Abstract
There is an increasing focus on researching children admitted to hospital with new variants of COVID-19, combined with concerns with hyperinflammatory syndromes and the overuse of antimicrobials. Paediatric guidelines have been produced in Bangladesh to improve their care. Consequently, the objective is to document the management of children with COVID-19 among 24 hospitals in Bangladesh. Key outcome measures included the percentage prescribed different antimicrobials, adherence to paediatric guidelines and mortality rates using purposely developed report forms. The majority of 146 admitted children were aged 5 years or under (62.3%) and were boys (58.9%). Reasons for admission included fever, respiratory distress and coughing; 86.3% were prescribed antibiotics, typically parenterally, on the WHO ‘Watch’ list, and empirically (98.4%). There were no differences in antibiotic use whether hospitals followed paediatric guidance or not. There was no prescribing of antimalarials and limited prescribing of antivirals (5.5% of children) and antiparasitic medicines (0.7%). The majority of children (92.5%) made a full recovery. It was encouraging to see the low hospitalisation rates and limited use of antimalarials, antivirals and antiparasitic medicines. However, the high empiric use of antibiotics, alongside limited switching to oral formulations, is a concern that can be addressed by instigating the appropriate programmes.
Keywords: antibiotics, antimicrobial stewardship programs, Bangladesh, children, COVID-19, guidelines, hospitals, outcomes
1. Introduction
The principal focus on children in low- and middle-income countries (LMICs) during the COVID-19 pandemic has been to address key issues, including the poor uptake of vaccinations and the envisaged impact as well as behavioural issues as a consequence of lockdowns and social distancing measures [1,2,3,4,5,6]. This resulted in Bangladesh organising immunisation outreach services for children and increasing the number of home visits to address concerns [3,7]. This is because children have a lower risk of infection with COVID-19 than adults, with typically milder clinical manifestations [8,9,10,11,12,13,14] and an appreciable number are asymptomatic [11,15]. Cough, fever, diarrhoea, nausea and respiratory infections are generally the most frequent clinical characteristics of children with COVID-19, with boys typically more prone than girls [8,11,12,13,15,16]. Overall, it is estimated that approximately 6% to 10% of infected children experience severe disease, lower than rates seen in adults [11,12]. Consequently, the focus among children during the current pandemic has been on other infectious diseases and their prevention.
However, the focus on children is changing with new variants and their potential implications. In addition, whilst a lower percentage of children with severe symptoms are admitted to intensive care units (ICUs) in LMICs, deaths among the hospitalised children are higher [12].
There are also increasing concerns with the inappropriate use of antimicrobials across LMICs. Patients with COVID-19 in hospitals are typically administered antibiotics despite only a limited number having concomitant bacterial or fungal infections [17,18,19,20,21,22]. This includes hospitals in Bangladesh [23,24]. However, high inappropriate prescribing will increase antimicrobial resistance (AMR) rates, increasing morbidity, mortality and costs [25,26,27,28,29,30,31,32], with high rates of AMR already a concern in Bangladesh [33,34,35,36]. Children with COVID-19 can also develop Kawasaki Disease (KD)-like symptoms/hyperinflammatory syndromes [8,37,38,39] as well as experience leucopoenia with marked lymphopenia, hyponatremia, hypoalbuminemia, gastrointestinal and respiratory changes [37], potentially increasing ICU admissions.
There have also been concerns generally with the level of misinformation surrounding the management of COVID-19. This includes issues with hydroxychloroquine, lopinavir/ritonavir, remdesivir and ivermectin, with robust studies suggesting limited clinical benefit, although systematic reviews have suggested remdesivir can accelerate clinical improvement [40,41,42,43,44,45,46,47,48,49]. This is a concern as misinformation can increase morbidity, mortality and costs [50,51,52,53]. Concerns with misinformation have resulted in groups, including the World Health Organization (WHO) and the British Medical Journal (BMJ), providing evidence-based guidance [54,55,56,57,58]. International guidelines have also been developed to improve the management of children with COVID-19 in paediatric intensive care unit (PICU) countries [59]. The Ministry of Health and Family Welfare in Bangladesh also made national guidelines available from May 2020 onwards [60]. Similarly, the Bangladesh Paediatric Association has published its guidelines as this patient population is inherently a vulnerable one (Table 1) [61].
Table 1.
Key recommendations for managing adults and children with COVID-19 in hospitals in Bangladesh (adapted from [60,61]).
| Adults/General (May 2020) |
Treatment (General)
Pharmacotherapy (moderate cases)
Severe disease
|
| Children (November 2020) |
Diagnosis and comorbidity
General treatment and ICU
Recommended medicines for treating children with COVID-19
|
ARDS—acute respiratory distress syndrome; ECMO—extracorporeal membrane oxygenation; ICU—Intensive Care Unit.
Consequently, we believe it is important to document the current management of children admitted to hospitals in Bangladesh with suspected or confirmed COVID-19, especially given concerns with antimicrobial use. In view of this, the objective of this study is to document current prevalence rates, treatments and outcomes of children with COVID-19, admitted to hospitals in Bangladesh in recent months. The findings can be used to suggest future quality improvement programmes in Bangladesh and other countries, if pertinent.
2. Results
We will first document the characteristics of patients admitted to hospitals across Bangladesh before documenting prescribed treatments and patient outcomes. One hundred and forty-six children were admitted with COVID-19 to the 24 hospitals taking part in this study, giving an overall prevalence of 3.7% (n = 3902, range = 0.55% to 29.5%) during the study period. COVID-19 was confirmed in the majority of admitted children by PCR tests (n = 111, 76%). The majority of admitted children were boys (n = 86, 58.9%) and were aged between 0 and 5 years (n = 91, 62.3%) (Table 2).
Table 2.
Patient characteristics among the 24 participating hospitals during the study period.
| Hospital | Date Survey Conducted | Total Number of Admitted Children during the Study Period | Total Number with COVID-19 (No.) | % of Admitted Children with COVID-19 | Number of Children with Confirmed COVID-19 (No. and %) | Number of Boys (No.) | Number of Girls (No.) | 0 to 5 Years of Age (no.) | 6 to 10 Years of Age (No.) | 11 to 18 Years of Age (No.) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1—Priv | 20 July 2021 | 14 | 2 | 14.3% | 2 (100%) | 0 | 2 | 1 | 0 | 1 |
| 2—Priv | 25 July 2021 | 18 | 1 | 5.6% | 1 (100%) | 1 | 0 | 0 | 1 | 0 |
| 3—Priv | 30 July 2021 | 19 | 2 | 10.5% | 1 (one susp.) | 1 | 1 | 1 | 1 | 0 |
| 4—Priv | 16 July 2021 | 32 | 2 | 6.3% | 0 (2 susp.) | 1 | 1 | 2 | 0 | 0 |
| 5—Priv | 11 August 2021 | 40 | 5 | 12.5% | 5 (100%) | 2 | 3 | 2 | 2 | 1 |
| 6—Priv | 30 July 2021 | 41 | 3 | 7.3% | 3 (100%) | 3 | 0 | 2 | 0 | 1 |
| 7—Pub | 04 February 2021 | 44 | 13 | 29.5% | 1 (12 susp.) | 5 | 8 | 11 | 2 | 0 |
| 8—Priv | 11 July 2021 | 50 | 5 | 10.0% | 0 (5 susp.) | 4 | 11 | 3 | 2 | 0 |
| 9—Priv | 24 July 2021 | 61 | 5 | 8.2% | 5 (100%) | 3 | 2 | 1 | 2 | 2 |
| 10—Priv | 11 August 2021 | 65 | 3 | 4.6% | 3 (100%) | 2 | 1 | 3 | 0 | 0 |
| 11—Priv | 01 August 2021 | 68 | 6 | 8.8% | 6 (100%) | 5 | 1 | 3 | 3 | 0 |
| 12—Priv | 30 July 21 | 75 | 15 | 20.0% | 15 (100%) | 9 | 6 | 4 | 9 | 2 |
| 13—Priv | 10 August 2021 | 85 | 4 | 4.7% | 4 (100%) | 2 | 2 | 3 | 1 | 0 |
| 14—Priv | 04 August 2021 | 103 | 14 | 13.6% | 0 (14 susp.) | 10 | 4 | 13 | 0 | 1 |
| 15—Pub | 31 July 2021 | 105 | 1 | 1.0% | 1 (100%) | 1 | 0 | 1 | 0 | 0 |
| 16—Pub | 31 August 2021 | 125 | 5 | 4.0% | 5 (100%) | 4 | 1 | 2 | 3 | 0 |
| 17—Pub | 04 August 2021 | 68 | 3 | 4.4% | 3 (100%) | 2 | 1 | 2 | 0 | 1 |
| 18—Pub | 31 July 2021 | 210 | 26 | 12.4% | 26 (100%) | 15 | 11 | 13 | 6 | 7 |
| 19—Pub | 11 July 2021 | 256 | 3 | 1.2% | 3 (100%) | 2 | 1 | 2 | 1 | 0 |
| 20—Pub | 08 October 2021 | 362 | 3 | 0.55% | 3 (100%) | 1 | 2 | 0 | 0 | 3 |
| 21—Pub | 11 July 2021 | 382 | 3 | 0.8% | 2 (1 susp) | 2 | 1 | 3 | 0 | 0 |
| 22—Pub | 08 November 2021 | 583 | 8 | 1.4% | 8 (100%) | 5 | 3 | 6 | 1 | 1 |
| 23—Pub | 08 November 2021 | 442 | 5 | 1.1% | 5 (100%) | 1 | 4 | 4 | 1 | 0 |
| 24—Pub | 17 July 2021 | 654 | 9 | 1.4% | 9 (100% | 5 | 4 | 9 | 0 | 0 |
| 3902 | 146 | 3.7% | 111—76% confirmed | 86 (58.9%) | 60 (41.1%) |
91 (62.3%) | 35 (24.0%) | 20 (13.7%) |
Column 3 includes children admitted during the study period and not on any specific day; Priv = private (including not for profit) hospital; Pub = public hospital; Confirmed—by PCR testing; susp = suspected.
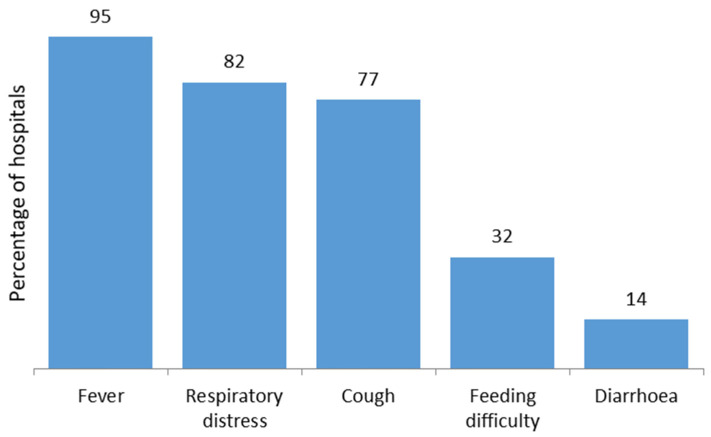
The consolidated principal reasons for hospital admission are documented in Table 3 based on pre-determined choices (Methodology Section 4.2), with only a limited number of children (n = 20, 13.7% including one referral) transferred to the PICU, typically with low oxygen saturation (Table 3, Figure 1). Children were typically admitted with more than one symptom, with Table 3 documenting the consolidated principal reasons for admission among participating hospitals. Potential reasons for admission to the PICU were also pre-determined and are documented in the Methodology Section 4.2. There were only a limited number of comorbidities among admitted children with COVID-19. These included bronchial asthma, dilated cardiomyopathy, congenital heart disease, dengue, nephrotic syndrome, malnutrition, pneumonia, sepsis and urinary tract infections.
Table 3.
Rationale for admission to the hospital and those subsequently admitted to the PICU.
| Hospital | Principal Documented Reasons for Hospital Admission for Children with Suspected COVID-19 during the Study Period | Total Number of Children Subsequently Admitted with COVID-19 to PICUs | Principal Reasons for PICU Admission |
|---|---|---|---|
| 1—Priv | Respiratory distress | 2 | Low oxygen saturation, comorbidities |
| 2—Priv | Fever, coughing, vomiting | 0 | Not applicable |
| 3—Priv | Fever, cough, low SPO2, respiratory distress, vomiting, diarrhoea | 0 | Not applicable |
| 4—Priv | Fever, cough, respiratory distress | 0 | Not applicable |
| 5—Priv | Prolonged fever, respiratory distress, diarrhoea | 1 | Low oxygen saturation, extensive involvement in high-resolution CT scan |
| 6—Priv | Prolonged fever, cough, respiratory distress | 0 | Not applicable |
| 7—Pub | Fever, cough, respiratory distress | 0 (1 referred) * | Unexplained bleeding |
| 8—Priv | Prolonged fever, breathing difficulties, diarrhoea | 1 | Shock |
| 9—Priv | Fever, cough, feeding difficulties | 1 | Shock, myocarditis |
| 10—Priv | Respiratory distress, cough, feeding difficulties | 0 | Not applicable |
| 11—Priv | Fever, cough, feeding difficulties | 2 | Low oxygen saturation, respiratory distress, shock |
| 12—Priv | Fever, respiratory distress, feeding difficulty | 0 | Not applicable |
| 13—Priv | Prolonged fever, cough, breathing difficulty/respiratory distress | 0 | Not applicable |
| 14—Priv | Fever, respiratory distress, feeding difficulty | 0 | Not applicable |
| 15—Pub | Fever, cough, respiratory distress | 0 | Not applicable |
| 16—Pub | Prolonged fever, cough, Breathing difficulties/respiratory distress, diarrhoea | 0 | Not applicable |
| 17—Pub | Fever, cough, respiratory distress | 0 | Not applicable |
| 18—Pub | Fever, cough, low SPO2, respiratory distress, vomiting | 9 | Fever, shock, low oxygen saturation, vomiting, feeding difficulties |
| 19—Pub | Fever, cough, respiratory distress | 0 | Not applicable |
| 20—Pub | Fever, cough, respiratory distress | 1 | Low oxygen saturation and comorbidities |
| 21—Pub | Prolonged fever, cough, respiratory distress | 0 | Not applicable |
| 22—Pub | Fever, cough, respiratory distress | 2 | Perinatal asphyxia, feeding difficulty, low oxygen saturation |
| 23—Pub | Fever, cough, respiratory distress | 0 | Not applicable |
| 24—Pub | Prolonged fever, respiratory distress, feeding difficulties | 0 | Not appliable |
PICU = Paediatric Intensive Care Unit; Priv = private hospital; Pub = public hospital; SPO2 = Saturation of peripheral oxygen; * referred to the PICU in another hospital.
Figure 1.
Reasons for hospital admission of children with COVID-19 (n = 146).
Table 4 contains the consolidated list of antibiotics (class or specific) prescribed for children with suspected or confirmed COVID-19 among the participating hospitals. As seen, there was a considerable prescribing of antibiotics, with 86.3% (126/146) of children prescribed them, with almost all antibiotics administered empirically (98.4%—124/126). In general, antibiotics were prescribed from the WHO Watch list as opposed to the Reserve list. In addition, antibiotics were typically administered parenterally with generally limited prescribing of oral antibiotics, including switching, for between 3 and 14 days. Children were generally assessed after 2 to 3 days (Table 4).
Table 4.
Clinical management of children with COVID-19 among the 24 hospitals.
| Hospital | Number and % Prescribed Antibiotics | Empiric or Following CST | Principal Antibiotics Prescribed (Actual or Class) for Children with COVID-19 | Antiviral Medicines Prescribed | Antiparasitic Medicines Prescribed | Duration of Antibiotic Prescribing (Days) | Clinical Assessment of Antibiotics (Days after Start of Treatment) |
|---|---|---|---|---|---|---|---|
| 1—Priv | 2 (100%) | All Empiric | Carbapenem, cephalosporins | No | No | 4–7 | 3 |
| 2—Priv | 1 (100%) | All Empiric | Ceftriaxone | No | No | 5 | 5 |
| 3—Priv | 2 (100%) | All Empiric | Ceftriaxone, ciprofloxacin | No | No | 3 and 5 | 3–5 |
| 4—Priv | 2 (100%) | All Empiric | Ampicillin, aminoglycosides, cephalosporins | No | No | 5 | 2 |
| 5—Priv | 5 (100%) | All Empiric | Cephalosporins, quinolones | Remdesivir (2 patients) | No | 7 | 2 |
| 6—Priv | 2 (66.7%) | All Empiric | Cephalosporins | No | No | 5–7 | 5–7 |
| 7—Pub | 13 (100%) | All Empiric | Ceftriaxone, amikacin, meropenem | No | No | 5–7 | 3 |
| 8—Priv | 5 (100%) | All Empiric | Carbapenem, aminoglycosides, cephalosporins | No | No | 5–7 | 3 |
| 9—Priv | 2 (40%) | All Empiric | Vancomycin, meropenem, co-amoxiclav | Remdesivir (1 patient) | No | 7–10 | 3 |
| 10—Priv | 3 (100%) | All Empiric | Ceftriaxone | No | No | 10 | 3 |
| 11—Priv | 4 (66.7%) | All Empiric | Ceftriaxone, ciprofloxacin, amikacin | Remdesivir (1 patient) | No | 3–7 | 3 |
| 12—Priv | 2 (13.3%) | 1 Empiric/1 CST | Aminoglycosides, cephalosporins, quinolones | No | No | 5–7 | Not recorded |
| 13—Priv | 4 (100%) | All Empiric | Aminoglycosides, carbapenems, cephalosporins | No | Ivermectin (1 patient) | 10 | 3–5 |
| 14—Priv | 14 (100%) | All Empiric | Aminoglycosides, penicillin, cephalosporins | No | No | 5–7 | 3 |
| 15—Pub | 1 (100%) | All Empiric | Cefixime | No | No | 7 | 3 |
| 16—Pub | 5 (100%) | All Empiric | Aminoglycosides, carbapenems, cephalosporins | No | No | 7–10 | 2–3 |
| 17—Pub | 3 (100%) | All Empiric | Ceftriaxone, ceftazidim, gentamicin | No | No | 3–7 | 3 |
| 18—Pub | 26 (100%) | All Empiric | Ceftriaxone, ceftazidim, meropenem, vancomycin, flucloxacillin, amikacin | No | No | 5–11 | 5–10 |
| 19—Pub | 3 (100%) | All Empiric | Ceftriaxone, meropenem | No | No | 7 | 3 |
| 20—Pub | 3 (100%) | All Empiric | Penicillin, carbapenem, aminoglycosides | Remdesivir (3 patients), acyclovir (1 patient) | No | 8–14 | 3 |
| 21—Pub | 3 (100%) | All Empiric | Amikacin, meropenem, clarithromycine | No | No | 7 | 2 |
| 22—Pub | 7 (87.5%) | 6 Empiric/1 CST | Ceftriaxone, amikacin, meropenem | No | No | 4–7 | 3 |
| 23—Pub | 5 (100%) | All Empiric | Ceftriaxone, amikacin, meropenem | No | No | 7–14 | 3 |
| 24—Pub | 9 (100%) | All Empiric | Aminoglycosides, carbapenem, macrolides | No | No | 7 days (minimum) | 3 |
| Total | 126 children—(86.3%) | 124 empiric (98.4%) | 8 children (5.5%) | 1 child (0.7%) |
Antibiotics could also be prescribed for underlying comorbidities; Priv = private (including not for profit) hospital; Pub = public hospital.
There was no prescribing of hydroxychloroquine for any child admitted with COVID-19 in the surveyed hospitals and very limited prescribing of either antivirals, including remdesivir, or antiparasitic medicines, including ivermectin (Table 4). Steroids were prescribed to children in 13 of the surveyed hospitals (n = 13, 54.2%), with immune boosters, including vitamins C and D, prescribed to children in 14 of the participating hospitals (n = 14, 58.3% of hospitals).
There were lower rates of prescribing of antibiotics among those hospitals where the clinicians stated that they had followed the Bangladesh Paediatric Association guidelines (Table 1 and Table 5). However, there was no significant statistical association (p = 0.127) between the administration of antibiotics and the clinicians in the participating hospitals who had stated they had complied with the Paediatric Association guidelines when prescribing antibiotics to children with suspected or confirmed COVID-19 and those who had not (Table 5).
Table 5.
Prescribing of antibiotics and adherence to Bangladesh Paediatric Association guidelines.
| Guideline adherence | Number of Children Administered Antibiotics | Number of Children Not Administered Antibiotics | % Administered Antibiotics |
|---|---|---|---|
| Hospitals where clinicians stated that they had followed the Paediatric Association guidelines | 74 | 15 | 83.1% (n = 89) |
| Hospitals where clinicians stated that they had not followed the Paediatric Association guidelines | 52 | 5 | 91.2% (n = 57) |
Total number of children = 146.
Encouragingly, at 1.4% (2/146), there were only a limited number of deaths among the children admitted with COVID-19, with 92.5% making a full recovery (135/146). The remainder still had complications during the study period.
3. Discussion
To the best of our knowledge, this is the first comprehensive study conducted among a range of both private and public hospitals across Bangladesh concerning the management of children hospitalised with suspected or confirmed COVID-19. Our findings build on the earlier study of Hussain et al. (2020), who found a higher mortality rate (13.3%) among children during the early stages of the pandemic [3], and the recent pilot studies conducted among children in Bangladesh and India [62,63]. The reduced mortality among children hospitalised with COVID-19 in our study (1.4%) compared with the study conducted by Hussain et al. (2020) may reflect improved clinical management as more knowledge is gained regarding the aetiology of COVID-19, especially with greater recognition of Kawasaki disease (KD)-like symptoms/hyperinflammatory syndromes among these children [8,37,38,39]. In addition, greater knowledge of treatment approaches has resulted in the development of updated guidelines (Table 1) as well as updated advice from groups such the WHO and the BMJ [54,58]. However, further research is needed on this topic before we can say anything with certainty. Having said this, only a low number of children were admitted to these general hospitals in Bangladesh with COVID-19 (Table 2), similar to the findings of other recent publications, including the recent pilot study in India [11,13,15,63].
Fever, cough and respiratory diseases were, again, the most frequent clinical characteristics of children admitted to the surveyed hospitals with COVID-19 (Table 3, Figure 1), with admission to hospital with suspected or confirmed COVID-19 also more common among boys (58.9%), similar to other studies [8,11,12,13,15,16,63].
Encouragingly there was limited prescribing of antiviral, antiparasitic and antimalarial medicines among the children with COVID-19 admitted to the surveyed hospitals (Table 4). This is in line with recommendations in the Bangladesh Paediatric guidelines (Table 1), with similar guidance in other countries [61,64,65]. This is important given concerns with the lack of effectiveness of these medicines in treating patients with COVID-19, despite the initial hype [40,41,43,44]. Encouragingly, the prescribing of immunomodulators such as vitamins and zinc also appeared to be in line with the paediatric guidelines (Table 1). However, we are beginning to see immunomodulators being discouraged in some LMICs [65].
However, there were concerns with the high empiric use of antibiotics among the children in the surveyed hospitals (Table 3), which is similar to their high use among adults admitted to hospitals with COVID-19 in Bangladesh [23,24]. These rates (86.3%) are higher than a recent pilot study undertaken in India, with rates of 69.4% [63]. This is despite, as mentioned, only a limited number of patients with COVID-19 across ages having concomitant bacterial or fungal infections [17,18,19,20,21,22,31,66]. In addition, the Paediatric Association guidelines advocate only the prudent use of antibiotics due to concerns with potential over-use (Table 1). However, the rates of antibiotic prescribing appear to be less among children where the clinicians have stated they followed paediatric guidelines (Table 5); however, this was not statistically significant. In any event, these high rates need to be addressed, going forward, to reduce future AMR.
Potential ways forward to improve the rational use of antibiotics among these children include instigating antimicrobial stewardship programmes (ASPs), combined with an additional COVID-19 module, and other educational activities; these currently do not exist. Such activities have been successful across LMICs to improve the rational use of antibiotics in a number of situations [28,29,32,36,67,68,69,70,71,72].
Future activities should also include encouraging antibiotic prescribing according to local antibiograms where initial prescribing is typically empiric, combined with instigating ASPs [35,73,74,75]. These combined activities should help reduce the extent of prescribing ‘Watch’ antibiotics in favour of the increased prescribing of ‘Access’ antibiotics, where pertinent (Table 2 and Table 4) [76,77]. ASPs could also potentially encourage earlier switching from parental to oral antibiotics where it is pertinent and practical to hasten earlier discharge and conserve resources [78,79,80]. This is important with the current practice of limited switching to oral antibiotics among the surveyed hospitals. We will be following up on these identified issues in future studies to improve antibiotic prescribing in Bangladesh, given continual concerns with high rates of AMR [33,34,35,81].
The seemingly low number of children admitted to these general hospitals in Bangladesh versus other conditions is important when considering future priorities with limited resources. This includes reviewing the clinical conditions of the other admitted children during this period without COVID-19 and whether resources should be focused on these children instead. In addition, there are key areas such as vaccinations to prevent future infections among children [28]. We are aware that in Bangladesh and other LMICs, vaccination rates have been appreciably compromised by lockdowns and other measures [1,2,3,4,5]. Whilst this was not a focus for this study, we are aware that the direct and indirect impact of COVID-19 on children has been underestimated. Children in LMICs are more likely to suffer from other comorbidities such as other respiratory tract infections, including pneumonia as well as malnutrition and HIV, which may complicate a COVID 19 diagnosis [82]. Consequently, we will also be following up on these issues as well in future research projects to provide additional guidance to all key stakeholder groups in Bangladesh and other countries.
We are aware of several limitations with this study. These included the fact that this was only a retrospective review of medical records without the ability to check with physicians regarding the rationale for any prescribing, especially of antibiotics. We were also limited by the extent of information that could be collected from the surveyed hospitals, and all cases of COVID-19 could not be confirmed by rt-PCR. We also could not analyse any differences between private and public hospitals as admission and other key criteria were not standardised. In addition, we extended the study period in each hospital in order to collect more data on the management of children with COVID-19. Consequently, our prevalence rates of admitted children could be overestimated. However, despite these limitations, we believe our findings are robust, given the number of participating hospitals, providing direction for future quality improvement programmes and other activities.
4. Materials and Methods
4.1. Study Setting and Design
The study was undertaken among a range of hospitals (n = 24) across Bangladesh, admitting children with suspected or confirmed COVID-19. Both private and public hospitals were included to ensure full representation, similar to point prevalence surveys conducted in other healthcare systems with a mixture of hospital types [83,84,85]. Initially, children in Bangladesh with COVID-19 were referred to public hospitals, mainly medical college hospitals. However, this changed, with private hospitals subsequently able to treat children admitted with COVID-19.
Private and public hospitals were not analysed separately due to anticipated different admission criteria, making comparisons problematic and difficult to justify. In addition, the aims of the study were to gain insights into current prevalence rates among children admitted with suspected COVID-19 to hospitals in Bangladesh, as well as insights into current management practices, rather than to assess differences in treatments and outcomes between different hospital types. This included the current prescribing of antimicrobials among hospitals in Bangladesh. The hospitals were purposely selected based on their ability to provide pertinent data as well as maximum variation in terms of their geography, location and size (Table 2).
This point prevalence survey was conducted based on standardised methodologies, with the data forms adapted to meet the specific requirements of the study and setting [62,83,84,85,86,87,88] (Supplementary Material File S1). Data were gathered from the medical records of children admitted to hospital with suspected or confirmed COVID-19 between July and November 2021. This included children subsequently admitted to PICUs, if such facilities existed in the hospital. Care in public hospitals in Bangladesh for these patients is typically provided without the need for co-payments. However, there are charges for patients admitted to private hospitals.
4.2. The Data Collection Tool and Analysis
A paper-based data collection tool was used by the investigators to collect the necessary information at the patient level. The specific forms were adapted from previous point prevalence surveys conducted in LMICs involving a number of co-authors [83,85,86,87,88,89,90,91,92]. This was combined with a synthesis of current guidelines for managing children with COVID-19 in Bangladesh (Table 1), a synthesis of the current literature [3,11,12,20,93] and a cognisance of the study aims and objectives. The data collection forms were pre-tested to ensure they would meet the study objectives. A pilot study was undertaken among a limited number of hospitals in Bangladesh [62] prior to full roll-out, with a similar pilot study undertaken in India [63]. The same forms were subsequently used in this multicentre study in Bangladesh as they were able to provide the relevant information to meet the aims and objectives of the study (Supplementary Material File S1).
There were no exclusion criteria. All children admitted on the day of data collection and during the previous nine days were included to give a combined total over a ten-day period.
The key data sets collected and the rationale for their inclusion in the data collection forms, where pertinent, are included in Table 6. The information was collected by the principal investigator in each hospital, with initial data collection taking place on one specific day, similar to other PPS studies. The medical records of patients admitted in the previous ten days were also viewed. Ten days were chosen for this study by the principal investigators because of the anticipated low numbers of children with COVID-19 actually admitted to general hospitals in Bangladesh on any specific day, with children with COVID-19 generally asymptomatic or with milder clinical manifestations versus adults [8,10,11,12,13]. Alongside this, there were anticipated low numbers of children with COVID-19 in the participating hospitals compared with other conditions of admitted children. In addition, as mentioned, the principal objective of the study was to gain knowledge of current management practices, including any concerns with antimicrobial use, rather than a robust assessment of prevalence rates. Children were also followed up, if necessary, to determine the average number of days they were prescribed antibiotics and when re-assessed.
Table 6.
Key data sets collected and their rationale.
| Key Data Sets | Rationale |
|---|---|
| Number of paediatric patients being treated in the hospital during the ten-day period and the number with COVID-19 | To gain insight into current prevalence rates among children admitted to hospitals in Bangladesh compared with other conditions |
| Whether COVID-19 was suspected or confirmed—confirmed with PCR tests | To gain additional insight into current COVID-diagnostic practices |
| The ages of admitted children | Patients’ ages were broken down into 3 bands: 0–5 years; 6–10 years; 11–18 years for comparative purposes based on the pilot study in both Bangladesh and India [62,63] |
| Principal reason for admission to hospital with actual/suspected COVID-19 |
|
| Comorbidities | Based on evidence amongst adults that certain comorbidities do have an impact on morbidity and mortality associated with COVID-19 [94,95] |
| Number of children admitted to PICU and the rationale |
|
| Number of children prescribed antibiotics and the antibiotics prescribed (by ATC Level 4 Grouping or individual antibiotics), and whether empiric or following CST findings |
|
| Route of administration, whether the rationale for antibiotic prescribing was re-assessed, and, if so, after how many days, and total length of antibiotic prescriptions |
|
| The extent of prescribing of antivirals, e.g., remdesivir, antimalarials, e.g., hydroxychloroquine, and antiparasitic medicines, e.g., ivermectin |
|
| The extent of prescribing of dexamethasone and other steroids, including methylprednisolone | Seen as potentially beneficial, especially among hospitalised patients, and endorsed in the guidelines [46,61,100] |
| Use of supplements/immune boosters including vitamins C or D or zinc | Discussed in the Bangladesh Paediatric Guidelines, with publications suggesting potential benefit [61,101,102] |
| Adherence to current guidelines, including those developed by the Bangladesh Paediatric Association —Table 1 [50] |
|
| Outcome—fully recovered, morbidity or mortality |
|
| Possible costs (principally private hospitals) (based on local currency) |
|
PICU = Pediatric Intensive Care Unit.
Data were collated by the principal investigator in each participating hospital and subsequently entered into Microsoft Excel® spreadsheets for analysis. As mentioned (Table 6), these contained drop-down menus with options for specific questions. The aggregated data for each hospital was subsequently transferred to the principal investigator collating the findings (BG) for analysis, with the ability to re-check and re-validate data when the need arose with the co-authors from the pertinent hospitals.
Descriptive statistics were typically used to summarise and collate the data using proportions (%) as appropriate, given the likely differences in admittance criteria between hospitals. However, the chi-square statistical test was used to assess whether there were significant differences in antibiotic prescribing between those hospitals that stipulated they followed current guidelines versus the remainder, with a p-value < 0.05 seen as significant (Table 5).
4.3. Patient and Hospital Anonymity and Ethical Approval
Patient anonymity was maintained throughout the study by the principal investigator in each hospital. Only aggregated anonymised data for each hospital was forwarded to the principal investigator collating the findings (BG) for analysis. Additionally, in the final analysis, hospitals were assigned a study identification number rather than each hospital being specifically identified (Hospitals 1 to 24).
Parents or guardians were not approached for consent since this was a retrospective study based on data collated from patients’ medical records, with no direct contact with children, their parents, or guardians. This is in line with previous point prevalence surveys undertaken by the co-authors [84,86,88,89,90,92,112].
The principal investigators orchestrated ethical approval for the hospitals and others involved in the study. The reference numbers are ibh/mirpur/2020/01; GSVMC/2021/346; BBEC, JU/M 2021/COVID-19/7 and AWMC/IRB-21 July 2021/028.
5. Conclusions
The low rates for the hospitalisation of children with COVID-19 among the surveyed hospitals in Bangladesh are encouraging and mirror the findings in other countries.
It was also encouraging to see limited prescribing of repurposed antimicrobials, including antimalarials, antivirals and antiparasitic medicines, given the hype that has surrounded these treatments, particularly hydroxychloroquine, which were without foundation once the findings from robust studies, including the WHO Solidarity and the UK Recovery studies, became known. This is important for promoting evidence-based approaches in the future.
However, high rates of the prescribing of antibiotics, often empiric, even among hospitals stating they are following the Paediatric Association guidelines, coupled with limited switching to oral formulations, are a concern. These issues need to be urgently addressed in Bangladesh, going forward, given the already high rates of AMR in the country coupled with ongoing resource pressures. This is important to reduce future morbidity, mortality, and costs. The instigation of local antibiograms and ASPs are key ways forward to improve future antibiotic use in hospitals in Bangladesh as well as LMICs generally. We will be monitoring such activities in the future.
Acknowledgments
The authors would like to thank Niam Chy from the University of Chittagong, Bangladesh, for his help with producing the figures.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11010105/s1, File S1: Bangladesh: COVID-19 Pediatric Case Management.
Author Contributions
Conception/design: K.C., M.H. (Mainul Haque), N.N. (Nadia Nusrat), N.A. (Nihad Adnan), N.S. (Natalie Schellack), A.K., M.G., J.C.M., S.O., B.G. Acquisition and analysis. K.C., M.H. (Mainul Haque), N.N. (Nadia Nusrat), N.A. (Nihad Adnan), S.I., A.B.L., D.B., A.R., E.K., A.M., N.J., J.A., S.A. (Sumala Ashraf), M.N.H., M.H. (Mahmuda Hassan), N.A. (Najnin Akhter), M.M., N.S. (Nazmus Sihan), N.N. (Nurun Naher), S.A. (Shaheen Akter), S.U.Z., T.C., J.N., S.B., M.D.I., A.M.H., H.R., P.K.B., M.S., F.C., A.K., N.S. (Natalie Schellack), J.C.M., B.G. Interpretation of the data: K.C., M.H. (Mainul Haque), N.N., N.A. (Nihad Adnan), S.I., A.B.L., S.K., A.K., Z.U.M., N.S. (Natalie Schellack), M.G., J.C.M., S.O., B.G. Drafting the work: K.C., M.H. (Mainul Haque), N.N. (Nadia Nusrat), S.K., A.K., N.S. (Natalie Schellack), M.G., J.C.M., S.O., B.G. Critically revising the paper: all authors. Accuracy of the data appropriately collected and included in the analysis as well as resolving queries: K.C., M.H. (Mainul Haque), N.N. (Nadia Nusrat), N.A. (Nihad Adnan), S.I., A.B.L., D.B., A.R., E.K., A.M., N.J., J.A., S.A. (Sumala Ashraf), M.N.H., M.H. (Mahmuda Hassan), N.A. (Najnin Akhter), M.M., N.S. (Nazmus Sihan), N.N. (Nurun Naher), S.A. (Shaheen Akter), S.U.Z., T.C., J.N., S.B., M.D.I., A.M.H., H.R., P.K.B., M.S., F.C., A.K., N.S. (Natalie Schellack), J.C.M., B.G. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding for this paper. The data collection and the development of the paper was funded by the co-authors.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The principal investigators orchestrated ethical approval for the hospitals and others involved in the study. The reference numbers are ibh/mirpur/2020/01, date: 31 August 2021; GSVMC/2021/346, date: 22 August 2021; BBEC, JU/M 2021/COVID-19/7, date: 18 July 2021, DLMCH/IEC2021/1, date: 06 October 2021, and AWMC/IRB-21 July 2021/028, date: 10 August 2021.
Informed Consent Statement
As mentioned, parents or guardians were not approached for consent since this was a retrospective study based on data collated from patients’ medical records, with no direct contact with children, their parents, or guardians. This is in line with previous point prevalence studies undertaken by the co-authors [84,86,88,89,90,92,112].
Data Availability Statement
Further data regarding the study is available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbas K., Procter S.R., van Zandvoort K., Clark A., Funk S., Mengistu T., Hogan D., Dansereau E., Jit M., Flasche S., et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: A benefit–risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob. Health. 2020;8:e1264–e1272. doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaythorpe K.A., Abbas K., Huber J., Karachaliou A., Thakkar N., Woodruff K., Li X., Echeverria-Londono S., Fouda A.A.B., Cutts F., et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. eLife. 2021;10:e67023. doi: 10.7554/eLife.67023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain M., Al Mamun M.A. COVID-19 in children in Bangladesh: Situation analysis. Asia Pac. J. Paediatr. Child Health. 2020;3:59–65. [Google Scholar]
- 4.Lassi Z., Naseem R., Salam R., Siddiqui F., Das J. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18:988. doi: 10.3390/ijerph18030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sama B.K., Kaur P., Thind P.S., Verma M.K., Kaur M., Singh D.D. Implications of COVID-19-induced nationwide lockdown on children’s behaviour in Punjab, India. Child Care Health Dev. 2020;47:128–135. doi: 10.1111/cch.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhoza P., Danovaro-Holliday M.C., Diallo M.S., Murphy P., Sodha S.V., Requejo J.H., Wallace A.S. Routine Vaccination Coverage—Worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1495–1500. doi: 10.15585/mmwr.mm7043a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rana S., Shah R., Ahmed S., Mothabbir G. Post-disruption catch-up of child immunisation and health-care services in Bangladesh. Lancet Infect. Dis. 2021;21:913. doi: 10.1016/S1473-3099(21)00148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A., Rinaldi E., Zusi C., Beatrice G., Saccomani M.D., Dalbeni A. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: A meta-analysis. Pediatr. Res. 2020;89:733–737. doi: 10.1038/s41390-020-1015-2. [DOI] [PubMed] [Google Scholar]
- 9.Kanthimathinathan H.K., Dhesi A., Hartshorn S., Ali S.H., Kirk J., Nagakumar P., Jyothish D. COVID-19: A UK Children’s Hospital Experience. Hosp. Pediatr. 2020;10:802–805. doi: 10.1542/hpeds.2020-000208. [DOI] [PubMed] [Google Scholar]
- 10.Mehta N.S., Mytton O.T., Mullins E.W.S., Fowler T.A., Falconer C.L., Murphy O.B., Langenberg C., Jayatunga W.J.P., Eddy D.H., Nguyen-Van-Tam J.S. SARS-CoV-2 (COVID-19): What do we know about children? A systematic review. Clin. Infect. Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhuiyan M.U., Stiboy E., Hassan M.Z., Chan M., Islam M.S., Haider N., Jaffe A., Homaira N. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021;39:667–677. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irfan O., Muttalib F., Tang K., Jiang L., Lassi Z.S., Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: A systematic review and meta-analysis. Arch. Dis. Child. 2021;106:440–448. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui X., Zhao Z., Zhang T., Guo W., Guo W., Zheng J., Zhang J., Dong C., Na R., Zheng L., et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2020;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque M., Gowere M., Nusrat N., Chowdhury K., Godman B. The response to COVID 19 across countries and the implications for future pandemics. Bangladesh J. Med. Sci. 2021;20:7–14. doi: 10.3329/bjms.v20i5.55417. [DOI] [Google Scholar]
- 15.Mansourian M., Ghandi Y., Habibi D., Mehrabi S. COVID-19 infection in children: A systematic review and meta-analysis of clinical features and laboratory findings. Arch. Pédiatrie. 2021;28:242–248. doi: 10.1016/j.arcped.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam M.A., Kundu S., Alam S.S., Hossan T., Kamal M.A., Hassan R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of 17515 patients. PLoS ONE. 2021;16:e0249788. doi: 10.1371/journal.pone.0249788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustafa Z.U., Salman M., Aldeyab M., Kow C.S., Hasan S.S. Antimicrobial consumption among hospitalized patients with COVID-19 in Pakistan. SN Compr. Clin. Med. 2021;3:1691–1695. doi: 10.1007/s42399-021-00966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini V., Jain C., Singh N., Alsulimani A., Gupta C., Dar S., Haque S., Das S. Paradigm Shift in Antimicrobial Resistance Pattern of Bacterial Isolates during the COVID-19 Pandemic. Antibiotics. 2021;10:954. doi: 10.3390/antibiotics10080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., Soucy J.-P.R., Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.-P.R., Westwood D., Daneman N., MacFadden D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L.S.-K., Chau S.K.-Y., Tso E.Y.-K., Tsang S.W.-C., Li I.Y.-F., Wong B.K.-C., Fung K.S.-C. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: Experience from a single hospital cluster. Ther. Adv. Infect. Dis. 2020;7 doi: 10.1177/2049936120978095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grau S., Hernández S., Echeverría-Esnal D., Almendral A., Ferrer R., Limón E., Horcajada J.P., On behalf of the Catalan Infection Control Antimicrobial Stewardship Program Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics. 2021;10:943. doi: 10.3390/antibiotics10080943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molla M.A., Yeasmin M., Islam K., Sharif M., Amin R., Nafisa T., Ghosh A.K., Parveen M., Arif M.H., Alam J.A.J., et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Infect. Prev. Pract. 2021;3:100134. doi: 10.1016/j.infpip.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mah-E-Muneer S., Hassan Z., Biswas A.A.J., Rahman F., Akhtar Z., Das P., Islam A., Chowdhury F. Use of Antimicrobials among Suspected COVID-19 Patients at Selected Hospitals, Bangladesh: Findings from the First Wave of COVID-19 Pandemic. Antibiotics. 2021;10:738. doi: 10.3390/antibiotics10060738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofer U. The cost of antimicrobial resistance. Nat. Rev. Genet. 2018;17:3. doi: 10.1038/s41579-018-0125-x. [DOI] [PubMed] [Google Scholar]
- 26.Founou R.C., Founou L.L., Essack S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0189621. doi: 10.1371/journal.pone.0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godman B., Egwuenu A., Haque M., Malande O., Schellack N., Kumar S., Saleem Z., Sneddon J., Hoxha I., Islam S., et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life. 2021;11:528. doi: 10.3390/life11060528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haque M., Meyer J.C., Godman B. Potential ways to address antimicrobial resistance across India and wider exacerbated by COVID-19. J. Appl. Pharm. Sci. 2021;11:i–vii. doi: 10.7324/JAPS.2021.11010ed. [DOI] [Google Scholar]
- 30.Hsu J. How COVID-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 31.Adebisi Y.A., Alaran A.J., Okereke M., Oke G.I., Amos O.A., Olaoye O.C., Oladunjoye I., Olanrewaju A.Y., Ukor N.A., Lucero-Prisno D.E. COVID-19 and Antimicrobial Resistance: A Review. Infect. Dis. Res. Treat. 2021;14 doi: 10.1177/11786337211033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumder A.A., Rahman S., Cohall D., Bharatha A., Singh K., Haque M., Hilaire M.G.-S. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020;13:4713–4738. doi: 10.2147/IDR.S290835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed I., Rabbi Md B., Sultana S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019;80:54–61. doi: 10.1016/j.ijid.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Global Antibiotic Resistance Partnership—Bangladesh, GARP-Bangladesh National Working Group Antibiotic Use and Resistance in Bangladesh—Situational Analysis and Recommendations. 2018. [(accessed on 1 December 2021)]. Available online: https://cddep.org/wp-content/uploads/2018/08/ANTIBIOTIC-USE-RESISTANCE-IN-BD_2018.pdf.
- 35.Nusrat T., Akter N., A Rahman N.A., Godman B., Rozario D.T.D., Haque M. Antibiotic resistance and sensitivity pattern of Metallo-β-Lactamase Producing Gram-Negative Bacilli in ventilator-associated pneumonia in the intensive care unit of a public medical school hospital in Bangladesh. Hosp. Pract. 2020;48:128–136. doi: 10.1080/21548331.2020.1754687. [DOI] [PubMed] [Google Scholar]
- 36.Haque M., Godman B. Potential Strategies to Improve Antimicrobial Utilisation in Hospitals in Bangladesh Building on Experiences Across Developing Countries. Bangladesh J. Med. Sci. 2021;20:469–477. doi: 10.3329/bjms.v20i3.52787. [DOI] [Google Scholar]
- 37.Mardi P., Esmaeili M., Iravani P., Abdar M.E., Pourrostami K., Qorbani M. Characteristics of Children With Kawasaki Disease-Like Signs in COVID-19 Pandemic: A Systematic Review. Front. Pediatr. 2021;9:625377. doi: 10.3389/fped.2021.625377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuhara J., Watanabe K., Takagi H., Sumitomo N., Kuno T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr. Pulmonol. 2021;56:837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobbs C.V., Khaitan A., Kirmse B.M., Borkowsky W. COVID-19 in Children: A Review and Parallels to Other Hyperinflammatory Syndromes. Front. Pediatr. 2020;8:593455. doi: 10.3389/fped.2020.593455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Who Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19. [(accessed on 10 October 2021)]. Available online: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
- 41.Horby P.W., Mafham M., Bell J.L., Linsell L., Staplin N., Emberson J., Palfreeman A., Raw J., Elmahi E., Prudon B., et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil Martínez V., Salas A.A., Ballestín S.S. Antiviral Therapeutic Approaches for SARS-CoV-2 Infection: A Systematic Review. Pharmaceuticals. 2021;14:736. doi: 10.3390/ph14080736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The RECOVERY Collaborative Group Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyer O. COVID-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371:m4057. doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 45.Charan J., Kaur R.J., Bhardwaj P., Haque M., Sharma P., Misra S., Godman B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev. Clin. Pharmacol. 2020;14:95–103. doi: 10.1080/17512433.2021.1856655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abubakar A.R., Sani I.H., Godman B., Kumar S., Islam S., Jahan I., Haque M. Systematic Review on the Therapeutic Options for COVID-19: Clinical Evidence of Drug Efficacy and Implications. Infect. Drug Resist. 2020;13:4673–4695. doi: 10.2147/IDR.S289037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadvalla B.-A. COVID-19: Ivermectin’s politicisation is a warning sign for doctors. BMJ. 2021;373:n747. doi: 10.1136/bmj.n747. [DOI] [PubMed] [Google Scholar]
- 48.Garegnani L.I., Madrid E., Meza N. Misleading clinical evidence and systematic reviews on ivermectin for COVID-19. BMJ Evid.-Based Med. 2021 doi: 10.1136/bmjebm-2021-111678. [DOI] [PubMed] [Google Scholar]
- 49.Cruciani M., Pati I., Masiello F., Malena M., Pupella S., De Angelis V. Ivermectin for Prophylaxis and Treatment of COVID-19: A Systematic Review and Meta-Analysis. Diagnostics. 2021;11:1645. doi: 10.3390/diagnostics11091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abena P.M., Decloedt E.H., Bottieau E., Suleman F., Adejumo P., Sam-Agudu N.A., TamFum J.J.M., Seydi M., Eholie S.P., Mills E.J., et al. Chloroquine and Hydroxychloroquine for the Prevention or Treatment of COVID-19 in Africa: Caution for Inappropriate Off-label Use in Healthcare Settings. Am. J. Trop. Med. Hyg. 2020;102:1184–1188. doi: 10.4269/ajtmh.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haque M., Godman B. Key findings regarding COVID 19 in Bangladesh and wider and their implications. Bangladesh J. Med. Sci. 2021;20:199–205. doi: 10.3329/bjms.v20i5.55616. [DOI] [Google Scholar]
- 52.Haque M., Islam S., Iqbal S., Urmi U.L., Kamal Z.M., Rahman A., Kamal M., Haque M., Jahan I., Islam Z., et al. Availability and price changes of potential medicines and equipment for the prevention and treatment of COVID-19 among pharmacy and drug stores in Bangladesh; findings and implications. Bangladesh J. Med. Sci. 2020;19:S36–S50. doi: 10.3329/bjms.v19i0.48106. [DOI] [Google Scholar]
- 53.Haque M., Abubakar A.R., O Ogunleye O., Sani I.H., Sefah I., Kurdi A., Islam S., Godman B. Changes in availability, utilization, and prices of medicines and protection equipment for COVID-19 in an Urban population of Northern Nigeria. J. Res. Pharm. Pract. 2021;10:17–22. doi: 10.4103/jrpp.jrpp_20_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organisation Operational Considerations for Case Management of COVID-19 in Health Facility and Community: Interim Guidance. Mar 19, 2020. [(accessed on 3 November 2021)]. Available online: https://apps.who.int/iris/handle/10665/331492.
- 55.World Health Organisation Coronavirus Disease (COVID-19) Advice for the Public: Myth Busters. 2020. [(accessed on 3 November 2021)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters.
- 56.WHO Countering Misinformation about COVID-19. 2020. [(accessed on 2 November 2021)]. Available online: https://www.who.int/news-room/feature-stories/detail/countering-misinformation-about-covid-19.
- 57.ECDC Vaccines and Treatment of COVID-19. 2020. [(accessed on 2 November 2021)]. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/vaccines-and-treatment.
- 58.BMJ Best Practice—Coronavirus Disease 2019 (COVID-19) 2021. [(accessed on 2 November 2021)]. Available online: https://bestpractice.bmj.com/topics/en-gb/3000201.
- 59.Kache S., Chisti M.J., Gumbo F., Mupere E., Zhi X., Nallasamy K., Nakagawa S., Lee J.H., Di Nardo M., de la Oliva P., et al. COVID-19 PICU guidelines: For high- and limited-resource settings. Pediatr. Res. 2020;88:705–716. doi: 10.1038/s41390-020-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ministry of Health and Family Welfare Government of the People’s Republic of Bangladesh. National Guidelines on Clinical Management of Coronavirus Disease 2019 (COVID-19) [(accessed on 1 November 2021)];2020 Available online: http://file.portal.gov.bd/files/cs.sylhet.gov.bd/notices/245c5244_369b_4305_958d_1f70d99d6681/4c021caf6cf2915278aa3598e74f5c09.pdf.
- 61.Bangladesh Paediatric Association (BPA) Management Guideline for Paediatric COVID-19. Nov, 2020. [(accessed on 1 November 2021)]. Available online: https://www.bpabd.org/asset/uploads/file/COVID_BPA_2nd%20ED_3.pdf.
- 62.Nusrat N., Haque M., Chowdhury K., Adnan N., Lutfor A.B., Karim E., Hassan M., Rabbany A., Begum D., Hasan M.N., et al. Pilot Study on the Current Management of Children with COVID-19 In Hospitals in Bangladesh; Findings and Implications. Bangladesh J. Med. Sci. 2021:188–198. doi: 10.3329/bjms.v20i5.55615. [DOI] [Google Scholar]
- 63.Kumar S., Haque M., Shetty A., Acharya J., Kumar M., Sinha V., Manohar B., Gowere M., Godman B. Current Management of Children with COVID-19 in Hospitals in India; Pilot Study and Findings. Adv. Hum. Biol. 2022;12:16–21. [Google Scholar]
- 64.Ministry of Health & Family Welfare Government of India Guidelines for Management of COVID-19 in Children (Below 18 Years) [(accessed on 1 November 2021)];2021 Available online: https://www.mohfw.gov.in/pdf/GuidelinesforManagementofCOVID19inCHILDREN18June2021final.pdf.
- 65.Ministry of Health Kenya Guidelines on the Case Management of COVID-19 in Kenya. [(accessed on 2 November 2021)];2021 Available online: https://www.health.go.ke/wp-content/uploads/2021/10/Final-guidelines-on-the-Management-of-Covid-19-in-Kenya-2021-Edition.pdf.
- 66.Abu-Rub L., Abdelrahman H., Johar A.-R., Alhussain H., Hadi H., Eltai N. Antibiotics Prescribing in Intensive Care Settings during the COVID-19 Era: A Systematic Review. Antibiotics. 2021;10:935. doi: 10.3390/antibiotics10080935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control. 2019;8:1–13. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akpan M.R., Isemin N.U., Udoh A.E., Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020;22:317–324. doi: 10.1016/j.jgar.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Van den Bergh D., Messina A.P., Goff D.A., van Jaarsveld A., Coetzee R., de Wet Y., Bronkhorst E., Brink A., Mendelson M., Richards G.A., et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents. 2020;56:106189. doi: 10.1016/j.ijantimicag.2020.106189. [DOI] [PubMed] [Google Scholar]
- 70.Mwita J.C., O Ogunleye O., Olalekan A., Kalungia A.C., Kurdi A., Saleem Z., Sneddon J., Godman B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021;14:515–530. doi: 10.2147/IJGM.S253216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sneddon J., Cooper L., Afriyie D.K., A Sefah I., Cockburn A., Kerr F., Cameron E., Goldthorpe J., Kurdi A., Seaton R.A. Supporting antimicrobial stewardship in Ghana: Evaluation of the impact of training on knowledge and attitudes of healthcare professionals in two hospitals. JAC-Antimicrob. Resist. 2020;2:dlaa092. doi: 10.1093/jacamr/dlaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee C.F., Cowling B.J., Feng S., Aso H., Wu P., Fukuda K., Seto W.H. Impact of antibiotic stewardship programmes in Asia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018;73:844–851. doi: 10.1093/jac/dkx492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morales A., Campos M., Juarez J.M., Canovas-Segura B., Palacios F., Marin R. A decision support system for antibiotic prescription based on local cumulative antibiograms. J. Biomed. Inform. 2018;84:114–122. doi: 10.1016/j.jbi.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Klinker K.P., Hidayat L.K., DeRyke C.A., DePestel D.D., Motyl M., Bauer K.A. Antimicrobial stewardship and antibiograms: Importance of moving beyond traditional antibiograms. Ther. Adv. Infect. Dis. 2021;8 doi: 10.1177/20499361211011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gauthier T.P. Antimicrobial Stewardship in Bangladesh: A Pharmacist’s Perspective. 2020. [(accessed on 1 November 2021)]. Available online: https://www.idstewardship.com/antimicrobial-stewardship-bangladesh-pharmacists-perspective/
- 76.Hsia Y., Lee B.R., Versporten A., Yang Y., Bielicki J., Jackson C., Newland J., Goossens H., Magrini N., Sharland M., et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 77.Sharland M., Pulcini C., Harbarth S., Zeng M., Gandra S., Mathur S., Magrini N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—be AWaRe. Lancet Infect. Dis. 2018;18:18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 78.Broom J., Broom A., Adams K., Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J. Antimicrob. Chemother. 2016;71:2295–2299. doi: 10.1093/jac/dkw129. [DOI] [PubMed] [Google Scholar]
- 79.Jones M., Huttner B., Madaras-Kelly K., Nechodom K., Nielson C., Goetz M.B., Neuhauser M.M., Samore M.H., Rubin M.A. Parenteral to Oral Conversion of Fluoroquinolones: Low-Hanging Fruit for Antimicrobial Stewardship Programs? Infect. Control. Hosp. Epidemiol. 2012;33:362–367. doi: 10.1086/664767. [DOI] [PubMed] [Google Scholar]
- 80.Cunha B.A. Beyond IV to PO Switch Therapy: Oral Antimicrobial Therapy. Infectious Disease News. 2015. [(accessed on 1 December 2021)]. Available online: https://www.healio.com/news/infectious-disease/20151014/beyond-iv-to-po-switch-therapy-oral-antimicrobial-therapy.
- 81.Hoque R., Ahmed S.M., Naher N., Islam M.A., Rousham E.K., Islam B.Z., Hassan S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE. 2020;15:e0227947. doi: 10.1371/journal.pone.0227947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simba J., Sinha I., Mburugu P., Agweyu A., Emadau C., Akech S., Kithuci R., Oyiengo L., English M. Is the effect of COVID-19 on children underestimated in low- and middle- income countries? Acta Paediatr. 2020;109 doi: 10.1111/apa.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paramadhas B.D.A., Tiroyakgosi C., Mpinda-Joseph P., Morokotso M., Matome M., Sinkala F., Gaolebe M., Malone B., Molosiwa E., Shanmugam M.G., et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Exp. Rev. Anti-Infect. Ther. 2019;17:535–546. doi: 10.1080/14787210.2019.1629288. [DOI] [PubMed] [Google Scholar]
- 84.Saleem Z., Hassali M.A., Versporten A., Godman B., Hashmi F.K., Goossens H., Saleem F. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Exp. Rev. Anti-Infect. Ther. 2019;17:285–293. doi: 10.1080/14787210.2019.1581063. [DOI] [PubMed] [Google Scholar]
- 85.Saleem Z., Hassali M.A., Godman B., Versporten A., Hashmi F.K., Saeed H., Saleem F., Salman M., Rehman I.U., Khan T.M. Point prevalence surveys of antimicrobial use: A systematic review and the implications. Exp. Rev. Anti-Infect. Ther. 2020;18:897–910. doi: 10.1080/14787210.2020.1767593. [DOI] [PubMed] [Google Scholar]
- 86.Skosana P., Schellack N., Godman B., Kurdi A., Bennie M., Kruger D., Meyer J. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Exp. Rev. Anti-Infect. Ther. 2021;19:1353–1366. doi: 10.1080/14787210.2021.1898946. [DOI] [PubMed] [Google Scholar]
- 87.Ogunleye O.O., Oyawole M.R., Odunuga P.T., Kalejaye F., Yinka-Ogunleye A.F., Olalekan A., Ogundele S.O., Ebruke B.E., Richard A.K., Paramadhas B.D.A., et al. A multicentre point prevalence study of antibiotics utilization in hospitalised patients in an urban secondary and a tertiary healthcare facilities in Nigeria: Findings and implications. Exp. Rev. Anti-Infect. Ther. 2021:1–10. doi: 10.1080/14787210.2021.1941870. [DOI] [PubMed] [Google Scholar]
- 88.Mustafa Z.U., Salman M., Yasir M., Godman B., Majeed H.A., Kanwal M., Iqbal M., Riaz M.B., Hayat K., Hasan S.S. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Exp. Rev. Anti-Infect. Ther. 2021:1–9. doi: 10.1080/14787210.2021.1986388. [DOI] [PubMed] [Google Scholar]
- 89.Okoth C., Opanga S., Okalebo F., Oluka M., Kurdi A., Godman B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 2018;46:128–136. doi: 10.1080/21548331.2018.1464872. [DOI] [PubMed] [Google Scholar]
- 90.Kurdi A., Hasan A.J., Baker K.I., Seaton R.A., Ramzi Z.S., Sneddon J., Godman B. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of Northern Iraq: The need for urgent action. Exp. Rev. Anti-Infect. Ther. 2020;19:805–814. doi: 10.1080/14787210.2021.1834852. [DOI] [PubMed] [Google Scholar]
- 91.Afriyie D.K., A Sefah I., Sneddon J., Malcolm W., McKinney R., Cooper L., Kurdi A., Godman B., Seaton R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC-Antimicrob. Resist. 2020;2:dlaa001. doi: 10.1093/jacamr/dlaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Momanyi L., Opanga S., Nyamu D., Oluka M., Kurdi A., Godman B. Antibiotic prescribing patterns at a leading referral hospital in Kenya: A point prevalence survey. J. Res. Pharm. Pract. 2019;8:149–154. doi: 10.4103/jrpp.jrpp_18_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panfili F.M., Roversi M., D’Argenio P., Rossi P., Cappa M., Fintini D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2020;44:27–35. doi: 10.1007/s40618-020-01327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caliskan T., Saylan B. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: A retrospective observational study. Rev. Assoc. Med. Bras. 2020;66:1679–1684. doi: 10.1590/1806-9282.66.12.1679. [DOI] [PubMed] [Google Scholar]
- 95.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharland M., Gandra S., Huttner B., Moja L., Pulcini C., Zeng M., Mendelson M., Cappello B., Cooke G., Magrini N., et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019;19:1278–1280. doi: 10.1016/S1473-3099(19)30532-8. [DOI] [PubMed] [Google Scholar]
- 97.Hsia Y., Sharland M., Jackson C., Wong I.C.K., Magrini N., A Bielicki J. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2018;19:67–75. doi: 10.1016/S1473-3099(18)30547-4. [DOI] [PubMed] [Google Scholar]
- 98.Van den Bosch C.M., Hulscher M.E., Akkermans R.P., Wille J., Geerlings S.E., Prins J.M. Appropriate antibiotic use reduces length of hospital stay. J. Antimicrob. Chemother. 2016;72:923–932. doi: 10.1093/jac/dkw469. [DOI] [PubMed] [Google Scholar]
- 99.Cyriac J.M., James E. Switch over from intravenous to oral therapy: A concise overview. J. Pharmacol. Pharmacother. 2014;5:83–87. doi: 10.4103/0976-500X.130042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar P., Kumar M., Bedi O., Gupta M., Kumar S., Jaiswal G., Rahi V., Yedke N.G., Bijalwan A., Sharma S., et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology. 2021;29:1001–1016. doi: 10.1007/s10787-021-00826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milani G., Macchi M., Guz-Mark A. Vitamin C in the Treatment of COVID-19. Nutrients. 2021;13:1172. doi: 10.3390/nu13041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Versporten A., Zarb P., Caniaux I., Gros M.-F., Drapier N., Miller M., Jarlier V., Nathwani D., Goossens H., Koraqi A., et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health. 2018;6:e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 104.Niaz Q., Godman B., Campbell S., Kibuule D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int. J. Clin. Pharm. 2020;42:1227–1236. doi: 10.1007/s11096-020-01056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakwatumbah S., Kibuule D., Godman B., Haakuria V., Kalemeera F., Baker A., Mubita M., Mwangana M. Compliance to guidelines for the prescribing of antibiotics in acute infections at Namibia’s national referral hospital: A pilot study and the implications. Exp. Rev. Anti-Infect. Ther. 2017;15:713–721. doi: 10.1080/14787210.2017.1320220. [DOI] [PubMed] [Google Scholar]
- 106.Olaru I.D., Meierkord A., Godman B., Ngwenya C., Fitzgerald F., Dondo V., Ferrand R.A., Kranzer K. Assessment of antimicrobial use and prescribing practices among pediatric inpatients in Zimbabwe. J. Chemother. 2020;32:456–459. doi: 10.1080/1120009X.2020.1734719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sefah I.A., Essah D.O., Kurdi A., Sneddon J., Alalbila T.M., Kordorwu H., Godman B. Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: Findings and implications for the future. JAC-Antimicrob. Resist. 2021;3:dlab080. doi: 10.1093/jacamr/dlab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gitaka J., Kamita M., Mureithi D., Ndegwa D., Masika M., Omuse G., Ngari M., Makokha F., Mwaura P., Mathai R., et al. Combating antibiotic resistance using guidelines and enhanced stewardship in Kenya: A protocol for an implementation science approach. BMJ Open. 2020;10:e030823. doi: 10.1136/bmjopen-2019-030823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campbell S.M., Meyer J.C., Godman B. Why Compliance to National Prescribing Guidelines is Important Especially across Sub-Saharan Africa and Suggestions for the Future. J. Biomed. Pharm. Sci. 2021;4:309. [Google Scholar]
- 110.MacBride-Stewart S., McTaggart S., Kurdi A., Sneddon J., McBurney S., Nascimento R.C.R.M., Mueller T., Kwon H.Y., Morton A., Seaton R.A., et al. Initiatives and reforms across Scotland in recent years to improve prescribing; findings and global implications of drug prescriptions. Int. J. Clin. Exp. Med. 2021;14:2563–2586. [Google Scholar]
- 111.Godman B., Fadare J., Kwon H.-Y., Dias C.Z., Kurdi A., Godói I.P.D., Kibuule D., Hoxha I., Opanga S., Saleem Z., et al. Evidence-based public policy making for medicines across countries: Findings and implications for the future. J. Comp. Eff. Res. 2021;10:1019–1052. doi: 10.2217/cer-2020-0273. [DOI] [PubMed] [Google Scholar]
- 112.Dlamini N.N., Meyer J.C., Kruger D., Kurdi A., Godman B., Schellack N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: A pilot study and implications. Hosp. Pract. 2019;47:88–95. doi: 10.1080/21548331.2019.1592880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further data regarding the study is available upon reasonable request.