Abstract
Background
Preliminary data suggest that the effectiveness of dalbavancin may be similar to current standard-of-care (SoC) treatment options for osteomyelitis with an advantageous dosing schedule.
Methods
This was a retrospective, observational cohort study of adult patients diagnosed with osteomyelitis. Patients were matched 1:2 to dalbavancin (administered as 2 doses separated by 1 week) or SoC treatment for osteomyelitis according to the Charlson Comorbidity Index, site of infection, and causative pathogen. The primary objective was to determine the incidence of treatment failure after a 1-year follow-up period. Secondary objectives included hospital length of stay (LOS), infection-related 1-year readmission rates, and treatment-related adverse events.
Results
A total of 132 patients received dalbavancin (n = 42) or SoC (n = 90). Baseline characteristics, including rates of surgical intervention, were similar between the 2 treatment groups. Treatment failure was similar between those who received dalbavancin and SoC (21.4% vs 23.3%; P = .81). Patients who received dalbavancin had a shorter hospital LOS (5.2 days vs 7.2 days; P = .01). There was no difference in the rates of infection-related readmission between the dalbavancin and the SoC group (31% vs 31.1%; P = .99). There were numerically fewer adverse events in the dalbavancin group compared with the SoC group (21.4% vs 36.7%; P = .08). Peripherally inserted central catheter line–related complications were reported in 17.8% of patients in the SoC group.
Conclusions
Dalbavancin administered as a 2-dose regimen is a safe and effective option for the treatment of osteomyelitis.
Keywords: dalbavancin, osteomyelitis
Osteomyelitis is a challenging infection to treat that often requires surgical intervention in addition to antimicrobial therapy. Antimicrobial therapy is often prescribed for 4–8 weeks and administered either intravenously (IV), orally, or using a combination of both routes [1–5]. Gram-positive organisms, such as Staphylococcus aureus, are among the most common organisms associated with osteomyelitis [6–8]. Effective treatment relies heavily on patient adherence. This can pose a significant challenge when therapies require multiple daily doses and becomes further complicated when multiple antimicrobials are required. In addition to adherence issues, outpatient IV therapy can result in complications such as infection and thrombosis, requires adequate patient education, and requires the ability to properly store parenteral antibiotics at home. Given these concerns, a less frequently administered antimicrobial would pose a significant benefit.
Dalbavancin, a long-acting lipoglycopeptide approved for the treatment of acute bacterial skin and skin structure infections (ABSSSIs), has recently emerged as a potential option for treating osteomyelitis [9]. Its terminal half-life of 14.4 days allows for less frequent administration [10]. Treatment for ABSSSI is dosed as either a single 1500-mg IV infusion or a 2-dose regimen (1000 mg IV, followed by 500 mg IV 1 week later) [9]. Dalbavancin had reasonable bone exposures in a pharmacokinetic study, which found that after a single 1000-mg dose, concentrations in cortical bone were 6.3 µg/g at 12 hours postinfusion and were maintained at 4.1 µg/g 2 weeks later [10]. The authors extrapolated these results to conclude that a dosing regimen of 1500 mg on day 1, followed by a second dose of 1500 mg on day 8, would result in dalbavancin concentrations at or above the MIC99.9 of 0.12 mg/L for staphylococcal species for ~1400 hours, or the equivalent of 8 weeks [10]. Given the promising pharmacokinetic data, a recent phase II randomized controlled trial was conducted comparing dalbavancin with standard of care (SoC) for the treatment of osteomyelitis; it found that dalbavancin was both an effective and well-tolerated treatment option [11].
Following the results of this phase II trial, our institution started utilizing dalbavancin as part of routine clinical care. The purpose of this study was to determine the incidence of treatment failure at 1 year in patients receiving either dalbavancin or SoC for the treatment of osteomyelitis in a real-world setting.
METHODS
Study Design
This was a retrospective, observational cohort study of adult patients diagnosed with either acute or chronic osteomyelitis who were treated with either dalbavancin or SoC at Allegheny General Hospital (AGH) and West Penn Hospital (WPH). Patients were reviewed from January 1, 2019, to November 27, 2020, as the first patient who received dalbavancin at our institution for the treatment of osteomyelitis did so in January 2019. Patient electronic medical records were reviewed for 1 year after the start of therapy to assess for the primary and secondary objectives. The study protocol was approved by the Allegheny Health Network Institutional Review Board.
Study Participants
Patients were included if they were 18–89 years of age, had a diagnosis of osteomyelitis defined by radiologic findings or histology, were treated with either dalbavancin or alternative antibiotics, and were admitted to either AGH or WPH. Radiographic imaging included either x-ray, computerized tomography, magnetic resonance imaging, or a combination of these at the discretion of the treating physicians. Patients were also included if they had received dalbavancin following diagnosis of osteomyelitis as an outpatient. Only the first osteomyelitis encounter was included. Patients were excluded if they received a curative amputation for the treatment of their osteomyelitis, had a monomicrobial infection caused by either gram-negative or anaerobic bacteria, had a polymicrobial infection without the recovery of gram-positive cocci, had concomitant endocarditis, were pregnant, or were incarcerated at the time of admission.
Study Intervention
Patients were matched 1:2 to dalbavancin or SoC based on Charlson Comorbidity Index score, site of infection, and causative pathogen. Dalbavancin was administered as a 1500-mg 30-minute IV infusion on day 1, followed by a second 1500-mg infusion on day 8 (range, 6–19 days). For patients with a creatinine clearance <30 mL/min not receiving intermittent hemodialysis, the dose of dalbavancin was reduced to 1000 mg administered on a schedule that has previously been defined [11]. Patients were permitted to receive either IV or oral therapy before initiating therapy with dalbavancin. If a patient received >1 course of dalbavancin within a 1-year period, only the initial treatment course was included. SoC therapy for osteomyelitis included either oral or IV therapy and was decided by the infectious diseases physician who followed the patient while admitted to the hospital. Patients with a polymicrobial infection with a gram-negative organism were permitted to receive targeted therapy with either oral or IV antibiotics in addition to dalbavancin.
Objectives
The primary objective of this study was to determine the incidence of treatment failure in patients receiving either dalbavancin or SoC for the treatment of osteomyelitis within a 1-year follow-up period. Treatment failure was defined as requiring additional antibiotics or a change in therapy due to a lack of treatment response, new sinus drainage or purulence at the site of infection, amputation due to progression of infection, isolation of a pathogenic organism from a deep tissue culture or biopsy, or the presence of inflammatory infiltrate or microorganisms.
Secondary objectives included hospital length of stay (LOS) during the index admission, infection-related readmission rates within a 1-year period, and pertinent safety information. Hospital LOS was defined as the total number of days the patient was admitted to the hospital, regardless of when appropriate antibiotics were initiated.
Statistical Analysis
The normality of continuous variables was assessed using the Kolmogorov-Smirnov test. Normally distributed variables were compared using Welch’s 2-sample t test, and non–normally distributed variables were compared using the Wilcoxon rank-sum test. Categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. P values <.05 were considered statistically significant. Backward stepwise logistic regression was used to identify patient variables correlated with treatment failure. All statistical analyses were performed using R, version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
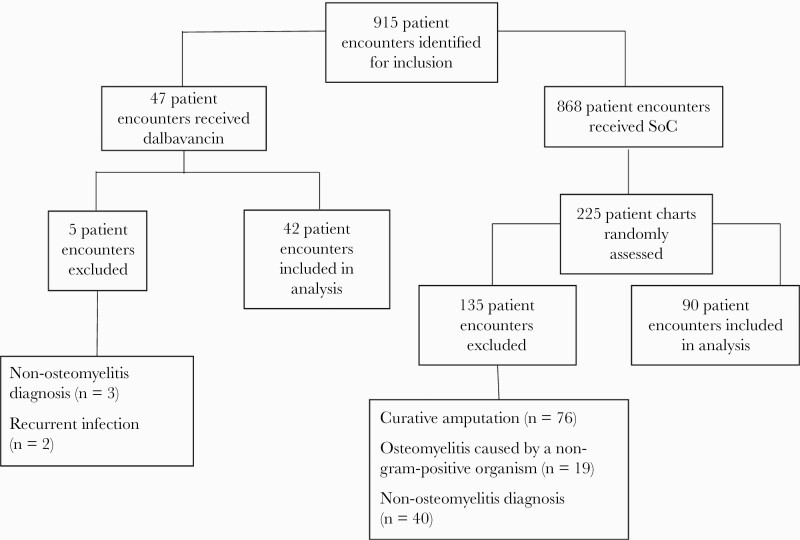
A total of 915 patient encounters were identified for study inclusion (Figure 1). After exclusions, a total of 42 patient encounters were analyzed in which patients received dalbavancin. There were 868 patient encounters identified in which an SoC regimen was administered. Of these, 225 medical records were randomly assessed to find a total of 90 matched patient encounters.
Figure 1.
Patient screening. Abbreviation: SoC, standard of care.
Baseline demographics were comparable between the 2 treatment groups, with the exception of white blood cell count (WBC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), all of which were more elevated in the SoC group (Table 1). The most common etiology of osteomyelitis was diabetic foot infection in both the dalbavancin and SoC arms. Staphylococcus aureus, either as a monomicrobial or polymicrobial infection, was the most commonly isolated pathogen for patients receiving dalbavancin or SoC. There was no difference in the number of patients who had any type of surgical intervention, and a similar number of patients in each group had retained hardware present for the duration of therapy (Table 1). Approximately 21% of patients in each treatment group received suppressive therapy after completing their initial treatment of osteomyelitis as planned by the infectious diseases (ID) physician, and there was no difference in the average number of days of suppressive therapy received between the dalbavancin group and the SoC group (Table 1).
Table 1.
Patient Demographics and Disease Characteristics at Baseline
| Characteristic | Dalbavancin (n = 42) | Standard of Care (n = 90) | p-value |
|---|---|---|---|
| Age, mean (SD), years | 59.5 (11.8) | 60.2 (11.9) | 0.77 |
| Male sex, no. (%) | 26 (61.9) | 61 (67.8) | 0.51 |
| Weight, mean (SD), kg | 94.5 (27.4) | 93.8 (26.4) | 0.91 |
| BUN, mean (SD), mg/dL | 20.4 (14.4) | 20 (12.8) | 0.88 |
| Scr, mean (SD), mg/dL | 1.07 (0.67) | 0.87 (1.75) | 0.18 |
| CrCl, mean (SD), mL/min | 95 (41.1) | 95.3 (37) | 0.97 |
| WBC, mean (SD), k/μL | 9.5 (3.9) | 11.7 (7.3) | 0.03 |
| CRP, mean (SD), mg/dL | 4.3 (6.9) | 9.2 (12.8) | 0.01 |
| ESR, median (IQR), mm/hr | 50 (22.3-84.8) | 66.5 (44-100.8) | 0.04 |
| Charlson Score, median (IQR) | 3 (2-5) | 3 (2-5) | 0.63 |
| Risk Factor, no. (%) | |||
| Hematogenous | 2 (4.8) | 6 (6.7) | 0.49 |
| Diabetic foot infection | 19 (45.2) | 42 (46.7) | |
| PVD | 3 (7.1) | 2 (2.2) | |
| Surgical site infection | 6 (14.3) | 17 (18.9) | |
| Physical injury | 12 (28.6) | 19 (21.1) | |
| Unknown | 0 (0) | 4 (4.4) | |
| Causative Pathogen, no. (%) | |||
| S. aureus | 11 (26.2) | 34 (37.8) | 0.74 |
| MRSA | 4 (36.4) | 7 (20.6) | |
| S. aureus + polymicrobial | 12 (28.6) | 23 (25.6) | |
| MRSA | 6 (50) | 11 (47.8) | |
| Other GPCa | 5 (11.9) | 9 (10) | |
| Other GPC + polymicrobial | 10 (23.8) | 16 (17.7) | |
| Culture negative | 4 (9.5) | 8 (8.9) | |
| Site of Infection, no. (%) | |||
| Spinal | 4 (9.5) | 10 (11.1) | 1 |
| Lower extremity | 32 (76.2) | 66 (73.3) | |
| Upper extremity | 6 (14.3) | 14 (15.6) | |
| Patients with any Surgical Intervention, no. (%) | 38 (90.5) | 77 (85.6) | 0.43 |
| Patients with any Retained Hardware, no. (%) | 4 (9.5) | 13 (14.4) | 0.58 |
| Antibiotics, no. (%) | |||
| Dalbavancin | 27 (64.3) | N/A | — |
| Dalbavancin + PO | 13 (31) | N/A | |
| Dalbavancin + IV | 2 (4.8) | N/A | |
| SoC IV | N/A | 48 (53.3) | |
| SoC PO | N/A | 17 (18.9) | |
| SoC IV + PO | N/A | 25 (27.8) | |
| Received Suppressive Therapy, no. (%) | 9 (21.4) | 19 (21.1) | 0.97 |
| Days of Suppressive Therapy, mean (SD) | 143 (156.7) | 112.8 (135.6) | 0.60 |
| ID Consultation, no. (%) | 42 (100) | 90 (100) | — |
Abbreviations: BUN, blood urea nitrogen; CrCl, creatinine clearance; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GPC, gram-positive cocci; ID, infectious disease; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PVD, peripheral vascular disease; Scr, serum creatinine; SoC, standard of care; WBC, white blood cell.
Other GPC include coagulase-negative Staphylococcus spp., Enterococcus spp., and Streptococcus spp.
Most patients in the dalbavancin arm received complete monotherapy with dalbavancin (64.3%). A total of 13 patients (31%) received additional oral antimicrobials, the most common being rifampin (n = 6), ciprofloxacin (n = 4), and metronidazole (n = 4). The average number of days of antimicrobial therapy before initiation of dalbavancin was 10.8 days, and the average number of days between dalbavancin doses (range) was 7.6 (6–19) days. Two patients received a single dose of dalbavancin. One patient originally started treatment in the SoC arm but was switched to dalbavancin after 28 days of therapy due to intolerable side effects. This patient was included in the dalbavancin arm during our analysis. Only 1 dose was administered as the patient deferred the second infusion of dalbavancin. This was determined to be a reasonable option as the patient had already completed approximately half of the treatment course with SoC before the initial dalbavancin infusion. This patient eventually failed therapy. The second patient declined the second dose of dalbavancin due to transient infusion-related reactions that occurred with the initial dose. This patient completed therapy with SoC and did not develop signs of treatment failure. Approximately 81.8% of patients in the SoC arm received definitive therapy with at least 1 IV antimicrobial, and 18.9% of patients received complete definitive therapy with oral antibiotics. The most common antimicrobials used in the SoC arm included vancomycin (n = 27), rifampin (n = 24), and cefazolin (n = 22). The median duration of therapy in the SoC group (interquartile range) was 43 (41–48) days. Of note, 100% of patients in each treatment group had an ID consultation during the treatment of their osteomyelitis.
Primary Objective
There was no difference in the incidence of treatment failure at 1 year between patients who received dalbavancin and patients who received an SoC regimen (21.4% vs 23.3%; P = .81). Additionally, we observed a higher number of days to treatment failure in the dalbavancin group as compared with the SoC group, although this difference was not significantly different (134.3 ± 70.1 days vs 95.3 ± 75.9 days; P = .17).
Secondary Objectives
There was no difference in the incidence of infection-related readmission during the study period between patients who had received dalbavancin or an SoC regimen (31% vs 31.1%; P = .99). However, patients who had received dalbavancin had a significantly shorter hospital LOS compared with patients who had received an SoC regimen (5.2 days vs 7.2 ± days; P = .01). Refer to Table 2 for the results of all primary and secondary objectives.
Table 2.
Primary and Secondary Outcomes in Patients Receiving Dalbavancin and Standard of Care
| Outcome | Dalbavancin (n = 42) | Standard of Care (n = 90) | P Value |
|---|---|---|---|
| Treatment failure, No. (%) | 9 (21.4) | 21 (23.3) | .808 |
| Days to treatment failure, mean (SD) | 134.3 (70.1) | 95.3 (75.9) | .169 |
| Infection-related readmission, No. (%) | 13 (31) | 28 (31.1) | .985 |
| Hospital LOS, mean (SD), d | 5.2 (4.2) | 7.2 (4.4) | .013 |
Abbreviation: LOS, length of stay.
Safety
The overall incidence of adverse events in patients who received dalbavancin was 21.4%, compared with 36.7% in the SoC arm (P = .08). Of the 9 patients in the dalbavancin group who had an adverse event documented in the electronic medical record, 4 patients experienced transient nausea and vomiting, 4 patients had infusion-related reactions, and 1 patient had an INR fluctuation documented. The patient who had a documented INR fluctuation was also receiving concomitant rifampin, which is known to cause INR fluctuations when administered with warfarin. Additionally, of the 73 patients in the SoC arm who were receiving therapy with an IV antimicrobial, 13 patients (17.8%) had a peripherally inserted central catheter (PICC) line–related complication. A further breakdown of adverse events can be seen in Table 3.
Table 3.
Adverse Events Reported in Patients Receiving Dalbavancin and Standard of Care
| Characteristic | Dalbavancin (n = 42) | Standard of Care (n = 90) | P Value |
|---|---|---|---|
| Any adverse event, No. (%) | 9 (21.4) | 33 (36.7) | .08 |
| Adverse event, No. (%) | |||
| PICC complication | 0 (0) | 13/73 (17.8) | .002 |
| Lab abnormality | 1 (2.4) | 10 (11.1) | .173 |
| Physical reaction | 8 (19) | 7 (7.8) | .057 |
| Medication-related error | 0 (0) | 3 (3.3) | .551 |
Abbreviation: PICC, peripherally inserted central catheter.
Backward Stepwise Regression
Factors that were identified as having a significant impact on the incidence of treatment failure included baseline WBC, BUN, and diabetic foot infection as the causative etiology of osteomyelitis (Table 4). A higher baseline WBC and BUN was protective against treatment failure. An etiology of diabetic foot infection was associated with greater odds of treatment failure. Treatment group was not identified as being associated with increased odds of treatment failure.
Table 4.
Variables included in Backward Stepwise Logistic Regression
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| Study regimen | 0.64 | 0.16–2.26 |
| Weight | 1.02 | 0.997–1.05 |
| BUN | 0.935 | 0.86–0.99 |
| WBC | 0.831 | 0.7–0.96 |
| ESR | 1.01 | 0.996–1.03 |
| Diabetic foot infection | 6.35 | 1.92–24.3 |
Abbreviations: BUN, blood urea nitrogen; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
DISCUSSION
In our real-world analysis, dalbavancin was equally effective in the treatment of osteomyelitis compared with SoC regimens. Adequate treatment of osteomyelitis typically requires 4–6 weeks of antimicrobial therapy and may last up to 8 weeks for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) osteomyelitis [3–5]. Dalbavancin offers a more convenient option compared with current SoC options in that a 2-dose regimen of 1500 mg separated by 1 week provides comparable effectiveness while significantly mitigating the risk of nonadherence to SoC regimens [9]. Additionally, 2-dose dalbavancin therapy avoids prolonged IV catheterization, which is associated with a 10%–60% rate of dangerous, potentially severe adverse events, including deep venous thrombosis, central line infection, catheter fracture and/or migration, and central venous stenosis [12–14]. Therefore, if data support the relative clinical effectiveness of therapy that avoids prolonged central venous catheter placement for osteomyelitis, it would be reasonable to consider this approach.
The use of dalbavancin for the treatment of osteomyelitis has been summarized elsewhere [15]. Previous reports of dalbavancin for this indication have been limited primarily to case reports and retrospective case series, with few randomized controlled trials or retrospective matched cohort studies. While these studies demonstrated satisfactory clinical success with dalbavancin for the treatment of osteomyelitis, there was large variability regarding dosing regimens and follow-up periods. Our analysis further validates these findings by using a consistent dosing regimen, a long follow-up period of 1 year, and by including a relatively large number of patients who received dalbavancin.
In our analysis, patients with diabetic foot infections were the only factor that was identified from backward stepwise regression to increase the odds of treatment failure. Patients in the SoC group had an elevated WBC, ESR, and CRP compared with patients in the dalbavancin group. Elevated WBC was found to be protective of treatment failure, and neither CRP nor ESR had an impact on the incidence of treatment failure. Therefore, although the SoC group had higher WBC, ESR, and CRP compared with the dalbavancin arm, this did not give an advantage to the dalbavancin group. Importantly, treatment with dalbavancin was not associated with treatment failure by logistic regression. It is possible that antibiotic therapy was futile in some of the included patients due to the severity of infection, poorly controlled diabetes, and patients with concomitant peripheral vascular disease. Indeed, our clinical success, while comparable to SoC, was much lower than that reported of dalbavancin by Rappo and colleagues (78.6% vs 96%, respectively) [11]. The main difference we identified in our patient populations was that our cohort had more diabetic foot infections (45.2% vs 5.7%) and less surgical debridement (90.5% vs 100%), indicating a potentially more real-world expectation for treatment success in this patient population.
The use of dalbavancin resulted in a 2-day shorter hospital LOS compared with patients who received SoC treatment options. This is similar to the study performed by Rappo and colleagues [11]. Potential explanations for this finding include that patients did not require prolonged hospitalizations to finalize antibiotic regimens according to susceptibilities, therapeutic drug monitoring for vancomycin, and patients receiving dalbavancin did not require home health resources.
Dalbavancin was well tolerated, with 9 patients experiencing an adverse drug event: 4 experienced transient nausea and vomiting, 4 experienced infusion-related reactions, and 1 patient experienced an INR fluctuation that could have been attributed to concomitant use of rifampin. We would consider these adverse drug events to be mild and perhaps less severe than some of the PICC line complications that resulted in emergency department (ED) visits.
The limitations of this study include its retrospective design. Given the retrospective nature of this study, we were unable to assess adherence in patients who received an SoC regimen. Additionally, patients presenting with treatment failure to facilities outside of our network may have been missed. Another weakness of this study is that patients in the dalbavancin arm received an average of 10.8 days of SoC therapy before receiving the initial dose of dalbavancin. Strengths of this study include the 1-year follow-up period, and all patients analyzed had an ID consultation. Having an ID consultation likely led to patients receiving an appropriate antimicrobial regimen for an appropriate duration of therapy, as evidenced by the median duration of therapy in the SoC arm of 43 days.
CONCLUSIONS
Dalbavancin was as equally efficacious as SoC options for the treatment of osteomyelitis and was associated with a shorter hospital LOS. Dalbavancin demonstrated adequate tolerability and resulted in the avoidance of PICC line–related complications. Dalbavancin offers an effective, safe, and convenient treatment option for patients with osteomyelitis.
Acknowledgments
Financial support. Neither this research, nor any of its investigators, received specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Potential conflicts of interest. D.C. is a member of the Merck Speaker’s Bureau, and T.W. has received speaking fees from Accelerate Diagnostics. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. All authors contributed significantly to the work presented and the preparation of the manuscript and have read and approved the final submitted version.
Patient consent. The study protocol (study 2020-317) was approved by the Allegheny Health Network institutional review board. Given the retrospective nature of our study, it did not include factors necessitating patient consent.
References
- 1. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spellberg, B, Lipsky, BA.. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54:132–73. [DOI] [PubMed] [Google Scholar]
- 4. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61:e26–46. [DOI] [PubMed] [Google Scholar]
- 5. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 6. Lew, DP, Waldvogel FA.. Osteomyelitis. Lancet 2004; 364:369–79. [DOI] [PubMed] [Google Scholar]
- 7. Sheehy SH, Atkins BA, Bejon P, et al. The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 2010; 60:338–433. [DOI] [PubMed] [Google Scholar]
- 8. Pfaller MA, Flamm RK, Castanheira M, et al. Dalbavancin in-vitro activity obtained against gram-positive clinical isolates causing bone and joint infections in US and European hospitals (2011-2016). Int J Antimicrob Agents 2018; 51:608–11. [DOI] [PubMed] [Google Scholar]
- 9. Dalbavancin. Package Insert. Durata Therapeutics; 2014. [Google Scholar]
- 10. Dunne MW, Puttagunta S, Sprenger CR, et al. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 2015; 59:1849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shingarev R, Allon M.. Peripherally inserted central catheters and other intravascular devices: how safe are they for hemodialysis patients? Am J Kidney Dis 2012; 60:510–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krein SL, Saint S, Trautner BW, et al. Patient-reported complications related to peripherally inserted central catheters: a multicenter prospective cohort study. BMJ Qual Saf 2019; 28:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grau D, Clarivet B, Lotthe A, et al. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control 2017; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almangour TA, Alhifany AA.. Dalbavancin for the management of osteomyelitis: a major step forward? J Antimicrob Chemother 2020; 75:2717–22. [DOI] [PubMed] [Google Scholar]