Abstract
Flavonoids and carotenoids are bioactive compounds that have protective effects against depressive symptoms. Flavonoids and carotenoids are the two main types of antioxidant phytochemicals. This study investigated the association between flavonoid and carotenoid intake and depressive symptoms in middle-aged Korean females. We analyzed the mechanism of these associations using an in silico method. Depressive symptoms were screened using the Beck Depression Inventory-II (BDI-II), and flavonoid and carotenoid intake were assessed using a semi-quantitative food frequency questionnaire. Using a multivariate logistic regression model, we found that flavones, anthocyanins, individual phenolic compounds, lycopene, and zeaxanthin were negatively associated with depressive symptoms. In silico analysis showed that most flavonoids have high docking scores for monoamine oxidase A (MAOA) and monoamine oxidase B (MAOB), which are two important drug targets in depression. The results of the docking of brain-derived neurotrophic factor (BDNF) and carotenoids suggested the possibility of allosteric activation of BDNF by carotenoids. These results suggest that dietary flavonoids and carotenoids can be utilized in the treatment of depressive symptoms.
Keywords: flavonoids, carotenoids, depression, in silico
1. Introduction
Depression is one of the most common mental illnesses that affect a person’s poor performance in education, work, and family life [1]. Several studies have reported that depressive symptoms are associated with an elevated risk of cardiovascular disease and heart failure [2,3]. In addition, the risk of depression is elevated in diseases such as angina, arthritis, asthma, cancer, and diabetes [4,5]. In a systematic analysis, depression was found to be associated with various chronic diseases, and the comorbidity of depression deteriorates health compared to depression alone [5]. The depression and depressive symptoms were also found to be associated with all-cause mortality and especially with cardiovascular diseases (CVD) mortality in studies conducted on the different races and populations [6,7]. There is a high prevalence of major depressive disorders, with approximately one in every 20 individuals affected. Up to 85% of residents in low- and middle-income countries did not receive treatment for mental disorders [8]. Therefore, effective prevention and treatment strategies are required to overcome depression. Several studies have shown that the antidepressant effects of polyphenols, especially dietary polyphenols, have the potential to be widely used in depression worldwide because their antidepressant effects can be cost-effective [9,10,11,12]. Most dietary polyphenols are associated with reduced symptoms of depression, and consumption of some polyphenols significantly reduces depressive symptoms [9]. Flavonoids are an important class of polyphenols with antidepressant properties reported in several research and review papers [13,14,15,16]. The anti-depressive effect of dietary carotenoids has also been observed in several studies [17,18]. Similarly, low concentrations of carotenoids in the blood are associated with depressive symptoms [19]. Therefore, the identification of dietary flavonoids and carotenoids with anti-depression properties and knowledge of the possible mechanisms are of great importance in establishing the use of flavonoids and carotenoids for depression prevention. Depression is a complex disorder, and different mechanisms are thought to explain its pathophysiology. These mechanisms include the biogenic amine (monoamine) hypothesis, dysregulation of the hypothalamic–pituitary–adrenal axis, abnormalities in the function of receptors (such as 5-hydroxytryptamine 1 (5-HT1), 5-HT2, and alpha2-adrenoceptors), neuroinflammation [20], genetic factors [21] antioxidant effects and anti-neuro-inflammation [16], and immune and environmental factors [22]. Other possible mechanisms for depression may include a lack or decrease in adult neurogenesis [23], abnormalities in the second messenger system, and elevated levels of corticotrophin-releasing factor (CRF) [24]. However, the exact mechanism underlying the initiation and progression of depression is unknown. Various natural flavonoids and carotenoids have been studied in various in vivo studies for their antidepressant properties in rats and mice, with mostly positive outcomes [25]. Natural flavonoids derived from plants such as rutin [26], quercetin [27,28], apigenin [29], epigallocatechin gallate [30], myricetin [31], hesperidin [32], kaempferol [28], naringenin [33], formononetin [34], beta-carotene [35], beta-cryptoxanthin [36], lutein [37] and genistein [38] have been shown to function as antidepressants in animal models. Recently, a high intake of dietary flavonoids has been associated with decreased depressive symptoms and improved general mental health in human studies [11,13].
To date, limited studies have been conducted to explore the possible mechanisms of the association of individual dietary flavonoid and carotenoid intake with antidepressant outcomes using an in silico analysis. Therefore, this study aimed to evaluate the effects of dietary flavonoid and carotenoid intake on depressive symptoms in middle-aged Korean females. In addition, flavonoids and carotenoids have also been used to explore possible mechanisms of action in molecular docking studies using an in silico analysis.
2. Materials and Methods
2.1. Subjects
The population in this diet-depression cohort study comprised participants recruited through hospital and community health centers in the Seoul and Gyeonggi areas of South Korea. This study was conducted from 2016 to 2018. Finally, 2201 females aged 45–69 years participated in the baseline survey. We estimated the sample size using STATCALS (https://www.cdc.gov/epiinfo/user-guide/statcalc/cohortandcrosssectional.html accessed on 10 April 2016) and previously study [39] (α = 0.05, β = 0.2, OR=0.65, prevalence 15%, drop rate 25%). Informed consent was obtained from all participants involved in the study. We excluded participants with implausible energy intakes [40] of <500 kcal/day (n = 8) and >3500 kcal/day (n = 3). As a result, a total of 2190 data were used for the final analysis.
The study was approved by the Institutional Review Board of the Gachon University Gil Medical Center (GDIRB2016-271) and was conducted in accordance with the Declaration of Helsinki.
2.2. Methods
2.2.1. Depressive Disorder Screening
Depressive disorder screening was conducted using the Beck Depression Inventory-II (BDI-II) and Center for Epidemiologic Studies-Depression Scale (CES-D). The BDI-II contains 21 questions, with each answer scored on a scale of zero to three, for a total score between zero and 63. A higher score was associated with severe depressive symptoms [41]. The Korean version of the BDI-II validated tool was used to assess depressive symptoms. We classified people with a BDI-II score of 14 or higher as those with depressive symptoms. The CES-D questionnaire consists of 20 questions, with a total score ranging from zero to 60, with higher scores indicating more severe depressive symptoms. Subjects with a CES-D score of 16 or higher were considered to have depressive symptoms [42]
2.2.2. Nutritional Assessment
Dietary intake, including macro-nutrient intake and flavonoid/carotenoid intake per day, were assessed using the previously validated 108-item semi-quantitative food frequency questionnaire (SQ-FFQ) [43]. The frequency of food intake was assessed over nine categories (three times/day, two times/day, one time/day, five to six times/week, two to four times/week, one time/week, two to three times/month, one time/month, almost never), and serving size was assessed as 0.5, 1, or 1.5 serving. Nutrient intake was calculated using the food composition database created by the Rural Development Administration of Korea [44]. Flavonoid and carotenoid content in foods was obtained from the tables of food functional composition by the National Academy of Agricultural Sciences of Korea [45] and the United States Department of Agriculture (USDA) database [46,47,48].
The subclass and individual phenolic compound of flavonoids are as follows: flavonols (kaempferol, myricetin, quercetin), flavones (luteolin, apigenin), flavanols, ((+)-catechin, (+)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin 3-gallate, theaflavin, theaflavin 3-gallate, theaflavin 3′-gallate, theaflavin 3,3′ digallate), flavanones (hesperptin, naringenin, eriodictyol), isoflavones (daidzein, genistein, glycitein, coumestrol, formonnetin, biochanin A), anthocyanins (cyanidin, delphinidine, pelargonidine, peonidine). We defined total flavonoids as the sum of all these subclasses. In addition, the intake of flavonols, flavones, flavanols, flavanones, isoflavones, and anthocyanins were summed up for individual phenolic compound intakes. Carotenoids and subclasses of carotenoids are as follows: α-carotene, β-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, and capsaicin. We defined total carotenoids as the sum of these subclasses.
2.2.3. Other Variables
The subject’s height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. The body mass index (BMI) was calculated as the weight in kilograms/height in meters squared.
The general characteristics and lifestyle data of the subjects were collected through face-to-face interviews via questionnaires. We considered education level (elementary school graduation or less, middle school graduation, high school graduation, and college graduation or higher), household income (<1000 dollar, 1000–2000 dollar, 2000–4000 dollar, >4000 dollar), current smoking (yes or no), current alcohol drinking (yes or no), marital status (married or other). The job type (white-collar worker, service worker, blue-collar worker, or housewife), chronic disease such as diabetes, hypertension, heart disease, or cancer (yes or no), physical activity (yes or no), menopausal status (yes or no), family history of depression (yes or no), use of antidepressant (yes or no), sleep duration (<6 h, 6–8 h, >8 h), stress (rarely, a litter, a lot, very much), age, BMI, and energy intake as potential confounding factors.
2.2.4. Molecular Docking (In Silico Analysis)
The crystal structures of three important targets in depression, monoamine oxidase A (MAOA) PDBID: 2Z5X [49], monoamine oxidase B (MAOB) PDBID: 4A79 [50] and brain-derived neurotrophic factor (BDNF) PDBID: 1B8M), were obtained from the PDB database. Inhibitors and molecules other than proteins were removed from the MAOA and MAOB structures, but flavin adenine dinucleotide (FAD), the co-enzyme present in MAOA and MAOB structures, was retained in the active site to mimic natural conditions. Proteins were prepared, and the docking grid was defined around the active site, which was the binding site of the known inhibitor (harmine and pioglitazone were the inhibitors present in MAOA and MAOB crystal structures, respectively [49,50]) using the AutoDock (version 4.2) Tool Kit (ADT version 1.5.6) and AutoGrid (version 4) [6], respectively. In the case of BDNF, the binding cavity was predicted using the CavityPlus web server [51]. The docking grid was defined to surround all predicted binding site residues that were distant from the loop region using AutoGrid through ADT. The compounds used in the docking study as ligands were extracted from PubChem, and format conversion was carried out using babel for the docking study [52]. Further ligand preparation was performed using ADT [53]. In the docking simulation, the number of evaluation steps was increased to 25,000,000 because some compounds had ten or more rotatable bonds, and finally, docking was carried using AutoDock 4.2 [53]. The binding affinity of ligands with targeted proteins was determined by the estimated free binding energy of binding (EFEB), which is considered to be better with a higher negative value. Interaction studies of ligands in protein–ligand complexes were carried out using LigPlot plus [54] and Chimera [55].
2.2.5. Statistical Analysis
The characteristics of the study subjects were expressed as the mean and standard deviation for continuous variables or as percentages for categorical variables. The differences between the control and depressive symptom groups were analyzed using independent t-tests (continuous variables), Mann–Whitney test (intakes of flavonoids and carotenoids), and chi-squared tests (categorical variables). The association between flavonoid and carotenoid intake and depressive symptoms using multivariable logistic regression analysis, education level, household income, marital status, age, BMI, job status, drinking, smoking, physical activity, family history of depression, stress, chronic disease status, sleep duration, menopause, and total energy intake were considered confounding factors. Flavonoid and carotenoid intake were categorized into quartiles, with the lowest quartile group considered as the reference group. Pearson correlation coefficient between the docking score of MAOA and MAOB was calculated using the SAS.
All statistical analyses were performed using SAS software (version 9.4 SAS institute Inc., Cary, NC, USA), and statistical significance was set at a p-value of <0.05.
3. Results
3.1. Characteristics of the Study Participants
The characteristics of the participants are summarized in Table 1. The sample comprised 2190 individuals with a mean age of 58.2 years (range 45–69 years), and the mean BMI was 24.1. Among the 2190 participants, 487 individuals (22.2%) were identified as having depressive symptoms using the BDI-II questionnaire (BDI-II score ≥ 14, mean score: 21.0 ± 7.0), and 363 subjects (16.6%) were classified as having depressive symptoms using the CES-D questionnaire (CES-D score ≥ 16, mean score: 23.7 ± 7.7).
Table 1.
Characteristics of the subjects.
| Variables | Total (n = 2190) |
|---|---|
| Age (years), mean ± SD | 58.2 ± 5.8 |
| BMI (kg/m2), mean ± SD | 24.1 ± 3.2 |
| BDI-II score, mean ± SD | 9.0 ± 7.8 |
| CES-D score, mean ± SD | 8.5 ± 8.5 |
| Depressive disorder prevalence, n (%) | |
| BDI-II score ≥ 14 | 487 (22.2) |
| CES-D score ≥ 16 | 363 (16.6) |
| Stage of depression using BDI-II score, n (%) | |
| Minimal (0–13) | 1703 (77.8) |
| Mild (14–19) | 270 (12.3) |
| Moderate (20–28) | 151 (6.9) |
| Severe (29–63) | 66 (3.0) |
| Stage of depression using CES-D score, n (%) | |
| Normal (0–15) | 1827 (83.4) |
| Probable depression (16–24) | 238 (10.9) |
| Definite depression(25–60) | 125 (5.7) |
| Education level, n (%) | |
| Elementary school | 322 (14.7) |
| Middle school | 569 (25.9) |
| High school | 967 (44.2) |
| College and higher | 332 (15.2) |
| Household income, n (%) | |
| <1000 dollar | 184 (8.4) |
| 1000–2000 dollar | 450 (20.5) |
| 2000–4000 dollar | 801 (36.6) |
| >4000 dollar | 755 (34.5) |
| Current Smoking, n (%) | |
| No | 2119 (96.8) |
| Yes | 71 (3.2) |
| Current alcohol drinking, n (%) | |
| No | 1530 (69.9) |
| Yes | 660 (30.1) |
| Physical activity, n (%) | |
| No | 907 (41.4) |
| Yes | 1283 (58.6) |
| Marital status, n (%) | |
| Married | 1689 (77.1) |
| Others | 501 (22.9) |
| Job, n (%) | |
| White-collar worker | 169 (7.7) |
| Service worker | 497 (22.7) |
| Blue-collar worker | 205 (9.4) |
| Housewife | 1319 (60.2) |
| Chronic disease, n (%) | |
| No | 1440 (65.8) |
| Yes | 750 (34.2) |
| Family history of depression, n (%) | |
| No | 2150 (98.2) |
| Yes | 40 (1.8) |
| Use of antidepressant, n (%) | |
| No | 2154 (98.4) |
| Yes | 36 (1.6) |
| Sleep duration, n (%) | |
| <6 h | 385 (17.5) |
| 6–8 h | 1420 (65.0) |
| >8 h | 385 (17.5) |
| Stress, n (%) | |
| Rarely | 549 (25.1) |
| A litter | 1088 (49.7) |
| A lot | 520 (23.7) |
| Very much | 33 (1.5) |
| Menopausal status | |
| No | 282 (12.9) |
| Yes | 1908 (87.1) |
SD, standard deviation; BDI-II, Beck Depression Inventory-II; CES-D, Center for Epidemiological Studies-Depression Scale. Chronic disease: diabetes, hypertension, heart disease, or cancer diagnosis.
3.2. Flavonoid and Carotenoid Intake between the Control and Depressive Symptoms Groups
Table 2 shows the intake of phytochemicals between control and depressive symptoms groups. The depressive symptoms group was classified as those with a BDI-II score of 14 or higher. The intake of total flavonoids was lower in the depressive symptom group, and similar results were observed in the flavonols, flavones, flavanols, flavanones, isoflavones, and anthocyanins. Subgroups of these compounds, the intake of myricetin, quercetin, luteolin, eriodictyol, daidzein, genestein, glycitein, coumesterol, cyanidin, delphinidine, pelargonidine, peonidin were lower in the depressive symptoms group than in the control group. The intakes of total carotenoids and most carotenoid subclasses (α-carotene, β-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, capsaicin) were significantly lower in the depressive symptoms group than in the control group.
Table 2.
Intake of flavonoids and carotenoids between the control and depressive symptom groups.
| Variables | Control (n = 1703) |
Depressive Symptoms * (n = 487) |
p-Value |
|---|---|---|---|
| Total flavonoids | 126.12 ± 1.44 | 113.71 ± 2.71 | <0.0001 |
| Flavonols | 13.39 ± 0.19 | 12.84 ± 0.38 | 0.0174 |
| Flavones | 1.45 ± 0.02 | 1.30 ± 0.03 | <0.0001 |
| Flavanols | 78.90 ± 1.08 | 70.51 ± 2.05 | 0.0001 |
| Flavanones | 7.44 ± 0.19 | 6.82 ± 0.31 | 0.046 |
| Isoflavonoids | 15.87 ± 0.30 | 14.68 ± 0.52 | 0.0322 |
| Anthocyanins | 8.74 ± 0.19 | 7.29 ± 0.31 | 0.0001 |
| Flavonols | |||
| Kaempferol | 1.53 ± 0.0.02 | 1.49 ± 0.05 | 0.2623 |
| Myricetin | 0.18 ± 0.00 | 0.17 ± 0.00 | 0.0004 |
| Quercetin | 11.69 ± 0.17 | 11.18 ± 0.34 | 0.0149 |
| Flavones | |||
| Luteolin | 1.23 ± 0.01 | 1.09 ± 0.03 | <0.0001 |
| Apigenin | 0.22 ± 0.00 | 0.21 ± 0.01 | 0.093 |
| Flavanols | |||
| (+)-Catechin | 3.71 ± 0.06 | 3.22 ± 0.11 | <0.0001 |
| (+)-Gallocatechin | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.003 |
| (−)-Epicatechin | 3.37 ± 0.07 | 2.88 ± 0.13 | <0.0001 |
| (−)-Epigallocatechin | 0.30 ± 0.01 | 0.27 ± 0.01 | 0.0003 |
| (−)-Epicatechin 3-gallate | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.8674 |
| Theaflavin | 9.67 ± 0.16 | 8.41 ± 0.29 | <0.0001 |
| Theaflavin 3-gallate | 6.21 ± 0.09 | 5.50 ± 0.17 | <0.0001 |
| Theaflavin 3′-gallate | 26.13 ± 0.36 | 23.43 ± 0.70 | 0.0003 |
| Theaflavin 3,3′ digallate | 29.49 ± 0.40 | 26.79 ± 0.78 | 0.0016 |
| Flavanones | |||
| Hesperidin | 6.58 ± 0.17 | 6.11 ± 0.30 | 0.0515 |
| Naringenin | 0.83 ± 0.03 | 0.69 ± 0.03 | 0.0012 |
| Eriodictyol | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.0215 |
| Isoflavones | |||
| Daidzein | 6.07 ± 0.12 | 5.63 ± 0.21 | 0.0487 |
| Genistein | 7.58 ± 0.14 | 6.99 ± 0.25 | 0.0251 |
| Glycitein | 2.12 ± 0.04 | 1.98 ± 0.07 | 0.0436 |
| Coumestrol | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.0216 |
| Formonnetin | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.1729 |
| Biochanin A | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.1777 |
| Anthocyanins | |||
| Cyanidin | 7.04 ± 0.17 | 5.80 ± 0.26 | <0.0001 |
| Delphinidine | 0.32 ± 0.02 | 0.28 ± 0.03 | 0.0019 |
| Pelargonidine | 0.21 ± 0.01 | 0.18 ± 0.01 | <0.0001 |
| Peonidine | 1.18 ± 0.03 | 1.04 ± 0.06 | 0.0046 |
| Total carotenoids | 24.69 ± 0.32 | 22.62 ± 0.59 | 0.0001 |
| α-carotene | 0.53 ± 0.01 | 0.45 ± 0.02 | 0.0042 |
| β-carotene | 6.46 ± 0.08 | 5.95 ± 0.15 | 0.0004 |
| Lycopene | 2.21 ± 0.06 | 1.91 ± 0.10 | 0.0015 |
| Lutein | 1.90 ± 0.03 | 1.79 ± 0.06 | 0.0391 |
| Zeaxanthin | 0.21 ± 0.00 | 0.19 ± 0.01 | 0.0038 |
| β-cryptoxanthin | 0.31 ± 0.00 | 0.28 ± 0.01 | 0.0025 |
| Capsaicin | 12.40 ± 0.20 | 11.44 ± 0.38 | 0.0015 |
* Depressive symptoms: BDI-II score ≥ 14; Values expressed as mean ± SE (standard error).
3.3. Association between Flavonoid Intake and Depressive Symptoms
Table 3 presents the results of the multivariable-adjusted regression analysis, showing that the risk of depressive symptoms was negatively associated with flavonoid intake, especially flavones (OR = 0.69, 95% CI: 0.48–0.99, p for trend = 0.0388) and anthocyanins (OR = 0.68, 95% CI: 0.48–0.96, p for trend = 0.0093) after adjusting for confounding factors. However, the total flavonoid intake was not statistically significant (OR = 0.69, 95% CI: 0.47–1.02, p for trend = 0.0604).
Table 3.
Association between total flavonoids and subclass intake and prevalence of depressive symptoms in multivariate-adjusted logistic regression analysis.
| Variables | Quartile | Median | No. of Total | No. of Cases | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI Lower Upper |
OR | 95% CI Lower Upper |
|||||||
| Total flavonoids | Q1 | 58.1 | 547 | 147 | 1.00 | 1.00 | ||||
| Q2 | 99.7 | 548 | 133 | 0.88 | 0.67 | 1.16 | 0.99 | 0.72 | 1.35 | |
| Q3 | 135.9 | 548 | 111 | 0.70 | 0.53 | 0.93 | 0.92 | 0.65 | 1.29 | |
| Q4 | 193.7 | 547 | 96 | 0.59 | 0.44 | 0.79 | 0.69 | 0.47 | 1.02 | |
| p-value for trend | 0.0001 | 0.0604 | ||||||||
| Flavonols | Q1 | 6.2 | 547 | 139 | 1.00 | 1.00 | ||||
| Q2 | 10.1 | 548 | 120 | 0.83 | 0.63 | 1.10 | 0.95 | 0.69 | 1.31 | |
| Q3 | 13.8 | 548 | 110 | 0.75 | 0.56 | 1.00 | 0.99 | 0.70 | 1.39 | |
| Q4 | 20.4 | 547 | 118 | 0.81 | 0.61 | 1.08 | 1.05 | 0.73 | 1.53 | |
| p-value for trend | 0.1602 | 0.7063 | ||||||||
| Flavones | Q1 | 0.7 | 547 | 158 | 1.00 | 1.00 | ||||
| Q2 | 1.2 | 548 | 124 | 0.73 | 0.55 | 0.95 | 0.86 | 0.63 | 1.17 | |
| Q3 | 1.6 | 548 | 106 | 0.60 | 0.45 | 0.79 | 0.78 | 0.57 | 1.09 | |
| Q4 | 2.2 | 547 | 99 | 0.55 | 0.41 | 0.74 | 0.69 | 0.48 | 0.99 | |
| p-value for trend | <0.0001 | 0.0388 | ||||||||
| Flavanols | Q1 | 21.7 | 547 | 148 | 1.00 | 1.00 | ||||
| Q2 | 61.0 | 548 | 121 | 0.77 | 0.58 | 1.02 | 0.96 | 0.70 | 1.31 | |
| Q3 | 87.9 | 548 | 124 | 0.80 | 0.61 | 1.06 | 1.12 | 0.81 | 1.55 | |
| Q4 | 128.5 | 547 | 94 | 0.57 | 0.42 | 0.77 | 0.77 | 0.54 | 1.11 | |
| p-value for trend | 0.0004 | 0.2801 | ||||||||
| Flavanones | Q1 | 1.2 | 547 | 138 | 1.00 | 1.00 | ||||
| Q2 | 3.4 | 548 | 112 | 0.76 | 0.58 | 1.01 | 0.82 | 0.60 | 1.13 | |
| Q3 | 7.7 | 548 | 126 | 0.89 | 0.67 | 1.17 | 0.83 | 0.60 | 1.13 | |
| Q4 | 13.5 | 547 | 111 | 0.76 | 0.57 | 1.00 | 0.80 | 0.57 | 1.12 | |
| p-value for trend | 0.1707 | 0.2967 | ||||||||
| Isoflavones | Q1 | 4.9 | 547 | 137 | 1.00 | 1.00 | ||||
| Q2 | 9.6 | 548 | 128 | 0.91 | 0.69 | 1.21 | 0.94 | 0.68 | 1.28 | |
| Q3 | 15.7 | 548 | 115 | 0.79 | 0.59 | 1.05 | 0.75 | 0.54 | 1.04 | |
| Q4 | 28.8 | 547 | 107 | 0.73 | 0.55 | 0.97 | 0.73 | 0.51 | 1.07 | |
| p-value for trend | 0.0217 | 0.0784 | ||||||||
| Anthocyanins | Q1 | 1.9 | 547 | 141 | 1.00 | 1.00 | ||||
| Q2 | 4.4 | 548 | 135 | 0.95 | 0.72 | 1.25 | 1.02 | 0.75 | 1.40 | |
| Q3 | 9.0 | 548 | 114 | 0.76 | 0.58 | 1.01 | 0.83 | 0.60 | 1.16 | |
| Q4 | 16.6 | 547 | 97 | 0.63 | 0.47 | 0.84 | 0.68 | 0.48 | 0.96 | |
| p-value for trend | 0.0006 | 0.0093 | ||||||||
Model 1 adjusted for age; model 2 adjusted for age, BMI, education level, household income, marital status, job, current alcohol drinking, current smoking, physical activity, chronic disease status (diabetes, hypertension, cancers, or cardiovascular diseases), sleep duration, family history of depression, stress, menopause status, and total energy intake; OR, odds ratio; CI, confidence interval; Q, quartile.
Adjusted ORs and 95% CIs of depressive symptoms related to individual phenolic compound intake are summarized in Table 4. In model 1, myricetin, luteolin, (+)-catechin, (+)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin, theaflavin, theaflavin 3-gallate, theaflavin 3′-gallate, theaflavin 3,3′ -digallate, naringenin, eriodictyol, daidzein, genistein, cyanidin, pelargonidine, and peonidine showed significant linear relationships. In model 2, compared with subjects in the lowest quartile of the phenolic compound intake, those in the highest quartile had a significantly lower odds of depressive symptoms (OR = 0.57, 95% CI: 0.39–0.82, p for trend = 0.004 for luteolin; OR = 0.65, 95% CI: 0.45–0.94, p for trend = 0.0128 for (+)-catechin; OR = 0.73, 95% CI: 0.53–1.00, p for trend = 0.0226 for (+)-gallocatechin; OR = 0.69, 95% CI: 0.48–0.98, p for trend = 0.0395 for theaflavin 3-gallate, OR = 0.66, 95% CI: 0.47–0.93, p for trend = 0.0055 for cyanidin; OR = 0.64, 95% CI: 0.46–0.90, p for trend = 0.0199 for pelargonidine).
Table 4.
Association between individual phenolic compound intake and prevalence of depressive symptoms in multivariate-adjusted logistic regression analysis.
| Variables | Quartile | Median | No. of Total | No. of Cases | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI Lower Upper |
OR | 95% CI Lower Upper |
|||||||
| Flavonols | ||||||||||
| Kaempferol | Q1 | 0.61 | 547 | 132 | 1.00 | 1.00 | ||||
| Q2 | 1.10 | 548 | 115 | 0.84 | 0.63 | 1.12 | 1.05 | 0.76 | 1.44 | |
| Q3 | 1.59 | 548 | 121 | 0.89 | 0.67 | 1.18 | 1.13 | 0.82 | 1.55 | |
| Q4 | 2.47 | 547 | 119 | 0.87 | 0.66 | 1.15 | 1.21 | 0.85 | 1.71 | |
| p-value for trend | 0.4562 | 0.2663 | ||||||||
| Myricetin | Q1 | 0.09 | 547 | 153 | 1.00 | |||||
| Q2 | 0.14 | 548 | 115 | 0.69 | 0.52 | 0.91 | 0.83 | 0.60 | 1.14 | |
| Q3 | 0.18 | 548 | 124 | 0.75 | 0.57 | 0.99 | 0.91 | 0.65 | 1.26 | |
| Q4 | 0.26 | 547 | 95 | 0.54 | 0.41 | 0.73 | 0.66 | 0.44 | 0.98 | |
| p-value for trend | 0.0001 | 0.0602 | ||||||||
| Quercetin | Q1 | 5.05 | 547 | 139 | 1.00 | |||||
| Q2 | 8.74 | 548 | 119 | 0.82 | 0.62 | 1.09 | 0.93 | 0.68 | 1.28 | |
| Q3 | 12.12 | 548 | 110 | 0.75 | 0.56 | 1.00 | 1.01 | 0.72 | 1.41 | |
| Q4 | 17.96 | 547 | 119 | 0.82 | 0.62 | 1.09 | 1.06 | 0.73 | 1.53 | |
| p-value for trend | 0.1858 | 0.6646 | ||||||||
| Flavones | ||||||||||
| Luteolin | Q1 | 0.59 | 547 | 161 | 1.00 | |||||
| Q2 | 0.96 | 548 | 122 | 0.69 | 0.53 | 0.91 | 0.81 | 0.60 | 1.11 | |
| Q3 | 1.32 | 548 | 114 | 0.64 | 0.48 | 0.84 | 0.83 | 0.60 | 1.15 | |
| Q4 | 1.87 | 547 | 90 | 0.48 | 0.36 | 0.64 | 0.57 | 0.39 | 0.82 | |
| p-value for trend | <0.0001 | 0.004 | ||||||||
| Apigenin | Q1 | 0.00 | 547 | 138 | 1.00 | |||||
| Q2 | 0.19 | 548 | 116 | 0.80 | 0.60 | 1.06 | 0.81 | 0.58 | 1.11 | |
| Q3 | 0.26 | 548 | 116 | 0.81 | 0.61 | 1.07 | 1.06 | 0.77 | 1.46 | |
| Q4 | 0.39 | 547 | 117 | 0.83 | 0.62 | 1.10 | 1.13 | 0.81 | 1.59 | |
| p-value for trend | 0.1531 | 0.5143 | ||||||||
| Flavanols | ||||||||||
| (+)-Catechin | Q1 | 1.23 | 547 | 152 | 1.00 | 1.00 | ||||
| Q2 | 2.45 | 548 | 127 | 0.79 | 0.60 | 1.04 | 0.98 | 0.72 | 1.34 | |
| Q3 | 3.70 | 548 | 114 | 0.69 | 0.52 | 0.92 | 0.84 | 0.60 | 1.16 | |
| Q4 | 6.38 | 547 | 94 | 0.55 | 0.41 | 0.73 | 0.65 | 0.45 | 0.94 | |
| p-value for trend | <0.0001 | 0.0128 | ||||||||
| (+)-Gallocatechin | Q1 | 0.00 | 562 | 140 | 1.00 | 1.00 | ||||
| Q2 | 0.00 | 381 | 92 | 0.96 | 0.71 | 1.30 | 1.02 | 0.73 | 1.44 | |
| Q3 | 0.01 | 540 | 123 | 0.90 | 0.68 | 1.19 | 0.90 | 0.66 | 1.24 | |
| Q4 | 0.02 | 707 | 132 | 0.70 | 0.54 | 0.92 | 0.73 | 0.53 | 1.00 | |
| p-value for trend | 0.0069 | 0.0226 | ||||||||
| (−)-Epicatechin | Q1 | 0.60 | 547 | 155 | 1.00 | 1.00 | ||||
| Q2 | 1.53 | 548 | 126 | 0.76 | 0.58 | 1.00 | 0.85 | 0.63 | 1.16 | |
| Q3 | 3.31 | 548 | 98 | 0.56 | 0.42 | 0.74 | 0.65 | 0.47 | 0.91 | |
| Q4 | 7.09 | 547 | 108 | 0.63 | 0.48 | 0.84 | 0.77 | 0.54 | 1.08 | |
| p-value for trend | 0.0033 | 0.169 | ||||||||
| (−)-Epigallocatechin | Q1 | 0.09 | 547 | 147 | 1.00 | 1.00 | ||||
| Q2 | 0.18 | 548 | 125 | 0.81 | 0.61 | 1.06 | 0.85 | 0.62 | 1.16 | |
| Q3 | 0.32 | 548 | 115 | 0.73 | 0.55 | 0.97 | 0.93 | 0.67 | 1.29 | |
| Q4 | 0.54 | 547 | 100 | 0.61 | 0.46 | 0.82 | 0.76 | 0.53 | 1.09 | |
| p-value for trend | 0.0011 | 0.2027 | ||||||||
| (−)-Epicatechin 3-gallate | Q1 | 0.00 | 547 | 125 | 1.00 | 1.00 | ||||
| Q2 | 0.00 | 548 | 113 | 0.87 | 0.65 | 1.16 | 0.90 | 0.65 | 1.25 | |
| Q3 | 0.01 | 548 | 112 | 0.85 | 0.64 | 1.14 | 1.05 | 0.76 | 1.46 | |
| Q4 | 0.01 | 547 | 137 | 1.10 | 0.83 | 1.46 | 1.36 | 0.97 | 1.92 | |
| p-value for trend | 0.6703 | 0.0742 | ||||||||
| Theaflavin | Q1 | 2.72 | 547 | 155 | 1.00 | 1.00 | ||||
| Q2 | 6.02 | 548 | 130 | 0.79 | 0.60 | 1.04 | 0.91 | 0.67 | 1.24 | |
| Q3 | 10.33 | 548 | 95 | 0.54 | 0.40 | 0.72 | 0.68 | 0.48 | 0.95 | |
| Q4 | 17.15 | 547 | 107 | 0.62 | 0.47 | 0.83 | 0.72 | 0.51 | 1.02 | |
| p-value for trend | 0.0004 | 0.0426 | ||||||||
| Theaflavin 3-gallate | Q1 | 1.81 | 547 | 152 | 1.00 | 1.00 | ||||
| Q2 | 4.59 | 548 | 128 | 0.80 | 0.61 | 1.05 | 0.93 | 0.68 | 1.26 | |
| Q3 | 6.72 | 548 | 111 | 0.67 | 0.50 | 0.89 | 0.90 | 0.64 | 1.25 | |
| Q4 | 10.54 | 547 | 96 | 0.56 | 0.42 | 0.75 | 0.69 | 0.48 | 0.98 | |
| p-value for trend | <0.0001 | 0.0395 | ||||||||
| Theaflavin 3′-gallate | Q1 | 6.17 | 547 | 145 | 1.00 | 1.00 | ||||
| Q2 | 20.06 | 548 | 124 | 0.82 | 0.62 | 1.08 | 1.04 | 0.76 | 1.42 | |
| Q3 | 29.28 | 548 | 123 | 0.82 | 0.62 | 1.08 | 1.12 | 0.81 | 1.55 | |
| Q4 | 42.43 | 547 | 95 | 0.60 | 0.44 | 0.80 | 0.80 | 0.56 | 1.14 | |
| p-value for trend | 0.0009 | 0.3429 | ||||||||
| Theaflavin 3,3′ digallate | Q1 | 5.27 | 547 | 142 | 1.00 | |||||
| Q2 | 24.35 | 548 | 118 | 0.79 | 0.60 | 1.05 | 0.91 | 0.67 | 1.25 | |
| Q3 | 36.33 | 548 | 130 | 0.91 | 0.69 | 1.20 | 1.22 | 0.89 | 1.69 | |
| Q4 | 47.04 | 547 | 97 | 0.63 | 0.47 | 0.85 | 0.84 | 0.59 | 1.20 | |
| p-value for trend | 0.0101 | 0.8335 | ||||||||
| Flavanones | ||||||||||
| Hesperidin | Q1 | 0.83 | 549 | 138 | 1.00 | 1.00 | ||||
| Q2 | 2.56 | 544 | 111 | 0.76 | 0.57 | 1.01 | 0.83 | 0.61 | 1.14 | |
| Q3 | 6.96 | 546 | 128 | 0.92 | 0.69 | 1.21 | 0.87 | 0.63 | 1.19 | |
| Q4 | 12.34 | 551 | 110 | 0.74 | 0.56 | 0.99 | 0.81 | 0.58 | 1.12 | |
| p-value for trend | 0.1644 | 0.3301 | ||||||||
| Naringenin | Q1 | 0.16 | 547 | 144 | 1.00 | 1.00 | ||||
| Q2 | 0.38 | 551 | 137 | 0.93 | 0.71 | 1.22 | 1.19 | 0.87 | 1.62 | |
| Q3 | 0.70 | 554 | 102 | 0.64 | 0.48 | 0.85 | 0.71 | 0.51 | 0.99 | |
| Q4 | 1.77 | 538 | 104 | 0.67 | 0.51 | 0.90 | 0.74 | 0.53 | 1.03 | |
| p-value for trend | 0.0055 | 0.0201 | ||||||||
| Eriodictyol | Q1 | 0.00 | 563 | 145 | 1.00 | 1.00 | ||||
| Q2 | 0.02 | 513 | 111 | 0.80 | 0.60 | 1.06 | 0.87 | 0.63 | 1.19 | |
| Q3 | 0.04 | 577 | 134 | 0.88 | 0.67 | 1.16 | 1.01 | 0.74 | 1.38 | |
| Q4 | 0.05 | 537 | 97 | 0.65 | 0.48 | 0.87 | 0.90 | 0.64 | 1.28 | |
| p-value for trend | 0.0186 | 0.8027 | ||||||||
| Isoflavones | ||||||||||
| Daidzein | Q1 | 1.78 | 547 | 135 | 1.00 | 1.00 | ||||
| Q2 | 3.60 | 548 | 129 | 0.94 | 0.72 | 1.25 | 0.95 | 0.69 | 1.30 | |
| Q3 | 5.96 | 548 | 116 | 0.81 | 0.61 | 1.08 | 0.83 | 0.59 | 1.15 | |
| Q4 | 11.19 | 547 | 107 | 0.74 | 0.56 | 0.99 | 0.74 | 0.51 | 1.07 | |
| p-value for trend | 0.0282 | 0.0832 | ||||||||
| Genistein | Q1 | 2.34 | 547 | 140 | 1.00 | 1.00 | ||||
| Q2 | 4.51 | 548 | 125 | 0.86 | 0.65 | 1.14 | 0.91 | 0.66 | 1.25 | |
| Q3 | 7.41 | 548 | 113 | 0.75 | 0.57 | 0.99 | 0.75 | 0.54 | 1.04 | |
| Q4 | 13.95 | 547 | 109 | 0.72 | 0.54 | 0.96 | 0.73 | 0.51 | 1.06 | |
| p-value for trend | 0.0278 | 0.0885 | ||||||||
| Glycitein | Q1 | 0.69 | 547 | 129 | 1.00 | 1.00 | ||||
| Q2 | 1.31 | 548 | 127 | 0.98 | 0.74 | 1.30 | 1.04 | 0.76 | 1.43 | |
| Q3 | 2.07 | 548 | 125 | 0.95 | 0.72 | 1.26 | 0.98 | 0.71 | 1.36 | |
| Q4 | 3.79 | 547 | 106 | 0.77 | 0.58 | 1.04 | 0.83 | 0.57 | 1.20 | |
| p-value for trend | 0.0626 | 0.2374 | ||||||||
| Coumestrol | Q1 | 0.02 | 547 | 139 | 1.00 | 1.00 | ||||
| Q2 | 0.03 | 548 | 114 | 0.77 | 0.58 | 1.02 | 0.77 | 0.56 | 1.06 | |
| Q3 | 0.05 | 548 | 126 | 0.87 | 0.66 | 1.15 | 0.95 | 0.68 | 1.31 | |
| Q4 | 0.11 | 547 | 108 | 0.72 | 0.54 | 0.95 | 0.72 | 0.50 | 1.04 | |
| p-value for trend | 0.0766 | 0.1683 | ||||||||
| Formonnetin | Q1 | 0.00 | 547 | 126 | 1.00 | 1.00 | ||||
| Q2 | 0.01 | 548 | 133 | 1.05 | 0.79 | 1.39 | 1.18 | 0.86 | 1.62 | |
| Q3 | 0.01 | 548 | 110 | 0.83 | 0.62 | 1.11 | 0.91 | 0.66 | 1.28 | |
| Q4 | 0.03 | 547 | 118 | 0.90 | 0.68 | 1.20 | 1.09 | 0.77 | 1.55 | |
| p-value for trend | 0.5079 | 0.5766 | ||||||||
| Biochanin A | Q1 | 0.00 | 547 | 129 | 1.00 | 1.00 | ||||
| Q2 | 0.01 | 548 | 130 | 0.99 | 0.75 | 1.31 | 1.03 | 0.75 | 1.42 | |
| Q3 | 0.02 | 548 | 110 | 0.81 | 0.61 | 1.08 | 0.86 | 0.61 | 1.20 | |
| Q4 | 0.03 | 547 | 118 | 0.88 | 0.66 | 1.17 | 1.03 | 0.72 | 1.48 | |
| p-value for trend | 0.1913 | 0.8488 | ||||||||
| Anthocyanins | ||||||||||
| Cyanidin | Q1 | 1.32 | 547 | 141 | 1.00 | 1.00 | ||||
| Q2 | 3.30 | 548 | 142 | 1.02 | 0.77 | 1.33 | 1.00 | 0.74 | 1.36 | |
| Q3 | 6.89 | 548 | 104 | 0.68 | 0.51 | 0.91 | 0.74 | 0.53 | 1.03 | |
| Q4 | 13.05 | 547 | 100 | 0.65 | 0.49 | 0.87 | 0.66 | 0.47 | 0.93 | |
| p-value for trend | 0.0004 | 0.0055 | ||||||||
| Delphinidine | Q1 | 0.01 | 547 | 151 | 1.00 | 1.00 | ||||
| Q2 | 0.07 | 546 | 121 | 0.75 | 0.57 | 0.98 | 0.77 | 0.57 | 1.05 | |
| Q3 | 0.20 | 552 | 97 | 0.56 | 0.42 | 0.75 | 0.65 | 0.47 | 0.91 | |
| Q4 | 0.54 | 545 | 118 | 0.73 | 0.55 | 0.96 | 0.77 | 0.56 | 1.07 | |
| p-value for trend | 0.0991 | 0.3163 | ||||||||
| Pelargonidine | Q1 | 0.02 | 547 | 156 | 1.00 | 1.00 | ||||
| Q2 | 0.09 | 546 | 124 | 0.73 | 0.56 | 0.96 | 0.83 | 0.61 | 1.13 | |
| Q3 | 0.16 | 552 | 109 | 0.62 | 0.47 | 0.82 | 0.67 | 0.49 | 0.92 | |
| Q4 | 0.48 | 545 | 98 | 0.55 | 0.41 | 0.73 | 0.64 | 0.46 | 0.90 | |
| p-value for trend | 0.0003 | 0.0199 | ||||||||
| Peonidine | Q1 | 0.10 | 530 | 139 | 1.00 | 1.00 | ||||
| Q2 | 0.48 | 513 | 117 | 0.83 | 0.62 | 1.10 | 1.02 | 0.74 | 1.40 | |
| Q3 | 0.83 | 576 | 118 | 0.72 | 0.55 | 0.95 | 0.80 | 0.58 | 1.10 | |
| Q4 | 2.50 | 571 | 113 | 0.70 | 0.52 | 0.92 | 0.78 | 0.56 | 1.08 | |
| p-value for trend | 0.029 | 0.115 | ||||||||
Model 1 adjusted for age; model 2 adjusted for age, BMI, education level, household income, marital status, job, current alcohol drinking, current smoking, physical activity, chronic disease status (diabetes, hypertension, cancers, or cardiovascular diseases), sleep duration, family history of depression, stress, menopause status, and total energy intake; OR, odds ratio; CI, confidence interval; Q, quartile.
3.4. Association between Carotenoid Intake and Depressive Symptoms
Table 5 shows that the association between depressive symptoms and total carotenoids and subclass of carotenoid intake. Lycopene and zeaxanthin were associated with lower prevalence of depressive symptoms after adjusting for multiple confounding factors (OR = 0.66, 95% CI: 0.47–0.92, p for trend = 0.0106 for lycopene and OR = 0.63, 95% CI: 0.44–0.90, p for trend = 0.028 for zeaxanthin, respectively).
Table 5.
Association between total carotenoid and carotenoid subclass intake and prevalence of depressive symptoms in multivariate-adjusted logistic regression analysis.
| Variables | Quartile | Median | No. of Total | No. of Cases | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI Lower Upper |
OR | 95% CI Lower Upper |
|||||||
| Total Carotenoids | Q1 | 11.61 | 396 | 151 | 1.00 | 1.00 | ||||
| Q2 | 18.03 | 423 | 125 | 0.77 | 0.58 | 1.01 | 0.72 | 0.53 | 0.99 | |
| Q3 | 25.65 | 444 | 104 | 0.61 | 0.46 | 0.82 | 0.67 | 0.48 | 0.94 | |
| Q4 | 38.29 | 440 | 107 | 0.64 | 0.48 | 0.84 | 0.70 | 0.47 | 1.04 | |
| p-value for trend | 0.0001 | 0.0604 | ||||||||
| α-carotene | Q1 | 0.15 | 412 | 135 | 1.00 | 1.00 | ||||
| Q2 | 0.30 | 418 | 130 | 0.94 | 0.71 | 1.24 | 0.85 | 0.62 | 1.17 | |
| Q3 | 0.48 | 428 | 120 | 0.84 | 0.63 | 1.11 | 0.82 | 0.59 | 1.15 | |
| Q4 | 0.83 | 445 | 102 | 0.69 | 0.51 | 0.92 | 0.74 | 0.51 | 1.09 | |
| p-value for trend | 0.0068 | 0.1635 | ||||||||
| β-carotene | Q1 | 3.27 | 397 | 150 | 1.00 | 1.00 | ||||
| Q2 | 4.99 | 429 | 119 | 0.74 | 0.56 | 0.98 | 0.82 | 0.59 | 1.12 | |
| Q3 | 6.75 | 437 | 111 | 0.68 | 0.51 | 0.90 | 0.90 | 0.64 | 1.27 | |
| Q4 | 9.56 | 440 | 107 | 0.65 | 0.49 | 0.87 | 0.82 | 0.55 | 1.22 | |
| p-value for trend | 0.0041 | 0.4396 | ||||||||
| Lycopene | Q1 | 0.28 | 406 | 141 | 1.00 | 1.00 | ||||
| Q2 | 0.82 | 416 | 132 | 0.91 | 0.69 | 1.20 | 0.92 | 0.68 | 1.26 | |
| Q3 | 2.00 | 434 | 114 | 0.76 | 0.57 | 1.00 | 0.80 | 0.58 | 1.10 | |
| Q4 | 4.30 | 447 | 100 | 0.64 | 0.48 | 0.86 | 0.66 | 0.47 | 0.92 | |
| p-value for trend | 0.0015 | 0.0106 | ||||||||
| Lutein | Q1 | 0.71 | 412 | 135 | 1.00 | 1.00 | ||||
| Q2 | 1.23 | 427 | 121 | 0.86 | 0.65 | 1.14 | 0.95 | 0.69 | 1.31 | |
| Q3 | 1.88 | 434 | 114 | 0.80 | 0.60 | 1.06 | 0.99 | 0.71 | 1.39 | |
| Q4 | 3.42 | 430 | 117 | 0.84 | 0.63 | 1.11 | 1.01 | 0.70 | 1.45 | |
| p-value for trend | 0.2813 | 0.8717 | ||||||||
| Zeaxanthin | Q1 | 0.07 | 403 | 144 | 1.00 | 1.00 | ||||
| Q2 | 0.13 | 431 | 117 | 0.76 | 0.58 | 1.01 | 0.75 | 0.55 | 1.04 | |
| Q3 | 0.22 | 423 | 125 | 0.83 | 0.63 | 1.10 | 0.86 | 0.62 | 1.19 | |
| Q4 | 0.37 | 446 | 101 | 0.64 | 0.48 | 0.86 | 0.63 | 0.44 | 0.90 | |
| p-value for trend | 0.0077 | 0.028 | ||||||||
| β-cryptoxanthin | Q1 | 0.12 | 416 | 131 | 1.00 | 1.00 | ||||
| Q2 | 0.21 | 406 | 142 | 1.11 | 0.85 | 1.47 | 1.05 | 0.77 | 1.44 | |
| Q3 | 0.32 | 434 | 114 | 0.84 | 0.63 | 1.12 | 0.76 | 0.54 | 1.07 | |
| Q4 | 0.50 | 447 | 100 | 0.72 | 0.53 | 0.96 | 0.75 | 0.52 | 1.09 | |
| p-value for trend | 0.004 | 0.0542 | ||||||||
| Capsaicin | Q1 | 4.70 | 401 | 146 | 1.00 | 1.00 | ||||
| Q2 | 8.02 | 424 | 124 | 0.79 | 0.60 | 1.05 | 0.82 | 0.60 | 1.13 | |
| Q3 | 12.90 | 436 | 112 | 0.70 | 0.53 | 0.93 | 0.77 | 0.55 | 1.08 | |
| Q4 | 20.52 | 442 | 105 | 0.65 | 0.49 | 0.86 | 0.70 | 0.48 | 1.02 | |
| p-value for trend | 0.0035 | 0.0782 | ||||||||
Model 1 adjusted for age; model 2 adjusted for age, BMI, education level, household income, marital status, job, current alcohol drinking, current smoking, physical activity, chronic disease status (diabetes, hypertension, cancers, or cardiovascular diseases), sleep duration, family history of depression, stress, menopause status, and total energy intake; OR, odds ratio; CI, confidence interval; Q, quartile.
3.5. Results of the In Silico Analysis
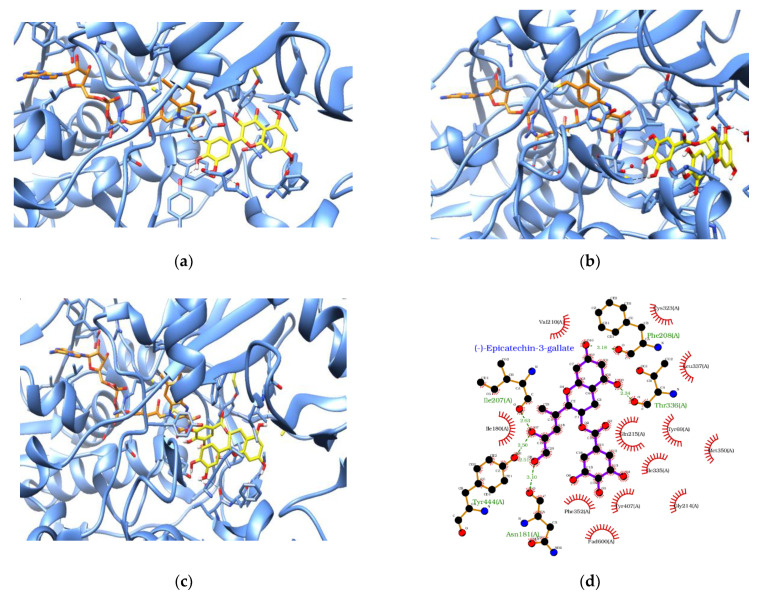
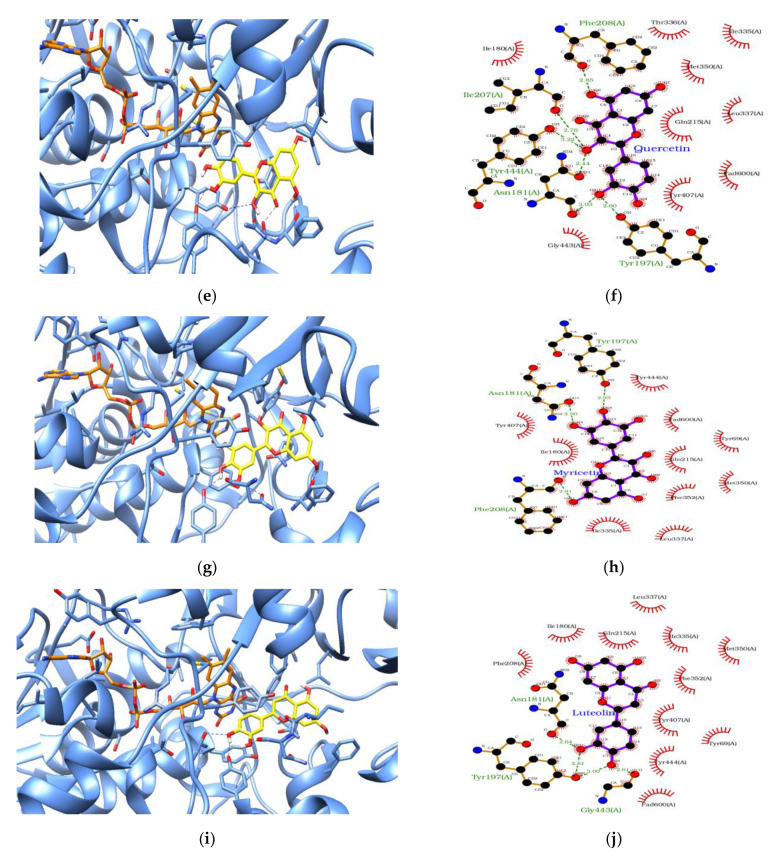
Docking results of all selected flavonoid and carotenoid compounds with MAO enzymes (MAOA and MAOB) and BDNF, respectively, were ranked according to the docking score, that is, EFEB. The top-scoring ligand in MAOA and MAOB was (−)-epicatechin-3-gallate, which had docking scores of −12.73 and −13.84, respectively (Table 6). A positive correlation between the EFEB docking scores of MAOA and MAOB 0.509 was observed. In the case of BDNF, the top-scoring molecule was alpha-carotene with an EFEB value of −7.24, and the minimum EFEB was −6.06 for lutein (Table 7). Further interaction studies of the top-scoring molecules in docked ligand–protein complexes of MAOA and MAOB revealed a similar binding pose. Multiple hydrogen bonds and hydrophobic interactions were present between the protein and ligand complexes (Figure 1). According to EFEB in MAOA, the top four flavonoids were (−)-epicatechin-3-gallate, quercetin, myricetin, and luteolin, which had 6 hydrogen bonds (HB) and 12 hydrophobic interactions (HPhoI), 4 HB and 9 HPhoI, 3 HB and 10 HPhoI, and 4 HB and 11 HPhoI, respectively (Figure 1 and Table 6).
Table 6.
Docking score (EFEB) of selected molecules with MAOA and MAOB.
| No | Name of Compound | EFEB in MAOA | EFEB in MAOB |
|---|---|---|---|
| 1 | (−) Epicatechin-3-gallate | −12.73 | −13.84 |
| 2 | Quercetin | −11.43 | −10.91 |
| 3 | Myricetin | −10.83 | −10.91 |
| 4 | Luteolin | −10.81 | −10.84 |
| 5 | Eriodictyol | −10.78 | −10.98 |
| 6 | Kaempferol | −10.37 | −9.97 |
| 7 | Delphinidine | −10.27 | −10.4 |
| 8 | Petunidine | −10.25 | −10.38 |
| 9 | Capsaicin | −10.24 | −10.43 |
| 10 | Biochanin A | −10.12 | −10.37 |
| 11 | Cyanidin | −9.98 | −9.86 |
| 12 | Naringenin | −9.89 | −10.05 |
| 13 | Apigenin | −9.84 | −9.94 |
| 14 | Peonidine | −9.69 | −10.41 |
| 15 | Glycitein | −9.68 | −9.59 |
| 16 | Formonnetin | −9.67 | −10.26 |
| 17 | Genistein | −9.65 | −10.28 |
| 18 | (+)-Catechin | −9.62 | −10.33 |
| 19 | (+)-Gallocatechin | −9.54 | −10.37 |
| 20 | Coumestrol | −9.47 | −10.3 |
| 21 | Epigallocatechin | −8.96 | −13.34 |
| 22 | Pelargonidine | −8.96 | −9.06 |
| 23 | Daidzein | −8.96 | −9.72 |
| 24 | Theaflavin | 59.64 | −7.74 |
EFEB: estimated free energy of binding, MAOA: monoamine oxidase A, MAOB: monoamine oxidase B.
Table 7.
Docking score (EBEF) of the selected carotenoids with BDNF.
| Sr. No | Name of Compound | EFEB in BDNF |
|---|---|---|
| 1 | α-Carotene | −7.24 |
| 2 | β-Cryptoxanthin | −6.79 |
| 3 | Lycopene | −6.47 |
| 4 | β-Carotene | −6.18 |
| 5 | Capsaicin | −6.17 |
| 6 | Zeaxanthin | −6.10 |
| 7 | Lutein | −6.06 |
EFEB: estimated free energy of binding, BDNF: brain-derived neurotrophic factor.
Figure 1.
(a): MAOA active site with ligand and co-enzyme (protein MAOA is shown in blue, compound in yellow and FAD co-enzyme in orange). (b) MAOB active site with ligand and co-enzyme (protein MAOB is shown in blue, compound in yellow and FAD co-enzyme in orange). (c,d) Interaction between the first ranked compound ((−)-epicatechin-3-gallate) with the target protein. (e,f) Interaction between the second-ranked compound (quercetin) with the target protein. (g,h) Interaction between the third-ranked compound (myricetin) with the target protein. (i,j) Interaction between the fourth-ranked compound (luteolin) with the target protein. (In 3D interaction figures, MAOA and MAOB are shown in blue, compounds in yellow, and FAD co-enzyme in orange).
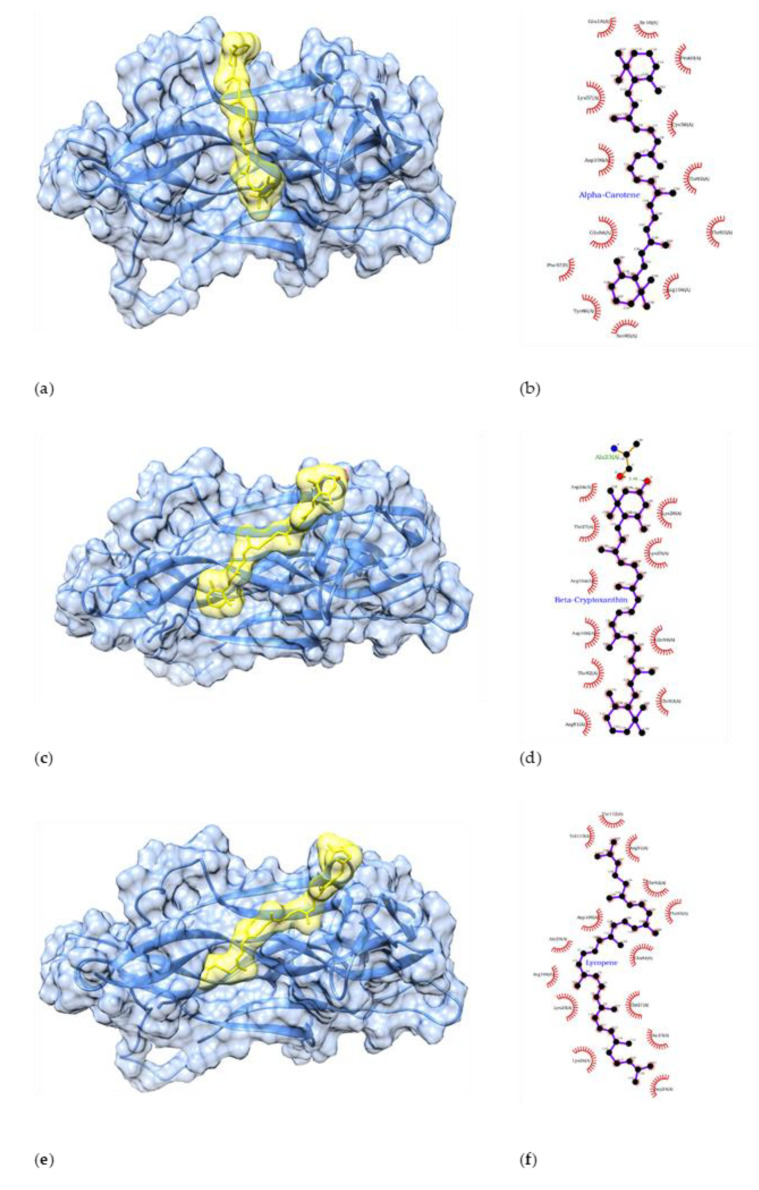
Similarly, an interaction study of BDNF with docked ligands revealed multiple hydrophobic interactions in protein–ligand complexes. A total of 13, 10, and 14 hydrophobic interactions were found for α-carotene, β-cryptoxanthin, and lycopene in the protein–ligand docked complex, and five residues (Thr82, Thr83, Gln84, Arg104, and Asp106) were found to be common among these three top-scoring ligands (Figure 2). As per the defined binding cavity, the ligands were docked in the middle region of the protein (Figure 2).
Figure 2.
Interaction between the first ranked compound (α-carotene) with BDNF (a,b). Interaction between the second-ranked compound (β-cryptoxanthin) with the target BDNF protein (c,d). Interaction between the third-ranked compound (lycopene) with BDNF protein (e,f). In 3D interaction figures, BDNF is shown in blue and compounds in yellow).
4. Discussion
This study was conducted to investigate the association between the dietary intake of flavonoids and carotenoids and depressive symptoms among middle-aged Korean females and to clarify the relevant mechanisms of the association using in silico analysis. To date, a variety of dietary flavonoids and carotenoids have shown antidepressant properties in numerous studies [13,15,16,26,29]. Additionally, these flavonoids and carotenoids are abundant in food and have potential therapeutic activities for depression, and can be used as a cost-effective means [14].
Although the antidepressant properties of several flavonoids and carotenoids have been studied to date, the complex nature of depression, including different mechanisms and pathways, hinders an accurate understanding of the mechanisms of antidepressant action [10,56]. However, different results of the in vivo studies suggest that some major flavonoids/carotenoids must be explored for their anti-depression effects and the most probable mechanisms for the development of antidepressants in the population [7,9,11].
In this study, subjects with depressive symptoms had fewer intakes of total flavonoids, subclass flavonoids, and individual flavonoids than control subjects. An intervention study reported that a high polyphenol diet (including six portions of fruit and vegetables and 50 grams of dark chocolate/day) for eight weeks reduced depressive symptoms in patients with mild hypertension [11]. In addition, an inverse association between subclass flavonoid (flavonol, flavone, and flavoanone) intake and depression risk has also been reported in a cohort study of middle-aged and older females in the United States [13]. A cross-sectional study showed that the highest dietary phytochemical index group had a lower prevalence of depressive symptoms among females in Iran [57].
In a multivariate-adjusted logistic regression analysis, dietary intake of flavones and anthocyanin subclasses was negatively associated with the risk of depressive symptoms in this study. Similarly, a recent study reported that flavanols, flavonols, flavononoes, flavones, and anthocyanin subclasses were inversely associated with depressive symptoms in adults living in the Mediterranean region [9]. However, the isoflavone subclass did not show any association with depressive symptoms, as observed in our study.
Among the individual compounds of flavonoids, luteolin, (+)-catechin, (+)-gallocatechin, theaflavin, and theaflavin 3-gallatecyanidin and pelargonidine were negatively associated with the risk of depressive symptoms in a logistic regression analysis. Naringin and quercetin intake were negatively associated with depression in a Mediterranean study [9].
Among the subclasses of carotenoids, lycopene and zeaxanthin intake showed significantly (34% and 37%, respectively) lower depressive symptom risk in this study. In the United States National Health and Nutrition Examination Survey, total carotenoid and all subgroup carotenoid intakes were inversely associated with depressive symptoms [17]. A cross-sectional study reported that α-carotene and β-carotene intake were inversely associated with the CES-D score [18]. In animal studies, lycopene administration (60 mg/kg) decreased plasma levels of lipopolysaccharide (LPS)-induced interleukin-1β (IL-1β) and heme oxygenase-1 (HO-1) and decreased interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in plasma [58]. In another animal study, IL-6, interleukin-1β (IL-1β), and TNF-α in the hippocampus were reduced by zeaxanthin treatment [59]. These results suggest that carotenoids could be used as potential therapeutics.
Individual compounds from flavonoids and carotenoids have been tested for mechanisms in important drug targets, MAO (MAOA and MAOB) and BDNF [15,50,60,61,62,63], which are known to be associated with depression through molecular docking studies for possible inhibitory roles. In flavonoids, MAO inhibition was selected to study the mechanism of anti-depression as some flavonoids are known to inhibit the MAO enzyme in the literature [57,59,61]. However, the docking score of the selected flavonoids had positive correlations in MAOA and MAOB, which is in line with the literature suggesting that a similar binding pocket is present in both these targets [64]. However, most of the individual flavonoid compounds showed high negative values of EFEB in MAOA and MAOB docking, which could be due to their inhibitory role in these enzymes. Contrary to the epidemiological data analysis results, theaflavin and theaflavin-3-gallate achieved high EFEB in the molecular docking study, suggesting their inability to inhibit MAOA and MAOB as drug targets. The high molecular weight (>500) of these flavonoids is expected to be the reason for the inability of these compounds to interact optimally with MAO. Therefore, these flavonoids may have different modes of action for their antidepressant properties, as intake of theaflavin and theaflavin 3-gallate are negatively associated with the risk of depressive symptoms (Table 4). Flavonoids with high negative values of EFEB have a high possibility of inhibiting MAO and the mode of anti-depression action through the MAO enzyme. Compounds with a docking score better than -9 EFEB could inhibit MAO as one of the important mechanisms for anti-depressive effects. Similar docking poses of molecules inside the binding pocket (Figure 1) and the literature also support our results, as flavonoids such as quercetin, luteolin, biochanin A, and cyanidine are known to inhibit MAO [58,59,62]. The top-scoring molecule, that is, (−)-epicatechin-3-gallate, has bioavailability issues as it has low absorption in the stomach when taken with food [65]. Bioavailability could be the main reason for the lack of significant association of (−)-epicatechin-3-gallate with anti-depressive symptoms in our study [65]. (−)-epicatechin-3-gallate is mainly contained in green tea. In our study, the intake of green tea, citron tea, and black tea was combined, so the intake of green tea may be underestimated. Furthermore, other compounds that had better docking scores but were not found to be significantly associated with depressive symptoms in our statistical analysis could be due to limitations of this study such as the small number of subjects and cross-sectional study design. Different flavonoids were found to be associated with direct and indirect mechanisms in the pathophysiology of depression, which is an active area of research for the development of therapeutics [66]. A complex mechanistic point was explored here to study the possible inhibition of MAOA and MAOB, which are known targets of flavonoids for anti-depressive effects in several cases [15,50,60,61,62]. The bioavailability of flavonoids is another important point because limited information regarding the bioavailability (reach of different flavonoids in the central nervous system) potential is not known [67]. Nevertheless, bioavailability and other important factors such as absorption, metabolism, and distribution of flavonoids are important questions to be considered in future research.
In the case of carotenoids, all ligands were docked in the middle part of BDNF, which is distant from the N-terminal region involved in tropomyosin receptor kinase B (TrkB) binding [68]. Hence, the binding of carotenoids does not directly interfere with TrkB binding and is expected to exert an allosteric effect on the TrkB binding region. However, in vitro experiments are required to confirm allosteric activation of the system. The top-scoring compound was α-carotene in the BDNF and carotenoid docking studies. However, all six carotenoids had slight differences in their docking scores (Table 7), which suggests a similar binding affinity of these carotenoids to BDNF.
The current study is meaningful in that it is the first to analyze the association between the intake of flavonoids and carotenoids and depressive symptoms using epidemiologic data and investigate the mechanism using an in silico analysis. This study also had several limitations. Because of its cross-sectional design, a causal relationship between the intake of flavonoids/carotenoids and depressive symptoms has not been identified. The docking results suggested that compounds with molecular weights greater than 500 could not inhibit MAO enzymes [15,50,60,61,62]. These results suggest that dietary flavonoids and carotenoids can be utilized in the treatment of depressive symptoms.
Author Contributions
Conceptualization, S.-J.P., V.J. and H.-J.L.; methodology, S.-J.P., V.J. and H.-J.L.; formal analysis, S.-J.P. and V.J.; data curation, S.-J.P. and V.J.; writing—original draft preparation, S.-J.P. and V.J.; writing—review and editing, H.-J.L.; supervision, H.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Food Research Institute(E0164500), Korea and partly carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014536022021)” Rural Development Administration, Korea.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Gachon University Gil Medical Center (GDIRB2016-271, approval date 4 October 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are reported in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daskalopoulou M., George J., Walters K., Osborn D.P., Batty G.D., Stogiannis D., Rapsomaniki E., Pujades-Rodriguez M., Denaxas S., Udumyan R. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: Data linkage study of 1.9 million women and men. PLoS ONE. 2016;11:e0153838. doi: 10.1371/journal.pone.0153838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Qian F., Hou C., Li X., Gao Q., Luo Y., Tao L., Yang X., Wang W., Zheng D. Longitudinal changes in depressive symptoms and risks of cardiovascular disease and all-cause mortality: A nationwide population-based cohort study. J. Gerontol. Ser. A. 2020;75:2200–2206. doi: 10.1093/gerona/glz228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold S.M., Köhler-Forsberg O., Moss-Morris R., Mehnert A., Miranda J.J., Bullinger M., Steptoe A., Whooley M.A., Otte C. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers. 2020;6:69. doi: 10.1038/s41572-020-0200-2. [DOI] [PubMed] [Google Scholar]
- 5.Moussavi S., Chatterji S., Verdes E., Tandon A., Patel V., Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 6.Kozela M., Bobak M., Besala A., Micek A., Kubinova R., Malyutina S., Denisova D., Richards M., Pikhart H., Peasey A. The association of depressive symptoms with cardiovascular and all-cause mortality in Central and Eastern Europe: Prospective results of the HAPIEE study. Eur. J. Prev. Cardiol. 2016;23:1839–1847. doi: 10.1177/2047487316649493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuijpers P., Vogelzangs N., Twisk J., Kleiboer A., Li J., Penninx B.W. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am. J. Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 8.Wang P.S., Aguilar-Gaxiola S., Alonso J., Angermeyer M.C., Borges G., Bromet E.J., Bruffaerts R., De Girolamo G., De Graaf R., Gureje O. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet. 2007;370:841–850. doi: 10.1016/S0140-6736(07)61414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godos J., Castellano S., Ray S., Grosso G., Galvano F. Dietary polyphenol intake and depression: Results from the mediterranean healthy eating, lifestyle and aging (meal) study. Molecules. 2018;23:999. doi: 10.3390/molecules23050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias G.P., Cavegn N., Nix A., do Nascimento Bevilaqua M.C., Stangl D., Zainuddin M.S.A., Nardi A.E., Gardino P.F., Thuret S. The role of dietary polyphenols on adult hippocampal neurogenesis: Molecular mechanisms and behavioural effects on depression and anxiety. Oxidative Med. Cell. Longev. 2012;2012:541971. doi: 10.1155/2012/541971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontogianni M.D., Vijayakumar A., Rooney C., Noad R.L., Appleton K.M., McCarthy D., Donnelly M., Young I.S., McKinley M.C., McKeown P.P. A high polyphenol diet improves psychological well-being: The polyphenol intervention trial (pphit) Nutrients. 2020;12:2445. doi: 10.3390/nu12082445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouayed J. Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr. Nutr. Food Sci. 2010;6:13–18. doi: 10.2174/157340110790909608. [DOI] [Google Scholar]
- 13.Chang S.-C., Cassidy A., Willett W.C., Rimm E.B., O’Reilly E.J., Okereke O.I. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am. J. Clin. Nutr. 2016;104:704–714. doi: 10.3945/ajcn.115.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko Y.-H., Kim S.-K., Lee S.-Y., Jang C.-G. Flavonoids as therapeutic candidates for emotional disorders such as anxiety and depression. Arch. Pharmacal Res. 2020;43:1128–1143. doi: 10.1007/s12272-020-01292-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Cheng C., Xin C., Wang Z. The Antidepressant-like Effect of Flavonoids from Trigonella Foenum-Graecum Seeds in Chronic Restraint Stress Mice via Modulation of Monoamine Regulatory Pathways. Molecules. 2019;24:1105. doi: 10.3390/molecules24061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hritcu L., Ionita R., Postu P.A., Gupta G.K., Turkez H., Lima T.C., Carvalho C.U.S., de Sousa D.P. Antidepressant flavonoids and their relationship with oxidative stress. Oxidative Med. Cell. Longev. 2017;2017:5762172. doi: 10.1155/2017/5762172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge H., Yang T., Sun J., Zhang D. Associations between dietary carotenoid intakes and the risk of depressive symptoms. Food Nutr. Res. 2020;64:3920. doi: 10.29219/fnr.v64.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D., Li Y. Associations of α-carotenoid and β-carotenoid with depressive symptoms in late midlife women. J. Affect. Disord. 2019;256:424–430. doi: 10.1016/j.jad.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Milaneschi Y., Bandinelli S., Penninx B.W., Corsi A.M., Lauretani F., Vazzana R., Semba R.D., Guralnik J.M., Ferrucci L. The relationship between plasma carotenoids and depressive symptoms in older persons. World J. Biol. Psychiatry. 2012;13:588–598. doi: 10.3109/15622975.2011.597876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoi T., Okuma Y., Nomura Y. The mechanisms of immune-to-brain communication in inflammation as a drug target. Curr. Drug Targets Inflamm. Allergy. 2002;1:257–262. doi: 10.2174/1568010023344599. [DOI] [PubMed] [Google Scholar]
- 21.León S.L., Croes E.A., Sayed-Tabatabaei F.A., Claes S., Van Broeckhoven C., van Duijn C.M. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: A meta-analysis. Biol. Psychiatry. 2005;57:999–1003. doi: 10.1016/j.biopsych.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Paykel E. The evolution of life events research in psychiatry. J. Affect. Disord. 2001;62:141–149. doi: 10.1016/S0165-0327(00)00174-9. [DOI] [PubMed] [Google Scholar]
- 23.Sahay A., Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 24.Jesulola E., Micalos P., Baguley I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet? Behav. Brain Res. 2018;341:79–90. doi: 10.1016/j.bbr.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Hryb A.B., Cunha M.P., Kaster M.P., Rodrigues A.L.S. Studies in Natural Products Chemistry. Volume 55. Elsevier; Amsterdam, The Netherlands: 2018. Natural Polyphenols and Terpenoids for Depression Treatment: Current Status; pp. 181–221. [Google Scholar]
- 26.Machado D.G., Bettio L.E., Cunha M.P., Santos A.R., Pizzolatti M.G., Brighente I.M., Rodrigues A.L.S. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008;587:163–168. doi: 10.1016/j.ejphar.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Samad N., Saleem A., Yasmin F., Shehzad M. Quercetin protects against stress-induced anxiety-and depression-like behavior and improves memory in male mice. Physiol. Res. 2018;67:795–808. doi: 10.33549/physiolres.933776. [DOI] [PubMed] [Google Scholar]
- 28.Park S.-H., Sim Y.-B., Han P.-L., Lee J.-K., Suh H.-W. Antidepressant-like effect of kaempferol and quercitirin, isolated from Opuntia ficus-indica var. saboten. Exp. Neurobiol. 2010;19:30–38. doi: 10.5607/en.2010.19.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng L., Guo X., Li Y., Yang X., Han Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016;774:50–54. doi: 10.1016/j.ejphar.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Lee B., Shim I., Lee H., Hahm D.-H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic–pituitary–adrenal Axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J. Med. Food. 2018;21:979–989. doi: 10.1089/jmf.2017.4161. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z., Wang G., Cui L., Wang Q. Myricetin attenuates depressant-like behavior in mice subjected to repeated restraint stress. Int. J. Mol. Sci. 2015;16:28377–28385. doi: 10.3390/ijms161226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antunes M.S., Jesse C.R., Ruff J.R., de Oliveira Espinosa D., Gomes N.S., Altvater E.E.T., Donato F., Giacomeli R., Boeira S.P. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur. J. Pharmacol. 2016;789:411–420. doi: 10.1016/j.ejphar.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Tayyab M., Farheen S., Khanam N., Hossain M.M., Shahi M.H. Antidepressant and neuroprotective effects of naringenin via sonic hedgehog-GLI1 cell signaling pathway in a rat model of chronic unpredictable mild stress. Neuromol. Med. 2019;21:250–261. doi: 10.1007/s12017-019-08538-6. [DOI] [PubMed] [Google Scholar]
- 34.Fu X., Qin T., Yu J., Jiao J., Ma Z., Fu Q., Deng X., Ma S. Formononetin Ameliorates Cognitive Disorder via PGC-1α Pathway in Neuroinflammation Conditions in High-Fat Diet-Induced Mice. CNS Neurol. Disord. Drug Targets. 2019;18:566–577. doi: 10.2174/1871527318666190807160137. [DOI] [PubMed] [Google Scholar]
- 35.Dhingra D., Bansal Y. Antidepressant-like activity of beta-carotene in unstressed and chronic unpredictable mild stressed mice. J. Funct. Foods. 2014;7:425–434. doi: 10.1016/j.jff.2014.01.015. [DOI] [Google Scholar]
- 36.Unno K., Noda S., Nii H., Kawasaki Y., Iguchi K., Yamada H. Anti-stress Effect of β-Cryptoxanthin in Satsuma Mandarin Orange on Females. Biol. Pharm. Bull. 2019;42:1402–1408. doi: 10.1248/bpb.b19-00325. [DOI] [PubMed] [Google Scholar]
- 37.Zeni A.L.B., Camargo A., Dalmagro A.P. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol. Biochem. Behav. 2019;179:63–72. doi: 10.1016/j.pbb.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Hu P., Ma L., Wang Y.-g., Ye F., Wang C., Zhou W.-H., Zhao X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017;108:426–435. doi: 10.1016/j.neuint.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Jacka F.N., Pasco J.A., Mykletun A., Williams L.J., Hodge A.M., O’Reilly S.L., Nicholson G.C., Kotowicz M.A., Berk M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 40.Rhee J.J., Sampson L., Cho E., Hughes M.D., Hu F.B., Willett W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015;181:225–233. doi: 10.1093/aje/kwu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck A., Steer R., Brown G. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonia, TX, USA: 1996. [Google Scholar]
- 42.Oh D.H., Kim S.A., Lee H.Y., Seo J.Y., Choi B.-Y., Nam J.H. Prevalence and correlates of depressive symptoms in korean adults: Results of a 2009 korean community health survey. J. Korean Med. Sci. 2013;28:128–135. doi: 10.3346/jkms.2013.28.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H., Park S., Kim J., Kim C., Chang K., Yim K., Kim K., Choi H. Development and validation of a computerized semi-quantitative food frequency questionnaire program for evaluating the nutritional status of the Korean elderly. J. Community Nutr. 2002;7:277–285. [Google Scholar]
- 44.National Rural Living Science Institute . Food Composition Table. 6th ed. Rural Development Administration; Suwon, Korea: 2006. [Google Scholar]
- 45.National Academy of Agricultural Sciences . Tables of Food Functional Composition. 1st ed. National Academy of Agricultural Sciences; Suwon, Korea: 2009. pp. 1–450. [Google Scholar]
- 46.U.S. Department of Agriculture. Agricultural Research Service USDA Database for the Flavonoid Content of Selected Foods, Release 3.2. [(accessed on 1 October 2016)];2015 Available online: https://data.nal.usda.gov/system/files/Flav3.2.pdf.
- 47.U.S. Department of Agriculture. Agricultural Research Service USDA Database for the Isoflavone Content of Selected Foods, Release 2.1. [(accessed on 1 October 2016)];2015 Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/isoflav/Isoflav_R2-1.pdf.
- 48.U.S. Department of Agriculture. Agricultural Research Service USDA Database for the Proanthocyanidin Content of Selected Foods, Release 2. [(accessed on 1 October 2016)];2015 Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/PA/PA02.pdf.
- 49.Son S.-Y., Ma J., Kondou Y., Yoshimura M., Yamashita E., Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binda C., Aldeco M., Geldenhuys W.J., Tortorici M., Mattevi A., Edmondson D.E. Molecular insights into human monoamine oxidase B inhibition by the glitazone antidiabetes drugs. ACS Med. Chem. Lett. 2012;3:39–42. doi: 10.1021/ml200196p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y., Wang S., Hu Q., Gao S., Ma X., Zhang W., Shen Y., Chen F., Lai L., Pei J. CavityPlus: A web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018;46:W374–W379. doi: 10.1093/nar/gky380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminf. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laskowski R.A., Swindells M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 55.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 56.Pathak L., Agrawal Y., Dhir A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs. 2013;22:863–880. doi: 10.1517/13543784.2013.794783. [DOI] [PubMed] [Google Scholar]
- 57.Darooghegi Mofrad M., Siassi F., Guilani B., Bellissimo N., Azadbakht L. Association of dietary phytochemical index and mental health in women: A cross-sectional study. Br. J. Nutr. 2019;121:1049–1056. doi: 10.1017/S0007114519000229. [DOI] [PubMed] [Google Scholar]
- 58.Zhang F., Fu Y., Zhou X., Pan W., Shi Y., Wang M., Zhang X., Qi D., Li L., Ma K., et al. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016;298:1–8. doi: 10.1016/j.jneuroim.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X., Gan T., Fang G., Wang S., Mao Y., Ying C. Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab. Brain Dis. 2018;33:705–711. doi: 10.1007/s11011-017-0179-x. [DOI] [PubMed] [Google Scholar]
- 60.Herraiz T., Guillén H. Monoamine oxidase-A inhibition and associated antioxidant activity in plant extracts with potential antidepressant actions. BioMed Res. Int. 2018;2018:4810394. doi: 10.1155/2018/4810394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandaruk Y., Mukai R., Terao J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014;1:639–649. doi: 10.1016/j.toxrep.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dreiseitel A., Korte G., Schreier P., Oehme A., Locher S., Domani M., Hajak G., Sand P.G. Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol. Res. 2009;59:306–311. doi: 10.1016/j.phrs.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Lee B.-H., Kim Y.-K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7:231. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finberg J.P., Rabey J.M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016;7:340. doi: 10.3389/fphar.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naumovski N., Blades B.L., Roach P.D. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants. 2015;4:373–393. doi: 10.3390/antiox4020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rendeiro C., Rhodes J.S., Spencer J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015;89:126–139. doi: 10.1016/j.neuint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Hu M., Wu B., Liu Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017;14:2861–2863. doi: 10.1021/acs.molpharmaceut.7b00545. [DOI] [PubMed] [Google Scholar]
- 68.Cazorla M., Prémont J., Mann A., Girard N., Kellendonk C., Rognan D. Identification of a low–molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Investig. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are reported in this manuscript.