Abstract
(1) This study tests hypothesis whether extracorporeal shock wave (ECSW) therapy effectively salvages mouse critical limb ischemia (CLI). In vitro result demonstrated that the angiogenesis parameters (i.e., tubular length/cluster/network formation) and protein expressions of EGFR/VEGFR2/RAS/c-Raf/MEK/ERK/VEGF/p-PI3K/p-Akt/p-m-TOR were significantly and progressively increased with stepwise augmentation of ECSW energy (0.1/0.14/0.20 mJ/mm2/140 impulses). On the other hand, they were suppressed by administration of Avastin (20 μM). Adult male B6 mice (n = 24) were equally categorized into group 1 (sham-operated control), group 2 (CLI), group 3 [CLI + ECSW (0.12 mJ/mm2/120 impulses/at days 1/3/7 after CLI induction)] and group 4 [CLI + ECSW (0.12 mJ/mm2/120 impulses) + Avastin (1 mg/intramuscular-injection)] at days 1/3/7 after CLI induction] and quadriceps were harvested by day 14. The laser Doppler result showed that the ratio of left (ischemia) to right (normal) limb blood flow was highest in group 1, lowest in group 2, and significantly higher in group 3 than in group 4 by days 7/14 after the CLI procedure (p < 0.0001). The protein expressions of cell proliferation/migration/angiogenesis receptors (EGFR/VEGFR2), angiogenesis biomarkers (VEGF/CXCR4/SDF-1) and cell proliferation/growth/survival (Ras/c-Raf/MEK/ERK)/(PI3K/Akt/m-TOR) and cell motility/proliferation (p-FAK/p-Scr) signaling biomarkers were significantly higher in group 3 than in groups 1/2/4, and significantly lower in group 1 than in groups 2/4, but they did not show a difference between groups 2 and 4 (all p < 0.001). The small vessel density and cellular levels of endothelial cell surface marker (CD31+) exhibited an identical pattern of blood flow, whereas the angiogenesis (CXCR4+/VEGF+) displayed an identical pattern of VEGFR2 among the groups (all p < 0.0001). The in vitro and in vivo studies found ECSW salvaged the CLI mainly through upregulating Ras-Raf-MEK/ERK/cell motility, cell proliferation/growth pathways and angiogenesis.
Keywords: critical limb ischemia, extracorporeal shock wave, cell proliferation, cell growth and cell motility signalings
1. Introduction
Peripheral arterial disease (PAD) is not only a major predictor of atherosclerotic cardiovascular disease [1,2] but is also accompanied with multiple comorbidities [3], leading to a high risk of amputation and mortality [4]. Epidemiological and clinical observational studies [5,6] have found that about 10% of the population less than 70 years in age, 15–20% of those aged 70–85 years and 50% in those aged more than 85 years have PAD, highlighting that PAD is much more common in the elderly. In our clinical practice, the estimated prevalence of either asymptomatic or symptomatic PAD was up to 13% in people aged over 50 years old [5]. Additionally, about one in three symptomatic patients will eventually progress into intermittent claudication or critical limb ischemia (CLI) [7]. Furthermore, more than 70% of PAD results from poorly controlled diabetes and chronic kidney disease [8,9]. Therefore, the socioeconomic and medical burdens from either PAD per se or its associated comorbidities are considerable [10].
PAD/CLI treatment remains a great challenge to surgeons and clinicians [11]. In addition to standard anti-platelet and anti-ischemic medical treatments [12,13], percutaneous transluminal angioplasty and peripheral bypass surgery are currently two common therapeutic procedural strategies for PAD/CLI [14,15]. However, owing to the high incidence of atherosclerotic restenosis, the victims of PAD/CLI often require repeated percutaneous transluminal angioplasty, secondary bypass surgery, or eventually amputation for severe CLI [16]. These could explain why the long-term outcomes remain regrettably unfavorable in these PAD/CLI patients [17,18]. In view of the lack of an effective treatment for PAD/CLI patients, the development of an innovative treatment for these disease entities is urgent and of paramount importance.
Extracorporeal shock wave (ECSW) treatment has been well documented as an effective and safe noninvasive therapy for musculoskeletal or non-musculoskeletal disorders [19,20,21], mainly through suppressing the inflammatory process, reducing painful sensations and strengthening tissue repair. Our preclinical studies have previously demonstrated that ECSW therapy effectively ameliorated ischemic related organ dysfunction [22,23,24,25,26,27] mainly through enhancing vasculogenesis and neovascularization, mobilizing EPCs from bone marrow into circulation and homing to ischemic organ/tissues for angiogenesis, upregulating the expression of SDF-1α in ischemic zones for tracking the CXCR4+ cells into the ischemic area for angiogenesis and suppressing inflammatory reaction in ischemic and peri-infarcted zones. However, the deep and exact underlying mechanism for how the ECSW therapy would salvage the CLI is still currently unclear.
A body of basic research [28,29] has identified that epithelial growth factor receptor (EGFR) plays a crucial role for initiation and upregulation of Ras-Raf-MEK-ERK signaling for cell proliferation and survival. Additionally, the cell proliferation, growth and survival pathways of PI3K/Akt/m-TOR are frequently activated by ischemic stimulation [30], and the p-FAK/p-Scr pathway plays an essential role in cell motility and proliferation [31]. On the other hand, our previous study showed that ECSW therapy upregulated angiogenesis through activation of VEGFR2 [32]. Therefore, we proposed that the way that ECSW therapy salvaged the CLI in rat might be through regulating the angiogenesis, cell proliferation/growth/survival and cell motility signalings.
2. Materials and Methods
2.1. Ethics Statement
Experimental protocols were approved by the Institutional Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2019032008). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved animal installation in our institute, with standard temperature and light cycles.
2.2. In Vitro Study for Assessment of ECSW Therapy on Upregulating the Protein Expression of (1) Cell-Proliferation/Growth/Survival and (2) Cell-Motility Signaling Pathways
To facilitate understanding of the mechanism of how ECSW therapy would enhance the angiogenesis, cell survival and salvage of CLI, an in vitro study using human umbilical vein endothelial cells (HUVECs) was utilized. The HUVECs were categorized into group A [HUVECs (i.e., as control group)], group B [HUVECs + ECSW (energy stepwise increased from 0.1, 0.14 to 0.2 mJ/mm2/140 impulses)], and group C [HUVECs + ECSW/0.20 mJ/mm2/140 impulses) + Avastin 20 μM (Bevacizumab, an angiogenic monoclonal antibody)]. The dosage of Avastin for the in vitro study was based on the previous report [33] with some modification.
2.3. Animal Model of CLI, Animal Grouping, and Treatment Strategy
The methodologies have been reported in our previous studies [34,35]. In detail, C57BL/6J (B6) male mice (Charles River Technology, BioLASCO, Taiwan) were anesthetized by inhalation of 2.0% isoflurane. Under sterile conditions, the left femoral artery, small arteries, and circumferential femoral artery were exposed and ligated over their proximal and distal portions prior to removal. For mice that served as sham-operated control group, the arteries were only separated without ligation.
For the purpose of the study, animals were categorized into four groups: (sham-operated control), group 2 (CLI), group 3 [CLI + ECSW (0.12 mJ/mm2/120 impulses/at days 1, 3 and 7 after CLI induction)] and group 4 [(CLI + ECSW (0.12 mJ/mm2/120 impulses) and Avastin (1 mg/intramuscular-injection) at days 1, 3 and 7 after CLI induction]. The ECSW energy applied to mice was based on our previous reports with some modifications [23,30]. The dosage of Avastin for the in vivo study was based on a previous report [36] with some modification.
2.4. Measurement of Blood Flow in CLI by Laser Doppler
The methodologies have been reported in our previous studies [34,35]. In detail, mice were anesthetized by inhalation of isoflurane (2.0%) before CLI induction and at days 1, 7, 14 after CLI induction. The mice were placed on supine position on a warming pad (37 °C) and blood flow was measured in both inguinal areas using a laser Doppler scanner (moorLDLS, Moor Instruments, UK). The ratio of blood stream in the left (ischemic) leg and right (normal) leg was computed. On day 14, the mice were euthanized and the quadriceps muscle in each animal was harvested for bench-work investigation.
2.5. Western Blot Analysis
The methodology for Western blot analysis was based on our recent reports [33,34]. In detail, equal amounts (50 μg) of protein extracts were segregated by SDS-PAGE. After electrophoresis, the segregated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. Nonspecific sites were blocked by incubation of the membrane in blocking buffer [5% nonfat dry milk in T-TBS (TBS containing 0.05% Tween 20)] at room temperature for one hour. Then the membranes were incubated with the indicated primary antibodies [vascular endothelial growth factor receptor 2 (VEGFR2) (1:1000, Abcam, Kaohsiung, Taiwan), epidermic growth factor receptor (EGFR) (1:1000, Cell Signaling, Kaohsiung, Taiwan), von Willebrand factor (vWF) (1:1000, Abcam), vascular endothelial growth factor (VEGF) (1:1000, Abcam), Ras (1:1000, Abcam), c-Raf (1:1000, Cell Signaling), phosphorylated (p)-MEK1/2(1:1000, Cell Signaling), p-ERK1/2 (1:1000, Sigma-Aldrich, Kaohsiung, Taiwan), total PI3K (1:5000, Abcam), p-PI3K (1:1000, Cell Signaling), total Akt (1:1000, Cell Signaling), p-Akt (1:1000, Cell Signaling), total m-TOR (1:1000, Cell Signaling), p-m-TOR (1:1000, Cell Signaling), p-FAK (1:1000, Cell Signaling), p-Src (1:1000, Cell Signaling), stromal cell-derived growth factor (SDF)-1α, CXCR4 (1:1000, Abcam), and β-actin (1:10000, Chemicon, Billerica, MA, USA)] for one hour at room temperature. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin IgG (1:2000, Cell Signaling) was used as a secondary antibody for one-hour incubation at room temperature.
2.6. Immunohistochemical (IHC) and Immunofluorescent (IF) Stains
The protocol of IF staining has been described in our previous reports [34,35]. For IHC and IF staining, rehydrated paraffin sections were first treated with 3% H2O2 for 10 min and incubated with Immuno-Block reagent (BioSB, Santa Barbara, CA, USA) for 30 min at room temperature. Sections were then incubated with primary antibodies specifically against alpha smooth muscle actin (α-SMA) (1/400, Sigma-Aldrich), CD31 (1/100, Bio-Rad, Kaohsiung, Taiwan), von Willebrand factor (vWF) (1/200, Sigma-Aldrich) and vascular endothelial cell (VEGF) (1:500, Abcam). Sections incubated with the use of irrelevant antibodies served as controls. Three sections of quadriceps specimen from each mouse were analyzed. For quantification, three randomly selected HPFs (200× or 400× for IHC and IF studies) were analyzed in each section. The mean number of positive-stained cells per HPF for each animal was then determined by summation of all numbers divided by nine.
2.7. Statistical Analysis
Quantitative data were presented as mean ± standard deviation (SD). Statistical analysis was carried by analysis of variance (ANOVA) followed by Bonferroni multiple comparison post hoc test. SAS statistical software for Windows version 8.2 was utilized. A p value < 0.05 was considered statistically significant.
3. Results
3.1. The Angiogenesis and Protein Expressions of EGFR/VEGFR2, and Cell Proliferation/Growth/Survival and Cell Motility Signaling Pathways
The in vitro study comprised several groups: the HUVECs were categorized into group A [HUVECs (i.e., as control group)], group B [HUVECs + ECSW (energy stepwise increased from 0.1, 0.14 to 0.2 mJ/mm2/140 impulses)], and group C [HUVECs + ECSW/0.20 mJ/mm2/140 impulses) + Avastin (20 μM)].
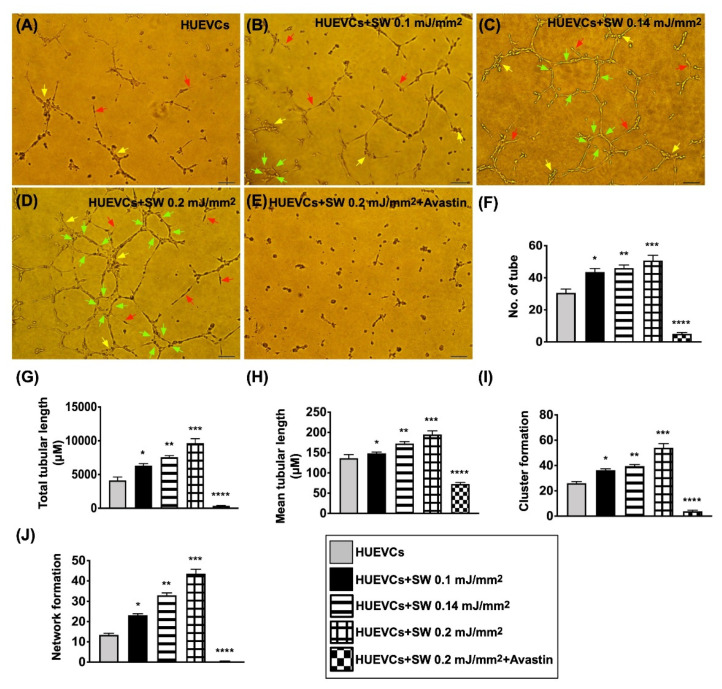
To elucidate the influence of ECSW therapy on angiogenesis, the Matrigel assay was utilized. The results showed that the angiogenesis parameters, i.e., tubular, cluster and network formation, were significantly and progressively higher in group B than in group A (i.e., control group) as the ECSW energy was stepwise increased (Figure 1). However, these increments in the angiogenesis markers were found to be significantly suppressed in group C, suggesting that Avastin inhibited angiogenesis (Figure 1).
Figure 1.
Impact of stepwise increased ECSW energy and Avastin on angiogenesis. (A–E) Illustrating the morphological features of Matrigel assay for identification of stepwise increase in ECSW energy on enhancing angiogenesis in human umbilical vein endothelial cells (HUVECs). The parameters of angiogenesis, including: (1) tubular formation (red arrows), (2) cluster formation (yellow arrows) and (3) network formation (green color). (F–J) Statistical analysis for angiogenesis parameters (F: number of tubules; G: total tubular length; H: mean tubular length; I: cluster formation; J: network formation). “*” represents in comparison with the control, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. Scale bar in right lower corner represents 50 µM. n = 6 for each group. HUVECs = human umbilical vein endothelial cells; SW = shock wave.
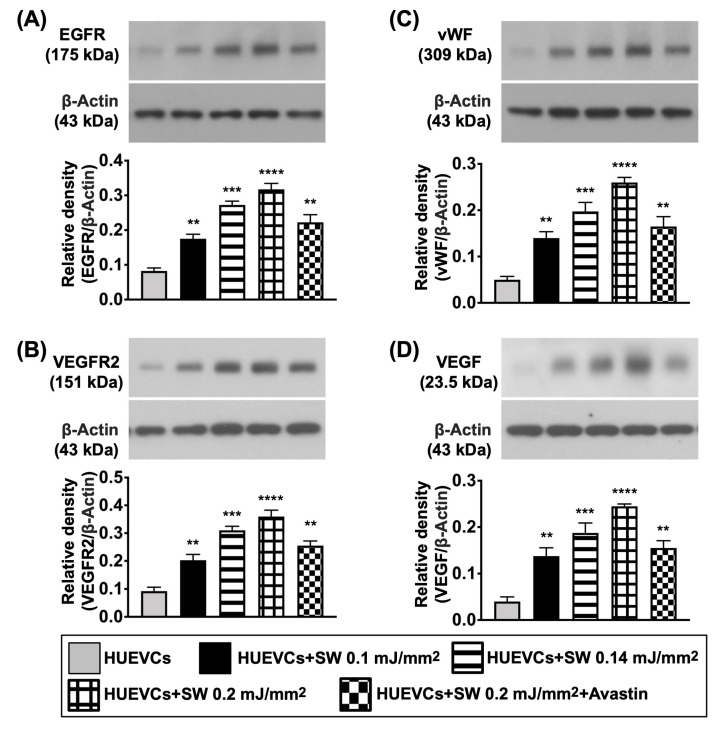
Next, to assess whether the ECSW therapy would upregulate the expressions of EGFR and VEGFR2, Western blot analysis was performed. As we expected, when compared to group A, the protein expressions of EGFR and VEGFR2 were significantly increased in group B (Figure 2). However, the protein expressions of these two parameters were significantly downregulated by Avastin treatment (Figure 2). Additionally, the protein expressions of vWF, an indicator of endothelial cell surface marker, and VEGF, an index of angiogenesis, were significantly increased in group B compared to group A, and were significantly reversed in group C (Figure 2).
Figure 2.
ECSW therapy upregulated the protein expressions of EGFR and VEGFR2, and angiogenic biomarkers in HUVECs. (A–D) Western blot analyses showed the results of protein expressions of epidermal growth factor receptor (EGFR) (A), vascular endothelial growth factor receptor 2 (VEGFR2) (B), von Willebrand factor (vWF) (C) and vascular endothelial growth factor (VEGF) (D). “*” represents in comparison with the control, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. n = 4 for each group. HUVECs = human umbilical vein endothelial cells; SW = shock wave.
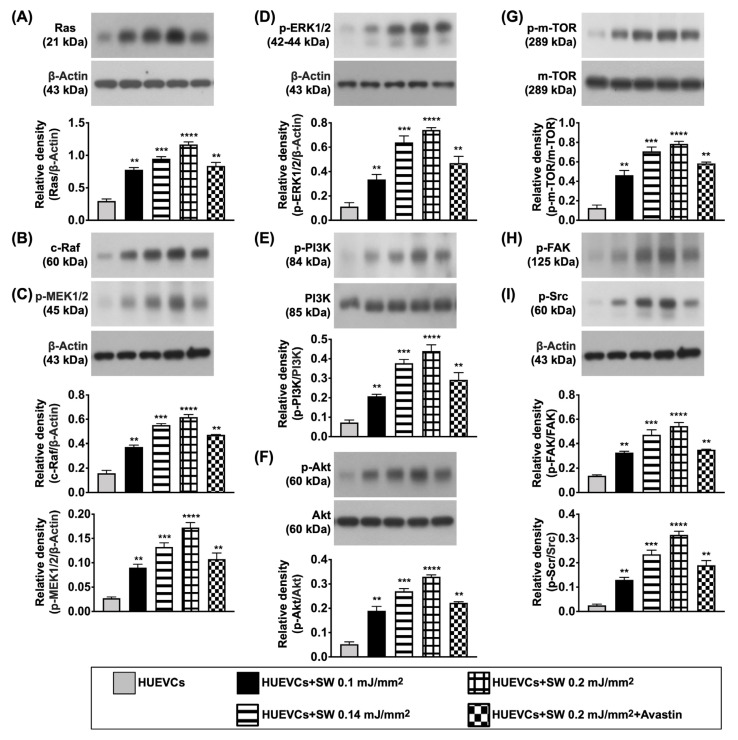
Furthermore, to clarify what cellular signalings were elicited after ECSW therapy, the Western blot was analyzed once again. The result demonstrated that the protein expressions of cell proliferation (Ras/c-Raf, p-MEK1/2, p-ERK1/2), cell proliferation/growth/survival (p-PI3K/p-Akt/p-m-TOR) and cell motility/proliferation (p-FAK/p-Scr) signaling pathways were significantly increased in group B as compared with group A, and were significantly reversed in group C (Figure 3), suggesting that ECSW therapy enhancement of angiogenesis might be via upregulating these signaling pathways.
Figure 3.
Protein expressions of cell proliferation/growth/survival, and cell motility signalings. (A–I) Western blot analyses showed the results of protein expressions of Ras (A), c-Raf (B), phosphorylated (p)-MEK1/2 (C), p-ERK1/2 (D), p-PI3K (E), p-Akt (F), p-m-TOR (G), p-focal adhesion kinase (FAK) (H) and p-Scr (I). “*” represents in comparison with the control, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. n = 4 for each group. HUVECs = human umbilical vein endothelial cells; SW = shock wave.
3.2. Ischemic to Normal Blood Flow (INBF) Ratio Measured by Laser Doppler Scan at Days 1, 7 and 14 after Left CLI Induction
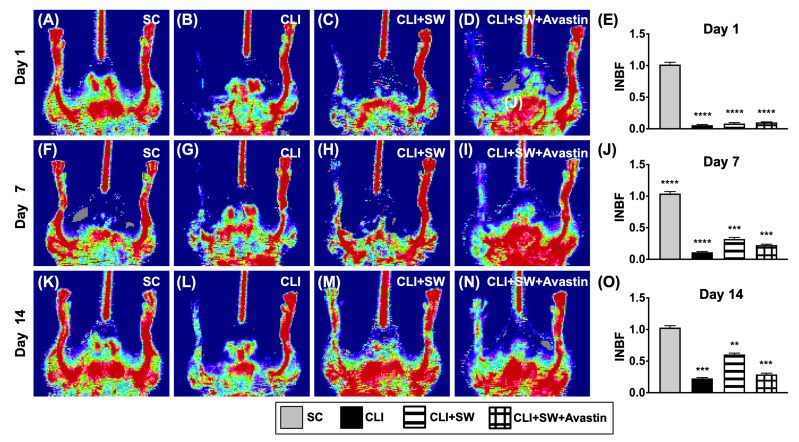
By day 1 after CLI induction, laser Doppler examination demonstrated a significantly higher INBF ratio in group 1 (SC) than in groups 2 (CLI), 3 (CLI + ECSW) and 4 (CLI + ECSW + Avastin), but there was not any obvious difference among the latter three groups (Figure 4). By days 7 and 14 after induction of CLI, the ratio of INBF was highest in group 1, lowest in group 2, and significantly higher in group 3 than in group 4 (Figure 4).
Figure 4.
Ischemic/normal blood flow (INBF) ratio measured by laser Doppler scan at days 1, 7 and 14 after left CLI induction. (A–D) Illustrating the laser Doppler finding of ratio of left limb (ischemia) to right limb (normal) blood flow (i.e., INBF) at day 1 after CLI procedure among the four groups. (E) Analytical result of ratio of INBF, **** for p < 0.0001. (F–I) Illustrating the laser Doppler finding of ratio of INBF at day 7 after CLI procedure among the four groups. (J) Analytical result of ratio of INBF, *** for p < 0.001, **** for p < 0.0001. (K–N) Illustrating the laser Doppler finding of ratio of INBF at day 14 after CLI procedure among the four groups. (O) Analytical result of ratio of INBF, ** for p < 0.01, *** for p < 0.001, n = 6 for each group. SC = sham-operated control; SW = shock wave.
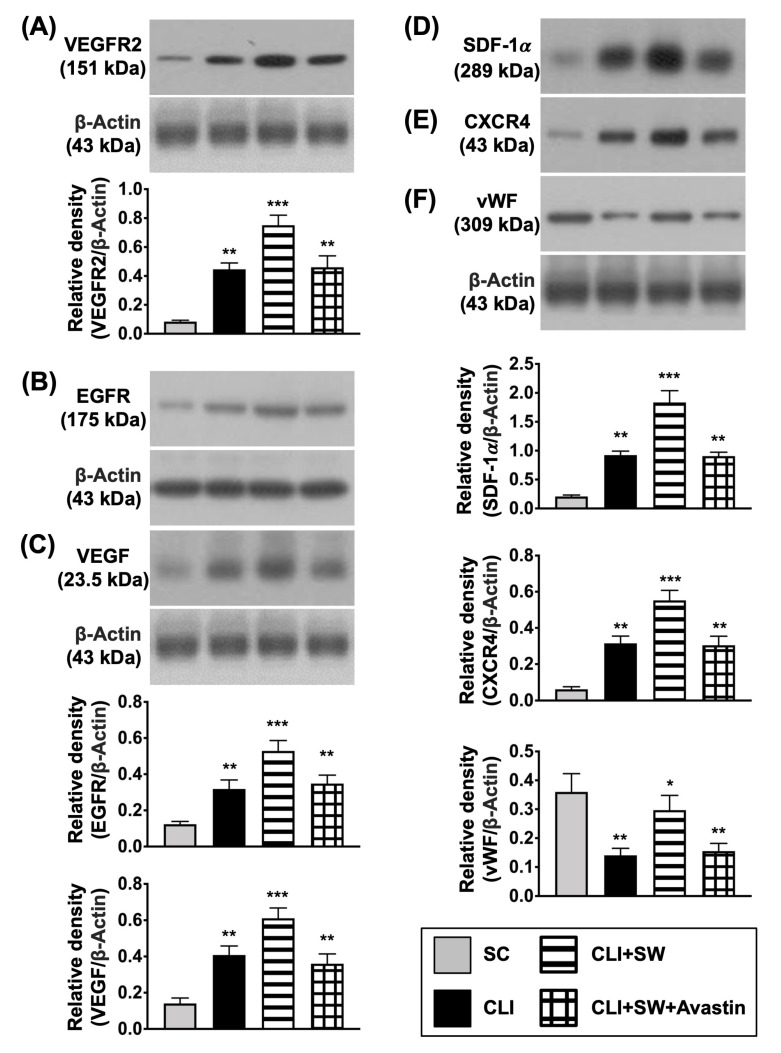
3.3. The Protein Expressions of Cell Growth/Angiogenesis Receptors and Angiogenic Factors in CLI Quadriceps Muscle by Day 14 after CLI Induction
First, to assess whether ECSW therapy would activate the expressions of angiogenesis/cell growth receptors and angiogenesis biomarkers, we utilized the tool of Western blot. The result demonstrated that the protein expressions of EGFR and VEGFR2, two indices of cell growth/angiogenesis receptors, were significantly higher in group 3 than in groups 1, 2 and 4, and significantly higher in groups 2 and 4 than in group 1, but they showed no difference between groups 2 and 4 (Figure 5). Additionally, the protein expressions of VEGF, SDF-1α and CXCR4, three indicators of angiogenesis biomarkers, displayed an identical pattern of VEGFR2, whereas the protein expression of vWF, an indicator of endothelial cell surface marker, exhibited an opposite pattern of VEGFR2 among the groups (Figure 5).
Figure 5.
The protein expressions of cell growth, angiogenesis receptor and angiogenic factors in CLI quadriceps muscle by day 14 after CLI induction. (A–F) Western blot analyses showed the results of protein expressions of vascular endothelial growth factor receptor 2 (VEGFR2) (A), epidermal growth factor receptor (EGFR) (B), vascular endothelial growth factor (VEGF) (C), (D) stromal cell derived factor (SDF)-1α, CXCR4 (E), and von Willebrand factor (vWF) (F), * for p < 0.05, ** for p < 0.01, *** for p < 0.001. n = 6 for each group. SC = sham-operated control; SW = shock wave.
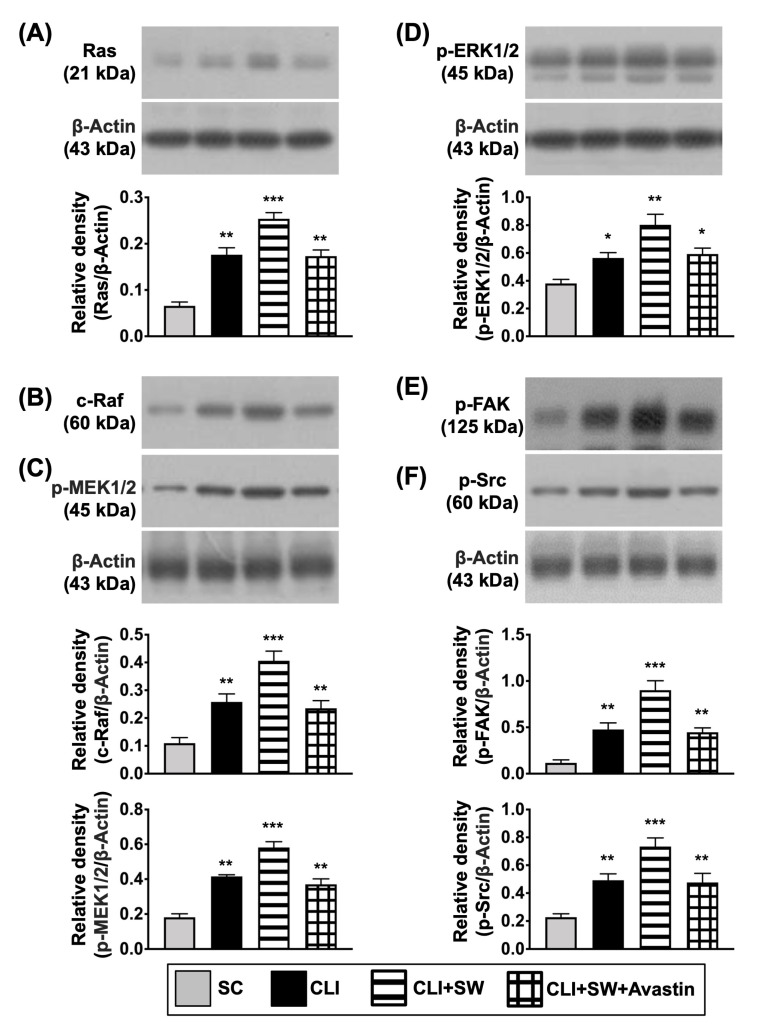
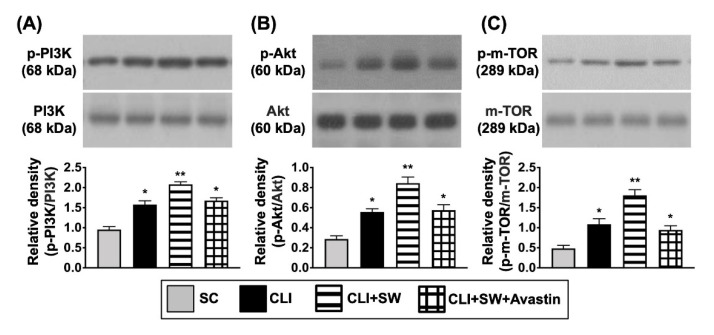
3.4. The Protein Expressions of Cell Proliferation/Growth/Survival and Cell Motility Signalings in CLI Quadriceps Muscle by Day 14 after CLI Induction
Next, based on our in vitro results, we planned to investigate whether ECSW therapy would also activate the cell proliferation and cell motility signalings in the in vivo study. As expected, the protein expressions of Ras, c-Raf, p-MEK1/2, and ERK1/2, four indicators of cell proliferation/survival signaling parameters, were significantly higher in group 3 than in other groups, significantly lower in group 1 than in groups 2 and 4, but similar between the latter two groups (Figure 6). Consistently, the protein expressions of p-FAK and p-Scr, two unique biomarkers of cell motility, displayed an identical pattern of Ras among the groups (Figure 7). In additional, the ratio of protein expressions of phosphorylated (p)-PI3K/p-Akt/p-m-TOR to the total PI3K/Akt/m-TOR, other biomarkers of proliferation/growth/survival signaling, also displayed an identical pattern of Ras (Figure 7). Taken together, our findings implicated that Avastin could inhibit therapeutic function of ECSW.
Figure 6.
The protein expressions of cell proliferation and cell mobility signalings in CLI quadriceps muscle by day 14 after CLI induction. (A–F) Western blot analyses showed the results of protein expressions of Ras (A), c-Raf (B), phosphorylated (p)-MEK1/2 (C), p-ERK1/2 (D), p-focal adhesion kinase (p-FAK) (E) and p-Scr (F), * for p < 0.05, ** for p < 0.01, *** for p < 0.001. n = 6 for each group. SC = sham-operated control; SW = shock wave.
Figure 7.
The protein expressions of cell proliferation/growth/survival signaling in CLI quadriceps muscle by day 14 after CLI induction. (A–C) Western blot analyses showed the results of protein expressions of phosphorylated (p)-PI3K (A), p-Akt (B) and p-m-TOR (C). * for p < 0.05, ** for p < 0.01. n = 6 for each group. SC = sham-operated control; SW = shock wave.
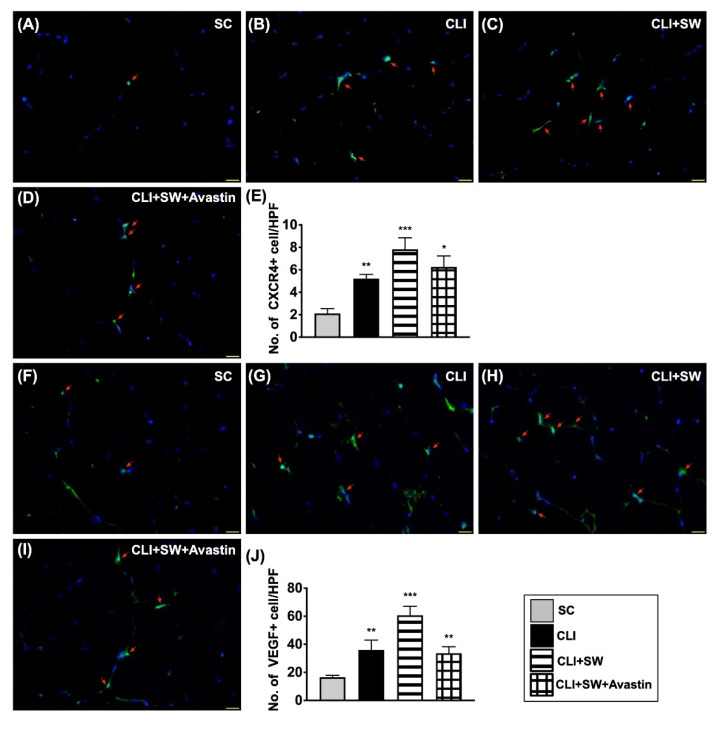
3.5. Cellular Expressions of Angiogenesis in CLI Quadriceps Muscle by Day 14 after CLI Induction
To evaluate whether the expression in cell level would be comparable with the protein level of angiogenesis, we utilized the IF microscopic instrument. As expected, the cellular expressions of CXCR4 and VEGF, two indicators of endothelial progenitor cell (EPC)/angiogenesis biomarkers, were significantly higher in group 3 than in groups 1, 2 and 4, and significantly higher in groups 2 and 4 than in group 1, but they showed no difference between groups 2 and 4 (Figure 8).
Figure 8.
Cellular expressions of angiogenesis in CLI quadriceps muscle by day 14 after CLI induction. (A–D) Illustrating the immunofluorescent (IF) microscopic finding (400×) for identification of cellular expression of CXCR4 (green color, red arrows). (E) Analytical result of number of CXCR4+ cells, * for p < 0.05, ** for p < 0.01, *** for p < 0.001. (F–I) Illustrating the IF microscopic finding (400×) for identification of cellular expression of vascular endothelial growth factor (VEGF) (green color, red arrows). (J) Analytical result of number of VEGF+ cells, ** for p < 0.01, *** for p < 0.001. All scale bars in right lower corner represent 20 µM. HPF = high-power field. n = 6 for each group. SC = sham-operated control; SW = shock wave.
3.6. The Microscopic Findings for Identification of Small Vessel Density and the Endothelial Cell Surface Marker in CLI Quadriceps Muscle by Day 14 after CLI Induction
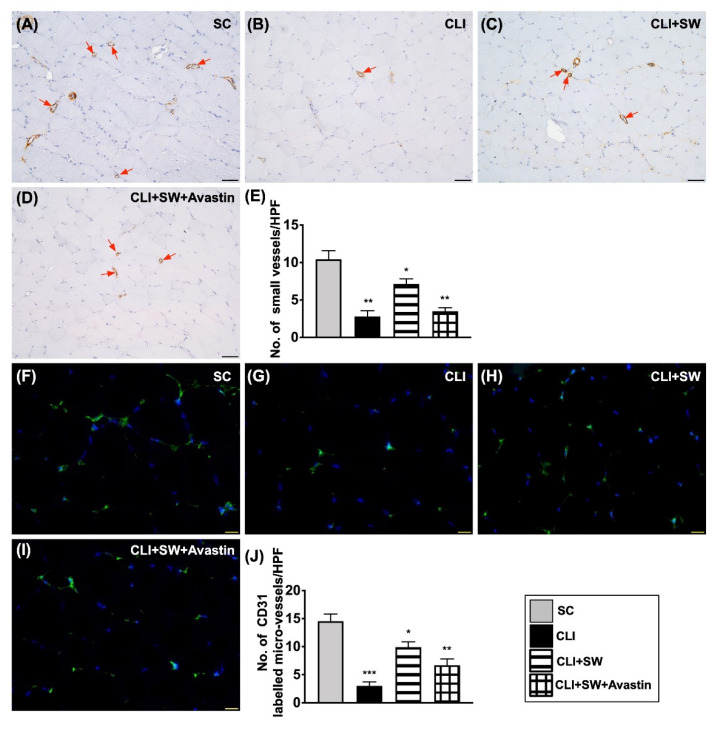
The number of small vessels (i.e., defined as diameter ≤25.0 μM) was significantly higher in group 1 than in other groups and significantly higher in group 3 than in groups 2 and 4, but it did not differ between the latter two groups (Figure 9). Additionally, the IF microscopic finding demonstrated that the number of CD31 cells, an indicator of endothelial cells, was highest in group 1, lowest in group 2 and significantly higher in group 3 than in group 4 (Figure 9).
Figure 9.
Small vessel density and the endothelial cell surface marker in CLI quadriceps muscle by day 14 after CLI induction. (A–D) Illustrating microscopic finding (200×) of alpha positively-stained smooth muscle actin (α-SMA) for identifying number of small vessels (i.e., defined as diameter ≤25.0 μM) (gray color) (red arrows). (E) Analytical result of number of small vessels, * for p < 0.05, ** for p < 0.01. All scale bars in right lower corner represent 50 µM. (F–I) Illustrating the immunofluorescent microscopic finding (400×) for identification of CD31+ cells (green color). (J) Analytical result of number of CD31+ cells, * for p < 0.05, ** for p < 0.01, *** for p < 0.001. All scale bars in right lower corner represent 20 µM. HPF = high-power field. n = 6 for each group. SC = sham-operated control; SW = shock wave.
4. Discussion
This study which investigated the therapeutic impact of ECSW on salvaging CLI yielded several striking preclinical implications. First, as compared with the CLI only group, the ratio of INBF (i.e., ratio of blood flow in CLI to normal limb) was remarkably improved in those CLI animals treated by ECSW, indicating that this therapy effectively salvaged the CLI in mice. Second, in vivo studies demonstrated that the salvage of CLI with ECSW therapy was mainly via neovascularization and angiogenesis effects. Third, in vitro and in vivo findings supported the idea that the underlying mechanism of ECSW therapy on enhancing angiogenesis and salvaging the CLI was related to three signaling pathways, including cell proliferation/growth/survival and cell motility.
Interestingly, our studies have previously demonstrated that ECSW therapy significantly preserved the ischemic related organ dysfunction in small and large animals [22,23,24,25,26,27,32]. These studies have further demonstrated that the salvage of ischemic related organ dysfunction in these small and large animals was mainly through enhancing the angiogenesis and SDF-1 angiogenic factors which attracted the EPC mobilization from circulation into the ischemia zone (i.e., a homing phenomenon) for angiogenesis [22,23,24,25,26,27,32], resulting in an increase of the blood flow in the ischemic region. The most important finding in the present study was that as compared with CLI animals without treatment, the blood flow in the CLI area and the molecular and cellular levels of angiogenic biomarkers were substantially increased in the CLI animals after receiving the ECSW treatment. Our findings corroborated the findings of our previous studies [22,23,24,25,26,27,32].
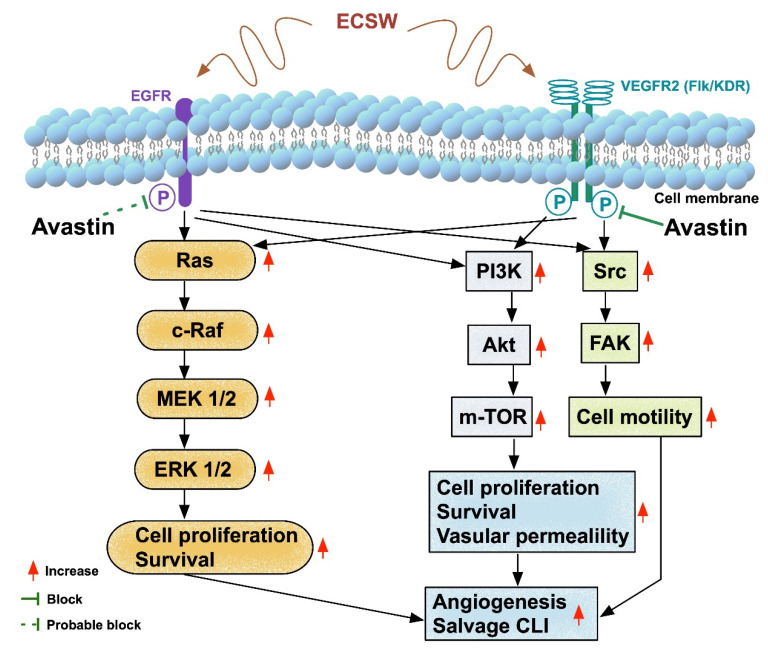
When looking at the results of our previous studies [22,23,24,26,27,32,35], we found that the limitations of these studies were that the exact underlying mechanisms for how the ECSW could salvage the CLI [23,32,35] and improve ischemic related heart function [22,24,26,27] have not been fully investigated. An essential finding in the in vitro study was that the ECSW promoted EGFR and VEGFR2 activation in the HUVECs. In additional, when we looked at the result of our in vivo study, we also found that the protein expressions of these two parameters were upregulated in CLI after ECSW treatment, suggesting the results in the in vitro and in vivo studies were comparable. Intriguingly, our previous study [32] has identified ECSW enhanced angiogenesis through VEGFR2 activation and recycling. Accordingly, our findings were comparable with the result from our previous study [32].
The essential findings of the present study, both in the in vitro and in vivo studies, demonstrated that the protein expressions of Ras/c-Raf/MEK/ERK (i.e., cell proliferation/survival pathway), PI3K/Akt/m-TOR (i.e., the signaling for cell proliferation/growth/survival) and p-FAK/p-Scr (i.e., cell motility/proliferation pathway) were significantly increased in the CLI group and further significantly in the CLI + ECSW group as compared to the control group, suggesting that upregulation of these signaling pathways through intrinsic response to stress/ischemic stimulation was remarkably augmented by ECSW therapy. On the other hand, the activation of these signalings was ameliorated by Avastin treatment, implicating that these downstream signalings activated by VEGFR and EGFR could be specifically blocked by the antiangiogenic monoclonal antibody, resulting in lowering the blood flow in the CLI area.
Some findings of this present study should be clarified to avoid misreading by the readers. First, in view of the Matrigel assay and Western blotting, we found that the protein and cellular levels of angiogenesis were inconsistent in terms of Avastin treatment. We suggested that this could be due to the Matrigel assay being focused on only morphological features through semi-quantitative analysis. Thus, it may not be as accurate as the Western blot analysis. Second, when we looked at the in vivo results, we found that angiogenesis biomarkers and protein levels of the aforementioned three cell signaling pathways were notably increased in the CLI group as compared to the SC group. We proposed that it could be an intrinsic response of cells/tissue in the CLI area to ischemic stimulation, especially in a situation of partial loss of microvasculature (i.e., a limited blood flow supplied to the ischemic cells/tissues).
Study Limitations
This study has limitations. First, the ECSW energy applied to mice was only based on our previous reports [23,28] without assessing the effect of stepwise increase in the dosage of ECSW on the CLI area. Therefore, the optimal in vivo energy of ECSW for improving the blood flow and salvaging the CLI in mice is currently unclear. Second, although the results were attractive and promising, the study period was only 14 days. Therefore, the long-term effect of ECSW therapy on CLI remains uncertain. Third, although extensive work has been performed, we cannot completely rule out the possibility that other signaling pathways may be involved in the angiogenesis. Accordingly, given the findings of the present study, we schematically illustrated the underlying mechanism of ECSW therapy on eliciting the signaling pathways that participate in angiogenesis and salvage of CLI in Figure 10. Finally, this study did not test the stepwise increase in the concentration of Avastin on the impact of cell viability and molecular-cellular levels of angiogenesis. Thus, we did not recommend what was the optimal dosage of Avastin for the in vitro and in vivo studies. Supplementary Figure S1 demonstrated how we assessed angiogenesis suppressed by Avastin in an ex vivo study of rat aortic ring. Supplementary Figure S2 showed cell viability progressively reduced with time after exposure to different dose of Avastin in MTT assay.
Figure 10.
Schematical illustration of the underlying mechanism of ECSW therapy on salvaging CLI in mice. ECSW = extracorporeal shock wave; EGFR = epidermic growth factor receptor; VEGFR = vascular endothelial growth factor receptor; CLI = critical limb ischemia.
In conclusion, the results of the present study showed that ECSW therapy promoted blood flow in CLI and salvaged the ischemic limb leg in mice mainly through activating the cell proliferation, growth and motility signalings, followed by enhancing angiogenesis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines10010117/s1. Figure S1. Ex vivo study of rat aortic ring assay for assessing the impact of Avastin on suppressing angiogenesis (A–H) Illustrating the results of 5-day exo vivo culture of aortic ring (AR) for determining the angiogenesis in groups 1 (A,B), 2 (C,D), 3 (E,F) and 4 (G,H), respectively. Scale bars in right lower corner represent 200 µm. The orange color (green arrows) indicated AR area. The blue color (red arrows) indicated sprout area. (I) Analytical result of AR area, p > 0.5. (J) Analytical result of sprout area, * for p < 0.05, ** for p < 0.01, *** for p < 0.001. (K) Analytical result of Mean sprouts front distance, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. (L) Analytical result of the ratio of sprout area to AR area, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001. Wimasis image analysis (Wimasis GmbH: Limited Liability Company) was utilized for quantitative analysis (n = 4 for each group). group 1 = Aortic ring (AR) (i.e., sham-control); group 2 = AR + Avastin (10 μM); group 3 = AR + Avastin (20 μM); group 4 = AR + Avastin (30 μM). Figure S2. MTT assay for determining the cell viability (A–C) By 24h (A), 48h (B) and 72h (C), cell culturing, the cell viability was significantly progressively reduced from groups 1 to 4, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001 (n = 4 for each group). MTT = [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. group 1 = Aortic ring (AR) (i.e., sham-control); group 2 = AR + Avastin (10 μM); group 3 = AR + Avastin (20 uM); group 4 = AR + Avastin (30 μM).
Author Contributions
P.-H.S., T.-C.Y. and H.-K.Y. designed the study. P.-H.S., T.-C.Y., H.-T.C., C.-H.C. and H.-K.Y. curated data. P.-H.S., T.-C.Y., C.-H.C., H.-K.Y. and H.-T.C. performed formal analysis. P.-H.S. was responsible for funding acquisition. P.-H.S., T.-C.Y., H.-T.C., C.-H.C., C.-R.H. and H.-K.Y. investigated experiments. H.-K.Y. administered and supervised the project. P.-H.S., T.-C.Y., J.Y.C. and H.-K.Y. wrote the first draft of the manuscript and all named authors contributed in revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by research grants from Chang Gung Memorial Hospital, Chang Gung University [CRRPG8J0091(1/3)/CRRPG8J0092(2/3)/RRPG8J0093(3/3)]
Institutional Review Board Statement
The study was approved by Institutional Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital 2019032008.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets of the present study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ. Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes F.G., Aboyans V., Fowkes F.J., McDermott M.M., Sampson U.K., Criqui M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 3.Aboyans V., Desormais I., Lacroix P., Salazar J., Criqui M.H., Laskar M. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. J. Am. Coll. Cardiol. 2010;55:898–903. doi: 10.1016/j.jacc.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 4.Amrock S.M., Abraham C.Z., Jung E., Morris P.B., Shapiro M.D. Risk Factors for Mortality Among Individuals With Peripheral Arterial Disease. Am. J. Cardiol. 2017;120:862–867. doi: 10.1016/j.amjcard.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 5.Andras A., Ferket B. Screening for peripheral arterial disease. Cochrane Database Syst. Rev. 2014:CD010835. doi: 10.1002/14651858.CD010835.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desormais I., Aboyans V., Guerchet M., Ndamba-Bandzouzi B., Mbelesso P., Dantoine T., Mohty D., Marin B., Preux P.M., Lacroix P., et al. Prevalence of peripheral artery disease in the elderly population in urban and rural areas of Central Africa: The EPIDEMCA study. Eur. J. Prev. Cardiol. 2015;22:1462–1472. doi: 10.1177/2047487314557945. [DOI] [PubMed] [Google Scholar]
- 7.Sigvant B., Wiberg-Hedman K., Bergqvist D., Rolandsson O., Andersson B., Persson E., Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc. Surg. 2007;45:1185–1191. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Huysman E., Mathieu C. Diabetes and peripheral vascular disease. Acta Chir. Belg. 2009;109:587–594. doi: 10.1080/00015458.2009.11680493. [DOI] [PubMed] [Google Scholar]
- 9.Garimella P.S., Hirsch A.T. Peripheral artery disease and chronic kidney disease: Clinical synergy to improve outcomes. Adv. Chronic Kidney Dis. 2014;21:460–471. doi: 10.1053/j.ackd.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 11.Sheu J.J., Lin P.Y., Sung P.H., Chen Y.C., Leu S., Chen Y.L., Tsai T.H., Chai H.T., Chua S., Chang H.W., et al. Levels and values of lipoprotein-associated phospholipase A2, galectin-3, RhoA/ROCK, and endothelial progenitor cells in critical limb ischemia: Pharmaco-therapeutic role of cilostazol and clopidogrel combination therapy. J. Transl. Med. 2014;12:101. doi: 10.1186/1479-5876-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momsen A.H., Jensen M.B., Norager C.B., Madsen M.R., Vestersgaard-Andersen T., Lindholt J.S. Drug therapy for improving walking distance in intermittent claudication: A systematic review and meta-analysis of robust randomised controlled studies. Eur. J. Vasc. Endovasc. Surg. 2009;38:463–474. doi: 10.1016/j.ejvs.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Singh P., Harper Y., Oliphant C.S., Morsy M., Skelton M., Askari R., Khouzam R.N. Peripheral interventions and antiplatelet therapy: Role in current practice. World J. Cardiol. 2017;9:583–593. doi: 10.4330/wjc.v9.i7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachs T., Pomposelli F., Hamdan A., Wyers M., Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: Angioplasty vs. bypass graft. J. Vasc. Surg. 2011;54:1021–1031.e1021. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 15.Reinecke H., Unrath M., Freisinger E., Bunzemeier H., Meyborg M., Luders F., Gebauer K., Roeder N., Berger K., Malyar N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015;36:932–938. doi: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 16.Aboyans V., Ricco J.B., Bartelink M.E.L., Bjorck M., Brodmann M., Cohnert T., Collet J.P., Czerny M., De Carlo M., Debus S., et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur. Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 17.Diehm C., Allenberg J.R., Pittrow D., Mahn M., Tepohl G., Haberl R.L., Darius H., Burghaus I., Trampisch H.J., German Epidemiological Trial on Ankle Brachial Index Study Group Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 18.Arain F.A., Ye Z., Bailey K.R., Chen Q., Liu G., Leibson C.L., Kullo I.J. Survival in patients with poorly compressible leg arteries. J. Am. Coll. Cardiol. 2012;59:400–407. doi: 10.1016/j.jacc.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notarnicola A., Moretti B. The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles Ligaments Tendons J. 2012;2:33–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Burneikaite G., Shkolnik E., Celutkiene J., Zuoziene G., Butkuviene I., Petrauskiene B., Serpytis P., Laucevicius A., Lerman A. Cardiac shock-wave therapy in the treatment of coronary artery disease: Systematic review and meta-analysis. Cardiovasc. Ultrasound. 2017;15:11. doi: 10.1186/s12947-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fode M., Lowenstein L., Reisman Y. Low-Intensity Extracorporeal Shockwave Therapy in Sexual Medicine: A Questionnaire-Based Assessment of Knowledge, Clinical Practice Patterns, and Attitudes in Sexual Medicine Practitioners. Sex Med. 2017;5:e94–e98. doi: 10.1016/j.esxm.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu M., Sun C.K., Lin Y.C., Wang C.J., Wu C.J., Ko S.F., Chua S., Sheu J.J., Chiang C.H., Shao P.L., et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: Molecular-cellular and functional assessment. PLoS ONE. 2011;6:e24342. doi: 10.1371/journal.pone.0024342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh K.H., Sheu J.J., Lin Y.C., Sun C.K., Chang L.T., Kao Y.H., Yen C.H., Shao P.L., Tsai T.H., Chen Y.L., et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit. Care Med. 2012;40:169–177. doi: 10.1097/CCM.0b013e31822d74d0. [DOI] [PubMed] [Google Scholar]
- 24.Sheu J.J., Lee F.Y., Yuen C.M., Chen Y.L., Huang T.H., Chua S., Chen Y.L., Chen C.H., Chai H.T., Sung P.H., et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int. J. Cardiol. 2015;193:69–83. doi: 10.1016/j.ijcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 25.Chai H.T., Chen K.H., Wallace C.G., Chen C.H., Sung P.H., Chen Y.L., Yuen C.M., Shao P.L., Sun C.K., Chang H.W., et al. Extracorporeal shock wave therapy effectively protects brain against chronic cerebral hypo-perfusion-induced neuropathological changes. Am. J. Transl. Res. 2017;9:5074–5093. [PMC free article] [PubMed] [Google Scholar]
- 26.Sheu J.J., Ali H.E.E., Cheng B.C., Chiang H.J., Sung P.H., Chen K.H., Yang C.C., Chen Y.T., Chiang J.Y., Lin P.Y., et al. Extracorporeal shock wave treatment attenuated left ventricular dysfunction and remodeling in mini-pig with cardiorenal syndrome. Oncotarget. 2017;8:54747–54763. doi: 10.18632/oncotarget.18287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung P.H., Yin T.C., Wallace C.G., Chen K.H., Shao P.L., Lee F.Y., Sun C.K., Sheu J.J., Chen Y.L., Omran M.M., et al. Extracorporeal Shock Wave-Supported Adipose-Derived Fresh Stromal Vascular Fraction Preserved Left Ventricular (LV) Function and Inhibited LV Remodeling in Acute Myocardial Infarction in Rat. Oxid. Med. Cell Longev. 2018;2018:7518920. doi: 10.1155/2018/7518920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts P.J., Der C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 29.Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheu J.J., Sung P.H., Wallace C.G., Yang C.C., Chen K.H., Shao P.L., Chu Y.C., Huang C.R., Chen Y.L., Ko S.F., et al. Intravenous administration of iPS-MSC(SPIONs) mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat. J. Cell Mol. Med. 2020;24:3593–3610. doi: 10.1111/jcmm.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee F.Y., Zhen Y.Y., Yuen C.M., Fan R., Chen Y.T., Sheu J.J., Chen Y.L., Wang C.J., Sun C.K., Yip H.K. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res. 2017;9:1603–1617. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang T.H., Sun C.K., Chen Y.L., Wang C.J., Yin T.C., Lee M.S., Yip H.K. Shock Wave Enhances Angiogenesis through VEGFR2 Activation and Recycling. Mol. Med. 2017;22:850–862. doi: 10.2119/molmed.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamili C., Kakaraparthy R.S., Vattikuti U.M. Anti-Angiogenic Activity of Flunarizine by In Ovo, In Vitro, and In Vivo Assays. Turk. J. Pharm. Sci. 2019;16:303–309. doi: 10.4274/tjps.galenos.2018.29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheu J.J., Chang M.W., Wallace C.G., Chiang H.J., Sung P.H., Tsai T.H., Chung S.Y., Chen Y.L., Chua S., Chang H.W., et al. Exendin-4 protected against critical limb ischemia in obese mice. Am. J. Transl. Res. 2015;7:445–459. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee F.Y., Sun C.K., Sung P.H., Chen K.H., Chua S., Sheu J.J., Chung S.Y., Chai H.T., Chen Y.L., Huang T.H., et al. Daily melatonin protects the endothelial lineage and functional integrity against the aging process, oxidative stress, and toxic environment and restores blood flow in critical limb ischemia area in mice. J. Pineal. Res. 2018;65:e12489. doi: 10.1111/jpi.12489. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q., Cheng Y., Huang L., Bai Y., Liang J., Li X. Inhibitory effect of carboplatin in combination with bevacizumab on human retinoblastoma in an in vitro and in vivo model. Oncol. Lett. 2017;14:5326–5332. doi: 10.3892/ol.2017.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of the present study are available from the corresponding author upon request.