Abstract
Simple Summary
During the COVID-19 pandemic, utilization of remote monitoring platforms was recommended. The HeartLogic algorithm identifies patients at risk of heart failure events, combining multiple sensors available on implantable cardioverter defibrillators. This analysis examined how multiple CIED sensors behave in periods of anticipated restrictions pertaining to physical activity. We demonstrated a significant drop in median activity level immediately after the implementation of stay-at-home orders, whereas there was no difference in the other contributing sensors. The weekly rate of heart failure alerts was significantly higher during the lockdown and post-lockdown than that reported in the pre-lockdown.
Abstract
Aims: The utilization of remote monitoring platforms was recommended amidst the COVID-19 pandemic. The HeartLogic index combines multiple implantable cardioverter defibrillator (ICD) sensors and has proved to be a predictor of impending heart failure (HF) decompensation. We examined how multiple ICD sensors behave in the periods of anticipated restrictions pertaining to physical activity. Methods: The HeartLogic feature was active in 349 ICD and cardiac resynchronization therapy ICD patients at 20 Italian centers. The period from 1 January to 19 July 2020, was divided into three phases: pre-lockdown (weeks 1–11), lockdown (weeks 12–20), post-lockdown (weeks 21–29). Results: Immediately after the implementation of stay-at-home orders (week 12), we observed a significant drop in median activity level whereas there was no difference in the other contributing parameters. The median composite HeartLogic index increased at the end of the Lockdown. The weekly rate of alerts was significantly higher during the lockdown (1.56 alerts/week/100 pts, 95%CI: 1.15–2.06; IRR = 1.71, p = 0.014) and post-lockdown (1.37 alerts/week/100 pts, 95%CI: 0.99–1.84; IRR = 1.50, p = 0.072) than that reported in pre-lockdown (0.91 alerts/week/100 pts, 95%CI: 0.64–1.27). However, the median duration of alert state and the maximum index value did not change among phases, as well as the proportion of alerts followed by clinical actions at the centers and the proportion of alerts fully managed remotely. Conclusions: During the lockdown, the system detected a significant drop in the median activity level and generated a higher rate of alerts suggestive of worsening of the HF status.
Keywords: ICD, CRT, heart failure, remote monitoring, multisensor
1. Introduction
The spread of the coronavirus disease 2019 (COVID-19) epidemic required a rapid response. The primary modes of disease prevention have involved limiting exposure and social distancing. At various times, stay-at-home orders were issued in many geographies and were shown to result in a rapid global reduction in physical activity [1]. Physical activity is an important determinant of health [2,3]. During the COVID-19 pandemic, hospital admissions for acute cardiac conditions markedly declined [4,5,6] as access to care was impacted by limited hospital resources and by the reluctance of patients to go to hospital. Guidance from the European Society of Cardiology (ESC) for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic [7] encouraged centers to consider telemedicine to provide patients medical advice and follow-ups. For patients with cardiovascular implantable electronic devices (CIEDs), in-person office visits had to be replaced by remote contact, using the device information obtained through remote monitoring [8,9].
To date, scant data exist on the consequences of the home confinement on the clinical status of heart failure (HF) patients with CIEDs [10]. Moreover, it is not known whether the physical activity decrease was associated with a worsening of physiological parameters. Modern CIEDs continuously monitor multiple clinical variables, in order to provide an early warning of changes in the clinical status. Therefore, we examined how multiple CIED sensors behave in the periods of anticipated restrictions pertaining to physical activity.
2. Materials and Methods
The home confinement for the COVID-19 pandemic in Italy was imposed from March 8th to May 18th. For this analysis we identified all of the HF patients with reduced left ventricular ejection fraction (≤35% at the time of implantation) who had received a HeartLogic-enabled implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy ICD (CRT-D) (RESONATE family, Boston Scientific) in accordance with standard indications [11] at 20 study centers (full list of participant centers in Supplemental Material section). Patients had to be on regular monitoring in the LATITUDE (Boston Scientific) platform, with diagnostic data available from at least 1 January 2020. As initialization is required, the HeartLogic index does not become available until 30–37 days after implantation. Thus, in this analysis, we included only devices implanted before November 2019. Before the lockdown, patients were followed up in accordance with the standard practice of the participating centers, based on current international recommendations [12]. The study protocol did not mandate any specific intervention algorithm and physicians were free to remotely implement clinical actions, to schedule extra in-office visits, or to adopt an active monitoring approach. Data were collected at the study centers in the framework of a prospective registry (Rhythm Detect Registry, ClinicalTrials.gov Identifier: NCT02275637) approved by the Institutional Review Board of the Coordinating Center (IRCCS Policlinico S. Matteo, Pavia, Italy) and all participant centers. The research was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent for data storage and analysis.
2.1. HeartLogic Index
The details of the HeartLogic algorithm have been reported previously [13]. Briefly, the algorithm combines data from multiple sensors: accelerometer-based first and third heart sounds, intrathoracic impedance, respiration rate, the ratio of respiration rate to tidal volume, night heart rate, and patient activity. Each day, the device calculates the degree of worsening in sensors from their moving baseline and computes a composite index. An alert is issued when the index crosses a programmable threshold.
2.2. Design of Analysis
We assessed the trend of all HeartLogic sensors from 1 January to 19 July. The period was divided in 3 phases: pre-lockdown (weeks 1–11), lockdown (weeks 12–20), post-lockdown (weeks 21–29). We calculated the change in all variables among phases, as well as the rate of alerts occurred, and the clinical actions or extra in-office visits performed to manage them. We also compared the sensed parameters that contributed to the calculation of the HeartLogic index in case of alerts among study phases.
2.3. Statistical Analysis
Descriptive statistics are reported as means ± SD for normally distributed continuous variables, or medians with 25th to 75th percentiles in the case of skewed distribution. Normality of distribution was tested by means of the nonparametric Kolmogorov–Smirnov test. Categorical data were expressed as percentages. Differences between continuous variables were performed using a Student’s T test for Gaussian variables, and a Mann–Whitney U test non-parametric test for non-Gaussian variables. Differences in proportions were compared by applying chi-squared analysis or Fisher’s exact test, as appropriate. Incidence rates with confidence intervals were calculated and compared, together with the incidence rate ratio. One-way analysis of variance for repeated measures was used to test for differences among phases. A p value < 0.05 was considered significant in all tests. All statistical analyses were performed by means of R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
In the analysis, we included 349 ICD and cardiac resynchronization therapy ICD patients who had received the device at the study centers before November 2019. Table 1 shows the baseline clinical variables of all patients in the analysis. No patients developed COVID-19 during the study period.
Table 1.
Demographics and baseline clinical parameters of the study population.
| Parameter | Total N = 349 |
|---|---|
| Male gender, n (%) | 283 (81) |
| Age, years | 69 ± 11 |
| Ischemic etiology, n (%) | 156 (45) |
| NYHA class | |
| − Class I, n (%) − Class II, n (%) − Class III, n (%) − Class IV, n (%) |
21 (6) 188 (54) 131 (37) 9 (3) |
| LV ejection fraction, % | 31 ± 8 |
| AF history, n (%) | 133 (38) |
| Valvular disease, n (%) | 63 (18) |
| Coronary artery disease, n (%) | 166 (48) |
| Diabetes, n (%) | 99 (28) |
| COPD, n (%) | 59 (17) |
| Chronic kidney disease, n (%) | 101 (29) |
| Hypertension, n (%) | 210 (60) |
| β-Blocker use, n (%) | 329 (94) |
| ACE-inhibitor, ARB or ARNI use, n (%) | 321 (92) |
| MRA use, n (%) | 209 (60) |
| Diuretic use, n (%) | 324 (93) |
| Antiarrhythmic use, n (%) | 84 (24) |
| Anticoagulant therapy use, n (%) | 142 (41) |
| Ivabradine use, n (%) | 28 (8) |
| CRT device, n (%) | 269 (77) |
| Primary prevention, n (%) | 329 (94) |
NYHA = New York Heart Association; LV = Left ventricle; AF = Atrial fibrillation; COPD = Chronic obstructive pulmonary disease; ACE = Angiotensin-converting enzyme; ARB = Angiotensin II receptor blockers; ARNI = Angiotensin receptor–neprilysin inhibitor; MRA = Mineralocorticoid receptor antagonists; CRT = Cardiac resynchronization therapy.
3.1. HeartLogic and Contributing Sensors Trends
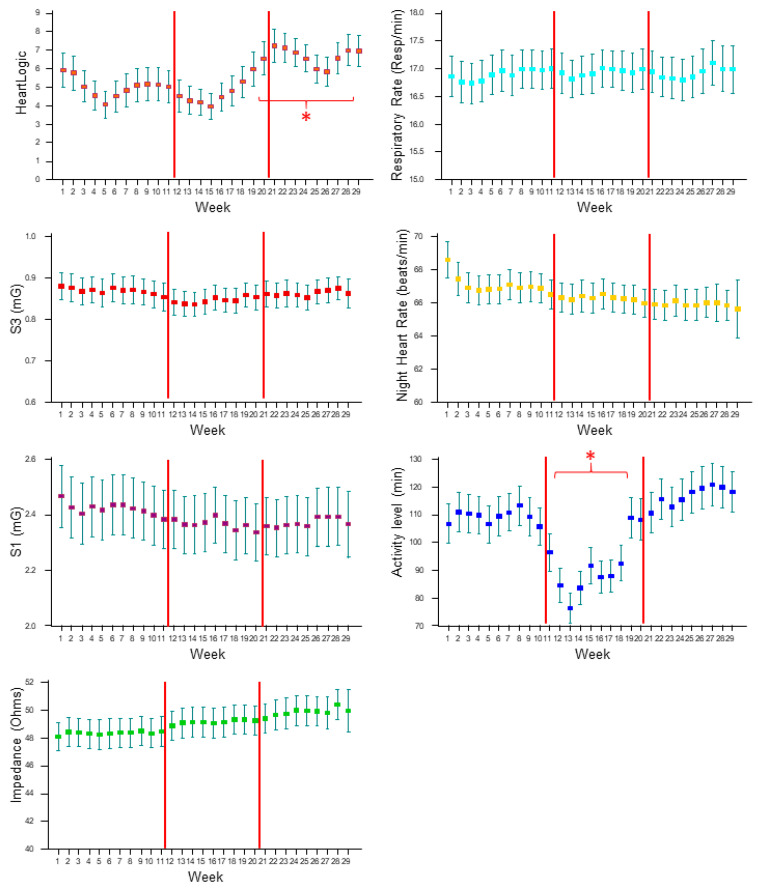
Figure 1 shows the weekly averages of the HeartLogic combined index, and all of the physiologic parameters collected by the devices from 1 January to 19 July. Immediately after the implementation of stay-at-home orders (from week 12), we observed a significant drop in the activity level that persisted until week 19, whereas there was no difference in the other contributing sensors. The composite HeartLogic index significantly increased at the end of the lockdown phase (from week 20), and the increase in the average index remained significant until week 28 in the post-lockdown phase.
Figure 1.
Weekly averages (with standard deviation) of the HeartLogic combined index and all physiologic parameters collected by the devices from 1 January to 19 July. The period was divided in 3 phases: pre-lockdown (weeks 1–11), lockdown (weeks 12–20), post-lockdown (weeks 21–29). S1: First heart sound; S3: Third heart sound; *: p < 0.05 versus pre-lockdown average.
3.2. HeartLogic Alerts, Characteristics and Management
The HeartLogic index crossed the threshold value 35 times in the pre-lockdown phase, 49 times during the lockdown phase (incidence rate ratio 1.71 [95% CI: 1.09–2.72] versus the pre-lockdown; p = 0.014), and 43 times during the post-lockdown phase (incidence rate ratio 1.50 [95% CI: 0.94–2.42] versus the pre-lockdown; p = 0.072) (Table 2).
Table 2.
HeartLogic alerts during the study phases.
| Alerts, n | Rate [95% CI], Alerts/100 pt-Weeks | Alert Duration, Days | Maximum Index Value | Alerts with Actions | Remote Management | |
|---|---|---|---|---|---|---|
| Pre-lockdown (weeks 1–11) | 35 | 0.91 (0.64–1.27) | 47 (29–60) | 28 ± 11 | 11 (31%) | 31 (89%) |
| Lockdown (weeks 12–20) | 49 | 1.56 (1.15–2.06) | 39 (28–57) | 30 ± 15 | 11 (22%) | 44 (90%) |
| Post-lockdown (weeks 21–29) | 43 | 1.37 (0.99–1.84) | 36 (25–57) | 24 ± 12 | 12 (28%) | 38 (88%) |
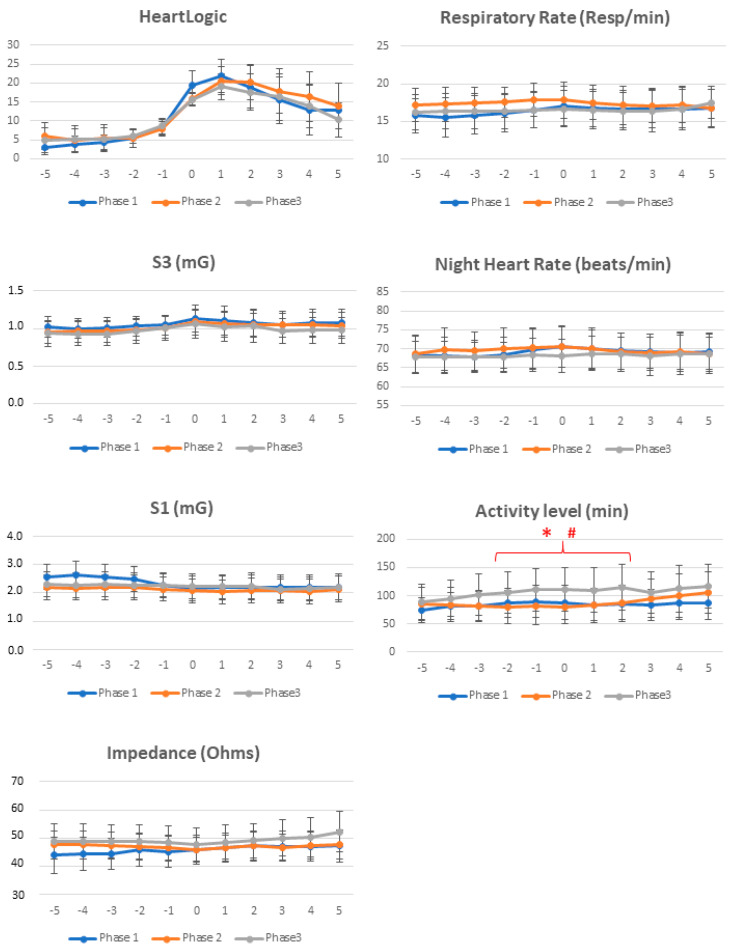
Of the 127 reported HeartLogic alerts, 113 (89%) did not require extra in-office visits and were managed remotely. Alert-triggered actions (e.g., drug adjustments, educational interventions) were reported in 34 (27%) cases. In the remaining cases, physicians adopted an active monitoring approach, intensifying the frequency of contacts but not intervening proactively. The proportion of alerts managed remotely, as well as the proportion of alerts triggering clinical actions, remained constant among study phases (Table 2). The average duration of the in-alert state was similar in the three study phases, as well as the maximum value of the index (Table 2). This was confirmed by comparable trends of HeartLogic index during the weeks immediately before and after the alert onset among study phases (Figure 2). On comparing the trends of all physiologic parameters among study phases, we noticed similar values, i.e., comparable contribution from all sensors to the combined HeartLogic index, except for higher activity levels before and after the alert onset in the post-lockdown phase than in the other phases.
Figure 2.
Comparison of trends collected immediately before and after the alerts among study phases. Weekly averages (with standard deviation) of HeartLogic index and all physiologic parameters are reported from 5 weeks before to 5 weeks after the alert onset (week 0). S1: First heart sound; S3: Third heart sound; *: p < 0.05 post-lockdown versus pre-lockdown; #: p < 0.05 post-lockdown versus lockdown.
4. Discussion
In this analysis, we showed a marked decrease in the ICD-measured physical activity, whereas no changes were detected in the other sensors, i.e., heart sounds, intrathoracic impedance, respiration parameters, heart rate. Indeed, the home confinement had no significant impact on single physiologic parameters beyond activity, but we observed an increased number of device-defined HF events, as detected by the combined HeartLogic index. This occurred at the end of the lockdown period and seemed to persist for some weeks after the end of home confinement.
The decline in physical activity during the lockdown was significant, as demonstrated through the use of smartphone accelerometers and algorithms for step counting in the general population [1] and in a small HF group [14]. In the present analysis, we confirmed this finding in a large HF population, although a low impact was expected in these patients, given the lower baseline activity level associated with their reduced functional capacity. The clinical relevance of measured physiologic parameters has been proven [13], as well as the meaningful association between individual sensors with changes in cardiac systolic and diastolic function [15], functional status [16], congestion [17], and prognosis [18,19]. Mitter et al. reported small changes in some parameters at the time of the lockdown in a smaller population of ICD and CRT-D patients [20]. Specifically, they demonstrated a decline in heart rate, an increase in intrathoracic impedance and a decrease in S3, and they interpreted this as a possible improvement linked to a decreased autonomic tone with less activity and potentially less frequent access to unhealthy food options. In our analysis, the night heart rate did not change; indeed, this parameter is a surrogate of resting heart rate and thus not directly associated with the activity level. Moreover, we did not confirm the increase in impedance described by Mitter et al. [20]. It is known that increases in CIED-measured impedance may not only be suggestive of less pulmonary congestion in HF, but may also be associated with the healing of the pocket hematoma or with the left ventricle volume changes induced by CRT early after implantation [21,22]. At the time of the lockdown, all our patients had received the device for at least 5 months; therefore, any effect linked with the initiation of the therapy had plausibly ended. The same applies to first and third heart sound amplitudes. They may improve early after CRT initiation, and we did not notice significant changes at the time of the home confinement in a population of patients implanted months before.
The rate of HeartLogic alerts was higher during the lockdown, as well as the average index after 8 weeks of home confinement. The analysis of the individual sensors during the weeks before the alert onset showed comparable contribution from all sensors to the combined HeartLogic index among study phases, except for higher activity levels in the post-lockdown phase. This suggests that the trigger mechanism of HF decompensation is the same under different activity conditions. Moreover, the decline in activity alone cannot lead to an increase in the index [13], but lower levels of activity could make the system more sensitive to other parameters. However, this would have resulted in different values among phases. Therefore, we tend to believe that the clinical relevance of diagnosed events was comparable among phases, and that patients may have experienced more frequent episodes of true HF decompensation. Indeed, the detrimental effects of the lockdown in patients with cardiovascular diseases have recently been demonstrated [23]. Our findings seem to disagree with the observations of a recent work that suggested potential beneficial effects of the lockdown in ICD patients for the reduction in real-life stressors [24].
In our centers with experts in the remote management of alerts, the COVID-19 restrictions had no impact on their standard practice. Indeed, the proportion of alerts managed remotely and alerts followed by clinical actions did not change between different phases of the pandemic. Consequently, the severity of the individual events was equivalent, since the in-alert states had similar durations and extents.
In previous works, the ability of HeartLogic to detect actionable HF events has been demonstrated, facilitating effective remote management [25,26,27,28,29]. In the context of the COVID-19 pandemic, the multisensor diagnostic platform was used effectively to facilitate the remote assessment of patient conditions. These findings confirm and extend previous anecdotal cases in which the HeartLogic algorithm provided critical data that allowed for the appropriate triage of patients, with reductions in unnecessary clinic visits during the COVID-19 pandemic [30].
The main limitation of this study is its observational non-randomized design. Moreover, as mentioned above, no predetermined actions were prescribed in response to HeartLogic alerts or to the individual subject’s reported signs or symptoms.
5. Conclusions
The HeartLogic multisensor platform detected the decrease in activity, although the home confinement had no impact on the other sensors. The increased number of alerts during the lockdown suggests that the home confinement had a negative effect on patients’ outcome. This must be taken into account in periods of prolonged social distancing and confirms the importance of ensuring safe and secure access to healthcare facilities for everybody who needs continuity of care.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11010120/s1, Full list of participant centers and investigators.
Author Contributions
Conceptualization, Writing—original draft preparation, Formal analysis, M.Z. and I.D.; Investigation, M.Z., L.C., A.D., M.M., A.D.R., L.S., G.G., C.C., V.E.S., G.S., C.L.G., G.A., A.T., E.P., M.G., A.P. and I.D.; Writing—review and editing, Data curation, S.V. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Institutional Ethics Committee of IRCCS Policlinico San Matteo—Pavia (Italy) on 5 May 2015; NCT: 02275637.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
M. Ziacchi has received speaker fees from Abbott, Biotronik, Boston Scientific, Medtronic; I. Diemberger has received minor speaker fees from Boston Scientific, Medtronic and Biotronik; M. Campari and S. Valsecchi are employees of Boston Scientific. The other authors report no conflict.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanganu-Bresch C., Zerbe M.J., Cutrufello G., Maci S.M. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann. Intern. Med. 2020;173:767–770. doi: 10.7326/M20-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T., Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2021;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmisano P., Guerra F., Ammendola E., Ziacchi M., Luigi Pisanò E.C., Dell’Era G., Aspromonte V., Zaccaria M., Di Ubaldo F., Capucci A., et al. Italian Association of Arrhythmology and Cardiac Pacing (AIAC). Physical Activity Measured by Implanted Devices Predicts Atrial Arrhythmias and Patient Outcome: Results of IMPLANTED (Italian Multicentre Observational Registry on Patients with Implantable Devices Remotely Monitored) J. Am. Heart Assoc. 2018;7:e008146. doi: 10.1161/JAHA.117.008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt A.S., Moscone A., McElrath E.E., Varshney A.S., Claggett B.L., Bhatt D.L., Januzzi J.L., Butler J., Adler D.S., Solomon S.D., et al. Fewer Hospitalizations for Acute Cardiovascular Conditions during the COVID-19 Pandemic. J. Am. Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Filippo O., D’Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A., Secco G.G., Campo G., Gallone G., Verardi R., et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boriani G., Palmisano P., Guerra F., Bertini M., Zanotto G., Lavalle C., Notarstefano P., Accogli M., Bisignani G., Forleo G.B., et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: Results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing) Intern. Emerg. Med. 2020;15:1445–1456. doi: 10.1007/s11739-020-02487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulchand S. COVID-19 and cardiovascular disease. BMJ. 2020;369:m1997. doi: 10.1136/bmj.m1997. [DOI] [PubMed] [Google Scholar]
- 8.Lakkireddy D.R., Chung M.K., Deering T.F., Gopinathannair R., Albert C.M., Epstein L.M., Harding C.V., Hurwitz J.L., Jeffery C.C., Krahn A.D., et al. Guidance for Rebooting Electrophysiology Through the COVID-19 Pandemic from the Heart Rhythm Society and the American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology: Endorsed by the American College of Cardiology. JACC Clin. Electrophysiol. 2020;6:1053–1066. doi: 10.1016/j.jacep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma N., Marrouche N.F., Aguinaga L., Albert C.M., Arbelo E., Choi J.I., Chung M.K., Conte G., Dagher L., Epstein L.M., et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. J. Arrhythm. 2020;36:813–826. doi: 10.1002/joa3.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Simone V., Guardalben S., Guarise P., Padovani N., Giacopelli D., Zanotto G. Home Monitoring trends during COVID-19 infection. J. Arrhythm. 2020;37:240–245. doi: 10.1002/joa3.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the, E.S.C. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 12.Slotwiner D., Varma N., Akar J.G., Annas G., Beardsall M., Fogel R.I., Galizio N.O., Glotzer T.V., Leahy R.A., Love C.J., et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Boehmer J.P., Hariharan R., Devecchi F.G., Smith A.L., Molon G., Capucci A., An Q., Averina V., Stolen C.M., Thakur P.H., et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Vetrovsky T., Frybova T., Gant I., Semerad M., Cimler R., Bunc V., Siranec M., Miklikova M., Vesely J., Griva M., et al. The detrimental effect of COVID-19 nationwide quarantine on accelerometer-assessed physical activity of heart failure patients. ESC Heart Fail. 2020;7:2093–2097. doi: 10.1002/ehf2.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calò L., Capucci A., Santini L., Pecora D., Favale S., Petracci B., Molon G., Bianchi V., Cipolletta L., De Ruvo E., et al. ICD-measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J. Interv. Card. Electrophysiol. 2020;58:95–101. doi: 10.1007/s10840-019-00668-y. [DOI] [PubMed] [Google Scholar]
- 16.Forleo G.B., Santini L., Campoli M., Malavasi M., Scaccia A., Menichelli M., Riva U., Lamberti F., Carreras G., Orazi S., et al. Long-term monitoring of respiratory rate in patients with heart failure: The Multiparametric Heart Failure Evaluation in Implantable Cardioverter-Defibrillator Patients (MULTITUDE-HF) study. J. Interv. Card. Electrophysiol. 2015;43:135–144. doi: 10.1007/s10840-015-0007-3. [DOI] [PubMed] [Google Scholar]
- 17.Yu C.M., Wang L., Chau E., Chan R.H., Kong S.L., Tang M.O., Christensen J., Stadler R.W., Lau C.P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 18.Cao M., Gardner R.S., Hariharan R., Nair D.G., Schulze C., An Q., Thakur P.H., Kwan B., Zhang Y., Boehmer J.P. Ambulatory Monitoring of Heart Sounds via an Implanted Device Is Superior to Auscultation for Prediction of Heart Failure Events. J. Card. Fail. 2020;26:151–159. doi: 10.1016/j.cardfail.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Fox K., Borer J.S., Camm A.J., Danchin N., Ferrari R., Lopez Sendon J.L., Steg P.G., Tardif J.C., Tavazzi L., Tendera M. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 20.Mitter S.S., Alvarez-Garcia J., Miller M.A., Moss N., Lala A. Insights from HeartLogic Multisensor Monitoring During the COVID-19 Pandemic in New York City. JACC Heart Fail. 2020;8:1053–1055. doi: 10.1016/j.jchf.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conraads V.M., Tavazzi L., Santini M., Oliva F., Gerritse B., Yu C.M., Cowie M.R. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: The SENSE-HF trial. Eur. Heart J. 2011;32:2266–2273. doi: 10.1093/eurheartj/ehr050. [DOI] [PubMed] [Google Scholar]
- 22.Maines M., Landolina M., Lunati M., Lonardi G., Pappone A., Proclemer A., Zanotto G., Santini M., Varbaro A., Vimercati M., et al. Intrathoracic and ventricular impedances are associated with changes in ventricular volume in patients receiving defibrillators for, C.R.T. Pacing. Clin. Electrophysiol. 2010;33:64–73. doi: 10.1111/j.1540-8159.2009.02579.x. [DOI] [PubMed] [Google Scholar]
- 23.Ogura A., Izawa K.P., Tawa H., Kureha F., Wada M., Harada N., Ikeda Y., Kimura K., Kondo N., Kanai M., et al. Impact of the COVID-19 pandemic on phase 2 cardiac rehabilitation patients in Japan. Heart Vessels. 2021;36:1184–1189. doi: 10.1007/s00380-021-01783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Shea C.J., Thomas G., Middeldorp M.E., Harper C., Elliott A.D., Ray N., Lau D.H., Campbell K., Sanders P. Ventricular arrhythmia burden during the coronavirus disease 2019 (COVID-19) pandemic. Eur. Heart J. 2021;42:520–528. doi: 10.1093/eurheartj/ehaa893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santini L., D’Onofrio A., Dello Russo A., Calò L., Pecora D., Favale S., Petracci B., Molon G., Bianchi V., De Ruvo E., et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin. Cardiol. 2020;43:691–697. doi: 10.1002/clc.23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capucci A., Santini L., Favale S., Pecora D., Petracci B., Calò L., Molon G., Cipolletta L., Bianchi V., Schirripa V., et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: A retrospective case series report. ESC Heart Fail. 2019;6:308–318. doi: 10.1002/ehf2.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calò L., Bianchi V., Ferraioli D., Santini L., Dello Russo A., Carriere C., Santobuono V.E., Andreoli C., La Greca C., Arena G., et al. Multiparametric Implantable Cardioverter-Defibrillator Algorithm for Heart Failure Risk Stratification and Management: An Analysis in Clinical Practice. Circ. Heart Fail. 2021;14:e008134. doi: 10.1161/CIRCHEARTFAILURE.120.008134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treskes R.W., Beles M., Caputo M.L., Cordon A., Biundo E., Maes E., Egorova A.D., Schalij M.J., Van Bockstal K., Grazioli-Gauthier L., et al. Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Fail. 2021;8:1541–1551. doi: 10.1002/ehf2.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Juan Bagudá J., Gavira Gómez J.J., Pachón Iglesias M., Cózar León R., Escolar Pérez V., González Fernández Ó., Rivas Gándara N., Goirigolzarri Artaza J., Díaz Molina B., Macías Gallego A., et al. Remote heart failure management using the HeartLogic algorithm. RE-HEART Registry. Rev. Esp. Cardiol. 2021 doi: 10.1016/j.rec.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Egolum U.O., Parikh K., Lekavich C., Wosik J., Frazier-Mills C., Fudim M. Applications of the Multisensor HeartLogic Heart Failure Monitoring Algorithm during the COVID-19 Global Pandemic. JACC Case Rep. 2020;2:2265–2269. doi: 10.1016/j.jaccas.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.