Abstract
Background:
We investigated the characteristics and outcomes of patients who underwent open partial nephrectomy (OPN) in the minimally invasive approach era.
Materials and methods:
We retrospectively reviewed 52 patients (55 cases) who underwent OPN from May 2009 to March 2016. We assessed perioperative change in estimated glomerular filtration rate (eGFR), complications, and oncological outcomes. Tumor complexity was evaluated using the R.E.N.A.L nephrometry score (NS) and the modified NS.
Results:
Fifteen cases (27%) had imperative indications and 40 (73%) had elective indications. The elective cases were more likely to have adverse tumor complexity based on NS. The perioperative complication rate defined as a Clavien-Dindo grade ≥IIIa was 11%. The rate of postoperative decline in eGFR at 1 month, 1 year, and 2 years was 22%, 20%, and 21%, respectively. Multivariate analysis revealed that male gender (odds ratio [OR] 11.8, p = 0.03), NS ≥9 (OR 13.9, p = 0.02), modified NS ≥11 (OR 13.5, p = 0.01), and cold ischemic time ≥40 minutes (OR 7.9, p = 0.04) were significantly associated with worsening eGFR at 1 year after surgery. During a median follow-up period of 52 months, the 5-year overall survival and recurrence-free survival rates were 93% and 84%, respectively.
Conclusions:
OPN is acceptable with regard to oncological outcomes and complications in the minimally invasive surgery era. We propose that OPN should be the preferred approach in cases in which it is technically difficult to preserve maximum renal function via a minimally invasive approach.
Keywords: Open partial nephrectomy, Renal cell carcinoma, Ischemic time, Minimally invasive approach, Renal function
Introduction
To preserve maximum renal function, partial nephrectomy (PN) is the considered choice for the management of renal cell carcinoma (RCC).[1] This procedure has acceptable surgical morbidity and an equivalent oncological outcome, leading to a paradigm shift away from radical nephrectomy. When technically feasible, PN is performed not only for patients with specific conditions such as a tumor in a solitary kidney, bilateral tumors, and patients at high risk for impaired renal function, but also for those with a normal contralateral kidney, depending on the size and location of the tumor.[1]
Recently, laparoscopic surgery and robot-assisted laparoscopic surgery have been increasingly employed. These minimally invasive approaches result in improved safety outcomes including reduced blood loss and shorter hospital stay.[2,3] The shift toward minimally invasive PN for RCC raises the question of whether an open surgical approach is no longer essential. In this study, in order to clarify when open partial nephrectomy (OPN) should be considered, we investigated the characteristics and outcomes of patients who underwent OPN in the minimally invasive approach era.
Materials and methods
Patient selection
This study was a retrospective, single institution study. We included 55 consecutive cases of 52 patients who underwent OPN from March 2009 to December 2016; 135 patients underwent laparoscopic PN during the same period.[3] This study was approved by our institutional review board (No. 312-40). The primary outcome assessed in this study was postoperative change in estimated glomerular filtration rate (eGFR) compared with preoperative data. Secondary outcomes included complication rate, and 5-year overall survival (OS) and recurrence-free survival (RFS) rates.
Preoperative evaluation of renal tumors
Renal tumors were assessed using cross-sectional imaging (three-dimensional computed tomography or magnetic resonance imaging). The author (SK) evaluated the renal tumors retrospectively using the R.E.N.A.L nephrometry score (NS), which takes into consideration tumor radius, exophytic/endophytic appearance, nearness to the collecting system, anterior/posterior position, and location relative to the polar line.[4] The modified NS we previously described was also used.[3] This is a modified scoring system in which 1, 2, and 3 points are given for tumor radii of ≤2.5, 2.5–4, and >4 cm, and for anterior, coronal plane, and posterior tumor positions, respectively. The decision to perform PN using an open, laparoscopic, or robot-assisted approach was made based upon tumor characteristics and the surgeon's preference. We decided to perform OPN if the patients had an imperative indication such as bilateral tumors, solitary kidney and chronic kidney disease, or if the patients had a stage T3a tumor, multiple tumors, or a high complexity tumor. We planned one-stage OPN in patients with unilateral multiple tumors. Patients with bilateral tumors underwent two-stage PN.
Open partial nephrectomy procedure
OPN was performed via a retroperitoneal approach in the lateral decubitus position, except for cases in which abdominal surgery was simultaneously performed. In almost all cases, we performed renal hilar clamping and used slushed ice to cool the kidney for a minimum of 10 minutes. After dissection of the tumor, we sutured the collecting system with absorbable sutures and performed renorrhaphy with felt-attached absorbable sutures. Then, we checked for urinary leakage via needle injection of indigo carmine directly into the ureter.
Follow-up schedule
Patients were allowed to walk and eat a meal on postoperative day (POD) 1. The drainage tube, epidural tube, and urethral catheter were removed on POD 1 to 3. Blood testing was usually performed shortly after the operation and on POD 1, 3, and 7. During follow-up, blood testing was performed at 1, 3, 6, 12, 18, and 24 months, and computed tomography was performed at 6, 12, 18, and 24 months.
Renal function and complications
We calculated eGFR using the following formula: 194 × [serum creatinine (mg/dL) − 1.094] × [age (years) − 0.287] × 0.739 (if female) mL/min/1.73 m2.[5] We assessed perioperative renal function, complications defined by the Clavien-Dindo classification, clinical and pathological findings, and oncological outcomes. We defined renal insufficiency as an eGFR of <60mL/min/1.73 m2, and impairment of postoperative renal function as an eGFR decline of more than 20% compared with preoperative data.
Statistical analysis
The OS and RFS rate were calculated using the Kaplan–Meier method. Logistic regression analysis was used to calculate risk factors for an eGFR decline of more than 20% compared with preoperative data. A p-value of <0.05 was considered statistically significant for all analyses. We used EZR (Jichi Medical University, Saitama, Japan) to analyze statistical parameters.
Results
Patient characteristics
Characteristics of the patients and cases are shown in Table 1. The median baseline estimated glomerular filtration rate (eGFR), NS, and modified NS were 73.2 mL/min/1.73 m2, 8, and 11, respectively. Clinical T1a disease was observed in 35 (64%) cases. Of the cases assessed in this study, 15 (27%) had imperative indications for PN (Table 2). The other 40 case (73%) had elective indications such as a past history of poly surgery, tumor characteristics, and simultaneous abdominal surgery including gastrectomy, hepatectomy and pancreatoduodenectomy. The NS and the modified NS were 8 and 10 in cases with imperative indications, and 8 and 11 in cases with elective indications, respectively. The total number of surgeons who performed the OPN procedures was 10, and none of them performed minimally invasive surgery. All of them had performed at least 30 cases of OPN.
Table 1.
Characteristics of all 52 patients (55 cases).
| Characteristics | Values |
|---|---|
| Age, yr, median (IQR) | 69 (57–74) |
| Male, n (%) | 34 (65) |
| Female, n (%) | 18 (35) |
| BMI, kg/m2, median (IQR) | 23.2 (21.8–26.3) |
| Baseline eGFR, mL/min/1.73 m2 | |
| Mean ± SD | 70.9 ± 19.1 |
| <60, n (%) | 16 (29.1) |
| ≥60, n (%) | 39 (70.9) |
| Clinical stage, n (%) | |
| T1aN0M0 | 35 (63.6) |
| T1bN0M0 | 15 (27.3) |
| T2aN0M0 | 2 (3.6) |
| T2bN0M0 | 0 |
| T3aN0M0 | 3 (5.5) |
| TanyNaMany | 0 |
| TanyNanyM1 | 0 |
| Tumor size, cm, median (IQR) | 3.4 (2.6–4.7) |
| NS, median | 8 |
| 4–6, n (%) | 10 (18.2) |
| 7–9, n (%) | 39 (70.9) |
| 10–12, n (%) | 6 (10.9) |
| Modified NS, median | 11 |
| 5–7, n (%) | 5 (9.1) |
| 8–11, n (%) | 35 (63.6) |
| 12–15, n (%) | 15 (27.3) |
| Operation time, min, median (IQR) | 200 (170–230) |
| Estimated blood loss, mL, median (IQR) | 200 (110–505) |
| Blood transfusion, n (%) | 3 (5.5) |
| Cold ischemic time, min, median (IQR) | 40 (33–54) |
BMI = body mass index; eGFR = estimated glomerular filtration rate; IQR = inter quartile range; NS = nephrometry score; SD = standard deviation.
Table 2.
Indications for open partial nephrectomy and nephrometry scores.
| Imperative indication casesn = 15 | Elective indication casesn = 40 | |
|---|---|---|
| Indications, n (%) | ||
| Bilateral tumors | 8 (53.3) | |
| Solitary kidney | 6 (40.0) | |
| Chronic kidney disease | 1 (6.7) | |
| History of abdominal poly-surgery | 7 (17.5) | |
| Simultaneous abdominal surgery | 4 (10.0) | |
| Tumor characteristics | 29 (72.5) | |
| T3a | 2 (5.0) | |
| Multiple tumors | 1 (2.5) | |
| Complexitya | 26 (65) | |
| NS, median | 8 | 8 |
| 4–6, n (%) | 5 (33.3) | 5 (12.5) |
| 7–9, n (%) | 10 (66.7) | 29 (72.5) |
| 10–12, n (%) | 0 (0) | 6 (15.0) |
| Modified NS, median | 10 | 11 |
| 5–7, n (%) | 3 (20.0) | 2 (5.0) |
| 8–11, n (%) | 10 (66.7) | 25 (62.5) |
| 12–15, n (%) | 2 (13.3) | 13 (32.5) |
Complexity = completely buried, large tumor size and/or renal hilar tumors; NS = nephrometry score.
Complications
Perioperative complications defined by Clavien-Dindo classification were observed in 18 cases (32%). Of these patients, grade IIIa or higher complications were observed in 6 cases (11%), which had NS scores ranging from 7 to 10 and modified NS scores ranging from 10 to 13. Acute kidney injury (AKI) was observed in 2 cases (4%), 1 (2%) undergoing temporary hemodialysis and 1 (2%) requiring permanent hemodialysis. Urinoma and renal abscess were each observed in 2 cases (4%), requiring placement of an indwelling drainage catheter. One case (2%) of ureteral injury that necessitated end-to-end anastomosis and 1 case (2%) of postoperative pseudo-aneurysm that required transcatheter arterial embolization occurred. Only 1 case (2%) had a positive surgical margin, but the patient had no recurrence of disease during the follow-up period. A total of 3 cases (6%) required transfusion during the operation. Grade II Clavien-Dindo classification complications were observed in 6 patients (11%), including 1 case (2%) each of urinary tract infection, AKI, increasing pleural effusion, pneumonia, renal infarction, and drug-induced acute liver injury. Grade I Clavien-Dindo classification complications were observed in 6 patients (11%), including 2 cases (4%) each of ileus and AKI, and 1 case (2%) each of drug-induced acute liver injury and macrohematuria. There were no cases of conversion to radical nephrectomy.
Pathological findings
Clear cell RCC was diagnosed in 44 cases (80%), type 1 papillary RCC in 2 cases (4%), type 2 papillary RCC in 2 cases (4%), chromophobe RCC in 2 cases (4%), oncocytoma in 4 cases (7%), and other histological types in 2 cases (4%). Pathologic T stage pT1a was observed in 37 cases (67%), T1b in 11 cases (20%), and T3a in 2 cases (4%).
Changes of renal function
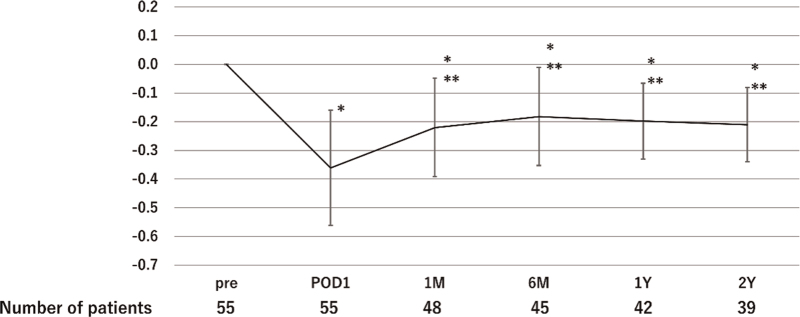
The rate of postoperative decline in eGFR compared with preoperative data at 1 month, 1 year, and 2 years was 22%, 20%, and 21%, respectively (Fig. 1). The rate of postoperative decline in eGFR was lowest at POD 1, and then gradually improved during the follow-up period. Because postoperative changes in eGFR reached a plateau, we evaluated factors associated with a decline of more than 20% in eGFR at 1 year after surgery compared with preoperative eGFR (Table 3). Using logistic regression analysis, male gender (odds ratio [OR] 11.8, 95% confidence interval [CI] 1.2–115.0, p = 0.03), NS ≥9 (OR 13.9, 95% CI 1.6–118.0, p = 0.02), modified NS ≥11 (OR 13.5, 95% CI 1.7–107.0, p = 0.01), and cold ischemic time ≥40 minutes (OR 7.9, 95% CI 1.0–60.8, p = 0.04) were found to be independent risk factors for a decline of more than 20% in eGFR at 1 year after surgery. The rate of postoperative decline in eGFR at 1 year in cases with no risk factors was 15%, whereas it was 35% in cases with 3 risk factors.
Figure 1.
The rate of postoperative decline in eGFR. eGFR = estimated glomerular filtration rate; M = months; Pre = preoperative; POD = postoperative day; Y = years. ∗p < 0.01, compared with eGFR at Pre; ∗∗p < 0.01, compared with eGFR at POD1.
Table 3.
Risk factors for decline in eGFR of more than 20% at 1 year after surgery.
| Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Model 1∗ | Model 2† | |||||||
|
|
|
|||||||
| Characteristics | Category | Univariate analysis (p) | OR | 95% CI | p | OR | 95% CI | p |
| Age, yr | ≥70 (Ref: <70) | 0.23 | 3.08 | 0.31–30.30 | 0.34 | 3.13 | 0.29–33.40 | 0.35 |
| Gender | Male (Ref: Female) | 0.11 | 11.80 | 1.20–115.0 | 0.03 | 11.60 | 1.15–117.0 | 0.04 |
| BMI, kg/m2 | ≥23 (Ref: <23) | 0.77 | 0.27 | 0.04–1.93 | 0.19 | 0.21 | 0.03–1.56 | 0.13 |
| Comorbiditya | Present (Ref: absent) | 0.43 | 5.79 | 0.47–70.80 | 0.17 | 3.05 | 0.32–29.00 | 0.33 |
| Baseline eGFR, mL/min/1.73 m2 | <60 (Ref: ≥60) | 0.85 | 0.45 | 0.04–5.04 | 0.52 | 0.37 | 0.04–3.64 | 0.40 |
| Solitary kidney | Present (Ref: absent) | 0.61 | 14.50 | 0.23–913.0 | 0.21 | 8.74 | 0.19–403.0 | 0.27 |
| NS | ≥9 (Ref: <9) | 0.04 | 13.90 | 1.64–118.0 | 0.02 | |||
| mNS | ≥11 (Ref: <11) | 0.01 | 13.50 | 1.70–107.0 | 0.01 | |||
| Cold ischemic time, min | ≥40 (Ref: <40) | 0.09 | 7.88 | 1.02–60.80 | 0.04 | 7.95 | 0.93–67.80 | 0.06 |
| Estimated blood loss, mL | ≥200 (Ref: <200) | 0.77 | 0.68 | 0.11–4.37 | 0.68 | 0.91 | 0.15–5.39 | 0.91 |
| Operation time, min | ≥200 (Ref: <200) | 0.36 | 1.31 | 0.16–10.90 | 0.80 | 0.57 | 0.08–4.05 | 0.58 |
CI = confidence interval; eGFR = estimated glomerular filtration rate; NS = nephrometry score; OR = odds ratio; Ref = reference.
Model 1 included NS.
Model 2 included modified NS.
Diabetes mellitus, hypertension, cardiovascular disease, systemic disease.
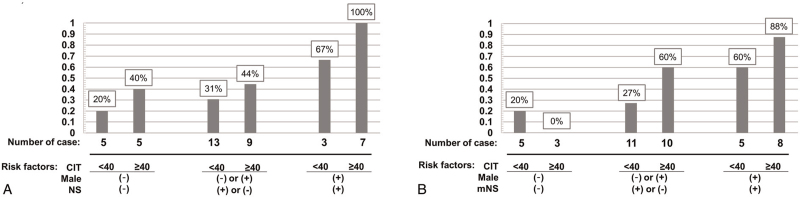
We also analyzed the proportion of cases with a decline of more than 20% in eGFR at 1 year after surgery according to their risk factors (Fig. 2A and B).
Figure 2.
Proportion of cases with eGFR decline of more than 20% at 1 year after surgery according to risk factors. (A) NS model, (B) modified NS model. Risk factors: cold ischemic time, male gender, NS of ≥9, or modified NS of ≥11. eGFR = estimated glomerular filtration rate; CIT = cold ischemic time; min = minutes; mNS = modified nephrometry score; NS = nephrometry score.
Based on these results, impaired postoperative renal function was expected in male patients with a NS ≥9 or modified NS ≥11 and a cold ischemic time of >40 minutes.
Survival outcomes
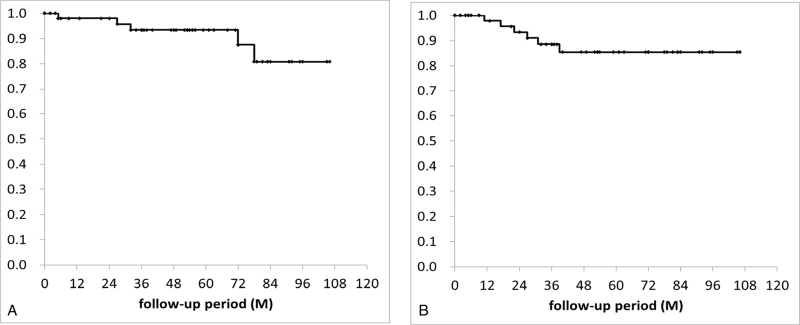
During a median follow-up period of 52 months, disease recurrence was observed in 6 cases (12%), including 3 (6%) in a local site, 2 (4%) in the contralateral kidney and 1 (2%) with lung and bone metastasis. Five patients (10%) died from other causes, including 4 (8%) due to other cancer and 1 (2%) due to acute respiratory distress syndrome. No patients died of RCC during study follow-up. The 5-year overall survival and recurrence-free survival rates were 93% and 84%, respectively (Fig. 3A and B).
Figure 3.
Kaplan–Meier probability curves for (A) overall survival and (B) recurrence-free survival. M = months.
Discussion
In the present study, we described the characteristics and outcomes of patients who underwent OPN during the minimally invasive approach era. Tumors in cases with elective indications for PN were more likely to have high complexity based on NS. Our results showed that OPN was acceptable in regard to oncological outcomes and complications in this era. In addition, we found that male gender, NS ≥9 or modified NS ≥11, and cold ischemic time of ≥40 minutes were significantly associated with worsening eGFR at 1 year after surgery. Although surgeons need to make efforts to shorten ischemic time, cold ischemia in OPN clearly appears to have an advantage in terms of preserving maximum renal function. These findings suggest that OPN in the minimally invasive era is beneficial for patients with adverse tumor complexity.
Clinical cases being considered for OPN may have shifted to a more challenging case mix, with minimally invasive approaches being increasingly employed for elective T1a and straightforward T1b lesions. Several studies have reported rates of cases with imperative indications for OPN ranging from 23% to 54%.[2,6–9] Likewise, the present study demonstrated an imperative indication such as a solitary kidney, bilateral tumor or impaired renal function in 27% of cases, whereas an elective indication was present in 73%, almost of these being due to tumor characteristics. These cases with elective indications were more likely to be categorized as high complexity according to NS. Thus, surgeons need to recognize that indications for OPN has expanded to include large and complex tumors.
In the present study, OPN was shown to be an effective and safe procedure based on good oncological outcomes and low complication rates. The 5-year OS and PFS rates were 93% and 84%, respectively, which was comparable with a previous report.[6] Several studies have reported complication rates after OPN, defined as a Clavien-Dindo classification of grade III or higher, ranging from 10% to 16%.[6,7] Similarly, the observed complication rate was 11% in the present study, which was associated with a high NS. Hence, notwithstanding the need to emphasize the utility of OPN, it is necessary to be especially cautious about complications in elective cases with increasingly adverse tumor complexity.
The role of OPN has evolved to permit treatment of increasingly complex tumors, enabling surgeons to push the boundaries of nephron-sparing surgery.[10] Cooling the kidney surface with slushed ice during hilar clamping is a traditional and important technique during OPN to preserve renal function.[11] This offers a great advantage in allowing extended ischemic time compared with minimally invasive approaches such as laparoscopic surgery and robot-assisted laparoscopic surgery with warm renal ischemia.[12] Recently, several studies showed that achievement of a “trifecta”, consisting of a warm ischemic time of <25 minutes, a negative surgical margin and no perioperative complications, should be a routine goal of minimally invasive surgery.[13] With regards to OPN surgery, Salah et al. reported good renal function after OPN using brief warm ischemia or zero-ischemia.[14] In contrast, cold renal ischemia in OPN was considered to be acceptable for up to 2 hours. Nevertheless, it is preferable to keep cold ischemia time as short as possible.[15] We demonstrated that a cold ischemic time of <40 minutes was one of the significant factors to predict renal function at 1 year after surgery. The duration of cold ischemic time during OPN permits surgeons to resect and suture carefully, which may lead to improved surgical margins and reductions in complications. We propose that OPN should be the preferred approach in cases where a minimally invasive approach may prove technically difficult to preserve maximum renal function.
The NS, which has been shown to be associated with the risk of complications, warm ischemic time, operative time, hospital stay, and estimated blood loss in cases involving laparoscopic or robot-assisted laparoscopic surgery, is also very useful in OPN because it can represent tumor complexity.[16,17] However, some urologists regard the original NS as not adequately reflecting the complexity of PN. Salah et al. showed the relationship between the original NS and a modified NS for OPN.[14] They described the newly modified NS was better at predicting outcomes in OPN patients. In our series, we used both the original NS and modified NS, and showed risk factors for impaired renal function after OPN using both nephrometry models. We clarified that gender, NS, and cold ischemic time were significantly associated with worsening eGFR at 1 year after surgery. The rate of decline in eGFR at 1 year after surgery was 15% in cases with no risk factors, whereas it was 35% in cases with 3 risk factors. When a cold ischemic time of ≥40 minutes was combined with other variables including male gender, an adverse NS, or both, postoperative renal function was expected to be impaired. Because cold ischemic time is the only modifiable factor that can be improved by surgeons, we believe that efforts should be made to shorten cold ischemic time without a decrease in surgical quality even for complex tumors.
There were several limitations to our study, including the retrospective study design, lack of a comparison group, short follow-up period, and small number of patients. In addition, we did not perform renal isotope studies. Moreover, the most significant limitation was selection bias regarding surgical approach. When PN was indicated, the surgeon's preference affected the decision to adopt a laparoscopic, robot-assisted, or open approach. Therefore, tumor complexity in the present study may not be generalizable. In addition, longer cold ischemic time was related to postoperative renal impairment in a previous study. However, prolonged ischemic time might simply reflect the difficulty of surgery and an associated greater loss of nephron mass. Thus, further study is needed to clarify the benefits of OPN in the setting of complex tumors.
Conclusions
We showed that OPN is acceptable with regard to oncological outcomes and complications in the minimally invasive surgery era. We propose that OPN should be the preferred approach in cases in which it is technically difficult to preserve maximum renal function via a minimally invasive approach. Cases with elective indications were more likely to have an adverse tumor complexity based on NS. Males with an NS ≥9 or a modified NS ≥11 and cold ischemic time of ≥40 minutes had worse renal function after surgery than patients without these risk factors. We should therefore make efforts to shorten ischemic time while maintaining surgical quality, because cold ischemic time is the only one of these factors that can be improved by surgeons. However, adequate cold ischemic time during OPN may permit surgeons to resect and suture carefully. These findings suggest that OPN is a valuable approach when a longer ischemic time and challenging reconstruction of the kidney are expected because of tumor complexity.
Acknowledgments
The authors expressed their appreciation to Shiro Hinotsu for his support in data analysis.
Statement of ethics
This study was approved by our institutional review board (No. 312-40). All participants provided opt-out or written informed consent for their participation and publication of this study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interest statement
The authors report no conflicts of interest.
Funding source
None.
Author contributions
Masumori N, Tanaka T, Kobayashi K, Kyoda Y: Study conception and design;
Shibamori K, Hashimoto K: Data acquisition, literature research, manuscript writing, manuscript editing, and manuscript revision;
Shibamori K, Hashimoto K, Shindo T, Tabata H: Data analysis and interpretation.
References
- [1].Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15(6):804–834. [DOI] [PubMed] [Google Scholar]
- [2].Gill IS, Matin SF, Desai MM, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol 2003;170(1):64–68. [DOI] [PubMed] [Google Scholar]
- [3].Masumori N, Ichihara K, Maehana T. Modified nephrometry score with body mass index more accurately predicts ischemic time in transabdominal laparoscopic partial nephrectomy for small renal masses. Urology 2018;122:104–109. [DOI] [PubMed] [Google Scholar]
- [4].Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182(3):844–853. [DOI] [PubMed] [Google Scholar]
- [5].Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53(6):982–992. [DOI] [PubMed] [Google Scholar]
- [6].Ghoneim TP, Sjoberg DD, Lowrance W, et al. Partial nephrectomy for renal tumors in solitary kidneys: Postoperative renal function dynamics. World J Urol 2015;33(12):2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ebbing J, Menzel F, Frumemto P, et al. Outcome of kidney function after ischaemic and zero-ischaemic laparoscopic and open nephron-sparing surgery for renal cell cancer. BMC Nephrol 2019;20(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Campbell SC, Novick AC, Streem SB, Klein E, Licht M. Complications of nephron sparing surgery for renal tumors. J Urol 1994;151(5):1177–1180. [DOI] [PubMed] [Google Scholar]
- [9].Heinze A, Larcher A, Umari P, et al. Assessing perioperative, functional and oncological outcomes of patients with imperative versus elective indications for robot-assisted partial nephrectomy: Results from a high-volume center. Int J Urol 2018;25(9):826–831. [DOI] [PubMed] [Google Scholar]
- [10].Anastasiadis E, O’Brien T, Fernando A. Open partial nephrectomy in renal cell cancer: Essential or obsolete? Int J Surg 2016;36(Pt C):541–547. [DOI] [PubMed] [Google Scholar]
- [11].Novick AC. Campbell MF, Wein AJ, Kavoussi LR. Open surgery of the kidney. Campbell-Walsh Urology 9th edPhiladelphia: WB Saunders; 2007;1686–1758. [Google Scholar]
- [12].Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178(1):41–46. [DOI] [PubMed] [Google Scholar]
- [13].Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol 2013;189(1):36–42. [DOI] [PubMed] [Google Scholar]
- [14].Salah M, ElSheemy MS, Ghoneima W, et al. Modified R.E.N.A.L nephrometry score for predicting the outcome following partial nephrectomy. Afr J Urol 2020;26:45. [Google Scholar]
- [15].Becker F, Van Poppel H, Hakenberg OW, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 2009;56(4):625–634. [DOI] [PubMed] [Google Scholar]
- [16].Hayn MH, Schwaab T, Underwood W, Kim HL. RENAL nephrometry score predicts surgical outcomes of laparoscopic partial nephrectomy. BJU Int 2011;108:876–881. [DOI] [PubMed] [Google Scholar]
- [17].Watts KL, Ghosh P, Stein S, Ghavamian R. Value of nephrometry score constituents on perioperative outcomes and split renal function in patients undergoing minimally invasive partial nephrectomy. Urology 2017;99:112–117. [DOI] [PubMed] [Google Scholar]