Abstract
Simple Summary
Listeria monocytogenes is the bacterium responsible for the majority of cases of human listeriosis, a foodborne infection that, in certain groups in the population (children, elderly, pregnant women and immunocompromised individuals), exhibits a high fatality rate (of up to 30%), and the need for hospital admission in more than 90% of cases. An awareness of the minimal concentrations for disinfectants and antibiotics necessary to destroy L. monocytogenes, may assist with choosing the most effective antimicrobials for controlling this microorganism, whether in the food industry or in the health system. The lethal concentrations of three disinfectants (sodium hypochlorite, benzalkonium chloride, and peracetic acid) and eight antibiotics (ampicillin, cephalothin, cefoxitin, erythromycin, chloramphenicol, gentamicin, tetracycline, vancomycin, and fosfomycin) for eight different strains of L. monocytogenes were determined in this research work. It was demonstrated that the lethal concentrations for the disinfectants tested were much lower than the concentrations customarily used of these compounds. The characteristics of the cell surface play an important role in the tolerance of L. monocytogenes to these biocides. A considerable prevalence of resistance to most of the antibiotics tested was noted, making it clear that the necessary measures to control resistance in L. monocytogenes must be adopted.
Abstract
When selecting effective doses of antimicrobials, be they biocides or antibiotics, it is essential to know the minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of these substances. The present research determined the MICs and MBCs for three biocides, sodium hypochlorite (SH), benzalkonium chloride (BC), and peracetic acid (PAA), and nine antibiotics in eight strains of Listeria monocytogenes of varying serotypes. Marked intra-species differences were observed in the resistance of L. monocytogenes to the biocides and antibiotics. The MICs (ppm) for the biocides ranged between 1750 and 4500 for SH, 0.25 and 20.00 for BC, and 1050 and 1700 for PAA. Their MBCs (ppm) ranged from 2250 to 4500 for SH, 0.50 to 20.00 for BC, and 1150 to 1800 for PAA. The MICs (ppm) for antibiotics lay between 1 and 15 for ampicillin, 8 and 150 for cephalothin, 20 and 170 for cefoxitin, 0.05 and 0.20 for erythromycin, 4 and 50 for chloramphenicol, 3 and 100 for gentamicin, 2 and 15 for tetracycline, 2 and 80 for vancomycin, and 160 and 430 for fosfomycin. The corresponding MBCs (ppm) were from 5 to 20 for ampicillin, 9 to 160 for cephalothin, 70 to 200 for cefoxitin, 4 to 5 for erythromycin, 9 to 70 for chloramphenicol, 5 to 100 for gentamicin, 3 to 30 for tetracycline, 3 to 90 for vancomycin, and 160 to 450 for fosfomycin. Notably, erythromycin showed considerable efficacy, demonstrated by the low values for both MIC and MBC. Based on EUCAST and the CLSI criteria, all strains were susceptible to erythromycin. All strains were resistant to cephalothin, cefoxitin, gentamicin, and fosfomycin. Further values for resistance were 87.50% for ampicillin and vancomycin, 75.00% for tetracycline, and 62.50% for chloramphenicol. The high prevalence of antibiotic resistance is a matter for concern. A positive correlation was found between MIC and MBC values for most of the biocides and antibiotics. The higher the hydrophobicity of the cell surface, the higher the susceptibility to biocides, suggesting that surface characteristics of bacterial cells influence resistance to these compounds.
Keywords: Listeria monocytogenes, minimum inhibitory concentration, minimum bactericidal concentration, antibiotics, biocides
1. Introduction
Bacteria of the genus Listeria are short, Gram-positive non-spore-producing rods that have the ability to grow in a wide range of temperatures (0.5 °C to 45 °C), pH values (4.7 to 9.2), and osmotic pressures. These characteristics, along with the fact that they are facultative anaerobes, allow these microorganisms to survive under adverse environmental conditions [1].
A total of 26 species have so far been identified within the genus Listeria (Table 1). Of all these species the most prominent is Listeria monocytogenes because it causes the most cases of listeriosis, be it in humans or in animals. Listeria ivanovii also sometimes triggers listeriosis, and a few sporadic cases have been described where listeriosis was caused by Listeria seeligeri [2].
Table 1.
Species in the genus Listeria. Adapted from Nwaiwu [3].
| Species | Year of Description | Reference |
|---|---|---|
| Listeria monocytogenes | 1940 | [4] |
| Listeria innocua | 1983 | [5] |
| Listeria seeligeri | 1983 | [6] |
| Listeria welshimeri | 1983 | [6] |
| Listeria ivanovii | 1984 | [7] |
| Listeria grayi | 1992 | [8] |
| Listeria marthii | 2010 | [9] |
| Listeria rocourtiae | 2010 | [10] |
| Listeria fleischmannii | 2013 | [11] |
| Listeria weihenstephanensis | 2013 | [12] |
| Listeria aquatica | 2014 | [13] |
| Listeria cornellensis | 2014 | [13] |
| Listeria floridensis | 2014 | [13] |
| Listeria grandensis | 2014 | [13] |
| Listeria riparia | 2014 | [13] |
| Listeria booriae | 2015 | [14] |
| Listeria newyorkensis | 2015 | [14] |
| Listeria goaensis | 2018 | [15] |
| Listeria costaricensis | 2018 | [16] |
| Listeria thailandensis | 2019 | [17] |
| Listeria valentina | 2020 | [18] |
| Listeria cossartiae | 2021 | [19] |
| Listeria farberi | 2021 | [19] |
| Listeria immobilis | 2021 | [19] |
| Listeria portnoyi | 2021 | [19] |
| Listeria rustica | 2021 | [19] |
Listeriosis is a food-borne zoonosis that most frequently and most seriously affects the risk groups, collectively known as YOPIs (the Young, Old, Pregnant, and Immunocompromised). Invasive listeriosis is an infection associated with a high rate of hospital admissions and is the food-borne disease with the greatest lethality rate [20]. Moreover, this infection can give rise to grave harm or sequelae, such as meningitis, encephalitis, septicaemia, endocarditis, and miscarriages [21]. For these reasons, L. monocytogenes is a major risk for the food industry, and in particular for producers of ready-to-eat (RTE) foods [22]. Thus, several measures are applied to reduce the prevalence and/or the levels of this bacterium in RTE foodstuffs [23,24].
Among the disinfectants most widely used in food-processing environments are sodium hypochlorite (SH), benzalkonium chloride (BC), and peracetic acid (PAA). Chlorine-based disinfectants like SH are inexpensive oxidizing compounds that show powerful, broad-spectrum bactericidal activity [25]. For their part, compounds derived from quaternary ammonium, like BC, are cationic surfactants that act by destroying the lipid bilayer membrane and have an antimicrobial effect on several types of microorganisms [26]. The antimicrobial activity of PAA is also based on the oxidation of cell components [27]. Both SH and PAA are approved for various uses in the European Economic Area (EEA) and Switzerland, including as food and feed area disinfectants (Product-Type 4). This is subject to the specifications and conditions for use established by the Commission Implementing Regulation (EU) 2017/1273 in the case of SH, and the Commission Implementing Regulation (EU) 2016/672 for PAA. At present, the use of BC in a range of types of biocidal products, including disinfectants for areas where food and feed are processed, is being reviewed in the EEA and Switzerland [28].
If disinfectants are to be effective, they must be utilized in appropriate doses. Use at sub-lethal concentrations is not only ineffective but can even be counterproductive since low doses of biocides are linked to an increase in tolerance of these substances and resistance to antibiotics, in addition to a heightened bacterial capacity to form biofilm [28,29,30,31,32]. For this reason, awareness of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of disinfectants is crucial.
In view of the seriousness of the infection, treatment with antibiotics is generally required for people suffering from invasive listeriosis. The increase in resistance to antibiotics over the last few decades has become a source of concern worldwide. Although various strategies are being devised to prevent and control this problem, bacterial resistance is becoming ever more frequent, both in clinical strains and in those found in the environment or in foodstuffs [33].
The aims of this study were: (1) to determine the minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) for nine antibiotics of clinical interest and three biocides used in food-processing plants relative to eight strains of L. monocytogenes belonging to different serotypes; (2) to establish the relationship between the MIC and MBC values of the biocides and antibiotics; and (3) to know the influence of cell surface hydrophobicity on the susceptibility to antimicrobials.
2. Materials and Methods
2.1. Strains and Culturing Conditions
Eight strains of L. monocytogenes were used: ATCC (American Type Culture Collection) 19111 (serotype 1/2a), ATCC 19112 (serotype 1/2c), ATCC 19114 (serotype 4a), ATCC 19117 (serotype 4d), ATCC 13932 (serotype 4b), STCC (Spanish Type Culture Collection) 936 (serotype 1/2b), STCC 937 (serotype 3b), and STCC 938 (serotype 3c). The bacterial cultures were kept in storage at a temperature of −50 °C in tryptone soy broth (TSB; Oxoid Ltd., Hampshire, UK) with 20% (vol/vol) of glycerol. Prior to each experiment, aliquots of approximately 20 μL of the frozen culture were transferred to tubes containing 5 mL of TSB (Oxoid) that had been incubated overnight at 37 °C. Thereafter, the cultures were inoculated onto tryptone soy agar (TSA, Oxoid Ltd., Hampshire, UK) plates and stored at 4 °C until required for use.
2.2. Determination of the Minimum Inhibitory Concentration (MIC)
The MIC of the antimicrobials was determined by the method involving microdilution in culture broth, as indicated by the Clinical and Laboratory Standards Institute of the United States of America [34]. In this process, different concentrations of twelve antimicrobials, comprising three biocides and nine antibiotics, were used. The biocides tested were sodium hypochlorite (SH), benzalkonium chloride (BC), and peracetic acid (PAA). All three were obtained from the Sigma-Aldrich Co. (Saint Louis, MO, USA). In preparing the solutions required, the initial substance contained 10% free chlorine in the case of the SH, 95% (on the assumption the product was pure) for the BC, and 39% acetic acid equivalent for the PAA. Dehydrated antibiotics were purchased from the Sigma-Aldrich Co. (Saint Louis, MO, USA). They were ampicillin (AMP), cephalothin (KF), cefoxitin (FOX), erythromycin (E), chloramphenicol (C), gentamicin (CN), tetracycline (TE), vancomycin (VA), and fosfomycin (FOS). Before the start of each experiment, solutions of each of these compounds were prepared under aseptic conditions in sterile distilled water (FOX, CN, TE, VA, FOS), in 95% ethanol (E, C), in phosphate buffered saline (PBS) at pH 8.0 (AMP) or in PBS at pH 6.0 (KF). Three replicates were performed for each strain and antimicrobial compound.
Five colonies of each strain were taken from the TSA (Oxoid) plates, inoculated into 9 mL of TSB (Oxoid), and incubated at 37 °C for 18 to 24 h. In this experimental work, polystyrene microtiter plates with one hundred wells (Oy Growth Curves Ab Ltd., Helsinki, Finland) were used. The wells were filled with a total volume of 200 μL, made up of 20 μL of the antimicrobial solution at a range of concentrations and 180 μL of the third dilution of the inoculum to obtain a final concentration in the well of approximately 105 cfu/mL. The concentration of the inoculum was confirmed by plating. Negative controls with 200 μL of TSB and 200 μL of the antimicrobial solutions and positive controls with 200 μL of the bacterial inoculum were used. Growth was determined by measuring the optical density of each sample in the range 480 to 520 nm (OD480–520) in a Bioscreen C MRB (Oy Growth Curves Ab). The value for MIC was set as the minimum concentration of the antimicrobial substance necessary to prevent bacterial growth after 48 h of incubation at 37 °C. The growth limit was deemed to be a value of 0.200 for OD480–520. Strains were classified as resistant, with reduced susceptibility (intermediate), or susceptible, based on given criteria. These were the guidelines set for L. monocytogenes in the case of AMP and E [35], the standards laid down for Staphylococcus spp. when considering C, TE, and FOS, for Staphylococcus aureus when considering CN and VA [35], and the norms used for S. aureus with respect to KF and FOX [36]. In certain cases, criteria established for another Gram-positive bacterium (Staphylococcus spp. or S. aureus) were employed because there were none for L. monocytogenes.
2.3. Determination of the Minimum Bactericidal Concentration (MBC)
The dilution in broth method was used to calculate the MBC for the antimicrobials [34]. A volume of 0.1 mL was removed from the wells in the microtiter plates (Oy Growth Curves Ab) where no growth was observed after 48 h of incubation at 37 °C, and was then inoculated onto the surface of TSA plates (Oxoid). They were incubated for 48 h at 37 °C, with MBC being taken to be the lowest concentration of the substance at which no colonies formed under these conditions. Since the limit of detection for this technique is 10 cfu/mL, the absence of any growth on a TSA plate indicated that the concentration lay below this value. The initial concentration of 105 cfu/mL had thus been reduced to below 10 cfu/mL. Consequently, the MBC was effectively deemed to be the minimum concentration of antimicrobial capable of inactivating more than 99.99% of the bacteria present. Three replicates were performed for each strain and antimicrobial compound.
2.4. Determination of Cell Surface Hydrophobicity (CSH)
The CSH of strains was determined by the microbial adhesion to solvents (MATS) test based on affinity to non-polar solvents [37]. Hexadecane was used as the hydrocarbon phase. L. monocytogenes cells were grown in TSB for 24 h at 37 °C. Cells were harvested by centrifugation (4000 rpm, 10 min, 4 °C), washed twice with sterile PBS (Merck KGaA, Darmstadt, Germany), and re-suspended in TSB at an initial concentration of 105 cfu/mL. After 24 h at 37 °C, the bacterial cells were centrifuged, washed twice with PBS, and re-suspended in 150 mM NaCl at a concentration of approximately 108 cfu/mL. An aqueous-phase sample (0.4 mL) was obtained and absorbance at 405 nm was determined (Bioscreen C MBR, Oy Growth Curves Ab). The cell suspension (2.0 mL) was vortexed with 0.33 mL of hexadecane (Merck KGaA, Darmstadt, Germany) for 60 s and then allowed to stand for 15 min at room temperature, resulting in the complete separation of the two phases. The percentage of cells present in the solvent was calculated using the following equation: % affinity = 100 × [1 − (A/A0)], where A0 is the absorbance of the original suspension at 405 nm prior to mixing, and A is the absorbance of the aqueous phase. Cell surface hydrophobicity was categorized as weak (<21%), moderate (21% to 50%), or strong (>50%) affinities [37]. All determinations were carried out eight times on four separate days (two replications were performed on the same day).
2.5. Statistical Analysis
A correlation analysis was performed to determine the relationship between the MIC and the MBC of the biocides and antibiotics and the percentages of affinity for hexadecane (hydrophobicity). The hydrophobicity values were compared for statistical significance using an analysis of variance techniques. Mean separations were obtained using Duncan’s multiple range test. Significant differences were established for a probability level of 5% (p < 0.05). All data processing in this study was carried out using the Statistica® 8.0 software package (Statsoft Ltd., Tulsa, OK, USA).
3. Results
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactricidal Concentration (MBC) for the Biocides
The MIC and MBC values for the three biocides tested on the eight strains of L. monocytogenes are shown in Table 2. SH was the substance requiring the greatest concentrations to inhibit the growth of strains after 48 h of incubation, with recorded MICs of between 1750 ppm (175 ppm of free chlorine) and 4500 ppm (450 ppm of free chlorine). The values noted for MBC were equal to or greater than those for MIC, ranging from 2250 ppm (225 ppm of free chlorine) to 4500 ppm (450 ppm of free chlorine).
Table 2.
Minimum inhibitory concentration (MIC; ppm) and minimum bactericidal concentration (MBC; ppm) for three biocides on eight strains of Listeria monocytogenes.
| Strain | Biocide | ||
|---|---|---|---|
| SH | BC | PAA | |
| ATCC 19111 | 1750 (2250) |
0.25 (1.50) |
1650 (1800) |
| ATCC 19112 | 2250 (2250) |
0.50 (0.50) |
1500 (1550) |
| ATCC 19114 | 3500 (3900) |
2.00 (4.00) |
1050 (1150) |
| ATCC 19117 | 3500 (3500) |
0.75 (3.00) |
1700 (1750) |
| ATCC 13932 | 3500 (3700) |
4.00 (5.00) |
1100 (1250) |
| STCC 936 | 3500 (3900) |
3.00 (5.00) |
1050 (1150) |
| STCC 937 | 4000 (4500) |
20.00 (20.00) |
1600 (1650) |
| STCC 938 | 4500 (4500) |
19.00 (19.00) |
1400 (1600) |
SH—sodium hypochlorite; BC—benzalkonium chloride; PAA—peracetic acid. LM—Listeria monocytogenes. ATCC—American Type Culture Collection; STCC—Spanish Type Culture Collection. The values not in brackets correspond to the minimum inhibitory concentration (MIC), whilst bracketed values indicate the minimum bactericidal concentration (MBC).
The MICs for BC were the lowest among the three substances tested, ranging between 0.25 ppm and 20.00 ppm. As in the case of SH, the MBCs for BC were very similar to or only slightly higher than the MICs, ranging between 0.50 ppm and 20.00 ppm. With respect to PAA, the values recorded for MIC were slightly lower than those for SH, falling in the range of 1050 ppm to 1700 ppm. In all cases, the MBCs for PAA were slightly higher than the MICs for this substance, with values of between 1150 ppm and 1800 ppm.
3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Antibiotics
The values for the MICs and MBCs of nine antibiotics of clinical interest with regards to the eight strains of L. monocytogenes studied are presented in Table 3. The MICs varied notably depending on the combination of antibiotic and strain under consideration. The values (ppm) for MICs ranged from 1 to 15 for ampicillin, 8 to 150 for cephalothin, 20 to 170 for cefoxitin, 0.05 to 0.20 for erythromycin, 4 to 50 for chloramphenicol, 3 to 100 for gentamicin, 2 to 15 for tetracycline, 2 to 80 for vancomycin, and 160 to 430 for fosfomycin.
Table 3.
Minimum inhibitory concentration (MIC; ppm) and minimum bactericidal concentration (MBC; ppm) for nine antibiotics of clinical interest on eight strains of Listeria monocytogenes.
| Strain | Antibiotic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| ATCC 19111 | 7 (10) |
150 (150) |
170 (180) |
0.05 (4) |
20 (70) |
45 (50) |
15 (30) |
80 (90) |
350 (350) |
| ATCC 19112 | 11 (11) |
140 (140) |
160 (170) |
0.10 (5) |
4 (10) |
45 (45) |
14 (18) |
45 (50) |
160 (160) |
| ATCC 19114 | 9 (9) |
80 (90) |
140 (180) |
0.05 (5) |
20 (70) |
20 (20) |
8 (11) |
20 (20) |
230 (290) |
| ATCC 19117 | 1 (5) |
8 (20) |
150 (200) |
0.20 (5) |
20 (55) |
4 (15) |
2 (10) |
2 (3) |
430 (450) |
| ATCC 13932 | 9 (9) |
70 (70) |
160 (160) |
0.20 (5) |
35 (60) |
20 (20) |
11 (11) |
20 (20) |
250 (260) |
| STCC 936 | 5 (6) |
8 (9) |
20 (70) |
0.20 (5) |
5 (30) |
3 (5) |
2 (3) |
20 (20) |
170 (180) |
| STCC 937 | 15 (15) |
150 (150) |
170 (200) |
0.20 (5) |
50 (70) |
50 (50) |
15 (30) |
35 (40) |
240 (280) |
| STCC 938 | 13 (20) |
150 (160) |
150 (150) |
0.20 (5) |
5 (9) |
100 (100) |
15 (30) |
50 (60) |
220 (230) |
| CUT-OFF POINTS S ≤ - R > |
1–1 | 0.12–0.50 * | 4–8 | 1–1 | 8–8 | 1–1 | 1–2 | 2–2 | 32–32 |
AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin. LM—Listeria monocytogenes. ATCC—American Type Culture Collection; STCC—Spanish Type Culture Collection. Values not in brackets correspond to minimum inhibitory concentrations (MICs), and those in brackets to minimum bactericidal concentrations (MBC). The cut-off points for MIC used to classify strains as susceptible (MIC ≤ lower cut-off), of reduced susceptibility (MIC > lower cut-off and ≤ upper cut-off), and resistant (MIC > upper cut-off) are indicated. The criteria for AMP and E were specifically for L. monocytogenes [35]. Those used for C, TE, and FOS were initially intended for Staphylococcus spp. [35], those used for CN and VA were intended for S. aureus [35] and those used for KF and FOX were also intended for S. aureus [36]. *—quality control range. Green shading shows susceptible strains, yellow shading shows strains with reduced susceptibility, and red shading indicates strains that are resistant, in accordance with the criteria applied.
The values for MBCs were greater than or equal to those for MICs with respect to all of the strains tested. Moreover, considerable differences were observed between strains. The recorded values for MBCs (ppm) ranged between 5 and 20 for ampicillin, 9 and 160 for cephalothin, 70 and 200 for cefoxitin, 4 and 5 for erythromycin, 9 and 70 for chloramphenicol, 5 and 100 for gentamicin, 3 and 30 for tetracycline, 3 and 90 for vancomycin, and 160 and 450 for fosfomycin. Notably, erythromycin demonstrated considerable efficacy, as shown by the low values for MIC and MBC.
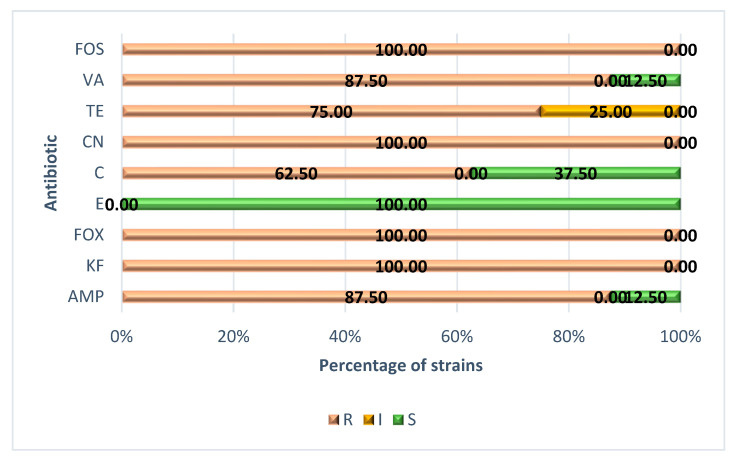
The percentages of strains that were resistant, intermediate, or susceptible to each of the antibiotics tested are shown in Figure 1. As can be seen, all of the strains presented resistance to cephalothin, cefoxitin, gentamicin, and fosfomycin. The prevalence of resistance was also high in the case of ampicillin and vancomycin (87.50%), tetracycline (75.00%), and chloramphenicol (62.50%). In contrast, all of the strains were susceptible to erythromycin.
Figure 1.
Percentage of strains of Listeria monocytogenes resistant (R), intermediate (I, with reduced susceptibility) or susceptible (S) to each of the nine antibiotics tested. AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin. LM—Listeria monocytogenes. American Type Culture Collection (ATCC) strains comprised ATCC 19111 (serotype 1/2a), ATCC 19112 (serotype 1/2c), ATCC 19114 (serotype 4a), ATCC 19117 (serotype 4d), and ATCC 13932 (serotype 4b). Those from the Spanish Type Culture Collection (STCC) comprised STCC 936 (serotype 1/2b), STCC 937 (serotype 3b), and STCC 938 (serotype 3c).
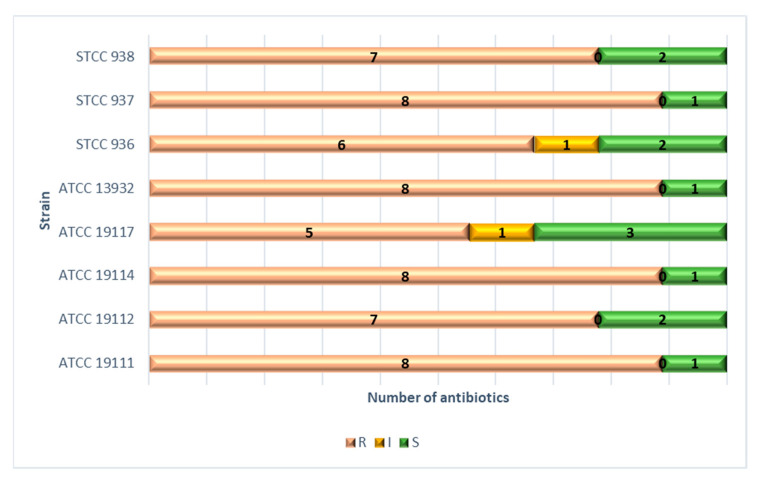
Lastly, the number of antibiotics to which each of the strains was resistant is shown in Figure 2. Four strains (ATCC 19111, ATCC 19114, ATCC 13932, and STCC 937) were resistant to eight antibiotics, two strains (ATCC 19112 and STCC 938) to seven, one strain (STCC 936) to six, and one strain (ATCC 19117) to five different antibiotics.
Figure 2.
Number of antibiotics to which each strain of L. monocytogenes was resistant, intermediate (with reduced susceptibility), or susceptible. ATCC—American Type Culture Collection; STCC—Spanish Type Culture Collection.
3.3. Relationship between MICs and MBCs of Biocides and Antibiotics
Twenty-three positive correlations (34.8%; p < 0.05) and three negative correlations (4.5%; p < 0.05) were found among the 66 correlations tested (Table 4). In the case of MBCs (Table 5), 27 (40.9%) positive correlations and five (7.6%) negative correlations were found. A positive correlation (p < 0.001) was obtained between the MIC and the MBC values for each antimicrobial tested (Table 6).
Table 4.
Coefficients of correlation between the MIC values of 12 biocides and antibiotics in eight strains of Listeria monocytogenes.
| Biocides (MIC) | Antibiotics (MIC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| SH | - | |||||||||||
| BC | 0.715 *** | - | ||||||||||
| PAA | −0.284 | 0.144 | - | |||||||||
| AMP | 0.273 | 0.734 *** | −0.016 | - | ||||||||
| KF | −0.182 | 0.483 * | 0.385 | 0.809 *** | - | |||||||
| FOX | −0.169 | 0.185 | 0.568 ** | 0.387 | 0.640 *** | - | ||||||
| E | 0.706 *** | 0.507 * | −0.018 | 0.000 | −0.348 | −0.265 | - | |||||
| C | 0.196 | 0.348 | 0.164 | 0.292 | 0.147 | 0.451 * | 0.194 | - | ||||
| CN | 0.220 | 0.696 *** | 0.297 | 0.732 *** | 0.829 *** | 0.438 * | 0.006 | −0.119 | - | |||
| TE | −0.166 | 0.471 * | 0.319 | 0.818 *** | 0.977 *** | 0.678 *** | −0.268 | 0.229 | 0.801 *** | - | ||
| VA | −0.513 * | 0.135 | 0.367 | 0.412 * | 0.808 *** | 0.342 | −0.492 * | 0.141 | 0.646 *** | 0.774 *** | - | |
| FOS | −0.155 | −0.235 | 0.590 ** | −0.576 ** | −0.230 | 0.380 | −0.011 | 0.271 | −0.236 | −0.231 | −0.080 | - |
SH—sodium hypochlorite; BC—benzalkonium chloride; PAA—peracetic acid; AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin; HYD—hydrophobicity, microbial adhesion to solvents (MATS) assay was used using hexadecane as hydrocarbon phase. ***— p < 0.001; **— p < 0.01; *— p < 0.05.
Table 5.
Coefficients of correlation between the MBC values of 12 biocides and antibiotics in eight strains of Listeria monocytogenes.
| Biocides (MBC) | Antibiotics (MBC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| SH | - | |||||||||||
| BC | 0.785 *** | - | ||||||||||
| PAA | −0.312 | 0.171 | - | |||||||||
| AMP | 0.374 | 0.798 *** | 0.289 | - | ||||||||
| KF | −0.128 | 0.435* | 0.471 * | 0.821 *** | - | |||||||
| FOX | 0.144 | 0.079 | 0.598 ** | 0.162 | 0.430 * | - | ||||||
| E | 0.601 ** | 0.301 | −0.475 * | 0.052 | −0.341 | −0.157 | - | |||||
| C | 0.047 | −0.094 | 0.027 | −0.342 | −0.104 | 0.479 * | −0.354 | - | ||||
| CN | 0.140 | 0.631 *** | 0.506 * | 0.937 *** | 0.833 *** | 0.209 | −0.158 | −0.414 * | - | |||
| TE | −0.017 | 0.566 ** | 0.698 *** | 0.799 *** | 0.913 *** | 0.473 * | −0.453 * | 0.017 | 0.854 *** | - | ||
| VA | −0.426 * | 0.126 | 0.517 ** | 0.549 ** | 0.807 *** | 0.108 | −0.749 *** | −0.127 | 0.694 *** | 0.805 *** | - | |
| FOS | −0.043 | −0.132 | 0.510 * | −0.325 | −0.210 | 0.618 ** | −0.325 | 0.633 *** | −0.178 | 0.063 | −0.156 | - |
SH—sodium hypochlorite; BC—benzalkonium chloride; PAA—peracetic acid; AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin. ***— p < 0.001; **— p < 0.01; *— p < 0.05.
Table 6.
Coefficients of correlation between MIC and MBC values of 12 antimicrobials in eight strains of Listeria monocytogenes.
| Biocides | Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS |
| 0.967 *** | 0.995 *** | 0.979 *** | 0.850 *** | 0.996 *** | 0.905 *** | 0.571 ** | 0.767 *** | 0.993 *** | 0.868 *** | 0.997 *** | 0.974 *** |
Values are the coefficient of correlation between the MIC and the MBC for each antimicrobial. SH—sodium hypochlorite; BC—benzalkonium chloride—PAA, peracetic acid; AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin. ***—p < 0.001; **—p < 0.01.
3.4. Cell Surface Hydrophobicity
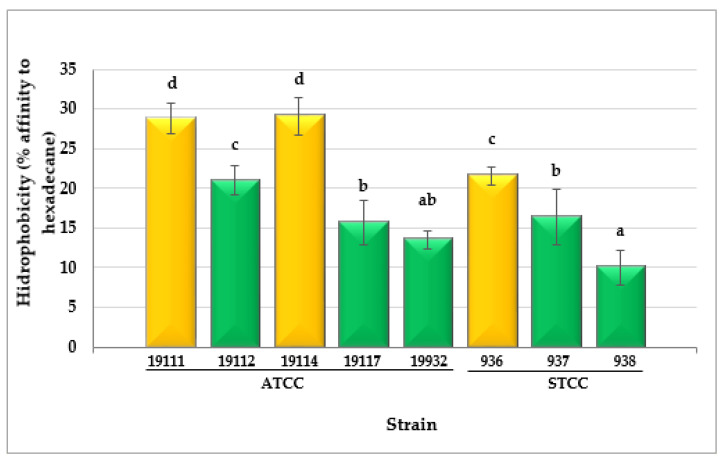
Substantial differences (p < 0.001) were observed between the values for hydrophobicity in the various strains of L. monocytogenes tested. The percentage of affinity for hexadecane ranged from 10.04 ± 2.17% in the case of strain LM STCC 938 to 29.11 ± 2.36% for LM ATCC 19114 (Figure 3). The highest values for cell surface hydrophobicity were reflected by a higher percentage of cells moving to the hydrophobic phase of the MATS assay.
Figure 3.
Cell surface hydrophobicity (CSH) values observed for cultures of eight Listeria monocytogenes strains. Data are means ± standard deviations (SD) for eight determinations. Mean values with no letters in common are significantly different (p < 0.05). ATCC—American Type Culture Collection; STCC—Spanish Type Culture Collection. The green columns represent weak CSH; the yellow columns represent moderate CSH.
Three strains (37.5% of the total) had moderate cell surface hydrophobicity (between 21% and 50% affinity for hexadecane), whilst five strains (62.5%) showed weak reactions (<21% affinity for hexadecane). Notably, the hydrophobicity values correlated negatively with the MICs and MBCs of the biocides (significantly for SH and BC) (Table 7).
Table 7.
Coefficients of correlation between hydrophobicity and MIC or MBC values of twelve biocides and antibiotics in eight strains of Listeria monocytogenes.
| Biocides | Antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| HYD | −0.504 * | −0.580 ** | −0.389 | 0.017 | 0.116 | 0.103 | −0.725 *** | −0.365 | −0.198 | 0.117 | 0.140 | −0.328 |
| (−0.532 **) | (−0.616 **) | (−0.387) | (−0.186) | (0.113) | (0.006) | (−0.082) | (−0.097) | (−0.155) | (−0.237) | (0.099) | (−0.313) | |
SH—sodium hypochlorite; BC—benzalkonium chloride; PAA—peracetic acid; AMP—ampicillin; KF—cephalothin; FOX—cefoxitin; E—erythromycin; C—chloramphenicol; CN—gentamicin; TE—tetracycline; VA—vancomycin; FOS—fosfomycin; HYD—hydrophobicity, microbial adhesion to solvents (MATS) assay was used using hexadecane as hydrocarbon phase. ***—p < 0.001; **—p < 0.01; *—p < 0.05. Values without brackets (first row) represent the correlations between hydrophobicity and MIC values. Values in brackets (second row) represent the correlations between hydrophobicity and MBC values.
4. Discussion
4.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Biocides
In previous works it has been demonstrated that contact with sub-inhibitory doses of various biocides habitually used in the food industry can trigger the adaptation of bacteria to the substances in question, as well as encourage the emergence of resistance to antibiotics and an increased ability to form biofilm [28,30,31,32,38,39]. This constitutes a challenge for food safety and public health. Thus, when establishing effective disinfection protocols, it is necessary to be aware of the MICs and MBCs of disinfectants in relation to the various microorganisms that may be present in food-processing environments. The current study determined the MICs and MBCs for three biocides widely used in the food industry, sodium hypochlorite (SH), benzalkonium chloride (BC), and peracetic acid (PAA), when applied to eight strains of L. monocytogenes of differing serotypes.
The disinfectant requiring the greatest concentrations as MICs was SH, needing between 1750 and 4500 ppm, which equates to 175 to 450 ppm of free chlorine. These values fall within the range observed by Lundén et al. [40], who quoted a value of 2500 ppm, and by Rodríguez-Melcón et al. [32], who recorded the value of 3500 ppm, both relating to strains of L. monocytogenes. A similar MIC of 5000 ppm was also noted by Buzón-Durán et al. [29] with respect to strains of another Gram-positive microorganism, specifically methicillin-resistant Staphylococcus aureus (MRSA). It should be noted that the comparison of our results with those of other research works should be carried out with caution, since the composition of the culture broth used for the determination of MIC and MBC (TSB in the study reported here) could influence the results obtained [41,42].
Benzalkonium chloride, a compound belonging to the group of derivatives of quaternary ammonium, was the disinfectant that produced inhibition at the lowest concentrations (between 0.25 ppm and 20.00 ppm). These values are similar to those noted by other authors for Gram-positive bacteria, such as the 2 ppm recorded by Buzón-Durán et al. [29] for MRSA and the range of between 3 and 13 ppm for different strains of L. monocytogenes reported by Rodríguez-Melcón et al. [32]. In the case of Gram-negative bacteria, results in line with those of the present research work have also been recorded, with values of 8 ppm for Salmonella enterica serotype Typhimurium [39], 15 ppm for Cronobacter sakazakii, and 20 ppm for Yersinia enterocolitica [28].
The MIC values for PAA observed in this study, ranging from 1050 ppm to 1700 ppm of 39% PAA, which equates approximately to 410 ppm and 660 ppm of the pure product, were higher than the MICs recorded for peroxyacids in previous research on L. monocytogenes, which fell in the range of 100 to 110 ppm [43]. The differences in the results of the various reports may be due to the fact that not all strains present the same susceptibility to biocides, as has been demonstrated previously [32]. Moreover, the varying composition of the mixtures of peroxyacids may also have been responsible for the marked differences in the results obtained by the various authors cited [44]. It must be pointed out that the MICs noted in the current work are in line with the values recorded for PAA in previous work on certain Gram-negative bacteria, where a value of 1200 ppm, equivalent to 468 ppm of the pure substance, was observed as the MIC for Cronobacter sakazakii, and a value of 1275 ppm, equating to 497.3 ppm of the product in its pure state, was observed as the MIC for Yersinia enterocolitica [28].
Unlike with antibiotics, no concentrations of disinfectants could be specified that allowed the bacteria to be classified as resistant, intermediate, or susceptible to these compounds. Various authors considered bacteria to be resistant when their MIC values are at least two to four times higher than those found in more susceptible strains [45]. Following this criterion, work done by Rodríguez-Melcón et al. [32] established two populations of strains of L. monocytogenes in terms of their susceptibility to BC. These were susceptible strains, with an average value of 3 ppm as the MIC, and resistant strains, where the values for the MIC were equal to or greater than 9 ppm. In the present research, strains of L. monocytogenes may be classified relative to SH as forming two groups, sensitive, with the MIC falling in the range of 1750 to 2250 ppm, and resistant, where the corresponding values were between 3500 and 4500 ppm. This could be similarly applied to BC, with sensitive strains of MICs ranging from 0.25 to 4 ppm, and resistant strains ranging from 19 to 20 ppm. Regarding PAA, the differences among strains were less marked and did not allow for a classification of this type.
In relation to MBCs, the values obtained for SH, ranging from 2250 ppm to 4500 ppm, coincide with the findings of earlier investigations [32], where the values observed were between 3500 ppm and 4500 ppm. Along these lines, it must also be kept in mind that the values for MBC may vary as a function of the growth mode of the bacteria. Thus, several studies, including Smith and Hunter [46], have highlighted the fact that MBCs for microorganisms like MRSA or Pseudomonas aeruginosa are between 10 and 1000 times higher for sessile bacteria forming part of biofilms than for planktonic bacteria (free-living).
It should be pointed out that the disinfectants tested are habitually used in concentrations much higher than their MBCs to achieve rapid, effective inactivation of microorganisms, making it highly unlikely for bacteria to survive and develop resistance under normal conditions [47]. The concentrations of free chlorine usually employed in the case of substances releasing chlorine such as SH are in the order of 800 to 2000 ppm of free chlorine [26,48]. They range between 1000 and 5000 ppm for quaternary ammonium compounds like benzalkonium chloride [26,49,50]. In the case of PAA, the values are 10,000 ppm to 150,000 ppm of the pure substance [51,52]. However, it is a known fact that under certain circumstances sub-lethal exposure to biocides does occur. This can be the outcome of incorrect calculations of the dosage to be used, inappropriate storage of disinfectants, an uneven distribution of the substances in use, or the presence of excessive amounts of residues of organic materials, which neutralize different biocidal substances, such as sodium hypochlorite [30]. Situations of this kind should be avoided because, as commented above, exposure of the bacteria present in food industry plants and equipment to sub-lethal doses of biocides poses a challenge to food safety since it favours adaptation to disinfectants, thus increasing the risk of the emergence of resistance to antibiotics and the capacity to form biofilm [28].
4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Antibiotics
Over recent decades there has been a marked increase in the prevalence of bacteria resistant to antibiotics, which is emerging as one of the principal threats to public health worldwide [33]. It is estimated that by 2050 infections by resistant bacteria will have become the prime cause of mortality around the world, leading to some ten million deaths annually, surpassing the values for cardiovascular disease and cancer. This estimated number of deaths foreseen within three decades contrasts with the 700,000 fatalities attributable to bacteria in 2014 [53]. Moreover, resistance to antibiotics has major financial repercussions since it is estimated that infections by these bacteria cost the health systems of the European Union and European Economic Area (EEA) approximately 1.1 thousand million euros every year [54].
In the work reported here, the prevalence of resistance depended on the antibiotic in question. Considerable percentages of strains, between 62.5% and 100%, were resistant to all the antibiotics tested, the sole exception being erythromycin, to which all the strains were susceptible. It is especially worrying that high levels of resistance were seen for ampicillin, chloramphenicol, gentamicin, tetracycline, and vancomycin, which are antibiotics used to treat invasive listeriosis. Beta-lactams are the antibiotics of choice for such treatments, principally ampicillin, administered alone or in combination with gentamicin. In the case of allergy to beta-lactams, it is customary to administer erythromycin, vancomycin, trimethoprim/sulfamethoxazole, or fluoroquinolones. Vancomycin is also used for treating listeriosis during pregnancy [55]. Other antibiotics that are sometimes used to treat this infection include rifampicin, tetracycline, and chloramphenicol [55]. It should be noted that resistance to the antibiotics indicated has been previously highlighted in strains of L. monocytogenes of different origins [56,57,58,59,60].
Furthermore, it must be stressed that the antibiotics to which the strains were resistant are hugely important in both human and veterinary medicine. The antibiotics ampicillin, cephalothin, cefoxitin, erythromycin, gentamicin, vancomycin, and fosfomycin are deemed “critically important”, while chloramphenicol and tetracycline are considered “highly important” in human medicine, according to the World Health Organization [61]. The World Organization for Animal Health [62] classifies ampicillin, erythromycin, gentamicin, and tetracycline as “critically important”, and cephalothin and fosfomycin as “highly important” in terms of veterinary medicine. In view of the clinical importance of these antibiotics, the considerable prevalence of resistance found is a cause for concern, including when antibiotics not directly used to treat listeriosis are affected, because of the possibility that resistance genes may be transferred horizontally to other genera of pathogenic bacteria [33].
Strains of L. monocytogenes were previously susceptible to most of the antibiotics effective against Gram-positive bacteria. In a research work carried out more than three decades ago by Wiggins et al. [63], the MIC values for ampicillin, penicillin, erythromycin, and tetracycline for 175 strains of L. monocytogenes were reported to be below the cut-off point established by the CLSI. However, in recent years a considerable increase has been observed in the prevalence of resistance in bacteria of this microbial species [57,64].
The selective pressure exerted by the use of antibiotics, particularly when incorrectly employed at sub-inhibitory doses, has been identified as the principal cause of the marked growth in the prevalence of resistance in recent decades [33,65]. Moreover, several recent works have highlighted the fact that changes in profiles of resistance to antibiotics may be due to the exposure of microorganisms to sub-inhibitory concentrations of biocides or other sub-lethal stressing factors [28,30,33,66]. Furthermore, the possibility of the horizontal transfer of mobile genetic elements, such as transposons or plasmids, between bacteria of the same or different genera, facilitates a rapid spread of resistance genes. This too is among the causes of the increase in the prevalence of resistance to antibiotics observed in recent decades [67].
4.3. Relationship between MICs and MBCs of Biocides and Antibiotics
Positive correlations were observed for the MICs and the MBCs between different classes of antimicrobials. Thus, a total of seven classes of antibiotics were used, including beta-lactams (ampicillin, cephalothin, and cefoxitin), macrolides (erythromycin), phenicols (chloramphenicol), aminoglycosides (gentamicin), tetracyclines (tetracycline), glycopeptides (vancomycin), and fosfomycin. The fact that such compounds have unrelated modes of action and mechanisms of resistance [68] suggest that different genes involved in antibiotic resistance are carried in the same mobile genetic elements, as previously reported [69]. Thus, it has been demonstrated that co-selection and co-transfer are a common phenomenon in antibiotic resistance emergence and spread [33].
4.4. Cell Surface Hydrophobicity
The values for hydrophobicity obtained for the various strains of L. monocytogenes (10.04 ± 2.17% to 29.11 ± 2.36%) fall in the range of previous studies (4.84 ± 1.11% to 31.82 ± 5.98%) using xylene as the hydrocarbon phase [70]. The negative correlations found between the hydrophobicity and the MICs or MBCs of the biocides should be noted. The higher the hydrophobicity, the higher the susceptibility to the biocides. These results reveal that cell surface plays an important role in the tolerance of L. monocytogenes to these antimicrobials, and especially to SH and BC. The relationship between high hydrophobicity and high susceptibility to hydrophobic antimicrobials (e. g. benzalkonium chloride or erythromycin) has been observed by other authors [71,72]. By contrast, these results do not coincide with several research works performed with Gram-negative bacteria, where bacterial cells with a high cell surface hydrophobicity have shown an increased tolerance to biocides [73], which is a consequence of the low number of charged (hydrophilic) binding sites for the biocides [30,74].
5. Conclusions
It was demonstrated that the MICs and MBCs for the biocides tested, sodium hypochlorite, benzalkonium chloride, and peracetic acid, relative to L. monocytogenes, were much lower than the concentrations of these disinfectants customarily used. For these compounds to be completely efficacious, they must exceed MBCs in all of the areas treated, with checks on aspects such as the correct calculation of the concentrations to be employed, even distribution of the disinfecting substances, and prior elimination of any residues of organic matter, the latter being of particular importance when chlorinated compounds are in use. A positive relationship was found between cell surface hydrophobicity and susceptibility to biocides, indicating that the characteristics of the cell surface play an important role in the tolerance of L. monocytogenes to these compounds.
A considerable prevalence of resistance to most of the antibiotics tested was noted, making it clear that the necessary measures to control resistance in L. monocytogenes must be adopted. Of the nine antibiotics included in the study, erythromycin had the greatest antimicrobial efficacy, since it had the lowest values for both MIC and MBC. The positive correlations observed for the MICs and MBCs between the biocides and the antibiotics with different modes of action suggest that resistance genes are carried in the same mobile genetic elements.
An awareness of the MICs and MBCs for biocides and antibiotics against L. monocytogenes may assist with choosing the most effective antimicrobials for controlling this microorganism, whether in the food industry or in the health system. Nevertheless, the marked intra-species differences observed make it clear that including various strains in any studies aimed at determining the resistance of L. monocytogenes to biocides and antibiotics is vital.
Author Contributions
Conceptualization, C.R.-M., C.A.-C., C.G.-F., J.C. and R.C.; data curation, C.R.-M. and R.C.; formal analysis, C.R.-M. and R.C.; funding acquisition, C.A.-C. and R.C.; investigation, C.R.-M., C.A.-C., C.G.-F., J.C. and R.C.; methodology, C.R.-M., C.A.-C., C.G.-F., J.C. and R.C.; project administration, C.A.-C. and R.C.; resources, C.A.-C. and R.C.; software, C.R.-M. and C.A.-C.; supervision, C.A.-C.; validation, C.A.-C. and R.C.; visualization, R.C.; writing—original draft, C.R.-M. and R.C.; writing—review and editing, C.A.-C. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the Junta de Castilla y León (Consejería de Educación, Spain, grant number LE018P20), and the Ministerio de Ciencia, Innovación y Universidades (Spain, grant number RTI2018-098267-R-C33).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skowron K., Kwiecińska-Piróga J., Grudlewska K., Świeca A., Paluszak Z., Bauza-Kaszewska B., Wałecka-Zacharska E., Gospodarek-Komkowska E. The occurrence, transmission, virulence and antibiotic resistance of Listeria monocytogenes in fish processing plant. Int. J. Food Microbiol. 2018;282:71–83. doi: 10.1016/j.ijfoodmicro.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M., McLauchin J. In: Handbook of Listeria Monocytogenes. Liu D., editor. CRC Press; Boca Raton, FL, USA: 2008. pp. 3–25. [Google Scholar]
- 3.Nwaiwu O. What are the recognized species of the genus Listeria? Access Microbiol. 2020;2:e000153. doi: 10.1099/acmi.0.000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirie J.H. The genus Listerella pirie. Science. 1940;91:383. doi: 10.1126/science.91.2364.383.a. [DOI] [PubMed] [Google Scholar]
- 5.Seeliger H.P.R. A pathogen listerien: L. innocua sp. n. (Seeliger et Schoofs, 1977) Zent. Bakteriol. Mikrobiol. Hygiene. 1. Abt. Originale. A Med. Mikrobiol. Infekt. Parasitol. 1981;249:487–493. [PubMed] [Google Scholar]
- 6.Rocourt J., Grimont P.A.D. Listeria welshimeri sp. nov. and Listeria seeligeri sp. nov. Int. J. Syst. Bacteriol. 1983;33:866–869. doi: 10.1099/00207713-33-4-866. [DOI] [Google Scholar]
- 7.Seeliger H.P.R., Rocourt J., Schrettenbrunner A., Grimont P.A.D., Jones D. Notes: Listeria ivanovii sp. nov. Int. J. Syst. Evol. Microbiol. 1984;34:336–337. doi: 10.1099/00207713-34-3-336. [DOI] [Google Scholar]
- 8.Rocourt J., Boerlin P., Grimont F., Jacquet C., Piffaretti J.C. Assignment of Listeria grayi and Listeria murrayi to a single species, Listeria grayi, with a revised description of Listeria grayi. Int. J. Syst. Evol. Microbiol. 1992;42:171–174. doi: 10.1099/00207713-42-1-171. [DOI] [PubMed] [Google Scholar]
- 9.Graves L.M., Helsel L.O., Steigerwalt A.G., Morey R.E., Daneshvar M.I., Roof S.E., Orsi R.H., Fortes E.D., Milillo S.R., Bakker H.C., et al. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int. J. Syst. Evol. Microbiol. 2010;60:1280–1288. doi: 10.1099/ijs.0.014118-0. [DOI] [PubMed] [Google Scholar]
- 10.Leclercq A., Clermont D., Bizet C., Grimont P.A.D., Le Flèche-Matéos A., Roche S.M., Buchrieser C., Cadet-Daniel V., Le Monier A., Lecuit M., et al. Listeria rocourtiae sp. nov. Int. J. Syst. Evol. Microbiol. 2010;60:2210–2214. doi: 10.1099/ijs.0.017376-0. [DOI] [PubMed] [Google Scholar]
- 11.Bertsch D., Rau J., Eugster M.R., Haug M.C., Lawson P.A., Lacroix C., Meile L. Listeria fleischmannii sp. nov., isolated from cheese. Pt 2Int. J. Syst. Evol. Microbiol. 2013;63:526–532. doi: 10.1099/ijs.0.036947-0. [DOI] [PubMed] [Google Scholar]
- 12.Lang Halter E., Neuhaus K., Scherer S. Listeria weihenstephanensis sp. nov., isolated from the water plant Lemna trisulca taken from a freshwater pond. Pt 2Int. J. Syst. Evol. Microbiol. 2013;63:641–647. doi: 10.1099/ijs.0.036830-0. [DOI] [PubMed] [Google Scholar]
- 13.Den Bakker H.C., Warchocki S., Wright E.M., Allred A.F., Ahlstrom C., Manuel C.S., Stasiewicz J., Burrell A., Roof S., Strawn L.K., et al. Listeria floridensis sp. nov., Listeria aquatica sp. nov., Listeria cornellensis sp. nov., Listeria riparia sp. nov. and Listeria grandensis sp. nov., from agricultural and natural environments. Pt 6Int. J. Syst. Evol. Microbiol. 2014;64:1882–1889. doi: 10.1099/ijs.0.052720-0. [DOI] [PubMed] [Google Scholar]
- 14.Weller D., Andrus A., Wiedmann M., Den Bakker H.C. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Pt 1Int. J. Syst. Evol. Microbiol. 2015;65:286–292. doi: 10.1099/ijs.0.070839-0. [DOI] [PubMed] [Google Scholar]
- 15.Doijad S.P., Poharkar K.V., Kale S.B., Kerkar S., Kalorey D.R., Kurkure N.V., Rawool D.B., Malik S.V.S.M., Ahmad R.Y., Hudel M., et al. Listeria goaensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018;68:3285–3291. doi: 10.1099/ijsem.0.002980. [DOI] [PubMed] [Google Scholar]
- 16.Núñez-Montero K., Leclercq A., Moura A., Vales G., Peraza J., Pizarro-Cerdá J., Lecuit M. Listeria costaricensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018;68:844–850. doi: 10.1099/ijsem.0.002596. [DOI] [PubMed] [Google Scholar]
- 17.Leclercq A., Moura A., Vales G., Tessaud-Rita N., Aguilhon C., Lecuit M. Listeria thailandensis sp. nov. Int. J. Syst. Evol. Microbiol. 2019;69:74–81. doi: 10.1099/ijsem.0.003097. [DOI] [PubMed] [Google Scholar]
- 18.Quereda J.J., Leclercq A., Moura A., Vales G., Gómez-Martín A., García-Muñoz A., Thouvenot P., Tessaud-Rita N., Bracq-Dieye H., Lecuit M. Listeria valentina sp. nov., isolated from a water trough and the faeces of healthy sheep. Int. J. Syst. Evol. Microbiol. 2020;70:5868–5879. doi: 10.1099/ijsem.0.004494. [DOI] [PubMed] [Google Scholar]
- 19.Carlin C.R., Liao J., Weller D., Guo X., Orsi R., Wiedmann M. Listeria cossartiae sp. nov., Listeria immobilis sp. nov., Listeria portnoyi sp. nov. and Listeria rustica sp. nov., isolated from agricultural water and natural environments. Int. J. Syst. Evol. Microbiol. 2021;71:004795. doi: 10.1099/ijsem.0.004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19:e06406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shourav H.A., Hasan M., Ahmed S. Antibiotic susceptibility pattern of Listeria spp. isolated from cattle farm environment in Bangladesh. J. Agric. Food Res. 2020;2:100082. doi: 10.1016/j.jafr.2020.100082. [DOI] [Google Scholar]
- 22.Rugna G., Carra E., Bergaminia F., Franzinia G., Faccini S., Gattuso A., Morgantia M., Baldi D., Naldia S., Serraino A., et al. Distribution, virulence, genotypic characteristics and antibiotic resistance of Listeria monocytogenes isolated over one-year monitoring from two pig slaughterhouses and processing plants and their fresh hams. Int. J. Food Microbiol. 2021;336:108912. doi: 10.1016/j.ijfoodmicro.2020.108912. [DOI] [PubMed] [Google Scholar]
- 23.Kraśniewska K., Kosakowska O., Pobiega K., Gniewosz M. The influence of two-component mixtures from Spanish origanum oil with Spanish marjoram oil or coriander oil on antilisterial activity and sensory quality of a fresh cut vegetable mixture. Foods. 2020;9:1740. doi: 10.3390/foods9121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA Control of Listeria monocytogenes in Ready-To-Eat Foods: Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition 2017. [(accessed on 3 December 2021)]; Available online: https://www.fda.gov/files/food/published/Draft-Guidance-for-Industry--Control-of-Listeria-monocytogenes-in-Ready-To-Eat-Foods-%28PDF%29.pdf.
- 25.Waghmare R.B., Annapure U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life. 2015;3:62–69. doi: 10.1016/j.fpsl.2014.11.001. [DOI] [Google Scholar]
- 26.Henriques A.R., Fraqueza M.J. Biofilm-forming ability and biocide susceptibility of Listeria monocytogenes strains isolated from the ready-to-eat meat-based food products food chain. LWT—Food Sci. Technol. 2017;81:180–187. doi: 10.1016/j.lwt.2017.03.045. [DOI] [Google Scholar]
- 27.Finnegan M., Linley E., Denyer S.P., McDonnell G., Simons C., Maillard J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010;65:108–115. doi: 10.1093/jac/dkq308. [DOI] [PubMed] [Google Scholar]
- 28.Capita R., Vicente-Velasco M., Rodríguez-Melcón C., García-Fernández C., Carballo J., Alonso-Calleja C. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci. Rep. 2019;9:15905. doi: 10.1038/s41598-019-51907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzón-Durán L., Alonso-Calleja C., Riesco-Peláez F., Capita R. Effect of subinhibitory concentrations of biocides on the architecture and viability of MRSA biofilms. Food Microbiol. 2017;65:294–301. doi: 10.1016/j.fm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Capita R., Riesco-Peláez F., Alonso-Hernando A., Alonso-Calleja C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014;80:1268–1280. doi: 10.1128/AEM.02283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Melcón C., Capita R., Rodríguez-Jerez J., Martínez-Suárez J., Alonso-Calleja C. Effect of low doses of disinfectants on the biofilm-forming ability of Listeria monocytogenes. Foodborne Path. Dis. 2019;16:262–268. doi: 10.1089/fpd.2018.2472. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Melcón C., Riesco-Peláez F., García-Fernández C., Alonso-Calleja C., Capita R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019;82:533–540. doi: 10.1016/j.fm.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Capita R., Alonso-Calleja C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013;53:11–48. doi: 10.1080/10408398.2010.519837. [DOI] [PubMed] [Google Scholar]
- 34.CLSI . M100 Performance Standars for Antimicrobial Susceptibility Testing. 29th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2019. [Google Scholar]
- 35.EUCAST European Committee on Antimicrobial Susceptibility Testing. V. 9.0. [(accessed on 14 July 2021)]. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 36.CLSI . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals. Approved Standard M31-A3. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2017. [(accessed on 3 December 2021)]. Available online: https://www.dbt.univr.it/documenti/OccorrenzaIns/matdid/matdid485539.pdf. [Google Scholar]
- 37.To M.S., Favrin S., Romanova N., Griffiths M.W. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 2002;68:5258–5264. doi: 10.1128/AEM.68.11.5258-5264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzón-Durán L., Capita R., Alonso-Calleja C. Antibiotic susceptibility of methicillin-resistant staphylococci (MRS) of food origin: A comparison of agar disc diffusion method and a commercially available miniaturized test. Food Microbiol. 2018;72:220–224. doi: 10.1016/j.fm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Capita R., Buzón-Durán L., Riesco-Peláez F., Alonso-Calleja C. Effect of sub-lethal concentrations of biocides on the structural parameters and viability of the biofilms formed by Salmonella Typhimurium. Foodborne Path. Dis. 2017;14:350–356. doi: 10.1089/fpd.2016.2241. [DOI] [PubMed] [Google Scholar]
- 40.Lundén J., Autio T., Markkula A., Hellström S., Korkeala H. Adaptive and cross-adaptive responses of persistent and nonpersistent Listeria monocytogenes strains to disinfectants. Int. J. Food Microbiol. 2003;82:265–272. doi: 10.1016/S0168-1605(02)00312-4. [DOI] [PubMed] [Google Scholar]
- 41.Sawer I.K., Berry M.I., Ford J.L. Effect of medium composition, agitation and the presence of EDTA on the antimicrobial activity of cryptolepine. Lett. Appl. Microbiol. 1997;25:207–211. doi: 10.1046/j.1472-765X.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 42.Dorey L., Lees P. Impact of growth matrix on pharmacodynamics of antimicrobial drugs for pig pneumonia pathogens. BMC Vet. Res. 2017;13:192. doi: 10.1186/s12917-017-1086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso-Hernando A., Capita R., Prieto M., Alonso-Calleja C. Adaptation and cross-adaptation of Listeria monocytogenes and Salmonella enterica to poultry decontaminants. J. Microbiol. 2009;47:142–146. doi: 10.1007/s12275-008-0237-5. [DOI] [PubMed] [Google Scholar]
- 44.EFSA Scientific Opinion on the evaluation of the safety and efficacy of peroxyacetic acid solutions for reduction of pathogens on poultry carcasses and meat. EFSA J. 2014;12:3599. [Google Scholar]
- 45.Soumet C., Ragimbeau C., Maris P. Screening of benzalkonium chloride resistance in Listeria monocytogenes strains isolated during cold smoked fish production. Lett. Appl. Microbiol. 2005;41:291–296. doi: 10.1111/j.1472-765X.2005.01763.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith K., Hunter I.S. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J. Med. Microbiol. 2008;57:966–973. doi: 10.1099/jmm.0.47668-0. [DOI] [PubMed] [Google Scholar]
- 47.Chen B., Han J., Dai H., Jia P. Biocide-tolerance and antibiotic-resistance in community environments and risk of direct transfers to humans: Unintended consequences of community-wide surface disinfecting during COVID-19? Environ. Pollut. 2021;283:117074. doi: 10.1016/j.envpol.2021.117074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norwood D.E., Gilmour A. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 2000;88:512–520. doi: 10.1046/j.1365-2672.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 49.Tamburro M., Ripabelli G., Vitullo M., Dallman T.J., Pontello M., Amar C.F.L., Sammarco M.L. Gene expression in Listeria monocytogenes exposed to sublethal concentration of benzalkonium chloride. Comp. Immunol. Microbiol. Infect. Dis. 2015;40:31–39. doi: 10.1016/j.cimid.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Poimenidou S.V., Chrysadakou M., Tzakoniati A., Bikouli V.C., Nychas G.J., Skandamis P.N. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int. J. Food Microbiol. 2016;237:164–171. doi: 10.1016/j.ijfoodmicro.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Gawande H.M., Dhotre A.V., Shendurse A.M., Khodwe N.M. Peroxyacetic acid: A potent food industry sanitizer. Indian Food Industry Mag. 2013;32:26–30. [Google Scholar]
- 52.Kitis M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004;30:47–55. doi: 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 53.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. [(accessed on 14 July 2021)]. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 54.OECD Antimicrobial Resistance. Tackling the Burden in the European Union. [(accessed on 14 July 2021)]. Available online: https://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf.
- 55.Charpentier E., Courvalin P. Antibiotic Resistance in Listeria spp. Antimicrob. Agents Chemother. 1999;43:2103–2108. doi: 10.1128/AAC.43.9.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso-Hernando A., Prieto M., García-Fernández C., Alonso-Calleja C., Capita R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control. 2012;23:37–41. doi: 10.1016/j.foodcont.2011.06.006. [DOI] [Google Scholar]
- 57.Álvarez-Fernández E., Domínguez-Rodríguez J., Capita R., Alonso-Calleja C. Influence of housing systems on microbial load and antimicrobial resistance patterns of Escherichia coli isolates from eggs produced for human consumption. J. Food Prot. 2012;75:847–853. doi: 10.4315/0362-028X.JFP-11-182. [DOI] [PubMed] [Google Scholar]
- 58.Aras K., Ardiç M. Occurrence and antibiotic susceptibility of Listeria species in turkey meats. Korean J. Food Sci. Anim. Resour. 2015;35:669–673. doi: 10.5851/kosfa.2015.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capita R., Felices-Mercado A., García-Fernández C., Alonso-Calleja C. Characterization of Listeria monocytogenes originating from the Spanish meat-processing chain. Foods. 2019;8:542. doi: 10.3390/foods8110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carvalho F.T., Vieira B.S., Vallim D.C., Carvalho L.A., Carvalho R.C.T., Pereira R.C.L., Figueiredo E.E.S. Genetic similarity, antibiotic resistance and disinfectant susceptibility of Listeria monocytogenes isolated from chicken meat and chicken-meat processing environment in Mato Grosso, Brazil. LWT. 2019;109:77–82. doi: 10.1016/j.lwt.2019.03.099. [DOI] [Google Scholar]
- 61.WHO (World Health Organization) Critically Important Antimicrobials for Human Medicine. 6th ed. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 62.OIE . World Organization for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance. World Organization for Animal Health; Paris, France: 2018. [Google Scholar]
- 63.Wiggins G.L., Albrittony L.W., Feeley J.C. Antibiotic susceptibility of clinical isolates of Listeria monocytogenes. Antimicrob. Agents Chemother. 1978;13:854–860. doi: 10.1128/AAC.13.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fallah A.A., Saei-Dehkordi S.S., Rahnama M., Tahmasby H., Mahzounieh M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control. 2012;28:327–332. doi: 10.1016/j.foodcont.2012.05.014. [DOI] [Google Scholar]
- 65.Cufaoglu G., Ambarcioglu P., Ayaz N.D. Meta-analysis of the prevalence of Listeria spp. and antibiotic resistant L. monocytogenes isolates from foods in Turkey. LWT. 2021;144:111210. doi: 10.1016/j.lwt.2021.111210. [DOI] [Google Scholar]
- 66.Molina-González D., Alonso-Calleja C., Alonso-Hernando A., Capita R. Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant Salmonella enterica strains. Food Control. 2014;40:329–334. doi: 10.1016/j.foodcont.2013.11.046. [DOI] [Google Scholar]
- 67.Olaimat A.N., Al-Holy M.A., Shahbaz H.M., Al-Nabusli A.A., Abu Goush M.H., Osaili T.M., Ayyash M.M., Holley R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Comprehen. Rev. Food Sci. Food Saf. 2018;17:1277–1292. doi: 10.1111/1541-4337.12387. [DOI] [PubMed] [Google Scholar]
- 68.Kapoor G., Saigal S., Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol Clin. Pharmacol. 2017;33:300–305. doi: 10.4103/joacp.JOACP_349_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Álvarez-Fernández E., Alonso-Calleja C., García-Fernández C., Capita R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: Comparison between 1993 and 2006. Int. J. Food Microbiol. 2012;153:281–287. doi: 10.1016/j.ijfoodmicro.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Rodríguez-Melcón C., Alonso-Calleja C., Capita R. Architecture and viability of the biofilms formed by nine Listeria strains on various hydrophobic and hydrophilic materials. Appl. Sci. 2019;9:5256. doi: 10.3390/app9235256. [DOI] [Google Scholar]
- 71.Chiu J., Han G., McCrystal K., Zuo M. Macrolide structures can confer differential susceptibility in Escherichia coli K30 deletions of group 1 capsule assembly genes. J. Exp. Microbiol. Immunol. 2017;3:50–56. [Google Scholar]
- 72.Jones I.A., Joshi L.T. Biocide use in the antimicrobial era: A review. Molecules. 2021;26:2276. doi: 10.3390/molecules26082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loughlin M.F., Jones M.V., Lambert P.A. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 2002;49:631–639. doi: 10.1093/jac/49.4.631. [DOI] [PubMed] [Google Scholar]
- 74.Braoudaki M., Hilton A.C. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004;42:73–78. doi: 10.1128/JCM.42.1.73-78.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.