Abstract
This study is to investigate the effect of high serum uric acid (UA) level on oxidative stress and semen quality of male infertility patients.
A cohort of 654 male individuals aged between 20 and 45 years old were included in this study, and their semen and venous blood samples were collected. The serum UA, blood glucose, blood lipids, and hormone levels were determined by chemiluminescence method. The changes in inflammatory factors, oxidative stress, adipokines, and biochemical indices in seminal plasma were determined by ELISA. Organic acids in seminal plasma were detected with reversed-phase ultra high performance liquid chromatography.
Compared with the control group, the amount of semen and the total number of sperm in the hyperuricemia group significantly reduced (P < .05). Semen volume decreased with the increase of serum UA level, and the total number of sperm also decreased. The level of luteinizing hormone increased and the level of testosterone decreased in the hyperuricemia group. The concentration of superoxide dismutase decreased and the concentration of endothelin increased in the hyperuricemia group (P < .05). The concentration of seminal plasma α-glucosidase and alkaline phosphatase in the hyperuricemia group decreased significantly (P < .05). Compared with the control group, the contents of ascorbic acid, tartaric acid, lactic acid, and UA in the seminal plasma were significantly reduced in the hyperuricemia group (P < .05).
Blood UA level may become a new risk predictor of semen quality in infertile men.
Keywords: inflammatory factors, organic acids, oxidative stress, semen quality, uric acid
1. Introduction
Uric acid (UA) is the final product of purine metabolism, and hyperuricemia is defined as a condition that in normal purine diet, the serum uric acid level is higher than 420 μmol/L in men and higher than 360 μmol/L in women.[1] About two-thirds of UA load is derived from internal sources (liver, muscle, intestine) and one-third from dietary sources. Kidneys are responsible for most of the daily UA excretion (65%–75%), with the remaining (25%–35%) being excreted through the gastrointestinal tract.[2–4] With continuous improvement of people's living conditions, great changes have taken place in their lifestyle and diet structure, and the prevalence of hyperuricemia is also increasing year by year. The most well-known disease induced by hyperuricemia is gout. However, many studies have reported that hyperuricemia also plays important roles in coronary atherosclerotic heart disease, hypertension, type II diabetes, obesity, and metabolic syndrome.[5–10] In addition, high blood UA levels are closely related to obesity and dyslipidemia.[11] In recent years, the prevalence of infertility is increasing, and the semen quality of men is declining. Studies have found that the decline of semen quality is related to a variety of metabolic diseases.[12–15] Therefore, in this study, we aimed to explore the relationship between hyperuricemia and semen quality and its possible mechanism, so as to provide reference for clinical diagnosis and treatment.
2. Materials and methods
2.1. Ethics approval
Written informed consent was obtained from every patient, and the study was approved by the ethics review board of Hebei Reproductive Medicine Center.
2.2. Patients
The study population consisted of 654 males (aged 20–45 years old) from 3 independent reproductive centers in China, including the Cangzhou Hospital of Integrated Traditional Chinese Medicine and Western Medicine of Hebei Province, the Hengshui Health School Affiliated Hospital, and the Second People's Hospital of Hengshui. All study subjects were Han, from Hebei Province, China. The exclusion criteria were as follows: male patients with mumps history, family history of hereditary disease, history of sexual dysfunction, external genital abnormalities, moderate or severe varicocele and azoospermia, or long-term medication history (such as diet pills and pain relief medicine) were excluded. Patients who lived or worked in a polluted environment (such as high temperatures or exposure to toxic substances) were also excluded. Hyperuricemia was diagnosed according to the international diagnostic criteria, with UA level in male ≥420 μmol/L and in female ≥360 μmol/L. According to the level of UA in the blood, the patients were divided into control group (UA level 330.09 ± 62.54 μmol/L; n = 391) and hyperuricemia group (UA level 483.35 ± 42.411 μmol/L; n = 263).
2.3. Blood biochemical analysis and blood pressure measurement
Serum was separated from the venous blood samples. Serum UA level was determined by chemical colorimetry, using the UA test kit supplied by Medical System Biotechnology Co., Ltd. (Ningbo, China). The blood glucose, blood total cholesterol, TGs, low-density lipoprotein, and high-density lipoprotein were detected by the chemiluminescencemicroparticle immunoassay on a Siemens CENTAUR XP electrochemimeter, using the test kits supplied by Medical System Biotechnology Co., Ltd. (Ningbo, China), and the follicle stimulating hormone, luteinizing hormone, estradiol, prolactin, and testosterone were measured using the kits provided by Siemens Healthcare Diagnostics Inc. (Shanghai, China).
The blood pressures of patients were all measured using a calibrated OMRON HEM-7012 (Omron Healthcare (China) Co., Ltd.) electronic sphygmomanometer. Before the measurement, the patients should empty the bladder and rest for more than 10 minutes. The patients were required not to drink alcohol, tea, coffee, etc within 1 hour, not wear tight clothes, and not take drugs that may affect blood pressure. The blood pressure values were measured 3 times (the difference between 2 measurements <4 mm Hg), and the average of the last 2 measurements was taken for analysis.
2.4. Analysis of semen samples
According to the 2010 World Health Organization Human Semen Examination and Handling Laboratory Manual (5th edition), semen samples were obtained by masturbation after sexual abstinence for 48 to 120 hours (2–5 days). The semen volume (SV) was measured by weighing, and then the semen parameters were routinely analyzed. The semen parameters in the current study included sperm concentration (SC), total sperm count (TSC), and percentage of proactive sperm (PR%). The seminal plasma α-glucosidase (α-GLU), alkaline phosphatase (AKP), and endothelin (ET) were detected by ELISA using the kit provided by Elisa Biotech Co., Ltd. (Shanghai, China). The seminal fructose was detected by the hexokinase method and the Zn concentration was detected by 5-BR-PAPS colorimetry. The levels of nitric oxide (NO), superoxide dismutase (SOD), and malondialdehyde (MDA) were determined by ELISA using the kits provided by Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). The levels of interleukin-1, interleukin-10 and tumor necrosis factor-α in seminal plasma were measured using ELISA kits provided by Hangzhou Lianke Biotechnology Co., Ltd. (Hangzhou, China).
2.5. Ultra-high performance liquid chromatograph
Semen sample (100 μL) was vortex mixed with 300 μL of 0.7 mol/L metaphosphoric acid solution and incubated at –4°C for 10 minutes. Then, after centrifugation at 10,000 r/min for 15 minutes, the supernatant was collected and filtered through a 0.2 μm nylon filter membrane. The ultra-high performance liquid chromatograph was performed on ACQUITY UPLC (Waters) with the ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm). The chromatograph parameters were: column temperature of 25°C; the mobile phase of 0.01 mol/L KH2PO4 (pH 2.3); the flow rate of 0.05 mL/min; the injection volume of 1 μL; and the wavelength of 215 nm. The Empower 3 chromatography software (Waters Corporation, USA) was used to calculate the content of organic acids, and the concentration of organic acids in the semen sample was determined according to the standard curve.
2.6. Statistical analysis
SPSS 21.0 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis. The Mann–Whitney U test was used to determine whether the data were normal distribution. Normal distribution measurement data were expressed as mean ± standard deviation. Single factor analysis of variance was used to test the difference between multiple groups. Two independent sample t test was used for the significant test on the difference between 2 groups. A P value less than .05 was considered statistically significant.
3. Results
3.1. General information of the patients
The clinical data of patients were analyzed. The body mass index, height, weight, fasting blood glucose, total cholesterol, TG, high-density lipoprotein, and low-density lipoprotein were significantly different between the control group and the hyperuricemia group (P < .05). There was no significant difference in age, abstinence time, systolic blood pressure, and diastolic blood pressure between these 2 groups (P > .05) (Table 1).
Table 1.
General information of the patients in this study.
| Age (years old) | Abstinence time (days) | BMI | Height (cm) | Weight (kg) | FBG (mmol/L) | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | TC (mmol/L) | TG (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | |
| Control (n = 391) | 29.3 ± 4.9 | 4.2 ± 1.9 | 25.38 ± 4.04 | 173.4 ± 5.1 | 76.34 ± 79.70 | 5.28 ± 0.73 | 120.5 ± 9.3 | 79.2 ± 7.0 | 4.58 ± 0.91 | 1.53 ± 1.20 | 1.31 ± 0.31 | 2.72 ± 0.76 |
| Hyperuricemia (n = 263) | 28.9 ± 4.3 | 4.2 ± 1.6 | 26.25 ± 4.37 | 173.6 ± 5.4 | 79.70 ± 16.14 | 5.56 ± 1.32 | 119.5 ± 9.2 | 79.3 ± 7.4 | 4.73 ± 0.94 | 1.84 ± 1.49 | 1.32 ± 0.34 | 2.86 ± 0.86 |
| P value | .285 | .912 | .009∗ | .550∗ | .004∗ | .001∗ | .187 | .858 | .041∗ | .004∗ | .761 | .029∗ |
3.2. Comparison of semen quality between the hyperuricemia and control groups
To determine the semen quality of hyperuricemia group, the SV and TSC were measured. Compared with the control group, the SV and TSC in the hyperuricemia group were significantly lower (P < .05), while the SC and PR were slightly decreased, but had no statistical difference (P > .05, Table 2). This indicates that the quality of semen decreased in the hyperuricemia group.
Table 2.
Comparison of semen quality.
| SV (mL) | SC (106/mL) | TSC (106) | PR (%) | |
| Control | 3.11 ± 1.27 | 74.30 ± 43.22 | 222.13 ± 153.61 | 41.73 ± 15.00 |
| Hyperuricemia | 2.88 ± 1.32∗ | 70.13 ± 44.42 | 192.85 ± 139.74∗ | 40.32 ± 15.06 |
| P value | .023 | .231 | .013 | .240 |
3.3. Comparison of semen quality at different UA levels
To compare the semen quality at different UA levels, quartile grouping was performed according to the serum UA level. With the increase of serum UA level, there was no significant difference in the SC and PR (P > .05), while the SV and TSC significantly decreased (P < .05, Table 3). This indicates that the semen quality is affected by the serum UA in a concentration dependent manner.
Table 3.
Impact of different serum UA levels on semen quality.
| ≤325 μmol/L (n = 163) | 325 μmol/L–392 μmol/L (n = 163) | 392 μmol/L–463 μmol/L (n = 164) | ≥463 μmol/L (n = 164) | P | |
| SV (mL) | 3.28 ± 1.33∗ | 3.03 ± 1.19∗ | 3.04 ± 1.26∗ | 2.73 ± 1.34 | .002 |
| SC (106/mL) | 73.48 ± 47.47 | 76.37 ± 40.36 | 72.32 ± 42.66 | 68.32 ± 44.12 | .417 |
| TSC (106) | 226.92 ± 174.01∗ | 219.69 ± 126.53∗ | 215.88 ± 146.91∗ | 178.86 ± 140.30 | .017 |
| PR (%) | 42.46 ± 16.25 | 41.68 ± 13.36 | 39.66 ± 15.38 | 40.71 ± 14.92 | .314 |
3.4. Comparison of serum reproductive hormone levels between the hyperuricemia and control groups
To determine the serum reproductive hormone levels, ELISA was performed. Compared with the control group, the luteinizing hormone and testosterone levels decreased in the hyperuricemia group (P < .05), while there was no significant difference in follicle stimulating hormone, prolactin, and E (P > .05, Table 4). This indicates that the hormone levels decreased in the hyperuricemia group.
Table 4.
Reproductive hormone levels in the study population.
| FSH (mIU/mL) | LH (mIU/mL) | PRL (ng/mL) | E2 (pg/mL) | T (ng/mL) | |
| Control | 4.84 ± 2.54 | 3.74 ± 1.83 | 10.44 ± 5.27 | 37.99 ± 13.49 | 4.54 ± 1.89 |
| Hyperuricemia | 4.53 ± 2.66 | 4.26 ± 2.22∗ | 11.21 ± 5.12 | 37.85 ± 15.74 | 4.05 ± 1.62∗ |
| P | .132 | .001 | .065 | .907 | .001 |
3.5. Comparison of the oxidative stress level and endothelial function between the hyperuricemia and control groups
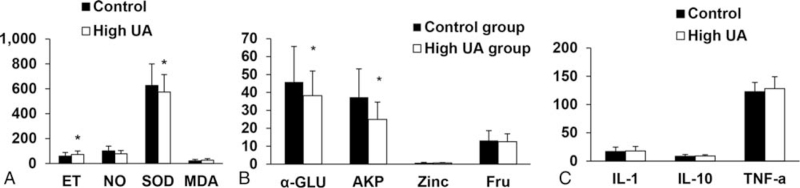
To observe the oxidative stress level and endothelial function in the hyperuricemia, ELISA was performed to detect the levels of SOD, ET, MDA, and NO. Compared with the control group, the concentration of SOD in the seminal plasma of the hyperuricemia group decreased, and the concentration of ET increased significantly (P < .05). There was no significant difference between the 2 groups in the concentrations of MDA and NO in the seminal plasma (P > .05, Fig. 1A). This indicates that the oxidative stress level and endothelial function have changed in the hyperuricemia group.
Figure 1.
Analysis of indicators. (A) Concentrations of SOD, MDA, ET, and NO in the seminal plasma. (B) Concentrations of Fru, Zinc, α-GLU, and AKP in the seminal plasma in the study population. (C) Concentrations of IL-1, IL-10, and TNF-α in the seminal plasma. α-GLU = α-glucosidase, AKP = alkaline phosphatase, ET = endothelin, Fru = fructose, IL-1 = interleukin-1, IL-10 = interleukin-10, MDA = malondialdehyde, NO = nitric oxide, SOD = superoxide dismutase, TNF-α = tumor necrosis factor-α, UA = uric acid. ∗P < .05, compared with control group. The value was expressed in terms of mean ± SD.
3.6. Comparison of the seminal plasma biochemical index between the hyperuricemia and control groups
The seminal plasma biochemical indices of α-GLU and AKP were measured by ELISA. Compared with the control group, the concentrations of α-GLU and AKP in the seminal plasma of the hyperuricemia group decreased significantly (P < .05). There was no significant difference between the 2 groups in the concentrations of fructose and zinc in the seminal plasma (P > .05, Fig. 1B). This indicates that the concentrations of α-GLU and AKP in the seminal plasma decreased in the hyperuricemia group.
3.7. Comparison of the inflammatory factors between the hyperuricemia and control groups
To determine the levels of inflammatory factors in the hyperuricemia, ELISA was performed. There were no significant differences in the concentrations of seminal plasma inflammatory factors (interleukin-1, interleukin-10, and tumor necrosis factor-α) between the 2 groups (P > .05, Fig. 1C).
3.8. Determination of 6 kinds of organic acids in seminal plasma
The content of organic acids in seminal plasma of 3 groups of samples was determined with ultra-high performance liquid chromatograph. The results showed that compared with the control group, the contents of ascorbic acid, tartaric acid, lactic acid, and UA in the seminal plasma were significantly reduced in the hyperuricemia group (P < .05, Table 5).
Table 5.
The content of 6 organic acids in human seminal plasma in control group and hyperuricemia group.
| Composition (n = 30) | Control (mg/mL) (n = 30) | Hyperuricemia (mg/mL) (n = 30) |
| Citric acid | 2.63 ± 0.90 | 2.33 ± 0.6 |
| Ascorbic acid | 0.17 ± 0.05 | 0.081 ± 0.019∗ |
| Succinic acid | 7.84 ± 3.83 | 7.21 ± 2.01 |
| Tartaric acid | 1.34 ± 0.54 | 0.55 ± 0.17∗ |
| Lactic acid | 1.72 ± 0.60 | 1.15 ± 0.25∗ |
| Uric acid | 0.0397 ± 0.0118 | 0.0298 ± 0.0079∗ |
4. Discussion
Usually, due to excessive intake of purine in the diet, insufficient excretion of kidney, hereditary metabolic disorders etc, excessive production of UA or reduced excretion will occur, leading to hyperuricemia. The present study showed that hyperuricemia was significantly associated with cardiovascular disease, metabolic syndrome, hypertension, diabetes, obesity, and dyslipidemia.[5–10] Nejatinamini et al[7] showed that serum UA had independent association with MetS components, and increased the risk of MetS by about 2 folds. Moreover, hyperuricemia is an independent risk factor for hypertension.[8] A meta-analysis[9] of 11 combined cohort studies found a significant relationship between elevated serum UA level and risk of developing type 2 diabetes, indicating a 17% increment in the risk of diabetes per 1 mg/dL increase in serum UA level. In this study, we found that the body weight, body mass index, fasting blood glucose, TC, TG, and low-density lipoprotein-C levels in the hyperuricemia group were higher than those in the control group, which was consistent with the above findings.
Similar to hyperuricemia, studies demonstrated a correlation between a decrease in the fertility rates and the increase in the incidence of metabolic disturbance, such as metabolic syndrome, hypertension, diabetes, obesity, and dyslipidemia.[11–14,16] However, whether hyperuricemia, as a metabolic disease, has an impact on the SV and sperm quality has not been reported yet. Guz et al[17] studied the difference between serum UA and semen UA levels between normal spermatozoa patients and abnormal sperm parameters patients (such as oligospermia, asthenospermia, oligospermia, and cryptozoospermia), but no significant difference was found between them. In this study, we compared the male patients with and without hyperuricemia, and found lower SV and TSC in men with hyperuricemia.
Many studies have shown that hyperuricemia-induced oxidative stress affects multiple organs and systems.[18–20] However, the change of oxidative stress level in seminal plasma caused by hyperuric acid has not been reported. Oxidative stress in male germ line is thought to affect male fertility and normal embryonic development.[21] The degradation products of lipid peroxidation, such as MDA, also affect sperm motility.[22] MDA accumulation can damage mitochondrial respiratory chain enzymes to varying degrees, resulting in mitochondrial damage. Therefore, oxidative stress causes rapid loss of ATP in sperm, damages sperm axons, induces sperm morphological defects, and decreases sperm motility. SOD is the main antioxidant enzyme in sperm, and its distribution and content may be closely related to semen quality.[23,24] In this study, the concentrations of SOD and MDA in seminal plasma were measured. The results showed that the concentration of SOD in the seminal plasma of the hyperuricemia group decreased significantly, indicating that there is oxidative stress in hyperuricemia. Therefore, the change of semen quality caused by hyperuricemia may be related to excessive oxidative stress. The detection of SOD activity is helpful for analyzing the reasons for the decline of semen quality and clinical treatment. In addition, ET plays a very important regulatory role in sperm maturation and penile erection. NO participates in the pathophysiological processes of various diseases, and has an important influence on spermatogenesis, sperm motility, fertilization ability, and the peroxidation reaction of sperm lipids. NO can inactivate superoxide anion to protect sperm membrane from damage by lipid peroxide, increase cGMP content in sperm cells, and facilitate sperm activation and capacitation.[25,26] NO can also inhibit the secretion of ET. This study found that the ET concentration in the hyperuricemia group was significantly reduced, NO decreased slightly, and ET/NO balance was destroyed, resulting in endothelial dysfunction, affecting the generation and capacitation of sperm and leading to decreased semen quality.
About 95% of semen is seminal plasma.[27] Seminal plasma, like serum, has complex components. Changes in the seminal plasma may affect the quality of semen.[28] Seminal fructose is a marker of seminal vesicle function, which is converted from blood glucose in seminal vesicle. Fructose is the main energy source for sperm motility, and the decrease of fructose content can lead to the decrease of sperm motility. Zinc in seminal plasma mainly comes from prostatic fluid, which reflects the function of prostate, and is mainly used for the in vitro diagnosis of prostatitis and male infertility.[29] In this study, we measured the levels of fructose and zinc in seminal plasma of patients with hyperuricemia and compared them with the control group. The results showed no significant difference between the 2 groups. The secretory function of epididymis and other accessory gonads also plays an important role in sperm quality.[30] The α-GLU secreted by epididymal epithelium can catalyze the decomposition of carbohydrates in polysaccharides or glycoproteins into glucose, which was the energy source for sperm motility. The α-GLU plays a critical role in energy supply during the whole process of sperm maturation, capacitation and fertilization, and also exerts important function in regulating the activity of glycosyl reaction. In this study, we found that the concentration of α-GLU in the hyperuricemia group decreased, suggesting that hyperuricemia may affect the quality of semen by affecting the secretory function of epididymis. AKP levels in seminal plasma were significantly correlated with SV and SC in boars[31] and stallions.[32] In bull,[33] after freezing and thawing semen, AKP level in seminal plasma increased significantly, sperm motility decreased and fertilization rate decreased, suggesting that AKP in seminal plasma may originate from the secretion of reproductive epithelial cells and the release of damaged sperm. However, there were few studies on the detection of AKP in human seminal plasma. The origin of AKP in seminal plasma and its role in male reproduction need to be further studied.
UA is one of the main antioxidants in seminal plasma, most of which is secreted by seminal vesicle gland epithelial cells. It can directly exert antioxidant function via combining with Fe2+ and Cu2+, and it can also directly remove singlet oxygen and hydroxyl free radicals. In the seminal system, the seminal plasma UA level of the normal group was significantly higher than that of the hyperuricemia group, that is, a higher level of UA in seminal plasma is beneficial to male reproductive health, which is opposite to the result of blood UA. At the same time, the correlation analysis between seminal plasma UA and blood UA was carried out, and the results showed that the two were not related. Therefore, we speculate that blood UA cannot penetrate the blood testis barrier, and patients with hyperuricemia affect the male reproductive system through oxidative stress damage. Seminal plasma citric acid content is related to testosterone levels, and it is the main indicator for evaluating androgen secretion and prostate function. Vitamin C has antioxidant effect, can reduce sperm aggregation, and prevent lipid peroxidation. It is shown that supplementation of vitamin C in idiopathic oligospermia can increase SC, sperm motility, and the percentage of sperms with forward motility.[34] Ascorbic acid as an antioxidant can protect sperm from damage caused by excessive ROS. Since ascorbic acid in semen is mainly produced by epididymal fluid, its content in seminal plasma can reflect epididymal function to a certain extent. Lactic acid in semen is produced by sperms and supporting cells,[35] which can be converted into pyruvate by LDH and plays a role in the production of ATP by sperm mitochondria. Thus, lactic acid plays a role in sperm energy metabolism. Tartaric acid participates in immune response, metabolic stress, and signal transduction in human body.[36] It prevents prostatitis by reducing the activity of prostatic acid phosphatase.[37] Therefore, the content of tartaric acid in semen is related to prostate function. At present, there is no report on succinic acid in seminal plasma. Dieudonne et al[38] reported that the citric acid content in patients with azoospermia and asthenospermia was lower than that of normal healthy individuals. Dunzendorfer et al[37] showed that after clinical treatment with tartaric acid in patients with chronic prostatitis, the sperm count increased significantly and the symptoms were significantly improved. Lay et al[39] reported that the lactic acid concentration of high, medium, and low fertility groups was significantly lower. The above results indicate that the contents of organic acids in seminal plasma of the normal fertility group were higher than that of the azoospermia group.[40] Consistently, our results showed that the contents of ascorbic acid, tartaric acid, lactic acid, and UA in hyperuricemia group were lower than the normal group. This indicates that high serum UA content not only affects sperm quality, but also reduces the organic acid content of seminal plasma. Organic acids provide energy in the body or indicate gonadal function. Gavella et al[41] reported that the content of UA in the semen of infertile men was not related to SC and motility, while that of ascorbic acid was not related to sperm quality. Lay et al[39] found no statistical difference between the level of fertility and the components of human seminal plasma (such as citric acid, lactic acid, and protein). This study showed that the 6 organic acids in the seminal plasma of normal men were not associated with SV, SC, and motility. Elevated serum UA may affect erectile function and sex hormone levels, leading to male reproductive dysfunction. Higher levels of UA in the physiological range of seminal plasma in the normal group are beneficial to male reproductive health. The data of organic acid content in human seminal plasma in this study may be slightly different from other research results, which may be caused by the differences in year of measurement, regional differences and differences in personal eating habits. However, the changing trends were basically the same.
5. Conclusions
In summary, we found that a high UA level may be a risk factor for decreased semen quality and concluded that hyperuricemia was an independent factor affecting SV and TSC. We further explored the possible causes in hyperuricemia patients affecting sperm quality. The results showed that hyperuricemia may affect sperm function by altering reproductive hormone levels, changing the levels of oxidative stress, destroying the balance of ET/NO (which results in endothelial dysfunction), and affecting the secretory function of epididymis and changing seminal microenvironment (such as decreasing the concentrations of α-GLU). These results may provide some references for clinical diagnosis and treatment of male infertility.
Author contributions
Conceptualization: Shusong Wang.
Data curation: Jing Ma, Ruiyu Han, Tong Cui, Chaoju Yang.
Formal analysis: Jing Ma, Ruiyu Han, Tong Cui.
Methodology: Jing Ma, Ruiyu Han, Tong Cui, Chaoju Yang.
Software: Jing Ma, Ruiyu Han, Tong Cui, Chaoju Yang.
Supervision: Shusong Wang.
Writing – original draft: Ruiyu Han, Jing Ma.
Writing – review & editing: Shusong Wang.
Footnotes
Abbreviations: α-GLU = α-glucosidase, AKP = alkaline phosphatase, ELISA = enzyme linked immunosorbent assay, ET = endothelin, MDA = malondialdehyde, NO = nitric oxide, PR% = percentage of proactive sperm, SC = sperm concentration, SOD = superoxide dismutase, SV = semen volume, TG = triglyceride, TC = Total cholesterol, TSC = total sperm count, UA = uric acid.
How to cite this article: Ma J, Han R, Cui T, Yang C, Wang S. Effects of high serum uric acid levels on oxidative stress levels and semen parameters in male infertile patients. Medicine. 2022;101:3(e28442).
The authors have no funding and conflicts of interest to disclose.
Department of Science and Technology of Hebei Province, Grant/Award Number: 2037715D, 2137796D.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, BP = blood pressure, FBG = fasting blood glucose, HDL = high-density lipoprotein, LDL = low-density lipoprotein, TC = total cholestero, TG = triglyceride.
P < .05, compared with the control group; the value was expressed in terms of mean ± standard deviation (SD).
PR = progressive motility, SC = sperm concentration, SD = standard deviation, SV = semen volume, TSC = total sperm count.
P < .05, compared with the control group; the value was expressed in terms of mean ± SD.
PR = progressive motility, SC = sperm concentration, SD = standard deviation, SV = semen volume, TSC = total sperm count, UA = uric acid.
The value was expressed in terms of mean ± SD.
P < .05, compared with the ≥463 group.
E2 = estradiol, FSH = follicle stimulating hormone, LH = luteinizing hormone, PRL = prolactin, SD = standard deviation, T = testosterone.
P < .05, compared with control group; the value was expressed in terms of mean ± SD.
Compared with control.
P < .05.
References
- [1].Remedios C, Shah M, Bhasker AG, Lakdawala M. Hyperuricemia: a reality in the Indian obese. Obes Surg 2012;22:945–8. [DOI] [PubMed] [Google Scholar]
- [2].Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol 2016;213:08–14. [DOI] [PubMed] [Google Scholar]
- [3].Burns CM, Wortmann RL. Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J. Disorders of purine and pyrimidine metabolism. McGraw-Hill Education, Harrison's Principles of Internal Medicine, 19e. New York, NY, USA:2015. [Google Scholar]
- [4].Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis 2008;67:960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krishnan E, Pandya BJ, Chung L, Dabbous O. Hyperuricemia and the risk for subclinical coronary atherosclerosis--data from a prospective observational cohort study. Arthritis Res Ther 2011;13:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nejatinamini S, Ataie-Jafari A, Qorbani M, et al. Association between serum uric acid level and metabolic syndrome components. J Diabetes Metab Disord 2015;14:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003;42:474–80. [DOI] [PubMed] [Google Scholar]
- [9].Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 2009;32:1737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen LY, Zhu WH, Chen ZW. Relationship between hyperuricemia and metabolic syndrome. J Zhejiang Univ Sci B 2007;8:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010;7:153–61. [DOI] [PubMed] [Google Scholar]
- [12].Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014;29:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pergialiotis V, Prodromidou A, Frountzas M, Korou LM, Vlachos GD, Perrea D. Diabetes mellitus and functional sperm characteristics: a meta-analysis of observational studies. J Diabetes Complications 2016;30:1167–76. [DOI] [PubMed] [Google Scholar]
- [14].Bhattacharya SM, Ghosh M, Nandi N. Diabetes mellitus and abnormalities in semen analysis. J Obstet Gynaecol Res 2014;40:167–71. [DOI] [PubMed] [Google Scholar]
- [15].Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med 1993;14:615–31. [DOI] [PubMed] [Google Scholar]
- [16].Martins AD, Majzoub A, Agawal A. Metabolic syndrome and male fertility. World J Mens Health 2019;37:113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guz J, Gackowski D, Foksinski M, et al. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One 2013;8:e68490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lytvyn Y, Perkins BA, Cherney DZ. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes 2015;39:239–46. [DOI] [PubMed] [Google Scholar]
- [19].Su HY, Yang C, Liang D, Liu HF. Research advances in the mechanisms of hyperuricemia-induced renal injury. Biomed Res Int 2020;2020:5817348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang L, Chang B, Guo Y, Wu X, Liu L. The role of oxidative stress-mediated apoptosis in the pathogenesis of uric acid nephropathy. Ren Fail 2019;41:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dobrakowski M, Kasperczyk S, Horak S, Chyra-Jach D, Birkner E, Kasperczyk A. Oxidative stress and motility impairment in the semen of fertile males. Andrologia 2017;49: [DOI] [PubMed] [Google Scholar]
- [22].Selley ML, Lacey MJ, Bartlett MR, Copeland CM, Ardlie NG. Content of significant amounts of a cytotoxic end-product of lipid peroxidation in human semen. J Reprod Fertil 1991;92:291–8. [DOI] [PubMed] [Google Scholar]
- [23].Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 2011;26:1628–40. [DOI] [PubMed] [Google Scholar]
- [24].Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2010;2010:686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kopalli SR, Hwang SY, Won YJ, et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol 2015;69:94–102. [DOI] [PubMed] [Google Scholar]
- [26].Nasimi P, Tabandeh MR, Roohi S. Busulfan-mediated oxidative stress and genotoxicity decrease in sperm of Satureja Khuzestanica essential oil-administered mice. Syst Biol Reprod Med 2018;64:348–57. [DOI] [PubMed] [Google Scholar]
- [27].Sharma R, Agarwal A, Mohanty G, et al. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod Biol Endocrinol 2013;11:38–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jayaraman V, Ghosh S, Sengupta A, Srivastava S, Sonawat HM, Narayan PK. Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J Assist Reprod Genet 2014;31:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lu JC, Huang YK, Zhang HK. Quick Manual of Clinical Test Reports. Shanghai: The Second Military Medical University Press; 2009. [Google Scholar]
- [30].Yin B, Liu HJ, Zhao M. Relationship between zinc, fructose and carnitine contents in seminal plasma and semen parameters. Zhong Hua Nan Ke Xue Za Zhi 2013;19:1051–3. [Google Scholar]
- [31].López Rodríguez A, Rijsselaere T, Beek J, Vyt P, Van Soom A, Maes D. Boar seminal plasma components and their relation with semen quality. Syst Biol Reprod Med 2013;59:05–12. [DOI] [PubMed] [Google Scholar]
- [32].Blanchard TL, Varner DD, Brinsko SP, Love CC. Azoospermia in stallions: determining the cause. Compend Contin Educ Vet 2012;34:E2. [PubMed] [Google Scholar]
- [33].Pesch S, Bergmann M, Bostedt H. Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology 2006;66:307–13. [DOI] [PubMed] [Google Scholar]
- [34].Colagar AH, Marzony ET. Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr 2009;45:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamamah S, Seguin F, Bujan L, Barthelemy C, Mieusset R, Lansac J. Quantification by magnetic resonance spectroscopy of metabolites in seminal plasma able to differentiate different forms of azoospermia. Hum Reprod 1998;13:132–5. [DOI] [PubMed] [Google Scholar]
- [36].Peti-Peterdi J, Gevorgyan H, Lam L, Riquier-Brison A. Metabolic control of renin secretion. Pflugers Arch 2013;465:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dunzendorfer U, Kruschwitz K, Feller H, Milbradt R. Na-tartrate in the treatment of chronic nonbacterial prostatitis. Andrologia 1983;15:135–40. [DOI] [PubMed] [Google Scholar]
- [38].Dieudonne O, Godin PA, Van-Langendonckt A, Jamart J, Galanti L. Biochemical analysis of the sperm and infertility. Clin Chem Lab Med 2001;39:455–7. [DOI] [PubMed] [Google Scholar]
- [39].Lay MF, Richardson ME, Boone WR, Bodine AB, Thurston RJ. Seminal plasma and IVF potential. Biochemical constituents of seminal plasma of males from in vitro fertilization couples. J Assist Reprod Genet 2001;18:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mehrparavar B, Minai-Tehrani A, Arjmand B, Gilany K. Metabolomics of male infertility: a new tool for diagnostic tests. J Reprod Infertil 2019;20:64–9. [PMC free article] [PubMed] [Google Scholar]
- [41].Gavella M, Lipovac V, Vucic M, Rocic B. Evaluation of ascorbate and urate antioxidant capacity in human semen. Andrologia 1997;29:29–35. [DOI] [PubMed] [Google Scholar]