SD = 7.53) | M = 0F = 26 | OA = 29.16 (SD = 6.65)C = 27.47 (SD = 4.20) | VAS (last 24 h)OA = 6.85 (SD = 0.38)C = 6.77 (SD = 0.43)WOMACOA = 54.92 (SD = 18.05)C = 52.46 (SD = 11.56)>6 months | III–IVN/A (not reported knee osteoarthritis laterality) |
Most studies tried to include primary knee osteoarthritis, without previous surgery on the evaluated limb, aiming at minimal brain neurophysiological changes, with no general restrictions on unilateral or bilateral knee osteoarthritis. Except by Preece et al,[43] most studies tried to exclude other influences than osteoarthritis on pain processing such as: previous surgery, peripheral/central nervous system diseases, infectious, inflammatory, rheumatic, psychiatric, or neoplastic diseases.[33,42,44,45]
Studies involving EEG were performed by Quante et al[42] and Preece et al.[43] Quante et al[42] performed a series of cases involving KL grade IV[46–49] large osteophytes, marked joint space narrowing, severe sclerosis, and definite bony deformity, with no control group, with biases in selection, detection, and reporting that were not identified due to lack of information in the article. They performed stimuli with intracutaneous electrical pulses (20 ms)[50] on the fingertips contralateral to knee with OA and verified changes in EEG brain activity (Table 3). However, there was only one loss in the intervention group and all measurements of the study were made on the same devices, by the same trained staff with the same parameters analyzed, which made the risks of biases due to losses and information low.
Table 3.
Study interventions.
| Author, publication date and country | Neurophysiological assessment | Types of stimuli | Analgesics during tests | Pre-test pain | Post-test pain |
| da Graça-Tarragó M et al (2016), Porto Alegre, Brazil[33] | TMS | CPM - non-dominant hand in cold water (0–1 °C) for 1 min) | No | N/A | N/A |
| Quante et al (2008), Neustadt, Holstein Germany[42] | EEG (2 32-channel amplifiers) | Intracutaneous electrical pulses (20 ms) | 10 mL of bupivacaine 0.5% + triancinolone 40 mg (6 patients) between pre- and post-intervention period | VAS = 5.83 ± 0.94. | NCi: VAS = 4.91 ± 1.01Ci: VAS = 4.93 ± 0.93P = .93 |
| Preece et al (2016), Manchester UK[43] | 64 channels EEG | Heat emissions by thulium laser stimulator (<150 ms). Auditory stimulus before physical stimulation. | No | WOMACs = 9.6WOMACc = 45 | IAIWOMACs 9.6 [3.0]–4.2 [2.7]; P < .01WOMACc = 45 [13]–21 [13]); P < .0115MPIWOMACs = 9.1 [3.2]–4.4 [2.7]); P < .01WOMACc = 43 [14]–25 [14]); P < .01 |
| Kittelson et al (2014), Colorado, USA[44] | TMS | Isometric torque and voluntary quadriceps activation | No | N/A | N/A |
| da Graça-Tarragó et al (2016), Porto Alegre, Brazil[45] | TMS | OA = a-EIMS 2 Hz;C = ElectroacupunctureCPM—non-dominant hand in cold water (0–1 °C for 1 min) | No | WOMACC = 52.46 (SD = 11.56)OA = 54.92 (SD = 18.05)VASC = 6.77(SD = 0.43)OA = 6.85 (SD = 0.38) | VASC = 4.32 (SD = 1.23)OA = 3.11 (SD = 1.54) |
Preece et al[43] performed an intervention study with Alexander's self-consciousness technique without randomization, evaluating patients’ parameters before and after interventions, using a group of patients with KL II–IV and a control group of healthy volunteers. His study presented an unknown risk of detection bias, with the possibility of blindness of the evaluators of outcomes not described in the article.
da Graça-Tarragó M et al[33,45] and Kittelson et al[44] performed studies involving TMS with KL grade III–IV. da Graça-Tarragó M et al[33,45] performed analyzes considering short interval intracortical inhibition with intervals between 2 ms stimuli and intracortical facilitation (ICF) with stimulus intervals of 12 ms. Using the tool developed by the da Graça-Tarragó M et al[33,45] presented low risks of bias.
Kittelson et al[44] made a comparative study between patients with knee OA on the waiting list for total knee prosthesis and healthy volunteers with unknown selection bias risk, since the authors did not mention the origin of the healthy volunteer population, in addition to presenting an imbalance in the initial variable (BMI—P = .018). Short interval intracortical inhibition with intervals of 3 ms and ICF with intervals of 15 ms were considered. In addition, they present unknown risk of detection bias, with the possibility of blindness of the evaluators of the outcomes, not described in the study.
The consensus between authors during analysis of the studies was reached at all times.
4.4. Cortex neurophysiological changes
The neurophysiological changes in cortex are displayed in Tables 4 and 5.
Table 4.
Neurophysiological changes.
| Author, publication date and country | Specific cortex neurophysiological changes |
| da Graça-Tarragó M et al (2016), Porto Alegre, Brazil[33] | Mean adjusted MEP amplitude was 13.53% higher in OA than in C (1.33 [0.49] vs 1.15 [0.13]) (P = .16).Mean CSP adjusted OA 23.43% lower than in healthy subjects (54.54 [16.10] vs 70.94 [22.87]) (P = .01).Pain modulator system evaluated by NPS (0–10) during a CPM task negatively correlated with cortical excitability (CSP) (P = .001).CSP negatively correlated with pain and disability. |
| Quante et al (2008), Neustadt, Holstein Germany[42] | NCi: amplitude N2/P2 (22.2 ± 6.0 μV)Ci: N2/P2 (9.6 ± 5.4 μV) P = .001). Decreased activation of the medial cingulate cortex |
| Preece et al (2016), Manchester UK[43] | No statistically significant changes in the late anticipation potential (P = .77) nor in the N2-P2 difference or in the laser evoked potential (P = .32). |
| Kittelson et al (2014), Colorado, USA[44] | Cortex motor—quadriceps area: association between RMT and pain (r = –0.575; P = .016);Associations between ICF and self-reported pain measures (r = –0.495, P = .043) and stiffness (r = –0.548, P = .023). |
| da Graça-Tarragó et al (2016), Porto Alegre, Brazil[45] | In the motor cortex - quadriceps-related area: a-EIMS compared with SHAM decreased MEP in 31.61% ([CI] 95% 2.34–60.98). Reduction of ICF 37.32% ([CI] 95% [69.93 a–5.00]). Increase of CSP 22.85% ([CI] 95% [10.90–34.79]). Reduction in VAS 68.08% ([CI] 95% [104–31.45]) and NPS 57.18% ([CI] 95% [104.14–10.21]) during CPM task. BDNF negatively correlated with PPT. |
Table 5.
Study analysis and outcomes.
| Author, publication date and country | Number of patients (total = 127) | Neurophysiological assessment | Neurophysiological changes | Studies conclusion |
| da Graça-Tarragó M et al (2016), Porto Alegre, Brazil[33] | Total = 31M = 0F = 31OA = 21C = 10 | TMS | Mean adjusted MEP amplitude was 13.53% higher in OA than in healthy subjects (1.33 [0.49] vs 1.15 [0.13]) (P = .16).Mean CSP adjusted OA was 23.43% lower than in healthy subjects (54.54 [16.10] vs 70.94 [22.87]) (P = .01). Pain modulator system evaluated by NPS (0–10) during a CPM task was negatively correlated with cortical excitability (CSP) (P = .001). CSP was negatively correlated with pain and disability assessed by WOMAC. | Change in cortical plasticity in OA is associated with lower intracortical inhibition, with levels of pain and disability, and decreased activation of the endogenous pain modulating system due to CPM. |
| Quante et al (2008), Neustadt, Holstein Germany[42] | Total = 12M = 3F = 9OA = 12C = 0 | EEG (2 32-channel amplifiers) | Significant reduction of cingulate gyrus activation and CPM/DNIC deficit in patients with knee OA | Conditioned pain modulation (CPM/DNIC) is subject to the neuronal plasticity of the descending pain inhibition systems and decreases in chronic pain cases |
| Preece et al (2016), Manchester UK[43] | Total = 21M = 10F = 11OA = 21C = 0 | 64 channels EEG | There were no statistically significant changes in the late anticipation potential (P = .77) nor in the N2–P2 difference nor in the laser evoked potential (P = .32). | Reductions in WOMAC after AT were associated with reductions in medial co-contraction during the gait precontact phase. |
| Kittelson et al (2014), Colorado, USA[44] | Total = 37M = 18F = 19OA = 17C = 20 | TMS | Association between RMT and pain (r = –0.575; P = .016); associations between ICF and self-reported pain measures (r = –0.495, P = .043) and stiffness (r = –0.548, P = .023) | In OA, there is an association between pain and motor response threshold; pain and intracortical facilitation. |
| da Graça-Tarragó et al (2016), Porto Alegre, Brazil[45] | Total = 26M = 0F = 26OA = 13C = 13 | TMS | a-EIMS compared to SHAM: decreased MEP in 31.61% ([CI] 95% 2.34–60.98]. Reduction of ICF 37.32% ([CI] 95% [69.93 to –5.00]) CSP increase 22.85% ([CI] 95% [10.90–34.79]). Reduction in VAS 68.08% ([CI] 95% [104–31.45]) and NPS 57.18% ([CI] 95% [104.14–10.21]) during the CPM task. BDNF was negatively correlated with PPT (r = 520.56) | a-EIMS enabled the corticospinal inhibition system at cortical and infracortical pain processing sites through a bottom-up mechanism. |
4.5. EEG studies
Quante et al[42] verified the decreased activation of the medial cingulate cortex in cases of OA pain stimulation, while secondary somatosensory cortex activity remained the same. The conditioned pain modulation is subject to the neuronal plasticity of the inhibiting systems descending of pain and decreases in chronic pain cases. Preece et al[43] did not find any associations changes in EEG, but verified reductions in WOMAC after application of Alexander Technnique which are associated to reductions in medial co-contraction during pre-contact phase in gait.
4.6. TMS studies
da Graça-Tarragó M et al[33] found an association between cortical changes in patients with knee OA and less intracortical inhibition, greater levels of pain such as decreased activation of the endogenous pain modulating system. Also, da Graça-Tarragó et al[45] studied the active electrical intramuscular stimulation in dermatomes corresponding to the nerve roots of the knee (L1–L5 and S1–S2). Active electrical intramuscular stimulation enabled the corticospinal inhibition system at cortical and intracortical pain processing sites probably through a bottom-up mechanism.
Kittelson et al[44] identified some associations between pain and motor response threshold such as pain and ICF in TMS in patients with knee OA.
5. Discussion
Neurophysiological changes related to pain are increasingly being explored to understand how the brain works towards pain. There are many studies analyzing brain changes in patients with knee OA such as Baliki et al[51] who studied changes in activation of brain areas which are responsible for evoked pain in OA. They are: bilateral thalamus, secondary somatosensory cortex, insula, supplementary motor area, medial frontal, and anterior cingulate gyrus, right and left amygdala. Only one of these areas is similar to those afflicted by chronic low back pain, another clinical condition associated with chronic pain. The less activated areas in chronic low back pain involve the anterior cingulate cortex, the prefrontal cortex, and the nucleus accumbens, suggesting a diminished function of the descending inhibitory system in those patients.[52]
Other studies with fMRI have found that chronic pain generates abnormal connectivity and activation between the dorsolateral prefrontal cortex and the knee OA pain matrix.[39] In addition, the cingulate cortex, thalamus and amygdala, areas involved in the processing of fear, emotions, and aversive conditioning are also closely related to pain in the knee OA patient.[53] It is also known that the dopaminergic system and the system involving opioids of the brain are involved with pain modulation.[54] And involve regions such as medial prefrontal cortex, anterior cingulate cortex,[55–57] and the nucleus accumbens.[58–60] Because of this, we can infer that there are different mechanisms of neuroplasticity involved in knee OA pain.
In addition to the results obtained in the literature, it is important to note the studies that used different methodologies to assess brain function related to knee OA pain.
Our systematic review examined the various functional central effects on neuroplasticity responsible for knee OA, changing parameters seen in EEG and TMS assessments. Our results can be compared and added to these previous studies in order to improve the understanding of the effects of neuroplasticity in people with knee OA.
Quante et al[42] verified in their study through EEG and magnetoencephalogram (MEG) findings that neuroplastic changes reduce the inhibition effect of pain promoted by the inhibitory descending systems of pain in patients with chronic pain. There was a reduction in the activity of the medium cingulate cortex (analyzed via EEG), while the activity of the secondary somatosensory cortex (analyzed via MEG) remained unchanged with the use of an external pain stimulus. In this study, no reduction of phasic pain with contraction, reduction of P2 in the EEG (less activity of the cingulate medium cortex), and maintenance of N1 and M1 in MEG (unchanged IBS) was observed in patients with unilateral knee OA. The changes were verified in the contralateral hemisphere to the electrical stimulus. The study could clarify whether there is a relationship between the hemisphere activated with knee OA, however, it prevailed for patients with unilateral knee OA.
The studies involving TMS[33,44,45] found a lower inhibition of the corticospinal system in patients affected by chronic pain, generating a dysfunctional state of modulatory pathways, among them, the descending inhibitory pathway of pain. It was observed that chronic and moderate to high intensity nociceptive stimulation in patients with knee OA caused a negative modulation in the pain inhibitory cortical pathways, leading to a lower excitability of the inhibitory neurons. The studies do not directly describe which hemisphere is activated or inhibited in patients with knee OA. However, one of the analyzed parameters was the cortical silent period, which evaluates the interruption of voluntary muscle contraction by transcranial stimulation of the contralateral motor cortex. In addition to the fact that most fibers of the corticospinal tract cross the pyramidal decussation,[61] we are led to believe that the changes occurred also in the contralateral hemisphere. In this case, the selection of patients with uni- or bilateral knee OA could influence the obtained results depending on where the electrodes were placed in those studies.
The included studies have a low risk of bias, however, some data were poorly reported, as seen in Table 1. Because of this, information such as quantification and duration of pain, as well as the knee OA radiological degree (KL scale)[46–49] were not homogeneously reported by the studies.
All included studies were original studies, conducted in research centers, without conflicts of interest.
The pain stimuli were not homogeneous in the included studies, ranging from intracutaneous electric pulses, intramuscular electrical stimulation,[62] cortical modulation of pain through cold thermal variation (CPM or formerly, pain inhibitory effect),[63] isometric torque/voluntary activation of the quadriceps muscle and heat emissions by thulium laser stimulator.
The main result of our review is to demonstrate through several studies how knee OA is able to modulate the neuronal pathways in order to generate chronic pain. In general, the central nervous system plays an important role in the generation and loss of pain inhibition, which can cause refractory pain leading to a clinically worse prognosis.
5.1. Limitations of studies
We performed a systematic review according to national and international guidelines and considered that our bibliographic research was meticulous. However, it was not possible to assess the risk of publication bias due to the limited number of studies related to the topic.
Considering also the limited number of studies, the authors chose to consider every kind of study and evidence published in order to carry out a complete analysis of possible studies to be developed in the future. Nevertheless, because of the mix of different kind of research articles, the analysis and synthesis results are biased.
The study by Quante et al[42] presented only a series of cases, with no control group paired with healthy volunteers with the normal pain-inhibitor phenomenon, which makes it difficult to compare and identify the regions actually altered in patients with knee OA. Among studies using EEG only, study by Quante et al found significant changes which recruited only 12 patients.
It is possible that the type of pain identified in the studies may be from other lesions unexplained in the studies, for example, soft tissue injuries, which may mimic gonalgia in patients with knee OA. Other descriptions are necessary in this type of study to identify other affections that can simulate gonalgia, such as: central sensitization, myofascial pain, etc.
Only studies by Quante et al,[42] Davis and Moayedi,[33] and da Graça-Tarragó et al[45] reported duration of pain, which was greater than or equal to 6 months (Table 2). WOMAC was analyzed only by Preece et al[43] and da Graça-Tarragó et al[45]; VAS was analyzed by Quante et al[42] and da Graça-Tarragó M et al[45] Unfortunately, the studies were not homogeneous and did not report all variables which could provide more important data.
Another limitation of the studies was the heterogeneity of the type of intervention performed throughout the studies: Quante et al[42] carried out a study using 10 mL of 0.5% bupivacaine + 40 mg triamcinolone to treat patients’ pain. The use of analgesics should be homogeneous in each study because analgesics can affect both peripheral and central function and therefore influence different EEG results.
5.2. Study design proposal
For a better understanding of the neurophysiological changes a standard intervention (pain treatment) should be performed because it can affect both peripheral and central function. To analyze the effect of these interventions, both EEG and TMS can be performed with special attention to the changes observed in the studies. On the matter of assessment times, it is important to carry out periodic and serial assessments to check for functional changes after standardized treatments and pain relief. Follow-up for at least 1 year can provide important evidence of how the brain behaves towards pain and also pain management.
It is important to the studies to contemplate both VAS, WOMAC, and also algometry (as a more objective measure) assessment methods to promote comparability between studies. Also it is important to gather information about comorbidities, given that these would provide insightful information on possible confounding factors.
It is surprising that after decades of studies, evidence available in the literature is still limited to these articles. Overall, it is not possible at the time to determine the exact changes observed in the brains of patients with knee OA. So far, the only assertion backed by the articles is that EEG and magnetoencephalogram findings showed that neuroplastic changes reduce the inhibition effect of pain promoted by the inhibitory descending systems of pain. Studies involving TMS found a lower inhibition of corticospinal system in chronic pain, generating dysfunctional state of modulatory pathways—the descending inhibitory pathway of pain. This should be an immediate call-to-arms for new double blinded clinical trials to be developed in the field involving patients with knee OA being treated homogeneously (with the same therapy or analgesics) and observing the functional changes in the brain with tests standardized: EEG or TMS.
All studies in the literature evaluated neurophysiological assessment methods alone. To better evaluate the neurophysiological changes that occur in patients with knee OA, we believe that associating measurement measures with fNIRS, EEG, and TMS may be beneficial.
6. Conclusion
Although there are not many studies currently performing neurofunctional assessments in patients with knee OA, it was possible to confirm some cortical changes verified in previous studies with brain fMRI.
This study contributes to a better understanding of the neurophysiological changes seen in the cingulate medium cortex, decrease in pain inhibitory effect and motor response threshold/intracortical pain facilitation. Advances in neuroplasticity studies may aid in the screening for early diagnosis of knee OA in the future. However, more studies are needed to prove such evidence, and may involve associations of assessment measures such as fNIRS, EEG, and TMS.
Author contributions
Conceptualization: Leandro Ryuchi Iuamoto, Wu Tu Hsing.
Data curation: Leandro Ryuchi Iuamoto, Fábio Ito.
Formal analysis: Leandro Ryuchi Iuamoto, Fábio Ito, Wu Tu Hsing.
Investigation: Leandro Ryuchi Iuamoto, Fábio Ito, Thales Tomé.
Methodology: Leandro Ryuchi Iuamoto, Thales Tomé.
Supervision: Wu Tu Hsing, Alberto Meyer, Marta Imamura, Linamara Battistella.
Validation: Alberto Meyer, Marta Imamura, Linamara Battistella.
Visualization: Wu Tu Hsing, Alberto Meyer, Marta Imamura.
Writing – original draft: Leandro Ryuchi Iuamoto, Thales Tomé, Alberto Meyer.
Writing – review & editing: Wu Tu Hsing, Alberto Meyer, Linamara Battistella.
Footnotes
Abbreviations: EEG = electroencephalogram/electroencephalography, fMRI = functional magnetic resonance imaging, fNIRS = functional near-infrared saturation, ICF = intracortical facilitation, KL = Kellgren–Lawrence, MEP = motor evoked potential, OA = osteoarthritis, PMCT = peripheral motor conduction time, TMS = transcranial magnetic stimulation, VAS = visual analogue scale, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
How to cite this article: Iuamoto LR, Ito FL, Tomé TA, Hsing WT, Meyer A, Imamura M, Battistella LR. Effects of neuroplasticity in people with knee osteoarthritis: a systematic review of the literature. Medicine. 2022;101:3(e28616).
Analysis of studies using neurophysiological assessment methods to identify changes in neural plasticity in knee osteoarthritis.
Role of the funding source: There are no sources of funding for this systematic review.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
N/A = Not available.
C = control group, F = female, M = male, N/A = Not available, OA = knee osteoarthritis group, SD = standard deviation, T = total, VAS = visual analogue scale, WOMACc = Western Ontario and McMaster Universities Osteoarthritis Index Complete, WOMACs = Western Ontario and McMaster Universities Osteoarthritis Index simplified questionnaire.
15MPI = 15 months post-intervention, a-EIMS = active electrical intramuscular stimulation, C = control group, Ci = “counterirritation on” group, DNIC = diffuse noxious inhibitory controls, CPM = conditioned pain modulation, EEG = electroencephalogram, IPI = immediately post-intervention, MEP = motor evoked potential, N/A = Not available, NCi = “counterirritation off” group, OA = knee osteoarthritis group, SD = standard deviation, TMS = transcranial magnetic stimulation, VAS = visual analogue scale, WOMACc = Western Ontario and McMaster Universities Osteoarthritis Index Complete, WOMACs = Western Ontario and McMaster Universities Osteoarthritis Index simplified questionnaire.
a-EIMS = active electrical intramuscular stimulation, BDNF = brain derived neurotrophic factor, C = control group, Ci = “counterirritation on” group, CI = confidence interval, CPM = conditioned pain modulation, CSP = cortical silent period, ICF = intracortical facilitation, MEP = motor evoked potential, NCi = “counterirritation off” group, NPS = numerical pain scale, OA = knee osteoarthritis group, PPT = pressure pain threshold, RMT = motor resting threshold, VAS = visual analogue scale.
a-EIMS = active electrical intramuscular stimulation, AT = Alexander technique, BDNF = brain derived neurotrophic factor, C = control group, CI = confidence interval, CPM = conditioned pain modulation, CSP = cortical silent period, DNIC = diffuse noxious inhibitory controls, EEG = electroencephalogram, F = female, ICF = intracortical facilitation, M = male, MEP = motor evoked potential, NPS = numerical pain scale, OA = knee osteoarthritis group, PPT = pressure pain threshold, RMT = motor resting threshold, T = total, TMS = transcranial magnetic stimulation, VAS = visual analogue scale, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
References
- [1].Kurek S, Rachwal T. Development of entrepreneurship in ageing populations of The European Union. Procedia Soc Behav Sci 2011;19:397–405. [Google Scholar]
- [2].Dos Santos WT, Rodrigues Ede C, Mainenti MR. Muscle performance, body fat, pain and function in the elderly with arthritis. Acta Ortop Bras 2014;22:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016;17:425.doi:10.1186/s12891-016-1286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hunter DJ, Lo GH. The management of osteoarthritis: an overview and call to appropriate conservative treatment. Rheum Dis Clin North Am 2008;34:689–712. [DOI] [PubMed] [Google Scholar]
- [5].Bierma-Zeinstra SM, Verhagen AP. Osteoarthritis subpopulations and implications for clinical trial design. Arthritis Res Ther 2011;13:213.doi:10.1186/ar3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bruyère O, Cooper C, Arden N, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015;32:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen OH, Laursen MB, Arendt-Nielsen L. Facilitation of pain sensitization in knee osteoarthritis and persistent post-operative pain: a cross-sectional study. Eur J Pain 2014;18:1024–31. [DOI] [PubMed] [Google Scholar]
- [8].Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken) 2016;68:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lluch Girbés E, Dueñas L, Barbero M, et al. Expanded distribution of pain as a sign of central sensitization in individuals with symptomatic knee osteoarthritis. Phys Ther 2016;96:1196–207. [DOI] [PubMed] [Google Scholar]
- [10].Lluch E, Nijs J, Courtney CA, et al. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disabil Rehabil 2018;40:2836–45. [DOI] [PubMed] [Google Scholar]
- [11].Beckwée D, De Hertogh W, Lievens P, Bautmans I, Vaes P. Effect of tens on pain in relation to central sensitization in patients with osteoarthritis of the knee: study protocol of a randomized controlled trial. Trials 2012;13:21.doi:10.1186/1745-6215-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res (Hoboken) 2015;67:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep 2015;13:225–34. [DOI] [PubMed] [Google Scholar]
- [14].Imamura M, Ezquerro F, Marcon Alfieri F, et al. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int J Inflam 2015;2015:329792.doi:10.1155/2015/329792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81. [DOI] [PubMed] [Google Scholar]
- [16].Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965–73. [DOI] [PubMed] [Google Scholar]
- [17].Skou ST, Roos EM, Simonsen O, et al. The effects of total knee replacement and non-surgical treatment on pain sensitization and clinical pain. Eur J Pain 2016;20:1612–21. [DOI] [PubMed] [Google Scholar]
- [18].Van Dijk GM, Dekker J, Veenhof C, van den Ende CH. Carpa Study Group. Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum 2006;55:779–85. [DOI] [PubMed] [Google Scholar]
- [19].Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 2009;61:1226–34. [DOI] [PubMed] [Google Scholar]
- [20].Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 2017;25:1926–41. [DOI] [PubMed] [Google Scholar]
- [21].Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 2001;93:107–14. [DOI] [PubMed] [Google Scholar]
- [22].Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012;64:2907–16. [DOI] [PubMed] [Google Scholar]
- [23].Arendt-Nielsen L. Pain sensitisation in osteoarthritis. Clin Exp Rheumatol 2017;35: (suppl): 68–74. [PubMed] [Google Scholar]
- [24].Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol 2014;66:3018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med 2011;2:205–12. [PMC free article] [PubMed] [Google Scholar]
- [26].Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606. [DOI] [PubMed] [Google Scholar]
- [27].Neogi T. Clinical significance of bone changes in osteoarthritis. Ther Adv Musculoskelet Dis 2012;4:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage 2011;19:647–54. [DOI] [PubMed] [Google Scholar]
- [29].Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol 2014;10:374–80. [DOI] [PubMed] [Google Scholar]
- [30].Haavik-Taylor H, Murphy B. Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study. Clin Neurophysiol 2007;118:391–402. [DOI] [PubMed] [Google Scholar]
- [31].Madry H, Kon E, Condello V, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24:1753–62. [DOI] [PubMed] [Google Scholar]
- [32].Pelletier R, Higgins J, Bourbonnais D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet Disord 2015;16:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tarragó M da G, Deitos A, Brietzke AP, Vercelino R, Torres IL, Fregni F, et al. Descending control of nociceptive processing in knee osteoarthritis is associated with intracortical disinhibition: an exploratory study. Medicine (Baltimore) 2016;95:e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Imamura M, Imamura ST, Kaziyama HHS, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum 2008;59:1424–31. [DOI] [PubMed] [Google Scholar]
- [35].Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 2008;51:S225–39. [DOI] [PubMed] [Google Scholar]
- [36].Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152: (3 suppl): S49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pelletier R, Higgins J, Bourbonnais D. Addressing neuroplastic changes in distributed areas of the nervous system associated with chronic musculoskeletal disorders. Phys Ther 2015;95:1582–91. [DOI] [PubMed] [Google Scholar]
- [38].Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01.doi:10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].University of Illinois at Chicago's Library of the Health Sciences at Peoria. Evidence Based Medicine - What is the PICO Model? [Internet]. Available at: https://researchguides.uic.edu/c.php?g=252338&p=3954402. Accessed Jan 03, 2019. [Google Scholar]
- [40].Study Quality Assessment Tools | National Heart, Lung, and Blood Institute (NHLBI) [Internet]. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed Jan 03, 2019. [Google Scholar]
- [41].Cochrane Methods Screening and Diagnostic Tests. Handbook for DTA Reviews. [Internet]. Available at: http://methods.cochrane.org/sdt/handbook-dta-reviews. Accessed Jan 03, 2019. [Google Scholar]
- [42].Quante M, Hille S, Schofer MD, Lorenz J, Hauck M. Noxious counterirritation in patients with advanced osteoarthritis of the knee reduces MCC but not SII pain generators: a combined use of MEG and EEG. J Pain Res 2008;1:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Preece SJ, Jones RK, Brown CA, Cacciatore TW, Jones AK. Reductions in co-contraction following neuromuscular re-education in people with knee osteoarthritis. BMC Musculoskelet Disord 2016;17:372.doi:10.1186/s12891-016-1209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kittelson AJ, Thomas AC, Kluger BM, Stevens-Lapsley JE. Corticospinal and intracortical excitability of the quadriceps in patients with knee osteoarthritis. Exp Brain Res 2014;232:3991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].da Graca-Tarragó M, Deitos A, Patrícia Brietzke A, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med 2016;17:877–91. [DOI] [PubMed] [Google Scholar]
- [46].Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ravaud P, Giraudeau B, Auleley GR, et al. Radiographic assessment of knee osteoarthritis: reproducibility and sensitivity to change. J Rheumatol 1996;23:1756–64. [PubMed] [Google Scholar]
- [48].Günther KP, Scharf HP, Puhl W, et al. Reproducibility of radiologic diagnosis in gonarthrosis. Z Orthop Ihre Grenzgeb 1997;135:197–202. [DOI] [PubMed] [Google Scholar]
- [49].Ravaud P, Auleley GR, Amor B. Radiographic assessment of progression in knee osteoarthritis. Rheumatol Eur 1995;24:129–31. [Google Scholar]
- [50].Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol 1984;6:405–10. [PubMed] [Google Scholar]
- [51].Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008;4:47.doi:10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Konno SI, Sekiguchi M. Association between brain and low back pain. J Orthop Sci 2018;23:03–7. [DOI] [PubMed] [Google Scholar]
- [53].Kulkarni B, Bentley DE, Elliott R, et al. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum 2007;56:1345–54. [DOI] [PubMed] [Google Scholar]
- [54].Kong J, Wang Z, Leiser J, et al. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: a functional neuroimaging study. Neuroimage Clin 2018;18:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014;17:1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Navratilova E, Xie JY, Meske D, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 2015;35:7264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Reckziegel D, Raschke F, Cottam WJ, Auer DP. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol Pain 2016;12:1744806916650690.doi:10.1177/1744806916650690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci 2008;9:314–20. [DOI] [PubMed] [Google Scholar]
- [59].Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. [DOI] [PubMed] [Google Scholar]
- [60].Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001;293:311–5. [DOI] [PubMed] [Google Scholar]
- [61].Welniarz Q, Dusart I, Roze E. The corticospinal tract: evolution, development, and human disorders. Dev Neurobiol 2017;77:810–29. [DOI] [PubMed] [Google Scholar]
- [62].Couto C, de Souza IC, Torres IL, Fregni F, Caumo W. Paraspinal stimulation combined with trigger point needling and needle rotation in treating myofascial pain: randomized sham-controlled clinical trial. Clin J Pain 2014;30:214–23. [DOI] [PubMed] [Google Scholar]
- [63].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–5. [DOI] [PubMed] [Google Scholar]