Abstract
A reverse transcriptase PCR (RT-PCR) was developed for use as a diagnostic screening test for the detection of bovine viral diarrhea virus (BVDV) in pooled bovine serum samples. Individual serum samples from 60 dairy cattle herds located in Pennsylvania were evaluated by the microplate virus isolation method, and pooled sera were analyzed by RT-PCR. RT-PCR was sensitive and specific and detected a single viremic serum sample in up to 100 pooled serum samples. RT-PCR analysis of pooled sera provides a rapid and cost-effective method for the screening of cattle herds for the presence of animals persistently infected with BVDV.
Bovine viral diarrhea (BVD) is a major disease of cattle that results in continued severe economic losses to the dairy and beef cattle industries primarily due to decreased reproductive performance. BVD virus (BVDV) is a widely occurring viral pathogen of cattle classified as a pestivirus in the family Flaviviridae (21). The virus consists of a single-stranded positive-sense RNA virus of approximately 12,308 bp in length flanked by 5′ and 3′ untranslated regions (UTRs). Considerable genomic heterogeneity, antigenic variation, and biological behavior exist among strains of BVDV. BVDV strains have been classified as genotype 1 or 2 on the basis of antigenic variation and differences of sequences in the 5′ UTR (20). The pathogenicity and clinical syndromes associated with in vivo infection with BVDV also vary by strain (3, 11). Infection of cattle with BVDV can result in gastrointestinal disease, immunosuppression with secondary infections, thrombocytopenia, and reproductive failure. In pregnant cows, transplacental infection with the virus occurs with a high degree of efficiency, leading to fetal loss, malformations, or birth of a persistently infected calf (7; T. R. Drake, D. A. Moore, R. H. Whitlock, A. E. Castro, A. L. Hattel, R. Reams, and W. Stroffegen, Int. Symp. Bovine Viral Diarrhea Virus, 1996).
Fetal infection with BVDV may result in the birth of a calf immunotolerant to BVDV with an inapparent persistent infection. Persistently infected (PI) animals remain viremic throughout their lifetimes, intermittently shed infectious BVDV, and serve as reservoirs of virus in cattle herds. Control programs for BVD consist of vaccination along with identification and removal of PI cows and with the institution of biosecurity measures to prevent reintroduction of BVDV into a herd. The cost of individually testing each cow in a herd has been a limiting economic factor for implementation of surveillance and control programs for BVDV. Testing of pooled sera has been proposed as a cost-efficient approach for detection of BVDV (17). The objective of this study was to develop a single-tube single-enzyme reverse transcriptase PCR (RT-PCR) assay with pooled serum for detection of BVDV. The assay was validated by comparison of results of RT-PCR evaluation with pooled sera with results from microplate virus isolation evaluation of individual serum samples.
Consensus primers were designed from the highly conserved region of the BVDV genome within the 5′ UTR of strain NADL (6) that shared maximum homology to BVDV genotypes 1 and 2 (10, 15). The locations of the primers and sequences were as follows: forward primer 103 (genomic positions 103 to 124), 5′-TAGCCATGCCCTTAGTAGGAC-3′; reverse primer 372 (genome positions 372 to 392), 5′-ACTCCATGTGCCATGTACAGC-3′. Viral RNA was extracted from serum with QIAamp Viral RNA purification kits (29504; Qiagen). RNA was extracted from 420 μl of serum and was stored at −70°C until used. Single-tube single-enzyme RT-PCR was performed with the Perkin-Elmer Gene Amp EZ rTth RNA PCR kit (N808-0179). The technique was modified to enhance its sensitivity. Briefly, each reaction mixture contained 1× buffer, each deoxynucleotide triphosphate at a concentration of 400 μM, 5 U of rTth DNA polymerase, 4 mM manganese acetate, each primer (primers 103 and 372) at a concentration of 0.60 μM, and 10 μl of extracted RNA brought to a volume of 50 μl with diethyl pyrocarbonate (DEPC)-treated water. After an initial incubation for 30 min at 62°C followed by denaturation at 94°C for 120 s, 40 cycles of amplification were performed by using a thermo profile of 94°C for 60 s and 62°C for 60 s, with a final extension at 65°C for 10 min. The amplification products were analyzed by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
The BVDV reference strains used were NADL (12), Singer (16), New York-1 (2), and type II-125 (National Animal Disease Center (18) [NADC], Ames, Iowa) and PA-1. The PA-1 strain was a genotype 2 BVDV strain isolated from clinical samples from a herd involved in an acute outbreak. The virus was characterized as type 2 BVDV by genotyping (J. Ridpath, NADC). Virus stocks were propagated in MDBK cells. The microplate virus isolation assay was performed as described previously (1, 5).
The specificities of the primers were tested with BVDV reference strains, BVDV field isolates, and other bovine viruses. Amplification products of 290 bp were obtained with strains NADL, Singer, NY-1, type II-125, and PA-1 (Fig. 1). The products derived from the reference strains were sequenced and confirmed to be BVDV specific. The RT-PCR also identified 50 field isolates of BVDV obtained from different locations in Pennsylvania, and their identities were confirmed by the microplate virus isolation method. In addition, 10 field isolates of BVDV from Wyoming and 13 field isolates from New York State were identified by RT-PCR, and their identities were confirmed by the microplate virus isolation method. No amplification products were obtained from RT-PCR evaluation of stocks of bovine respiratory syncytial virus, parainfluenza virus type 3, infectious bovine rhinotracheitis virus, bovine rotavirus, bovine coronavirus, bluetongue virus type 17, and bovine cytomegalovirus.
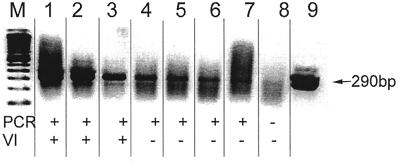
FIG. 1.
Sensitivity of RT-PCR versus that of microplate virus isolation method for detection of BVDV. The gel shows amplicons from 10-fold serial dilutions in DEPC-treated water of RNA extracted from NADL strain BVDV. Lanes 1 to 8, 1:101 to 1:108 dilutions, respectively; lane 9, kit control; lane M, molecular mass marker. The presence of 290-bp band indicated positivity for BVDV. Results were summarized in the row PCR across the bottom of the gel. The results of the microplate virus isolation method with the same serial 10-fold dilutions are listed in the row labeled VI.
The sensitivity of RT-PCR was determined with serial 10-fold dilutions of virus reference strains in DEPC-treated water. Microplate virus isolation was performed with each of the serial dilutions, and viral RNA was extracted for RT-PCR. The sensitivity of detection of the NY-1 and Singer BVDV strains by RT-PCR was equivalent to that by the microplate virus isolation method. However, RT-PCR was 100 times more sensitive than the microplate virus isolation method for detection of the NADL (genotype 1) and PA-1 (genotype 2) strains of BVDV (Table 1). The comparative differences in the sensitivities of detection of different strains of BVDV were hypothesized to be due to virus viability or in vitro growth characteristics in the microplate virus isolation method rather than to differences in amplification by RT-PCR (14). The microplate virus isolation method requires infectious BVDV, while RT-PCR requires extracted viral RNA. The microplate virus isolation method did not detect nonviable BVDV strain PA-1 in a sample preparation that had undergone several freeze-thaw cycles. However, RT-PCR was still able to detect viral RNA in the sample at a dilution of 1:100.
TABLE 1.
Sensitivity of RT-PCR versus that of microplate virus isolation method for detection of multiple strains of BVDV
| BVD strain (no. of TCID50/ml) | Test | Detection of BVDV in samples diluted:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Neat | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | 10−6 | ||
| NY-1 (103.4) | VIa | + | + | + | + | + | − | − |
| PCR | + | + | + | + | + | − | − | |
| PA-1 (100)b | VI | − | − | − | − | − | − | − |
| PCR | + | + | + | − | − | − | − | |
| NADL (103) | VI | + | + | + | − | − | − | − |
| PCR | + | + | + | + | + | + | − | |
| Singer (105) | VI | + | + | + | + | + | − | − |
| PCR | + | + | + | + | + | − | − | |
VI, microplate virus isolation method.
The sample was exposed to multiple freeze-thaw cycles.
To study the effect of the virus neutralization (VN) antibody titer (4) on the detection of BVDV by RT-PCR, a single BVDV-positive serum sample was diluted with BVDV-negative sera with VN titers of 1:128, 1:256, 1:512, 1:1,024, and 1:2,048. The sera were pooled in lots of 10, 25, 50, and 100 samples each, and the pooled sera were held at 4°C for approximately 1 h prior to RNA extraction. These conditions were similar to those encountered during the pooling of diagnostic samples. RT-PCR with extracted RNA showed that the high antibody titers in pooled serum samples had no appreciable effect on the sensitivity of detection of BVDV by RT-PCR. Similar observations were reported for bulk tank milk samples (19).
The effects of time and temperature on the stability of BVDV RNA in serum were determined with sera from persistently infected cows. A BVDV-positive serum sample with a virus titer of 103 50% tissue culture infective doses (TCID50)/ml was divided into two portions. One portion was stored at 4°C, and the other was stored at ambient temperature. Viral RNA was extracted from each serum sample at 0, 24, 48, 72, and 120 h. RT-PCR was performed with RNA extracted at each time interval. Viral RNA was detected by RT-PCR from the serum sample stored at ambient temperature for 24 h but was not detected at 48 h. Viral RNA extracted from serum stored at 4°C was detected at 72 h but was not detected at 120 h. The rate of degradation of the viral RNA and the stability of BVDV in serum under various conditions were dependent on the initial virus titer, sample handling, and the purity of the serum, e.g., hemolysis.
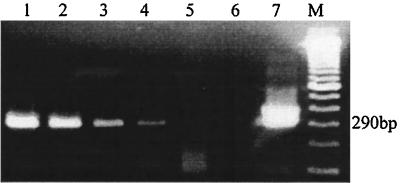
The optimized RT-PCR assay was used to screen pooled sera under diagnostic laboratory conditions. A total of 8,637 blood samples were collected from 60 individual herds from 16 counties in Pennsylvania. The herds were selected on the basis of clinical histories of reproductive problems. Samples of blood were collected at least 3 months after vaccination for BVD. A volume of 100 μl of serum from each cow was pooled in groups of approximately 30, 50, and 100 samples and RNA was extracted. RT-PCR identified every BVDV-positive sample in pools of 30, 50, and 100 samples. The animal with the lowest serum BVDV titer (100.22 TCID50/ml) tested suspicious by the microplate virus isolation assay but was clearly positive by RT-PCR when that serum sample was incorporated into pooled serum lots of 30, 50, and 90 samples (Fig. 2).
FIG. 2.
RT-PCR evaluation of pooled sera for BVDV. Lane 1, amplicons from RNA extracted from serum from a cow persistently infected with BVDV; lanes 2 to 4, amplicons from RNA extracted from serum from lane 1 pooled with BVDV-negative serum in lots of 30, 50, and 100 samples, respectively; lane 5, water (negative control); lane 6, BVDV-negative serum; lane 7, kit control; lane M, molecular size marker. The presence of a 290-bp band indicated positivity for BVDV.
Pooled serum samples from 13 of 60 herds (∼22%) were positive for BVDV by RT-PCR. RT-PCR results were compared to results for individual serum samples by the microplate virus isolation method. The rate of agreement between the results of the RT-PCR test with pooled sera and those of the microplate virus isolation test with individual serum samples was 100%. The VN antibody titers of pooled serum samples from 11 BVDV-positive herds and 10 randomly selected BVDV-negative herds were determined (Table 2). Ten of the 11 BVDV-positive herds had comparatively high antibody titers (1:512 or greater). The antibody titers for the 10 BVDV-negative herds had an average VN titer of 1:256, with a range of titers of 1:64 to 1:512. The higher levels of antibodies in herds containing viremic cows were attributed to exposure to BVDV via shedding of virus by PI cows rather than to vaccination. Genotype 1 BVDV was isolated from 10 of 11 BVDV-positive herds. Genotype 2 BVDV was isolated from the one BVDV-positive herd with a low antibody titer of 1:64 (herd 1). The VN antibody titer assay used the Singer strain (genotype 1) of BVDV as the indicator virus. It is likely that the one herd with a low VN titer to genotype 1 BVDV had a high VN antibody titer to genotype 2 BVDV (serum not tested). The virus titers for positive animals were calculated (Table 2).
TABLE 2.
Numbers of samples, VN antibody titers, and serum BVDV titers for herds containing BVDV-infected cows
| Herd no. | No. of serum samples pooled | Antibody titer of pooled serum | No. of positive samples in pooled serum | TCID50 of virus/ ml of positive sample |
|---|---|---|---|---|
| 1 | 55 | 1:64 | 5 | 102.4a |
| 2 | 63 | 1:2,048 | 1 | 101.7 |
| 3 | 48 | 1:512 | 1 | NAb |
| 4 | 46 | 1:2,048 | 1 | 102 |
| 5 | 23 | 1:1,024 | 7 | 102.4a |
| 6 | 50 | 1:2,048 | 1 | 102.3 |
| 7 | 50 | 1:2,048 | 1 | 100.33 |
| 8 | 100 | 1:1,024 | 14 | NA |
| 9 | 50 | 1:512 | 9 | 101.64a |
| 10 | 50 | 1:1,024 | 7 | 102.2a |
| 11 | 40 | 1:1,024 | 1 | 100.22 |
Average TCID50 per milliliter of sera from all cows positive for BVD.
NA, not available.
A single-tube single-enzyme RT-PCR assay for the detection of BVDV in pooled serum samples was developed for use as an economical diagnostic test for the screening of cattle herds. Initial studies focused on the design of primers in the conserved 5′ UTR to detect all strains of BVDV. Identification of many divergent strains of BVDV including reference strains and field isolates from Pennsylvania, New York, and Wyoming, including genotype 1 and type 2 BVDV strains, indicated that the target sequences in the 5′ UTR were specific for BVDV. A single-tube single-enzyme system (10) was used to reduce the risk of contamination of samples, which could lead to potentially false-positive reactions. Although sensitivity may have been sacrificed relative to that of a two-enzyme system, the potential loss in sensitivity was deemed acceptable in contrast to the risk of contamination that could be encountered in a high-volume diagnostic environment.
The optimal size for pooled serum lots was dependent on herd size and the ages of the cows to be tested. Since each serum sample in a pool of serum samples that tests positive must be individually tested by the microplate virus isolation method, the number of samples from animals per serum pool affects the ultimate cost for evaluation of the entire herd (17). Although the expected prevalence of PI animals in herds is 1 to 2% (13), relative risk for infection with BVDV can be assigned to different age groups of animals. Sera from older multiparous cows with a lower prevalence of BVDV infection can be pooled into larger lots of serum samples than sera from heifers 2 years old or less, which have a higher expected prevalence of BVDV infection.
A quality assurance program was necessary to set strict guidelines for sample quality and adherence to sample-processing protocols. Specimen storage conditions, time, and temperature proved to have significant impacts on nucleic acid recovery and the efficiency of the RT-PCR (8). To enhance the opportunities to detect BVDV-infected animals in herds, the following procedures are recommended: blood samples should arrive within 24 to 48 h of collection. Samples should be stored cold and shipped on wet ice. Initial sample processing, i.e., pooling and freezing, should occur on the day of receipt of specimens. Pre-PCR and post-PCR procedures should be done in separate areas (9).
The single-tube single-enzyme RT-PCR assay was shown to be a sensitive and specific test for the detection of BVDV in bovine serum pooled in lots of up to 100 samples. Each serum sample within a positive pool must be retested individually to identify the BVDV-infected animals. Quality assurance and quality control programs are essential components for RT-PCR evaluation of serum to avoid false-positive or false-negative results. RT-PCR reduces the testing cost per cow and provides the opportunity for widespread participation in BVD surveillance and control programs.
Acknowledgments
We thank Hana Van Campen, University of Wyoming, for providing BVDV field isolates and Edward Dubovi, Cornell University, for providing monoclonal antibodies and field isolates of BVDV. Susan Gordon, Kay Palchak, and Geraldine Brigman provided excellent technical assistance.
This research was funded by the Pennsylvania Department of Agriculture, Harrisburg, Pa.
REFERENCES
- 1.Afshar A, Dulac G C, Dubuc C, Howard H. Comparative evaluation of the fluorescent antibody test and microtiter immunoperoxidase assay for detection of bovine viral diarrhea virus from bull semen. Can J Vet Res. 1991;55:91–93. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker J A, York C J, Gillespie J H, Mitchell G B. Virus diarrhea in cattle. Am J Vet Res. 1954;15:525–531. [PubMed] [Google Scholar]
- 3.Bolin S R. The current understanding about the pathogenesis and clinical forms of BVD. Vet Med. 1990;85:1124–1132. [Google Scholar]
- 4.Bolin S R, Ridpath J F. Range of virus neutralizing activity and molecular specificity of antibodies induced in cattle by inactivated bovine viral diarrhea vaccine. Am J Vet Res. 1990;51:703. [PubMed] [Google Scholar]
- 5.Castro M D, Stoffregen W C, Brigman G P, Kathleen H A. A method to detect bovine viral diarrhea virus contamination in cell cultures using immunoperoxidase staining. J Vet Diagn Investig. 1997;9:427–431. doi: 10.1177/104063879700900417. [DOI] [PubMed] [Google Scholar]
- 6.Collet M S, Larson R, Gold C, Strick D. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988;165:191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- 7.Cortese S V. Food animal practice, current vet therapy. W. B. Philadelphia, Pa: Saunders Company; 1999. Bovine virus diarrhea virus and mucosal disease; pp. 286–290. [Google Scholar]
- 8.Cuypers H T M, Bresters D, Winkel N, Reesink H W, Weiner A J, Houghton M, vander Poel C L, Lelie P N. Storage conditions of blood samples and primer selection affect the yield of cDNA polymerase chain reaction products of hepatitis C virus. J Clin Microbiol. 1992;30:3220–3224. doi: 10.1128/jcm.30.12.3220-3224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieffenbach C W, Dveksler G S. Setting up a PCR laboratory. PCR Methods Appl. 1993;3:S2. doi: 10.1101/gr.3.2.s2. [DOI] [PubMed] [Google Scholar]
- 10.Drew T W, Yapp F, Paton D J. The detection of bovine viral diarrhea virus in bulk milk samples by the use of a single tube RT-PCR. Vet Microbiol. 1999;64:145–154. doi: 10.1016/s0378-1135(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie J H, Madin S H, Darby N B. Cellular resistance in tissue culture, induced by non-cytopathogenic strains, to a cytopathogenic strain of virus diarrhea virus of cattle. Proc Soc Exp Biol Med. 1962;110:248–250. doi: 10.3181/00379727-110-27481. [DOI] [PubMed] [Google Scholar]
- 12.Gutenkust D E, Malmquist W A. Complement-fixing and neutralizing antibody response to bovine viral diarrhea and hog cholera antigens. Can J Comp Med Vet Sci. 1964;28:19–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Houe H. Epidemiology of bovine viral diarrhea virus. Vet Clin N Am Food Anim Pract. 1995;11:521–548. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- 14.Laamanen U I, Neuvonen E P, Yliviuhkola E M, Veijalainen P M L. Comparison of RT-PCR assay and virus isolation in cell cultures for the detection of bovine viral diarrhea in field samples. Res Vet Sci. 1997;63:199–203. doi: 10.1016/s0034-5288(97)90020-5. [DOI] [PubMed] [Google Scholar]
- 15.Letellier C, Kerkhofs P, Wellemans G, Vanopdenbosch E. Detection of genotyping of bovine diarrhea virus by reverse transcription-polymerase chain amplification of the 5′ untranslated region. Vet Microbiol. 1999;64:155–167. doi: 10.1016/S0378-1135(98)00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClurkin A W, Normann J O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Med Vet Sci. 1966;30:190–198. [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz-Zanzi C A, Johnson W O, Thurmond M C, Hietala S K. Pooled-sample testing as a herd-screening tool for detection of bovine viral diarrhea virus persistently infected cattle. J Vet Diagn Investig. 2000;12:195–203. doi: 10.1177/104063870001200301. [DOI] [PubMed] [Google Scholar]
- 18.Pellerin C, Van Den Hurk J, Lecomte J. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994;203:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- 19.Radwan G S, Brock K V, Hogan J S, Smith L K. Development of a PCR amplification assay as a screening test using bulk milk samples for identifying dairy herds infected with bovine viral diarrhea virus. Vet Microbiol. 1995;44:77–92. doi: 10.1016/0378-1135(94)00121-c. [DOI] [PubMed] [Google Scholar]
- 20.Ridpath J F, Bolin S R, Dubovi E J. Segregation of bovine viral diarrhea virus into genotypes. J Virol. 1994;205:66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- 21.Wengler G, Bradley D W, Collett M S, Heinz F X, Schlesinger R W, Strauss J H. Family flaviviridae. In: Murphy F A, Fauquet C M, Bishop C M, et al., editors. Viral taxonomy. New York, N.Y: Springer-Verlag; 1995. pp. 415–427. [Google Scholar]