Grafting is a vegetative propagation technique wherein the root system of one species (stock) is joined with the shoot (scion) of another species to form a new plant variety. Successful grafting involves the joining of both vascular (xylem and phloem) and non-vascular tissues (epidermis, cortex/pith) between the stock and scion. Grafting is generally used to develop plant varieties that are resistant to diseases, abiotic stresses, and for desired plant stature (Willingham et al., 2001; Sánchez-Rodríguez et al., 2012). Generally, grafts tend to be more compatible between species of the same genus than between more distantly related species (Goldschmidt, 2014). In certain cases, an incompatible graft can survive for months without establishing a proper vascular connection, called delayed graft incompatibility. Delayed graft incompatibility causes economic burden and time loss to farmers and commercial growers. Hence, it is important to know the molecular events that occur during delayed graft incompatibility.
In this issue of The Plant Cell, Hannah Thomas, Lisa Van den Broeck, and colleagues (Thomas et al., 2021) inferred gene regulatory networks (GRNs) for compatible versus incompatible grafts using tomato (Solanum lycopersicum var. M82) and pepper (Capsicum annum var. Big Dipper) plants correlated with major anatomical changes during grafting. The authors established that self-grafted tomato or pepper plants exhibited 100% survival and 0–6% fragility across the graft junctions. By contrast, hetero-grafted pepper:tomato (scion:stock) and tomato:pepper plants exhibited 75% and 37% survival at 30 days after grafting (DAG), respectively, and the majority of the heterografts broke in the fragility test. Anatomical observations at 30 DAG showed a successful vascular connection in self-grafted plants and discontinuous vascular connection consistent with delayed graft incompatibility in heterografted plants.
To understand delayed graft incompatibility, the authors constructed an anatomical timeline for the compatible self-grafts and delayed graft incompatibility heterografts. This timeline showed that self-grafted plants developed callus around the stem periphery at the graft junction by 3 DAG, which eventually differentiated into xylem between 3 and 6 DAG. However, the heterografted plants developed callus and differentiated into xylem only at the tomato half of junction suggesting that the pepper half stalled in establishing a vascular connection even at 6 DAG.
To identify the genes involved in a successful vascular connection at the graft junction, the authors performed RNA sequencing with graft junction tissue at 1, 3, and 5 DAG for both self- and hetero-grafts and generated GRNs. Comparing the GRNs between the self-grafted tomato and pepper plants revealed that these two plants use a distinct gene network for the successful graft connection, even though these networks converge to similar downstream genes. To further identify the shared genetic components between the self- and hetero-grafted plants, the authors identified orthologs of graft-related transcription factors from Arabidopsis in both tomato and pepper and observed that their dynamics are perturbed in heterografts compared to self-grafted plants.
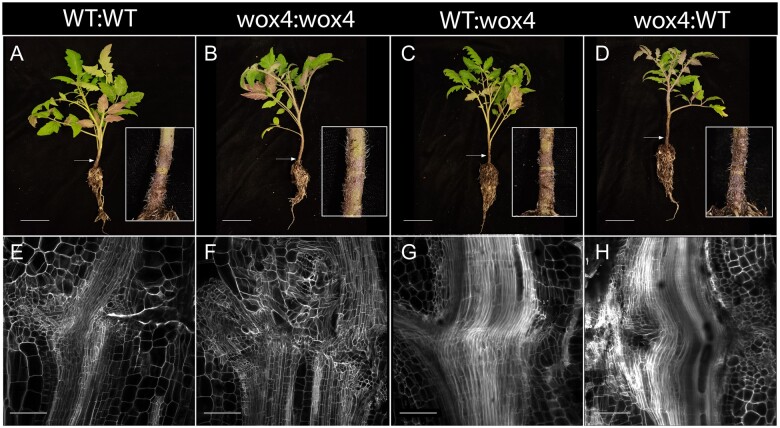
Based on the observation that some of the common transcription factors between the self- and hetero-grafted plants are regulated by WUSCHEL RELATED HOMEOBOX 4 (WOX4), a gene involved in procambial specification, the authors studied SlWOX4 and CaWOX4 in more detail. While the expression of the WOX4 orthologs in self-grafted plants shows a steady increase, their expression in heterografts is disrupted, suggesting that WOX4 plays a crucial role in establishing the vascular connection. To test this, the authors obtained a CRISPR-Cas9 mutant of tomato WOX4 and made self- and hetero-grafts with wild-type (WT) plants. While Slwox4 self-grafts failed to form a successful graft junction, heterografts between Slwox4:WT and WT:Slwox4 succeeded in establishing vascular connections, suggesting a requirement for WOX4 in at least one of the graft partners (see Figure). It will be interesting to determine if WOX4 homologs play a key role in successful graft formation in other species.
Figure.
WOX4 is essential for graft compatibility. A–D, Representative images for the self- (A and B) and hetero-grafts (C and D) between the WT and wox4 mutant. E–H, Representative anatomical sections through the graft junction for the graft combinations shown above at 30 DAG showing a discontinuous vascular connection in wox4 self-grafts (F) and a continuous vascular connection in WT self-grafts (A) and WT and wox4 heterografts (C and D). Reprinted from Thomas et al. (2021), Figure 7.
In a nutshell, this study shows that delayed graft incompatibility may be related to distinct GRNs employed by incompatible species in the graft junction connection. As pointed out by the authors, the observation that grafting is not controlled by a genetically conserved regulatory pathway is consistent with the fact that grafting is a human intervention exploiting plants’ innate ability to regenerate after healing. Thus, it follows that certain components related to the wound response and regeneration may be conserved, but regulation of a successful graft junction may be species-specific.
References
- Goldschmidt EE (2014) Plant grafting: new mechanisms, evolutionary implications. Front Plant Sci 5: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez E, Leyva R, Constán-Aguilar C, Romero L, Ruiz JM (2012) Grafting under water stress in tomato cherry: improving the fruit yield and quality. Ann Appl Biol 161: 302–312 [Google Scholar]
- Thomas H, Van den Broeck L, Spurney R, Sozanni R, Frank M (2021) Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 34: 535–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SL, Pegg KG, Cooke AW, Coates LM, Langdon PWB, Dean JR (2001) Rootstock influences postharvest anthracnose development in ‘Hass’ avocado. Aust J Agric Res 52: 1017–1022 [Google Scholar]