Abstract
The ability to sense and respond to physical forces is critical for the proper function of cells, tissues, and organisms across the evolutionary tree. Plants sense gravity, osmotic conditions, pathogen invasion, wind, and the presence of barriers in the soil, and dynamically integrate internal and external stimuli during every stage of growth and development. While the field of plant mechanobiology is growing, much is still poorly understood—including the interplay between mechanical and biochemical information at the single-cell level. In this review, we provide an overview of the mechanical properties of three main components of the plant cell and the mechanoperceptive pathways that link them, with an emphasis on areas of complexity and interaction. We discuss the concept of mechanical homeostasis, or “mechanostasis,” and examine the ways in which cellular structures and pathways serve to maintain it. We argue that viewing mechanics and mechanotransduction as emergent properties of the plant cell can be a useful conceptual framework for synthesizing current knowledge and driving future research.
We review the mechanical properties of the plant cell wall, the plasma membrane, and the vacuole, as well as two classes of mechanosensors that perceive and transduce changes in plant cell mechanics.
Introduction
In its simplest form, a plant cell can be visualized as a water balloon trapped inside a cardboard box (Fester Kratz, 2011). The box represents the cell wall, and the water balloon represents the protoplast. The protoplast is crowded with molecules, ions, macromolecular structures, and organelles. As a result, water readily moves in, causing it to swell and generate turgor pressure—the force that pushes the plasma membrane (PM) against the inner side of the cell wall. As long as the wall is stiff enough to counteract this force, the system remains in equilibrium. If the cell wall is too weak, then it will either stretch to accommodate the contents of the protoplast or break open. While the balloon-in-a-box analogy neatly summarizes the essential players in plant cell mechanics, the reality is much more complicated. In this review, we introduce the mechanical properties of the cell wall, the PM, and the protoplast, and discuss the ways in which the dynamic and inter-connected material properties of each of these components produce and maintain the overall mechanics of the plant cell.
Both exogenous (touch, wounding, dehydration, flooding, pathogen entry, and gravity) and endogenous (growth, cell movement, division, and morphogenesis) sources cause changes to cellular mechanics which must be sensed and dealt with (Monshausen and Haswell, 2013; Haswell and Verslues, 2015; Moulia et al., 2021; Robinson, 2021; Trinh et al., 2021). Here we outline several mechanisms by which the plant cell is thought to sense mechanical signals at (1) the PM, (2) the cell wall, and (3) inside the cell. We also consider how information from these different cellular compartments may be integrated to inform adaptive responses and maintain mechanical homeostasis at the cellular level.
While we focus here on plant cell mechanobiology, it is important to keep in mind that measurements and observations made at the single-cell level would most certainly be altered by cell–cell signaling, close connections via plasmodesmata, and shared cell walls. The mechanics are even more complicated at the tissue and organismal levels, where cell–cell and tissue tissue interactions add additional forces, constraints, and feedback. Several recent reviews address these important and intriguing issues (Sassi and Traas, 2015; Echevin et al., 2019; Moulia et al., 2019; Long et al., 2020; Trinh et al., 2021). Readers interested in the ways in which cell-level events are integrated into supracellular mechanical responses are referred to a recent review (Moulia et al., 2021).
The mechanics of the cell wall, the PM, and the vacuole
Below, we discuss three components of the cell that contribute to cell mechanics: the primary cell wall, the PM, and the vacuole. The mechanical contributions of the cytoskeleton are addressed in several recent reviews (Xiao and Anderson, 2016; Hamant et al., 2019) and are not discussed here.
The cell wall
Cell wall mechanics is a highly active area of study, particularly in relation to growth, anisotropy, and development. Several recent reviews cover our current understanding in more detail than possible here (Braybrook and Jönsson, 2016; Höfte and Voxeur, 2017; Fruleux et al., 2019; Grones et al., 2019). Briefly, the cell wall must be able to (1) counteract the high pressure generated by the protoplast within, yet (2) allow for cell expansion and growth. To do both, it must be simultaneously strong and flexible. In fact, biophysical models of the cell wall have characterized this material as “viscoelastoplastic” (Braybrook and Jönsson, 2016; Fruleux et al., 2019), meaning that it is flexible enough to return to its original shape after a deforming force is removed, unless the force exceeds a yield threshold. When the latter occurs, the “plastic” part of the material description comes into play and the cell wall is permanently stretched to a new shape (Cosgrove, 2018). The importance of tuning the yield threshold of the cell wall is illustrated by the opening and closing of mature guard cells. The guard cell can expand and contract repeatedly without its wall undergoing plastic deformation—at least, at relevant turgor pressures (Rui et al., 2018).
Mechanical differences between cell types and temporal/geometric mechanical tuning within the same cell produce the varied shapes, sizes, and growth patterns of plant cells. For an excellent example of how dynamic changes in cell wall mechanics play out during growth, see recent reviews on pollen tube tip-growth (Cameron and Geitmann, 2018; Cascallares et al., 2020; Ma et al., 2021). As detailed below, the tunable viscoelastoplastic behavior of the plant cell wall is achieved by a heterogeneous mix of materials that are dynamically interconnected and whose material properties can be locally modulated by divalent cations, biosynthesis of new materials, and enzymatic modifications (Anderson and Kieber, 2020). The primary components of the cell wall are cellulose, hemicelluloses, pectins, and callose (Lampugnani et al., 2018).
In its crystalline state, cellulose is the strongest material in the primary cell wall and is organized into microfibrils, which are composed of multiple straight chains of β(1→4) linked D-glucose (Polko and Kieber, 2019). Mechanically speaking, the main role of this material is to resist tensile (stretching) forces. Noncovalent contacts between cellulose microfibrils create a network that holds much of the stress in the wall (Zhang et al., 2021). The mechanical contribution of this network is tuned during synthesis by the orientation of cellulose deposition (Höfte and Voxeur, 2017), rate of synthesis (Kesten et al., 2017, Polko and Kieber, 2019), crystallinity (Li et al., 2014), and possibly the degree of polymerization (Fang et al., 2020). While there is some evidence for postdeposition enzymatic remodeling of the cellulose structure via plant-secreted cellulases (Tsabary et al., 2003; Glass et al., 2015), cellulosic strength is primarily controlled through modification of its interactions with other cell wall components, as explained below.
Unlike the conserved uniformity of cellulose, hemicelluloses are a diverse group of polysaccharides that vary widely in type and abundance across tissues and species, and include heteromannans, heteroxylans, xyloglucans, and mixed linkage glucans (Scheller and Ulvskov, 2010; Pauly et al., 2013). A common structural element amongst these polymers is the presence of a β(1→4) linked backbone, which is thought to form noncovalent interactions with cellulose (Scheller and Ulvskov, 2010). The exact roles and interactions of hemicelluloses, particularly xyloglucans, have been questioned in the last several years as part of an effort to understand cell wall organization. One current hypothesis is that there are distinct areas—termed “biomechanical hotspots”—where the xyloglucan binds tightly to cellulose (Park and Cosgrove, 2012b; Zhao et al., 2014; Nili et al., 2015). This idea is supported by genetic evidence; for example, Arabidopsis plants lacking xyloglucan (xxt1 xxt2) have only mild cell wall disruption but do exhibit defects in the organization of cellulose deposition (Cavalier et al., 2008; Anderson et al., 2010; Park and Cosgrove, 2012a; Xiao et al., 2016). New cell walls developed by xxt1 xxt2 protoplasts showed no effect on cellulose organization, implying that xyloglucan does not play a major role during initial wall formation (Kuki et al., 2020). The interactions between cellulose and hemicellulose may be modulated in multiple ways. Expansin proteins, which can loosen the cell wall and lead to cell expansion (McQueen-Mason et al., 1992), have been hypothesized to alter the bonds between cellulose and hemicellulose (McQueen-Mason and Cosgrove 1995; Ma et al., 2013; Wang et al., 2013). Lastly, enzymes such as xyloglucan endotransglucosylase/hydrolases (Miedes et al., 2013) and xylosidases (Shigeyama et al., 2016) may further influence the mechanical contribution of hemicelluloses, though direct evidence is largely lacking (Stratilová et al., 2020). Future work will refine our understanding of the spatial layout of the cell wall and the role of hemicelluloses. Multidisciplinary approaches, such as computational modeling (e.g. Nili et al., 2015; Zhang et al., 2021), will likely be crucial here.
Pectins comprise a large group of polysaccharides typically with homogalacturonan or rhamnogalacturonan-based backbones that can be decorated with an impressive array of substitutions and sidechains (Harholt et al., 2010; Atmodjo et al., 2013). Mechanically, pectins contribute to the porosity and water content of the cell wall and resist compressive forces (Bidhendi and Geitmann, 2016). A notable material property of pectins is their controllable and reversible gel formation in vitro, which is the result of crosslinking of de-esterified pectin in the presence of divalent calcium (Ca2+; see Peaucelle et al., 2012; Bidhendi and Geitmann, 2016) for recent reviews). The esterification status of pectin has been found to influence overall cell shape (Haas et al., 2020). However, it is worth noting that the ultimate effect of de-esterification on wall mechanics is not always straightforward, as it is determined by the de-esterification patterning and the concentration of cations. Discontinuous pectin de-esterification or low Ca2+ concentrations can result in a weakened wall (Bidhendi and Geitmann, 2016). De-esterification of pectin may also negatively affect expansin-mediated wall loosening (Wang et al., 2020). Moreover, pectin likely does not passively surround the cellulose/hemicellulose network as previously proposed, but instead interacts with them and the other cell wall polymers (Phyo et al., 2017; Rongpipi et al., 2018). The nature of these interactions has recently been investigated in vivo where it was found that reduced pectin synthesis in Arabidopsis quasimodo2 mutants negatively influences cellulose deposition and organization (Du et al., 2020). The effect of the pectin–cellulose network on mechanics has been noted in vitro where it was shown that the addition of a pectin hydrogel after cellulose deposition resulted in a material with higher load-bearing capabilities. A weaker material results if the pectin is present during cellulose deposition (Lopez-Sanchez et al., 2016, 2017).
Callose is a β-1,3-glucan chain with occasional β-1,6 branches (Chen and Kim, 2009). Commonly found in healthy somatic cells, callose is often referred to as a “leak sealant” as it is deposited by cells after injury (Bacete et al., 2018). However, callose deposition is also associated with a variety of other processes including pollen development, germination, and tube growth (Ma et al., 2021), cell plate formation (Drakakaki, 2015), plasmodesma permeability regulation (De Storme and Geelen, 2014), and pathogen response (Wang et al., 2021). An in vitro analysis of callose–cellulose hydrogels suggests that callose adds flexibility to a cell wall, helping to avoid stress-induced fractures (Abou-Saleh et al., 2018). In pollen tubes, callose has been found to increase the load-bearing capability of the wall (Parre and Geitmann, 2005). Further analysis of the mechanical contributions of callose in these settings as well as its in vivo interactions with other cell wall polymers remains to be explored.
In summary, the mechanics of the cell wall change dynamically in time and space and in response to developmental and stress-related cues. Dynamic control is accomplished at least in part by fine-tuning the assembly of and the interactions between a diverse array of structural components including cellulose, hemicellulose, pectin, and callose. Proteins such as arabinogalactan proteins (Silva et al., 2020) are also proposed to contribute to cell wall mechanics (Lamport et al., 2018). Secondary cell wall components also add mechanical complexity. For example, the phenolic compound lignin increases cell wall resistance to deformation (Özparpucu et al., 2017). Because these components do not function independently, but strongly influence one another, it is challenging to understand the mechanical effect of a certain material in vivo through classical genetic approaches. Moreover, cell age, cell type, species, and exposure to abiotic/biotic stresses all affect cell wall mechanics, complicating comparisons between studies and leaving ample room for further inquiry.
The PM
Compared to the cell wall, the PM does not contribute as directly to cell mechanics because it is far more flexible and less resistant to forces. However, it can act as a mediator between the inside and the outside of the cell, and as a signaling platform that senses mechanical cues from either direction (Le Roux et al., 2019; Ackermann and Stanislas, 2020). Both fluid and transient solid behaviors give the cellular membrane its key physical properties, including compression, lateral tension, and curvature (Le Roux et al., 2019)—and these properties are likely to be highly dynamic. For example, the extent to which the PM is compressed between the resistant cell wall and the protoplast pressing outward changes with turgor. Osmotic swelling and shrinking (Le Roux et al., 2019) and modifications to the cell wall (Jaillais and Ott, 2020) are also likely to dynamically impact membrane tension, thickness, and curvature.
Proteins embedded in the PM and the presence of specialized lipids can influence the overall mechanical properties of the cell, sometimes in a highly localized fashion (Gronnier et al., 2018; Le Roux et al., 2019). For example, while it is often assumed that localized stretching can quickly diffuse across the membrane because the lipids in a membrane behave like a fluid, the degree of tension diffusion can vary due to local differences in lipid composition, membrane curvature, or anchoring proteins (Kozlov and Chernomordik, 2015; Cohen and Shi, 2020). Furthermore, the cell wall likely stabilizes proteins in the membrane and minimizes lipid diffusion (Martiniere et al., 2012), perhaps through chemical interactions between extracellular residues of membrane proteins and residues in the wall (Feraru et al., 2011; McKenna et al., 2019; Daněk et al., 2020; Li et al., 2021).
The PM is physically connected both to the cell wall and to the components inside the cell. A direct connection between the PM and cell wall is revealed by Hechtian strands, thin strings of PM that become visible when the cell is plasmolyzed. The exact composition, effects, and function of these attachments remain mysterious, though a recent study found that Hechtian strand removal via laser microdissection leads to increased callose in the wall of that cell (Yoneda et al., 2020). This result points to a potential function of PM–cell wall connections in sensing and responding to cell wall composition. On the cytoplasmic side, the PM engages in dynamic physical connections with the endoplasmic reticulum (ER) at locations called ER–PM contact sites (discussed further below).
The physical integrity of the PM must be tightly controlled (Schapire et al., 2008; Perez-Sancho et al., 2015), as a breach due to osmotic shock, freezing (Yamazaki et al., 2008), drying, or puncture could result in losing cellular contents, exposing organelles to harmful conditions, and a loss of turgor. For example, to protect membranes from tearing due to freezing, lipid composition is modulated, and membrane surface area is controlled (Yamazaki et al., 2008; Takahashi et al., 2016). Control of membrane surface area also occurs in response to changes in turgor, with high turgor inducing exocytosis, and low turgor inducing endocytosis (Zonia and Munnik, 2007; Zwiewka et al., 2015). Changes in turgor are also proposed to affect the deposition of cell wall material (Proseus and Boyer, 2005), and the presence/clustering of membrane proteins like PIN-FORMED1, an auxin efflux carrier (Nakayama et al., 2012; Zwiewka et al., 2015), and aquaporins, which allow for water diffusion through the membrane (Martinière et al., 2019). The relationship between mechanically influenced endo/exocytosis and the resulting modifications in membrane volume or composition—which in turn regulate the mechanics of the cell—is another example of the emergent properties of plant mechanobiology. Future research should also consider how cell type-specific differences in PM properties could affect the mechanical attributes of the PM as well as its interactions with the cell wall and intracellular components.
The vacuole
Turgor pressure is a crucial contributor to the stiffness of the overall plant (Beauzamy et al., 2014). The turgor of a plant cell averages around 0.44 MPa (Beauzamy et al., 2015) and can be as high as 2 MPa (Weber et al., 2015). For reference, a car tire has a hydrostatic pressure of around 0.25 MPa! While all internal components may affect the mechanics of the cell to a degree and at various time scales (Bashline et al., 2014), one organelle with a high degree of influence is the vacuole. The vacuole is the largest organelle in most plant cells and can serve a variety of functions. It is key for turgor regulation, ion storage and homeostasis, and the degradation of cellular components (Tan et al., 2019; Kaiser and Scheuring, 2020). There are two main types: protein storage vacuoles, which are specialized storage for seeds, and lytic vacuoles, which are more generally found across plant cells (Shimada et al., 2018; Cui et al., 2019). Lytic vacuoles hold a solution of proteins, sugars, and metabolites in a compartment separate from the rest of the cell, drawing water in and allowing the cell to control turgor without interfering with cytoplasmic contents or cytoplasmic volume.
The relationship between turgor pressure, vacuoles, and cell expansion is complicated. It is thought that wall loosening is followed by water uptake, resulting in an increase in vacuolar volume during cell growth. Recent evidence suggests that increasing vacuolar volume serves to restrict cytoplasmic volume in an already expanding cell, not to drive that expansion as previously thought (for more details, see Dünser and Kleine-Vehn, 2015; Kaiser and Scheuring, 2020; Cui et al., 2020). The mechanisms by which vacuole expansion and remodeling are integrated with cell wall loosening and stress relaxation during growth are still being studied (Dünser et al., 2019, see below). In guard cells, vacuole expansion and stomata opening require an inward pumping of solutes followed by water entry (Eisenach and De Angeli, 2017). During development, extra vacuolar membrane may be derived from the ER (Viotti et al., 2013) or via fusion between smaller vacuoles (Cui et al., 2019).
While inhibition of vacuole expansion has been shown to correlate with a reduction in cell expansion (Kaiser et al., 2019), a dramatically expanded vacuole is not strictly required for cell growth. Tubulated or fragmented vacuoles are seen across many cell types, including actively dividing cells, growing root cells, guard cells, and tip-growing cells like pollen tubes and root hairs (Cui et al., 2020). How vacuolar tubulation or fragmentation affects the overall mechanical properties of a plant cell is not yet clear, though there is a close association between vacuolar dynamics and guard cell closing (Gao et al., 2005). Future studies should take a closer look at vacuole morphology, what controls it, and how it affects cellular mechanics in both growing and nongrowing cells. As vacuole morphology changes reversibly along with stomatal opening and closing, guard cells may be a good model system for asking these questions in the future (Tanaka et al., 2007; Zheng et al., 2014).
Mechanotransduction and mechanostasis
The process of sensing and responding to mechanical stimuli can be understood as a version of signal transduction, which we term mechanotransduction. According to this paradigm, changes to a cell’s mechanical environment trigger mechanosensors to initiate signaling events that lead to adaptive responses. Such adaptive responses include rapid movements, like the closing of a Venus flytrap (Mano and Hasebe, 2021), or more slowly revealed adaptations like thigmomorphogenesis in response to repeated touch (Braam and Chehab, 2017). At the cellular level, mechanotransduction can induce altered hormone signaling, changes in gene expression (Chehab et al., 2011), defense or stress responses (Coutand, 2020; Ghosh et al., 2021), or a permanent decision such as programmed cell death (Basu and Haswell, 2020).
Another outcome of mechanotransduction is a return to mechanical homeostasis (or, as we term it here, “mechanostasis,” (Chan et al., 2011)). When a cell is in mechanostasis, the strength of the cell wall and the strength of turgor pressure are in balance, and membrane deformations are at basal levels. Figure 1 illustrates the concept of mechanostasis in response to hypo-osmotic cell swelling. The cell might return to its original size through osmotic processes that decrease turgor or through a change in the mechanical properties of the cell, such as strengthening the cell wall. Alternatively, the cell might expand, decreasing turgor by virtue of an increased cell volume. All these responses return the cell to a state where turgor and cell wall strength are in balance. If a mechanosensor fails to sense swelling and one of these downstream outcomes is not activated, the cell can lose integrity and potentially lyse. Below we will examine signaling pathways that respond to changes in membrane tension and to changes in cell wall mechanics that are thought to maintain mechanostasis of the cell.
Figure 1.
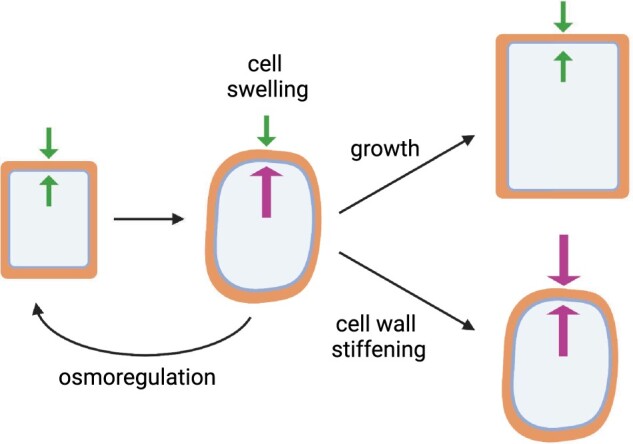
Restoration of cellular mechanostasis after hypo-osmotic swelling. Hypo-osmotic cell swelling is caused by an increase in turgor pressure (inner arrows), which disrupts the homeostatic balance between turgor and the cell wall. The cell could regain balance between turgor and cell wall stiffness by returning to its previous size through osmoregulation, by undergoing expansion, or by stiffening the cell wall to counter the increased turgor.
Mechanical stimuli can be transient (i.e. touch or wind) or continuous (i.e. gravity or turgor), and it remains unknown if sustained stimuli continually activate the same mechanosensors used to sense transient stimuli (Toyota and Gilroy, 2013). In the following section, we review our current understanding of mechanotransduction with a focus on the roles of two types of membrane-embedded molecular mechanosensors: mechanosensitive (MS) ion channels and receptor-like kinases (RLKs). However, we note that mechanosensors come in a range of scales, and that structures like the cytoskeleton, entire cells like trichomes, and even tissues can be MS (see Hamant and Haswell, 2017 for more discussion).
MS ion channels: sensors of membrane mechanics
The PM is a critical platform for the perception of mechanical stimuli in plant cells (Ackermann and Stanislas, 2020). For a plant cell, monitoring the lateral tension in the PM provides one way to perceive osmotic cell swelling (Haswell and Verslues, 2015), cell expansion (Kell and Glaser, 1993), cell wall loosening (Frachisse et al., 2020), vibrations (Ghosh et al., 2016; Tran et al., 2021), plant cell invasion (i.e. by fungal hyphae [Westman et al., 2019], or even dehydration, when the PM pulls away from the cell wall and forms Hechtian strands [Haswell and Verslues, 2015]).
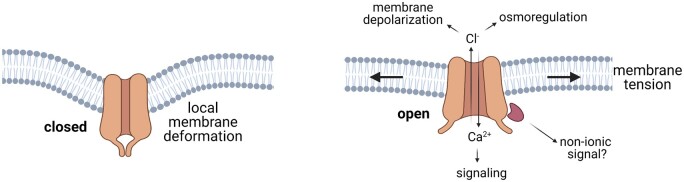
Membrane tension is perceived and signaled through the action of MS ion channels. In response to increased lateral membrane tension, MS ion channels open, conducting ions between cellular compartments or into and out of cells. Most are proposed to open due to their interactions with the lipid bilayer in which they are embedded, according to the general principle of “force-from-lipids” (Cox et al., 2017). Recent structures of plant MS channels (Jojoa-Cruz et al., 2018; Liu et al., 2018; Zhang et al., 2018; Maity et al., 2019; Deng et al., 2020; Li et al., 2020) also support a biophysical model wherein changes in the energetics of lipid deformation drive channel behavior (Wiggins and Phillips, 2005; Ursell et al 2008). Recent cryoEM structures of the open and closed states of Arabidopsis MscS-Like1 (MSL1) provide evidence for a striking molecular mechanism: the channel imposes a dramatic local membrane curvature when closed, and membrane stretch flattens both the membrane and transmembrane domains of the channel, causing it to open (Figure 2; Li et al., 2020; Deng et al., 2020). Similarly, the cryoEM structure of the Arabidopsis REDUCED HYPEROSMOLALITY-INDUCED CA2+ INCREASE1.2 (OSCA1.2) channel revealed local deformation of the membrane at a region of the protein hypothesized to be MS, although other models have been proposed (Jojoa-Cruz et al., 2018; Liu et al., 2018; Zhang et al., 2018). Once opened, MS channel ion conductance could lead to osmoregulation, intracellular Ca2+ signaling, membrane depolarization, and/or changes in extracellular pools of ions (Figure 2). There is further evidence for a nonconducting function that relies on dephosphorylation of MSL10 (Veley et al., 2014; Maksaev et al., 2018), indicating that posttranslational modifications and protein–protein interactions can also contribute to MS channel signal transduction.
Figure 2.
Activation through membrane flattening and potential signaling outputs of MS ion channels. Once opened by membrane tension, MS ion channels mediate ion movement according to their electrochemical gradient. Some MS channels allow Ca2+ to enter the cytoplasm from the apoplast which could serve as a secondary messenger for mechanotransduction signaling cascades. Other MS channels release anions which could theoretically depolarize the membrane and/or function in osmoregulation by reducing cytoplasmic ion concentrations. Nonselective MS channels conduct both cations and anions. Some channels may have nonconducing functions, such as interacting with and activating other signaling partners.
MS channels are found in all domains of life; in plant cells, they localize to the PM as well as organellar membranes and are expressed in a range of cell types and developmental stages (Hamilton et al., 2015b). The classes of MS channels or MS channel homologs encoded in plant genomes include the OSCA, MSL, MID1-COMPLEMENTING ACTIVITY (MCA), TWO-PORE K+ (TPK; Frachisse et al., 2020) and PIEZO families (Zhang et al., 2019b; Radin et al., 2021). There are additional MS activities detected in plant membrane patches, such as the Rapid Mechanically Activated (RMA) current (Guerringue et al., 2018), that has yet to be attributed to any gene (Haswell, 2007).
PM MS channels as cell swelling sensors
While MS ion channels are obvious candidates for cell swelling sensors due to their ability to respond to increased membrane tension, the molecular mechanism by which they do so is not fully understood. One possibility is that MS channels take up a larger area as their pore opens (Phillips et al., 2009), so simply opening could help relieve increased membrane tension caused by cell swelling. Alternatively, MS ion channels could contribute to mechanostasis by directly ameliorating osmotic cell swelling through the release of osmolytes. In fact, this was the first discovered role of MS channels; the release of osmolytes by bacterial MS channels is thought to protect cells from rupturing during hypo-osmotic shock (Levina et al., 1999; Boer et al., 2011; Buda et al., 2016). The pollen-specific nonselective MS ion channel MSL8 likely serves an analogous role during pollen hydration and germination (Hamilton et al., 2015a; Hamilton and Haswell, 2017).
In addition, it has long been hypothesized that MS Ca2+ channel(s) sense cell swelling and activate intracellular responses by mediating cytoplasmic Ca2+ transients (Cazalé et al., 1998; Pauly et al., 2001; Nakagawa et al., 2007; Nguyen et al., 2018). The hypothesis has gained molecular understanding in recent years. The MCA1 channel enhances Ca2+ influx in response to hypo-osmotic but not hyperosmotic shock (Nakagawa et al., 2007; Stephan et al., 2016), and was recently reported to be inherently MS (Yoshimura et al., 2021). The Ca2+-permeable channel responsible for the RMA current, which requires the DEFECTIVE KERNEL 1 protein is stimulated by membrane tension (Tran et al., 2017), and could be activated during cell swelling. Finally, cell swelling could activate an MS channel that does not conduct Ca2+ directly, but that subsequently activates a voltage-gated Ca2+ channel through membrane depolarization (Frachisse et al., 2020), which may be the case for MSL10 (Basu and Haswell, 2020).
MS channels in the response to cell shrinking
OSCA1.1 was discovered in a screen for decreased cytosolic Ca2+ levels in response to hyperosmotic stress (Hou et al., 2014; Yuan et al., 2014), and, along with OSCA3.1 and OSCA1.2, is a PM stretch-activated channel with nonselective cation conductance (Murthy et al., 2018; Zhang et al., 2018). How might OSCAs be activated by hyperosmotic stress, a condition that might be predicted to decrease rather than increase membrane tension? It is possible that membrane tension could be produced locally at Hechtian strands during dehydration, but OSCA1.1 also opens in response to hyperosmolarity when heterologously expressed in mammalian cells (Yuan et al., 2014). This suggests that OSCAs can also respond directly to stimuli other than membrane tension, such as PM hyperpolarization or changes in osmolarity. Thus, how OSCA1.1 mechanosensitivity is related to its ability to promote resistance to hyperosmotic stress remains an exciting topic for future study.
Adaptive responses to MS ion channel activity
Arabidopsis and rice MCA1 and MCA2 (Nakagawa et al., 2007; Kurusu et al., 2012; Mori et al., 2018), and Arabidopsis OSCAs (Yuan et al., 2014; Thor et al., 2020), PIEZO (Mousavi et al., 2021; Radin et al., 2021), and MSL10 (Basu and Haswell, 2020) all promote Ca2+ transients, and MCA1 and MSL10 have been reported to promote reactive oxygen species accumulation (Kurusu et al., 2012; Basu and Haswell, 2020). How these signaling intermediates are connected to downstream outcomes is still unknown. In the long term, some MS channel signaling pathways are expected to result in adaptive responses that lead to a reestablishment of mechanostasis, such as strengthening the cell wall to reinforce against potential future mechanical challenges. In support of this idea, when cell walls are softened pharmacologically with cellulose synthase inhibitors, MCA1 promotes lignification (Denness et al., 2011; Engelsdorf et al., 2018) which could lead to strengthening of the cell wall. Perhaps a lack of lignification and cell wall stiffening is what prevents mca1 roots from penetrating hard agar (Nakagawa et al., 2007; Yamanaka et al., 2010). MCA1 and MSL10 promote the expression of TOUCH-INDUCED (TCH) genes (Nakagawa et al., 2007; Basu and Haswell, 2020). TCH gene induction in response to cell swelling may have adaptive consequences for cell mechanics as TCH4 is a xyloglucan endotransferase/hydrolase (Shinohara et al., 2017), though the magnitude of the effect of xyloglucans on cell wall mechanics is debated (Park and Cosgrove, 2015). In response to cell swelling, changes in cytosolic Ca2+ levels may stimulate exocytosis, which could alleviate membrane stretching (Frachisse et al., 2020) to restore PM mechanostasis.
MS channels promote adaptive responses beyond re-establishing mechanostasis. For example, OSCAs promote survival in hyperosmotic conditions (Yuan et al., 2014; Cao et al., 2020; Zhai et al., 2020) and contribute to stomatal closure during immune signaling (Thor et al., 2020), Piezo contributes to defense against systemic viruses (Zhang et al., 2019b), and MCA1 and MCA2 promote cold tolerance (Mori et al., 2018). MSL10 promotes programmed cell death in response to cell swelling (Basu and Haswell, 2020), which might be adaptive if it allows for the recovery of materials from cells with excessive membrane damage after swelling. Transcripts of MSL10 and OSCA homologs are expressed in the sensory cells of Venus flytrap trigger hairs and tentacles of Cape sundew (another carnivorous plant). An exciting hypothesis is that these MSL10 homologs open when trigger hairs are stimulated, leading to the depolarization of sensory cells, generating an action potential to close the Venus flytrap (Iosip et al., 2020; Procko et al., 2021).
RLKs: candidate sensors of cell wall mechanics
Plant cells must continuously sense and mitigate changes in cell wall mechanics during normal growth and in response to environmental and developmental stresses (Beauzamy et al., 2014; Rui and Dinneny, 2020). Losing the wall’s structural support threatens PM integrity and can cause cell bursting and cell death (Hamann et al., 2009; Feng et al., 2018). But what is the relevant trigger for plant cells to respond to changes in cell wall mechanics? It could be stress—the tension within the wall arising from turgor pressure acting on it. Alternatively, it could be strain, which is the cell wall’s deformation due to that stress (Fruleux et al., 2019), or the displacement of the PM relative to the cell wall (Vaahtera et al., 2019; Bacete and Hamann, 2020).
Another stimulus for cell wall sensors is often referred to as “cell wall integrity.” Cell wall integrity signaling has been studied by genetically or pharmacologically inhibiting the biosynthesis of wall components or by treating plants with cell wall-degrading enzymes. The problem with such manipulations is that they alter both the mechanical properties of the cell wall (like stress and strain) and the chemical composition of the cell wall, and “cell wall integrity sensors” could be responding to either stimulus. Even if the stimulus is mechanical, cell wall integrity is itself a challenging concept. What is being perceived—just a weakening of the cell wall, or complete cell wall rupture? The ultimate outcome of defective cell wall integrity pathways is often cell bursting (Boisson-Dernier et al., 2009; Ge et al., 2017; Feng et al., 2018), but it is not clear what the normal trigger for such pathways is. Nor are the adaptive responses that these pathways lead to fully understood. It is believed that they protect cell integrity through cell wall strengthening, and this has been proposed to take place via a range of mechanisms: deposition of general cell wall materials (Boisson-Dernier et al., 2013), modification of pectin crosslinking (Feng et al., 2018), ectopic lignification (Hamann et al., 2009) or callose deposition (Mecchia et al., 2017), but direct evidence that these modifications are responsible for cell wall stiffening in these contexts is lacking.
For sensors that do indeed respond to cell wall mechanical properties, “cell wall mechanosensor” might be a more appropriate term. To establish that a protein is a cell wall mechanosensor, researchers should first test if it is required for responses to exogenous mechanical manipulations like compression or indentation, which in the short term should not modify the chemical components of the cell wall. How to obtain direct evidence of a cell wall sensor’s mechanosensitivity is a future question for the field and may in the end require studies in a heterologous or in vitro system, similar to what is used to establish the mechanosensitivity of ion channels. We focus below on proteins that appear to be direct cell wall mechanosensors, while acknowledging that the situation is complicated by the fact that they also bind cell wall epitopes and/or peptides.
One important class of candidate cell wall mechanosensors are RLKs (Galindo-Trigo et al., 2016; Nissen et al., 2016; Doblas et al., 2018; Gigli-Bisceglia et al., 2020). RLKs have an intracellular kinase domain and diverse extracellular domains separated by a single transmembrane domain. There are hundreds of RLKs in plant genomes with a multitude of functions (Dievart et al., 2020). Several from the Catharanthus roseus RLK1-Like (CrRLK1L) family have been implicated in cell wall mechanosensing; THESEUS1 (THE1) does so in roots and cotyledons (Hamann et al., 2009; Engelsdorf et al., 2018), FERONIA (FER) in roots (Shih et al., 2014; Engelsdorf et al., 2018; Feng et al., 2018), and Buddha’s Paper Seal (BUPS)1 in pollen tubes (Zhou et al., 2021). Other CrRLK1Ls (Galindo-Trigo et al., 2016) and members of other RLK families (Xu et al., 2008; Wolf et al., 2014; Van der Does et al., 2017) participate in related processes like cell expansion, tip growth, and cell wall modification. These processes involve many changes in cell biomechanics, and it is possible that some of these other RLKs are mechanosensory although that has yet to be demonstrated.
FER, THE1, and BUPS1 as cell wall–PM proximity sensors
CrRLK1L signal transduction has primarily been studied upon activation with extracellular peptide ligands called rapid alkalinization factors (RALFs). However, RALFs are not required for the FER- and THE1-dependent response to cell wall damage in roots (Feng et al., 2018; Gonneau et al., 2018). Instead, the extracellular domain of FER can bind pectin in vitro (Feng et al., 2018; Lin et al., 2018), theoretically allowing FER and other CrRLK1Ls to report cell wall deformation to the cytoplasm. FER is required for cytosolic Ca2+ transients and apoplastic alkalinization when roots are perturbed by bending, touched with a glass micropipette, or subjected to hypo-osmotic shock (Shih et al., 2014), and for cell integrity and Ca2+ transients in response to salinity stress. The latter causes a decrease in the stiffness of epidermal cell walls in the elongation zone of Arabidopsis roots due to impaired pectin crosslinking (Feng et al., 2018). BUPS1 promotes pollen tube survival upon exit from the constrictive style tissue into the more open transmitting tract (as well as thin, compressive microchannels that widen), perhaps by promoting a stiffening of the cell wall (Zhou et al., 2021).
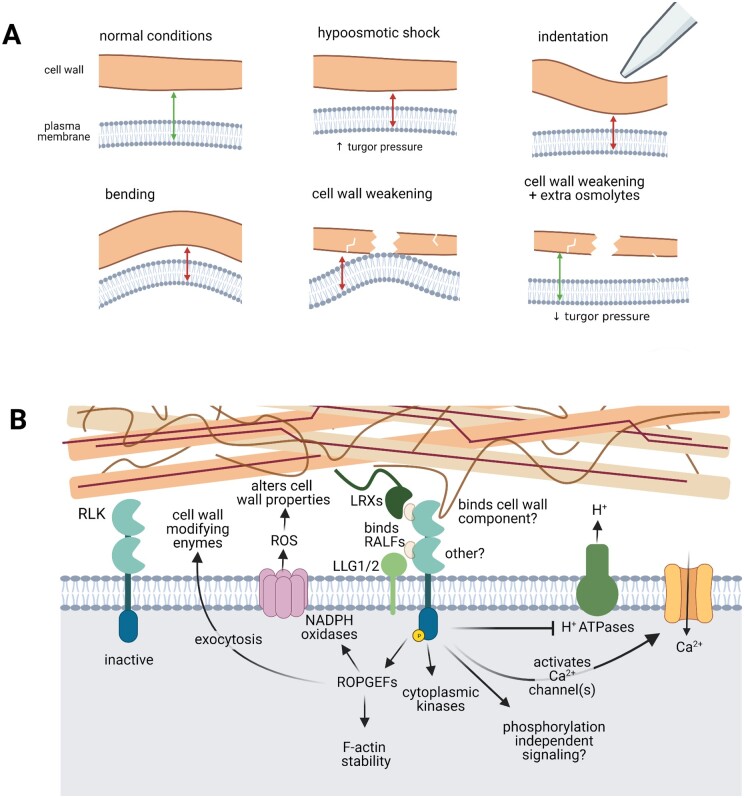
THE1 is required for the deposition of lignin, production of ROS, and jasmonic acid and salicylic acid accumulation in response to cellulose deficiency throughout the plant, whether induced genetically (Hématy et al., 2007; Merz et al., 2017) or pharmacologically (Denness et al., 2011; Merz et al., 2017; Engelsdorf et al., 2018). These responses can be suppressed by lowering turgor pressure using high osmolarity media (Hamann et al., 2009; Engelsdorf et al., 2018), which has led to speculation that THE1 senses the displacement of the PM toward the cell wall in response to local cell wall softening (Figure 3A; Vaahtera et al., 2019; Bacete and Hamann, 2020). It is also possible that other perturbations that activate FER—hypo-osmotic shock, bending, and indentation—also lead to PM displacement, but more work is required to determine the specific stimulus or stimuli in each of these cases. In the case of BUPS1, perhaps there is also a transient decrease in the distance between the PM and cell wall as pollen tubes with soft apical cell walls emerge into the transmitting tract and lose the compressive forces imposed by style cells (Zhou et al., 2021).
Adaptive responses to CrRLK1L activity
Binding to RALFs triggers CrRLK1L autophosphorylation (Haruta et al., 2014) and transphosphorylation by cytoplasmic kinases (Du et al., 2016). Hallmarks of RALF/CrRLK1L signaling in roots are alkalinization of the apoplast, cytoplasmic Ca2+ fluxes, and ROS accumulation (Boisson-Dernier et al., 2013; Haruta et al., 2014; Gonneau et al., 2018; Figure 3B). These signal transduction events are likely triggered by the CrRLK1L phosphorelay, although the kinase activity of CrRLK1s is not always required (Shih et al., 2014; Haruta et al., 2018; Gronnier et al., 2020). FER promotes the phosphorylation of H+-ATPase 2, which is thought to inhibit H+-ATPase activity and lead to alkalinization of the apoplast (Haruta et al., 2014). Additionally, activated FER signals through plant Rho-GTPases (RAC/ROPs) and activates NADPH oxidase-dependent ROS accumulation (Duan et al., 2010, 2014) and positively regulates the stability of the F-actin cytoskeleton (Dong et al., 2019). BUPS1 activates ROP1, whose activation is correlated with pectin de-methylesterification and wall thickening, likely stiffening the cell wall of pollen tubes (Zhou et al., 2021) by increasing rates of pectin methylesterase exocytosis (Luo et al., 2017). The Ca2+ channel directly or indirectly activated by CrRLK1Ls remains unknown. FER is also thought to serve as a scaffold for immune receptors, with binding to RALFs inhibiting its scaffolding function (Stegmann et al., 2017).
Figure 3.
Possible activators and signaling outputs of RLKs. A, Expected changes in cell wall–PM proximity in response to mechanical signals. Some RLKs are required for responses to hypo-osmotic shock, indentation, and bending (FER) and to cell wall weakening (FER and THE1). They might respond to the altered distance between the PM and certain cell wall components during these manipulations, and/or the compression of the PM against the cell wall. Lowering turgor pressure using osmolytes, which would be predicted to relieve the PM compression or displacement that occurs when cell walls are impaired, suppresses RLK signaling. B, PM-localized CrRLK1Ls are hypothesized to be activated by cell wall mechanics through their interaction with cell wall components, although this is less well-understood than their activation by RALF peptide ligands. RALFs mediate the interaction of CrRLK1Ls with LORELEI-like glycophosphatidylinositol-anchored protein co-receptors (LLGs) and Leucine-Rich-Repeat Extensins (LRXs) (Mecchia et al., 2017; Xiao et al., 2019). Activated CrRLK1Ls promote apoplastic alkalinization (perhaps by inhibiting the activity of H+-ATPases), the opening of unknown Ca2+ channel(s), cytoplasmic kinase cascades, and ROPGEF signaling, which activates ROS production through NADPH oxidases and is thought to promote exocytosis of cell wall components (Zhou et al., 2021). CrRLK1L kinase activity is not required for all signaling outputs and may depend on the stimulus. Components are not drawn to scale.
Impairing cell wall integrity in seedlings leads to growth arrest, presumably to prevent cell expansion until the cell wall can be reinforced through cell wall modifications (Cano-Delgado et al., 2003; Hématy et al., 2007; Hamann et al., 2009; Tsang et al., 2011; Engelsdorf et al., 2018). Put another way, growth arrest may allow cells with softened cell walls to return to mechanostasis without the confounding mechanical challenges of normal growth (Feng et al., 2018). Even under standard conditions, fer roots grow aberrantly, sometimes faster and sometimes slower than wild-type roots, and experience greater fluctuations in strain in the elongation zone. This aberrant growth pattern suggests that fer roots have impaired mechanosensory feedback, consistent with the role of FER as a mechanosensor (Shih et al., 2014). In contrast, THE1 seems to require cell wall damage for activation (Hématy et al., 2007; Merz et al., 2017; Vaahtera et al., 2019). Perhaps, CrRLK1Ls have different thresholds of activation, which would allow plants to fine tune their responses to different degrees of cell wall impairment, allowing plant cells to differentiate large, short-term increases in cell wall stress/strain and the smaller increases associated with long-term growth processes.
Interactions between MS channel and RLK signaling
The mechanosensing pathways of the cell wall and the PM may be integrated beyond the fact that both compartments are inextricably linked during mechanical manipulations (Figure 3A). For example, RLKs may modify the activity of MS channels. MCA1 is genetically downstream of THE1 in promoting lignification and hormone accumulation in response to isoxaben treatment, which inhibits cellulose biosynthesis (Engelsdorf et al., 2018). When WAK1, a member of the WALL-ASSOCIATED KINASE RLK family, binds oligogalacturonides, MSL6 is phosphorylated (Kohorn et al., 2016). Conversely, future studies may reveal that MS ion channel conductance can alter cell wall mechanics, and therefore RLK activity, by delivering or removing ions that affect polymer crosslinking, enzyme activity, or electrostatic interactions between different cell wall components. Additionally, ions present or complexed in the cell wall could influence ion flux through MS channels (Volkov, 2015). For all the reasons listed above, it is difficult, or even impossible to untangle signaling pathways that are begun by mechanical perturbations to the PM or to the cell wall. For the plant cell, it may not be critical to differentiate them.
Subcellular mechanotransduction
Additional mechanosensory components in the cell wall–PM continuum almost certainly await discovery. However, the search for mechanosensors should not be focused solely on the cell periphery. As discussed below, our current understanding of mechanotransduction in multiple subcellular compartments illustrates the need for an integrated vision of plant cell mechanobiology at the cellular scale.
Vacuolar mechanosensors
There has long been evidence for vacuolar mechanosensing; excised patches of vacuolar membrane, or tonoplast, from red beet and onion cells display MS currents (Alexandre and Lassalles, 1991; Badot et al., 1992). The tonoplast TPK1 channel protects isolated vacuoles from lysis during hypo-osmotic shock (Maathuis, 2011). Modeling of water flux through vacuolar aquaporins suggests that they are mechanosensitive and predicts that they close when membrane tension is high (Leitao et al., 2014; Goldman et al., 2017), providing protection to vacuolar integrity during hypo-osmotic shock by limiting further water influx and helping the vacuole maintain turgor. Plant PIEZOs localize to the tonoplast where they promote vacuole tubulation, tonoplast internalization, and/or fission in tip-growing moss protonema and Arabidopsis pollen tubes (Radin et al., 2021). Cell wall damage transcriptionally downregulates aquaporin expression (Hamann et al., 2009), and through FER, triggers vacuolar fragmentation (Dünser et al., 2019). Root growth through stiff media also promotes vacuolar fragmentation (Dünser et al., 2019), indicating that vacuolar reorganization may be a general response to more than one mechanotransduction pathway.
Plastid mechanosensors
Two MS ion channel homologs found in the plastid envelope, MSL2 and MSL3, are implicated in plasmid osmoregulation (Haswell and Meyerowitz, 2006). The abnormal swelling of nongreen plastids in msl2 msl3 mutant leaf and root epidermis can be rescued by growth on media supplemented with osmolytes that presumably enter the cytoplasm and reduce the hypo-osmolarity of the plastid stroma (Veley et al., 2012). The same phenomenon is observed in mutants lacking functional members of the K+ EXCHANGE ANTIPORTER family (Kunz et al., 2014). Downstream effects of MSL2 and MSL3 deficiency include callus formation and superoxide accumulation in the shoot apical meristem, and these phenotypes can likewise be partially suppressed by growing seedlings on media with osmotic support (Wilson et al., 2016). Interestingly, plastid mechanosensation is involved in downstream responses to cell wall–PM disruption, as MSL2 and MSL3 contribute to the accumulation of JA after isoxaben treatment (Engelsdorf et al., 2018). Furthermore, MSL2 is required for the systemic spread of H2O2 after high light treatment (Fichman et al., 2021). Perhaps, MSL2 plays a similar role in chloroplasts as MSL1 in mitochondria, which contributes to redox homeostasis and the dissipation of excess membrane potential, and may be activated by ROS (Lee et al., 2016). We are only just beginning to understand organellar osmoregulation and mechanobiology, and they are topics that warrant future study.
Mechanotransduction in the ER and plasmodesmata
Evidence is growing that the ER plays a role in mechanotransduction in plants. Contact sites between the PM and the ER are places of connection to the actin and microtubule cytoskeletons (Wang et al., 2014, 2016), and may serve as important signaling hubs wherein mechanical information from the cell wall–PM–cytoskeleton continuum is conveyed to the ER. SYNAPTOTAGMIN 1 (SYT1), an integral ER membrane protein enriched at ER–PM contact sites, helps maintain PM integrity in response to salinity stress as well as mechanical pressure (Schapire et al., 2008; Perez-Sancho et al., 2015). SYT1 and other ER–PM contact site proteins, such as VAP27s, preferentially localize to Hechtian strands (Wang et al., 2016; Lee et al., 2020). Plasmodesmata contain tight connections between the ER and PM (Tilsner et al., 2016) and close in response to mechanical perturbation (Jaffe et al., 1985) and to differences in turgor between neighboring cells (Oparka and Prior, 1992). Modeling suggests that the plasmodesmal structure itself is inherently mechanosensitive, as the tethered desmotubules could move due to pressure differentials between cells and occlude the pores (Park et al., 2019).
Nuclear mechanotransduction
The biomechanics of and mechanotransduction in the nucleus, while well-studied in animals (Janota et al., 2020), is barely studied in plants (Goswami et al., 2020a). Hyperosmotic stress leads to a stiffening of the nucleus in root cells accompanied by a decrease in nuclear size and circularity. This shrinking and stiffening may result from chromatin condensation (Goswami et al., 2020b) as it does in animals (Irianto et al., 2013). During development, nuclei at the shoot apical meristem–organ boundary are compressed due to tissue folding and display changes in chromatin architecture and linker histone expression compared to neighboring, noncompressed cells (Fal et al., 2021). Future biomechanical studies of the plant nucleus should address whether cell wall-derived mechanical changes can be conveyed to the nucleus, and what, if any, role nuclear shape has on gene expression changes that contribute to reestablishing nuclear and cellular mechanostasis. The nuclear Ca2+ transients long associated with mechanical signaling (van Der Luit et al., 1999; Pauly et al., 2001), may derive from as-yet-unidentified MS Ca2+ channels in the nuclear membrane (Itano et al., 2003; Xiong et al., 2004; Enyedi and Niethammer, 2016).
Summary and perspective
Here we have described our current understanding of the material properties of three key components of the plant cell: the PM, the cell wall, and the protoplast. Their mechanics are interdependent, connected through direct and indirect physical connections and through mechanotransduction pathways. As a result, physical changes to one component often dynamically lead to the alteration of other components through osmoregulation, cell wall modifications, or membrane trafficking. We have outlined two major PM-based mechanotransduction systems (based on MS ion channels and RLKs as mechanosensors) and several known or suspected intracellular mechanisms for conveying mechanical information, including the vacuole, plastids, the ER, plasmodesmata, and the nucleus. We have highlighted multiple interactions between these various pathways, such as synergy between MS channels and RLKs, and between RLKs and vacuoles.
We also introduce the concept of mechanostasis, wherein the strength of the cell wall and the strength of turgor pressure are in balance. We argue that many mechanotransduction processes serve as feedback loops designed to bring the cell back into a state of mechanostasis. Considering its location between the protoplast and the cell wall, the PM might be considered the de facto organizing center for mechanostasis; yet the vacuole and other intracellular organelles are likely also involved.
Thus, in many ways, plant cell mechanics can be viewed as an emergent property of all the components, rather than individual contributions that are simply summed together. Even when considering an individual cellular component, there is a multitude of factors that influence mechanics and mechanotransduction—such as the interactions between different cell wall polymers, whose properties are constantly being tuned in space and in time. In some cases, this interconnectedness is an experimental asset, for instance being able to suppress cell wall integrity defects with osmotic support (Hamann et al., 2009; Engelsdorf et al., 2018). However, the interdependence of the mechanical properties of cell components can also be experimentally challenging. While one can measure the stiffness of a plant cell or tissue using atomic force microscopy, it can be difficult to separate the effects of turgor and of cell wall properties (Braybrook, 2015). Future plant cell mechanobiology research will reveal how these many components and mechanotransduction pathways work together dynamically to create a holistic, homeostatic entity capable of maintaining mechanostasis or undergoing adaptive changes, as appropriate.
Acknowledgments
We are grateful to the members of the Haswell Lab, Thorsten Hamman, Charlie Anderson, Siobhan Braybrook, and Marcela Rojas-Pierce for insightful comments on early drafts of this manuscript. Figures were created using BioRender.com.
Funding
Our research is currently supported by National Science Foundation Graduate Research Fellowship DGE-1745038 to J.M.C. and K.M.; a Howard Hughes Medical Institute-Simons Foundation Faculty Scholar grant and National Science Foundation MCB1929355 to E.S.H., and the National Science Foundation Center for Engineering Mechanobiology CMMI1548571.
Conflict of interest statement. The authors declare no conflicts of interest.
Contributor Information
Jennette M Codjoe, Department of Biology and Center for Engineering Mechanobiology, Washington University in St Louis, St Louis, Missouri, 63130, USA.
Kari Miller, Department of Biology and Center for Engineering Mechanobiology, Washington University in St Louis, St Louis, Missouri, 63130, USA.
Elizabeth S Haswell, Department of Biology and Center for Engineering Mechanobiology, Washington University in St Louis, St Louis, Missouri, 63130, USA.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Elizabeth S. Haswell (ehaswell@wustl.edu).
References
- Abou-Saleh RH, Hernandez-Gomez MC, Amsbury S, Paniagua C, Bourdon M, Miyashima S, Helariutta Y, Fuller M, Budtova T, Connell SD, et al. (2018) Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures. Nat Commun 9: 4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann F, Stanislas T (2020) The plasma membrane—an integrating compartment for mechano-signaling. Plants 9: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre J, Lassalles JP (1991) Hydrostatic and osmotic pressure activated channel in plant vacuole. Biophys J 60: 1326–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Carroll A, Akhmetova L, Somerville C (2010) Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 152: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Kieber JJ (2020) Dynamic construction, perception, and remodeling of plant cell walls. Annu Rev Plant Biol 71: 39–69 [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 [DOI] [PubMed] [Google Scholar]
- Bacete L, Hamann T (2020) The role of mechanoperception in plant cell wall integrity maintenance. Plants 9: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacete L, Mélida H, Miedes E, Molina A (2018) Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J 93: 614–636 [DOI] [PubMed] [Google Scholar]
- Badot PM, Ding JP, Pickard BG (1992) Mechanically activated ion channels occur in vacuoles of onion bulb scale parenchyma. C R Acad Sci Paris 315: 437–443 [Google Scholar]
- Bashline L, Lei L, Li S, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7: 586–600 [DOI] [PubMed] [Google Scholar]
- Basu D, Haswell ES (2020) The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr Biol 30: 2716–2728.e6 [DOI] [PubMed] [Google Scholar]
- Beauzamy L, Louveaux M, Hamant O, Boudaoud A (2015) Mechanically, the shoot apical meristem of Arabidopsis behaves like a shell Inflated by a pressure of About 1 MPa. Front Plant Sci 6: 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauzamy L, Nakayama N, Boudaoud A (2014) Flowers under pressure: ins and outs of turgor regulation in development. Ann Bot 114: 1517–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidhendi AJ, Geitmann A (2016) Relating the mechanics of the primary plant cell wall to morphogenesis. J Exp Bot 67: 449–461 [DOI] [PubMed] [Google Scholar]
- Boer M, Anishkin A, Sukharev S (2011) Adaptive MscS gating in the osmotic permeability response in E. coli : the question of time. Biochemistry 50: 4087–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanaraja S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, Grossniklaus U (2009) Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136: 3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J, Chehab EW (2017) Thigmomorphogenesis. Curr Biol 27: R863–R864 [DOI] [PubMed] [Google Scholar]
- Braybrook SA (2015) Measuring the elasticity of plant cells with atomic force microscopy. In Pauluch EK, ed, Biophysical Methods in Cell Biology, Methods in Cell Biology. Academic Press, Cambridge, MA, pp 237–254 [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Jönsson H (2016) Shifting foundations: the mechanical cell wall and development. Curr Opin Plant Biol 29: 115–120 [DOI] [PubMed] [Google Scholar]
- Buda R, Liu Y, Yang J, Hegde S, Stevenson K, Bai F, Pilizota T (2016) Dynamics of Escherichia coli ’s passive response to a sudden decrease in external osmolarity. Proc Natl Acad Sci USA 113: 5838–5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C, Geitmann A (2018) Cell mechanics of pollen tube growth. Curr Opin Genet Dev 51: 11–17 [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34: 351–362 [DOI] [PubMed] [Google Scholar]
- Cao L, Zhang P, Lu X, Wang G, Wang Z, Zhang Q, Zhang X, Wei X, Mei F, Wei L, et al. (2020) Systematic analysis of the maize OSCA genes revealing ZmOSCA family members involved in osmotic stress and ZmOSCA2.4 confers enhanced drought tolerance in transgenic Arabidopsis. Int J Mol Sci 21: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascallares M, Setzes N, Marchetti F, López GA, Distéfano AM, Cainzos M, Zabaleta E, Pagnussat GC (2020) A complex journey: cell wall remodeling, interactions, and integrity during pollen tube growth. Front Plant Sci 11: 1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al. (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalé AC, Rouet-Mayer MA, Barbier-Brygoo H, Mathieu Y, Laurière C (1998) Oxidative burst and hypoosmotic stress in tobacco cell suspensions. Plant Physiol 116: 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DD, Van Dyke WS, Bahls M, Connell SD, Crister P, Kelleher JE, Kramer MA, Pearce SM, Sharma S, Neu CP (2011) Mechanostasis in apoptosis and medicine. Prog Biophys Mol Biol 106: 517–524 [DOI] [PubMed] [Google Scholar]
- Chehab EW, Wang Y, Braam J (2011) Mechanical force responses of plant cells and plants. In Wojtaszek P, ed, Mechanical Integration of Plant Cells and Plants, Signaling and Communication in Plants. Springer, Berlin, Heidelberg, pp 173–194 [Google Scholar]
- Chen XY, Kim JY (2009) Callose synthesis in higher plants. Plant Signal Behav 4: 489–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AE, Shi Z (2020) Do cell membranes flow like honey or jiggle Like jello? BioEssays 42: 1900142. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2018) Diffuse growth of plant cell walls. Plant Physiol 176: 16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutand C (2020) The effect of mechanical stress on plant susceptibility to pests: a mini opinion review. Plants 9: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B (2017) Origin of the force: the force-from-lipids principle applied to Piezo channels. In Gottlieb PA, ed, Current Topics in Membranes, Piezo Channels. Academic Press, Cambridge, MA, pp 59–96 [DOI] [PubMed] [Google Scholar]
- Cui Y, Cao W, He Y, Zhao Q, Wakazaki M, Zhuang X, Gao J, Zeng Y, Gao C, Ding Y, et al. (2019) A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat Plants 5: 95–105 [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhao Q, Hu S, Jiang L (2020) Vacuole biogenesis in plants: how many vacuoles, how many models? Trends Plant Sci 25: 538–548 [DOI] [PubMed] [Google Scholar]
- Daněk M, Angelini J, Malínská K, Andrejch J, Amlerová Z, Kocourková D, Brouzdová J, Valentová O, Martinec J, Petrášek J (2020) Cell wall contributes to the stability of plasma membrane nanodomain organization of Arabidopsis thaliana FLOTILLIN2 and HYPERSENSITIVE INDUCED REACTION1 proteins. Plant J 101: 619–636 [DOI] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2014) Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Front Plant Sci 5: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Maksaev G, Schlegel AM, Zhang J, Rau M, Fitzpatrick JAJ, Haswell ES, Yuan P (2020) Structural mechanism for gating of a eukaryotic mechanosensitive hannel of small conductance. Nat Commun 11: 3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156: 1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dievart A, Gottin C, Perin C, Ranwez V, Chantret N (2020) Origin and diversity of plant receptor-like kinases. Annual Rev Plant Biol 71: 131–156 [DOI] [PubMed] [Google Scholar]
- Doblas VG, Gonneau M, Höfte H (2018) Cell wall integrity signaling in plants: malectin-domain kinases and lessons from other kingdoms. Cell Surf 3: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Zhang Z, Liu Y, Tao LZ, Liu H (2019) FERONIA regulates auxin-mediated lateral root development and primary root gravitropism. FEBS Lett 593: 97–106 [DOI] [PubMed] [Google Scholar]
- Drakakaki G (2015) Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci 236: 177–184 [DOI] [PubMed] [Google Scholar]
- Du C, Li X, Chen J, Chen W, Li B, Li C, Wang L, Li J, Zhao X, Lin J, et al. (2016) Receptor kinase complex transmits RALF peptide signal to inhibit root growth in Arabidopsis. Proc Natl Acad Sci 113: 8326–8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Kirui A, Huang S, Wang L, Barnes WJ, Kiemle SN, Zheng Y, Rui Y, Ruan M, Qi S, et al. (2020) Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell 32: 3576–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünser K, Gupta S, Herger A, Feraru MI, Ringli C, Kleine-Vehn J (2019) Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J 38: e100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünser K, Kleine-Vehn J (2015) Differential growth regulation in plants — the acid growth balloon theory. Curr Opin Plant Biol 28: 55–59 [DOI] [PubMed] [Google Scholar]
- Echevin E, Le Gloanec C, Skowrońska N, Routier-Kierzkowska AL, Burian A, Kierzkowski D (2019) Growth and biomechanics of shoot organs. J Exp Bot 14: 3573–3585 [DOI] [PubMed] [Google Scholar]
- Eisenach C, De Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsdorf T, Gigli-Bisceglia N, Veerabagu M, McKenna JF, Vaahtera L, Augstein F, Van der Does D, Zipfel C, Hamann T (2018) The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci Signal 11: 1–15 [DOI] [PubMed] [Google Scholar]
- Enyedi B, Niethammer P (2016) A case for the nuclear membrane as a mechanotransducer. Cell Mol Bioeng 9: 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fal K, Korsbo N, Alonso-Serra J, Teles J, Liu M, Refahi Y, Chabouté ME, Jönsson H, Hamant O (2021) Tissue folding at the organ–meristem boundary results in nuclear compression and chromatin compaction. Proc Natl Acad Sci 118: e20178591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Li B, Liu Y, Zhu J, Li G, Hou G, Zhou J, Qiu X (2020) Critical role of degree of polymerization of cellulose in super-strong nanocellulose films. Matter 2: 1000–1014 [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu MC, Maman J, Steinhorst L, Schmitz-Thom I, et al. (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ Signaling. Curr Biol 28: 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Kleine-Vehn J, Martinière A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J (2011) PIN polarity maintenance by the cell wall in Arabidopsis. Curr Biol 21: 338–343 [DOI] [PubMed] [Google Scholar]
- Fester Kratz R (2011) Botany for Dummies, 1st ed. John Wiley & Sons Inc., Hoboken, NJ. [Google Scholar]
- Fichman Y, Myers RJ, Grant DG, Mittler R (2021) Plasmodesmata-localized proteins and ROS orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci Signal 14: eabf0322. [DOI] [PubMed] [Google Scholar]
- Frachisse JM, Thomine S, Allain JM (2020) Calcium and plasma membrane force-gated ion channels behind development. Curr Opin Plant Biol 53: 57–64 [DOI] [PubMed] [Google Scholar]
- Fruleux A, Verger S, Boudaoud A (2019) Feeling stressed or strained? A biophysical model for cell wall mechanosensing in plants. Front Plant Sci 10: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Trigo S, Gray JE, Smith LM (2016) Conserved roles of CrRLK1L receptor-like kinases in cell expansion and reproduction from algae to angiosperms. Front Plant Sci 7: 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC (2005) The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba. Plant Physiol 139: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Luo X, Ruan H, García-Valencia LE, Zhong S, et al. (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358: 1596–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Barbacci A, Leblanc-Fournier N (2021) Mechanostimulation: a promising alternative for sustainable agriculture practices. J Exp Bot 72: 2877–2888 [DOI] [PubMed] [Google Scholar]
- Ghosh R, Mishra RC, Choi B, Kwon YS, Bae DW, Park SC, Jeong MJ, Bae H (2016) Exposure to sound vibrations lead to transcriptomic, proteomic and hormonal changes in Arabidopsis . Sci Rep 6: 33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli-Bisceglia N, Engelsdorf T, Hamann T (2020) Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell Mol Life Sci 77: 2049–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Barkwill S, Unda F, Mansfield SD (2015) Endo-β-1,4-glucanases impact plant cell wall development by influencing cellulose crystallization: endoglucanses alter cell wall formation. J Integr Plant Biol 57: 396–410 [DOI] [PubMed] [Google Scholar]
- Goldman RP, Jozefkowicz C, Canessa Fortuna A, Sutka M, Alleva K, Ozu M (2017) Tonoplast (BvTIP1;2) and plasma membrane (BvPIP2;1) aquaporins show different mechanosensitive properties. FEBS Lett 591: 1555–1565 [DOI] [PubMed] [Google Scholar]
- Gonneau M, Desprez T, Martin M, Doblas VG, Bacete L, Miart F, Sormani R, Hématy K, Renou J, Landrein B, et al. (2018) Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis. Curr Biol 28: 2452–2458 [DOI] [PubMed] [Google Scholar]
- Goswami R, Asnacios A, Hamant O, Chabouté ME (2020a) Is the plant nucleus a mechanical rheostat? Curr Opin Plant Biol 57: 155–163 [DOI] [PubMed] [Google Scholar]
- Goswami R, Asnacios A, Milani P, Graindorge S, Houlné G, Mutterer J, Hamant O, Chabouté ME (2020b) Mechanical shielding in plant nuclei. Curr Biol 30: 1–17 [DOI] [PubMed] [Google Scholar]
- Grones P, Raggi S, Robert S (2019) FORCE-ing the shape. Curr Opin Plant Biol 52: 1–6 [DOI] [PubMed] [Google Scholar]
- , Gronnier J, Franck CM, Stegmann M, DeFalco TA, Cifuentes AA, Dünser K, Lin W, Yang Z, Kleine-Vehn J, Ringli C, et al. (2020) FERONIA regulates FLS2 plasma membrane nanoscale dynamics to modulate plant immune signaling. bioRxiv: 2020.07.20.212233 (July 22, 2020)
- Gronnier J, Gerbeau-Pissot P, Germain V, Mongrand S, Simon-Plas F (2018) Divide and rule: plant plasma membrane organization. Trends Plant Sci 23: 899–917 [DOI] [PubMed] [Google Scholar]
- Guerringue Y, Thomine S, Frachisse JM (2018) Sensing and transducing forces in plants with MSL10 and DEK1 mechanosensors. FEBS Lett 592: 1968–1979 [DOI] [PubMed] [Google Scholar]
- Haas KT, Wightman R, Meyerowitz EM, Peaucelle A (2020) Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 367: 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Bennett M, Mansfield J, Somerville C (2009) Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J 57: 1015–1026 [DOI] [PubMed] [Google Scholar]
- Hamant O, Haswell ES (2017) Life behind the wall: sensing mechanical cues in plants. BMC Biol 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant O, Inoue D, Bouchez D, Dumais J, Mjolsness E (2019) Are microtubules tension sensors? Nat Commun 10: 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ES, Haswell ES (2017) The tension-sensitive ion transport activity of MSL8 is critical for its function in pollen hydration and germination. Plant Cell Physiol 58: 1222–1237 [DOI] [PubMed] [Google Scholar]
- Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES (2015a) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350: 438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ES, Schlegel AM, Haswell ES (2015b) United in diversity: mechanosensitive ion channels in plants. Annu Rev Plant Biol 66: 113–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J, Suttangkakul A, Vibe Scheller H (2010) Biosynthesis of pectin. Plant Physiol 153: 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gaddameedi V, Burch H, Fernandez D, Sussman MR (2018) Comparison of the effects of a kinase-dead mutation of FERONIA on ovule fertilization and root growth of Arabidopsis. FEBS Lett 592: 2395–2402 [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell ES (2007) MscS‐Like proteins in plants. Mechanosensitive Ion Channels, Part A. Elsevier, Amsterdam, Netherlands, pp 329–359 [Google Scholar]
- Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16: 1–11 [DOI] [PubMed] [Google Scholar]
- Haswell ES, Verslues PE (2015) The ongoing search for the molecular basis of plant osmosensing. J Gen Physiol 145: 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Höfte H, Voxeur A (2017) Plant cell walls. Curr Biol 27: R865–R870 [DOI] [PubMed] [Google Scholar]
- Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, Hao Y, Luan S, Li L (2014) DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res 24: 632–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosip AL, Böhm J, Scherzer S, Al-Rasheid KAS, Dreyer I, Schultz J, Becker D, Kreuzer I, Hedrich R (2020) The Venus flytrap trigger hair–specific potassium channel KDM1 can reestablish the K+ gradient required for hapto-electric signaling. PLoS Biol 18: e3000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irianto J, Swift J, Martins RP, McPhail GD, Knight MM, Discher DE, Lee DA (2013) Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys J 104: 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Okamoto S, Zhang D, Lipton SA, Ruoslahti E (2003) Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc Natl Acad Sci USA 100: 5181–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Ott T (2020) The nanoscale organization of the plasma membrane and its importance in signaling: a proteolipid perspective. Plant Physiol 182: 1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janota CS, Calero-Cuenca FJ, Gomes ER (2020) The role of the cell nucleus in mechanotransduction. Curr Opin Plant Biol 63: 204–211 [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Huberman M, Johnson J, Telewski FW (1985) Thigmomorphogenesis: the induction of callose formation and ethylene evolution by mechanical perturbation in bean stems.. Physiologia Plantarum 64: 271–279 [Google Scholar]
- Jojoa-Cruz S, Saotome K, Murthy SE, Tsui CCA, Sansom MS, Patapoutian A, Ward AB (2018) Cryo-EM structure of the mechanically activated ion channel OSCA1.2. eLife 7: e41845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Eisa A, Kleine-Vehn J, Scheuring D (2019) NET4 modulates the compactness of vacuoles in Arabidopsis thaliana. Int J Mol Sci 20: 4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Scheuring D (2020) To lead or to follow: contribution of the plant vacuole to cell growth. Front Plant Sci 11: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell A, Glaser RW (1993) On the mechanical and dynamic properties of plant cell membranes: their role in growth, direct gene transfer and protoplast fusion. J Theor Biol 160: 41–62 [Google Scholar]
- Kesten C, Menna A, Sánchez-Rodríguez C (2017) Regulation of cellulose synthesis in response to stress. Curr Opin Plant Biol 40: 106–113 [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Hoon D, Minkoff BB, Sussman MR, Kohorn SL (2016) Rapid oligo-galacturonide induced changes in protein phosphorylation in Arabidopsis. Mol Cell Proteomics 15: 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, Chernomordik LV (2015) Membrane tension and membrane fusion. Curr Opin Struct Biol 33: 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuki H, Yokoyama R, Kuroha T, Nishitani K (2020) Xyloglucan is not essential for the formation and integrity of the cellulose network in the primary cell wall regenerated from Arabidopsis protoplasts. Plants 9: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Gierth M, Herdean A, Satoh-Cruz M, Kramer DM, Spetea C, Schroeder JI (2014) Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc Natl Acad Sci USA 111: 7480–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H, Yamanaka T, Iida K, Nakagawa Y, Saji H, et al. (2012) Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport DTA, Tan L, Held M, Kieliszewski MJ (2018) The role of the primary cell wall in plant morphogenesis. Int J Mol Sci 19: 2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani ER, Khan GA, Somssich M, Persson S (2018) Building a plant cell wall at a glance. J Cell Sci 131: jcs207373. [DOI] [PubMed] [Google Scholar]
- Le Roux AL, Quiroga X, Walani N, Arroyo M, Roca-Cusachs P (2019) The plasma membrane as a mechanochemical transducer. Philos Trans R Soc B Biol Sci 374: 20180221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Maksaev G, Jensen GS, Murcha MW, Wilson ME, Fricker M, Hell R, Haswell ES, Millar AH, Sweetlove LJ (2016) MSL1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J 88: 809–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Vila Nova Santana B, Samuels E, Benitez-Fuente F, Corsi E, Botella MA, Perez-Sancho J, Vanneste S, Friml J, Macho A, et al. (2020) Rare earth elements induce cytoskeleton-dependent and PI4P-associated rearrangement of SYT1/SYT5 ER-PM contact site complexes in Arabidopsis. J Exp Bot 71: 3986–3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao L, Prista C, Loureiro-Dias MC, Moura TF, Soveral G (2014) The grapevine tonoplast aquaporin TIP2;1 is a pressure gated water channel. Biochem Biophys Res Commun 450: 289–294 [DOI] [PubMed] [Google Scholar]
- Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18: 1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, von Wangenheim D, Zhang X, Tan S, Darwish-Miranda N, Naramoto S, Wabnik K, De Rycke R, Kaufmann WA, Gütl D, et al. (2021) Cellular requirements for PIN polar cargo clustering in Arabidopsis thaliana. New Phytol 229: 351–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bashline L, Lei L, Gu Y (2014) Cellulose synthesis and its regulation. Arabidopsis Book 12: e0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu Y, Wang J, Liu X, Zhang W, Sun L (2020) Structural insights into a plant mechanosensitive ion channel MSL1. Cell Rep 30: 4518–4527 [DOI] [PubMed] [Google Scholar]
- Lin W, Tang W, Anderson CT, Yang Z (2018) FERONIA’s sensing of cell wall pectin activates ROP GTPase signaling in Arabidopsis. bioRxiV. doi: 10.1101/269647v2
- Liu X, Wang J, Sun L (2018) Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2. Nat Commun 9: 5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Cheddadi I, Mosca G, Mirabet V, Dumond M, Kiss A, Traas J, Godin C, Boudaoud A. (2020) Cellular heterogeneity in pressure and growth emerges from tissue topology and geometry. Curr Biol 30: 1504–1516 [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez P, Martinez-Sanz M, Bonilla MR, Wang D, Gilbert EP, Stokes JR, Gidley MJ (2017) Cellulose-pectin composite hydrogels: intermolecular interactions and material properties depend on order of assembly. Carbohydr Polym 162: 71–81 [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez P, Martinez-Sanz M, Bonilla MR, Wang D, Walsh CT, Gilbert EP, Stokes JR, Gidley MJ (2016) Pectin impacts cellulose fibre architecture and hydrogel mechanics in the absence of calcium. Carbohydr Polym 153: 236–245 [DOI] [PubMed] [Google Scholar]
- Luo N, Yan A, Liu G, Guo J, Rong D, Kanaoka MM, Xiao Z, Xu G, Higashiyama T, Cui X, et al. (2017) Exocytosis-coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nat Commun 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Wang Y, Qiu S, Kang Z, Che S, Wang G, Huang J (2013) Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS One 8: e75997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wu Y, Zhang G (2021) Formation pattern and regulatory mechanisms of pollen wall in Arabidopsis. J Plant Physiol 260: 153388. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM (2011) Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol 191: 84–91 [DOI] [PubMed] [Google Scholar]
- Maity K, Heumann JM, McGrath AP, Kopcho NJ, Hsu PK, Lee CW, Mapes JH, Garza D, Krishnan S, Morgan GP, et al. (2019) Cryo-EM structure of OSCA1.2 from Oryza sativa elucidates the mechanical basis of potential membrane hyperosmolality gating. Proc Natl Acad Sci 116: 14309–14318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksaev G, Shoots JM, Ohri S, Haswell ES (2018) Nonpolar residues in the presumptive pore‐lining helix of mechanosensitive channel MSL10 influence channel behavior and establish a nonconducting function. Plant Direct 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Hasebe M (2021) Rapid movements in plants. J Plant Res 134: 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniere A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta-Peyret L, Luu DT, Botchway SW, Webb SED, Mongrand S, Maurel C, et al. (2012) Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc Natl Acad Sci USA 109: 12805–12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Fiche JB, Smokvarska M, Mari S, Alcon C, Dumont X, Hematy K, Jaillais Y, Nollmann M, Maurel C (2019) Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol 179: 1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JF, Rolfe DJ, Webb SED, Tolmie AF, Botchway SW, Martin-Fernandez ML, Hawes C, Runions J (2019) The cell wall regulates dynamics and size of plasma-membrane nanodomains in Arabidopsis. Proc Natl Acad Sci USA 116: 12857–12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Martínez-Bernardini A, Fabrice TN, Ringli C, Muschietti JP, et al. (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358: 1600–1603 [DOI] [PubMed] [Google Scholar]
- Merz D, Richter J, Gonneau M, Sanchez-Rodriguez C, Eder T, Sormani R, Martin M, Hématy K, Höfte H, Hauser MT (2017) T-DNA alleles of the receptor kinase THESEUS1 with opposing effects on cell wall integrity signaling. J Exp Bot 68: 4583–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedes E, Suslov D, Vandenbussche F, Kenobi K, Ivakov A, Van Der Straeten D, Lorences EP, Mellerowicz EJ, Verbelen JP, Vissenberg K (2013) Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J Exp Bot 64: 2481–2497 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Haswell ES (2013) A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot 64: 4663–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Renhu N, Naito M, Nakamura A, Shiba H, Yamamoto T, Suzaki T, Iida H, Miura K (2018) Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci Rep 8: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulia B, Bastien R, Chauvet-Thiry H, Leblanc-Fournier N (2019) Posture control in land plants: growth, position sensing, proprioception, balance, and elasticity. J Exp Bot 70: 3467–3494 [DOI] [PubMed] [Google Scholar]
- Moulia B, Douady S, Hamant O (2021) Fluctuations shape plants through proprioception. Science 372: eabc6868. [DOI] [PubMed] [Google Scholar]
- Mousavi SAR, Dubin AE, Zeng WZ, Coombs AM, Do K, Ghadiri DA, Keenan WT, Ge C, Zhao Y, Patapoutian A (2021) PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc Natl Acad Sci USA 118: e2102188118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A (2018) OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. eLife 7: e41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104: 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Smith RS, Mandel T, Robinson S, Kimura S, Boudaoud A, Kuhlemeier C (2012) Mechanical regulation of auxin-mediated growth. Curr Biol 22: 1468–1476 [DOI] [PubMed] [Google Scholar]
- Nguyen HTH, Bouteau F, Mazars C, Kuse M, Kawano T (2018) The involvement of calmodulin and protein kinases in the upstream of cytosolic and nucleic calcium signaling induced by hypoosmotic shock in tobacco cells. Plant Signal Behav 13: e1494467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nili A, Yi H, Crespi VH, Puri VM (2015) Examination of biological hotspot hypothesis of primary cell wall using a computational cell wall network model. Cellulose 22: 1027–1038 [Google Scholar]
- Nissen KS, Willats WGT, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 21: 516–527 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM (1992) Direct evidence for pressure-generated closure of plasmodesmata. Plant J 2: 741–750 [Google Scholar]
- Özparpucu M, Rüggeberg M, Gierlinger N, Cesarino I, Vanholme R, Boerjan W, Burgert I (2017) Unravelling the impact of lignin on cell wall mechanics: a comprehensive study on young poplar trees downregulated for CINNAMYL ALCOHOL DEHYDROGENASE (CAD). Plant J 91: 480–490 [DOI] [PubMed] [Google Scholar]
- Park K, Knoblauch J, Oparka K, Jensen KH (2019) Controlling intercellular flow through mechanosensitive plasmodesmata nanopores. Nat Commun 10: 3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012a) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 158: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012b) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases, Plant Physiol 158: 1933–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2015) Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol 56: 180–194 [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A (2005) More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol 137: 274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M, Gille S, Liu L, Mansoori N, de Souza A, Schultink A, Xiong G (2013) Hemicellulose biosynthesis. Planta 238: 627–642 [DOI] [PubMed] [Google Scholar]
- Pauly N, Knight MR, Thuleau P, Graziana A, Muto S, Ranjeva R, Mazars C (2001) The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 30: 413–421 [DOI] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook S, Höfte H (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci 3: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sancho J, Vanneste S, Lee E, McFarlane HE, Esteban del Valle A, Valpuesta V, Friml J, Botella MA, Rosado A (2015) The Arabidopsis Synaptotagmin1 is enriched in endoplasmic reticulum-plasma membrane contact sites and confers cellular resistance to mechanical stresses. Plant Physiol 168: 132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R, Ursell T, Wiggins P, Sens P (2009) Emerging roles for lipids in shaping membrane-protein function. Nature 459: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo P, Wang T, Xiao C, Anderson CT, Hong M (2017) Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: insights from solid-state NMR. Biomacromolecules 18: 2937–2950. [DOI] [PubMed] [Google Scholar]
- Polko JK, Kieber JJ (2019) The regulation of cellulose biosynthesis in plants. Plant Cell 31: 282–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Murthy S, Keenan WT, Mousavi SAR, Dabi T, Coombs A, Procko E, Baird L, Patapoutian A, Chory J (2021) Stretch-activated ion channels identified in the touch-sensitive structures of carnivorous Droseraceae plants. eLife 10: e64250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proseus TE, Boyer JS (2005) Turgor pressure moves polysaccharides into growing cell walls of Chara corallina. Ann Bot 95: 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin I, Richardson RA, Coomey JH, Weiner ER, Bascom CS, Li T, Bezanilla M, Haswell ES (2021) Plant Piezo homologs modulate vacuole morphology during tip growth. Science 373: 586–590 [DOI] [PubMed] [Google Scholar]
- Robinson S (2021) Mechanobiology of cell division in plant growth. New Phytol 231: 559–564 [DOI] [PubMed] [Google Scholar]
- Rongpipi S, Ye D, Gomez ED, Gomez EW (2018) Progress and opportunities in the characterization of cellulose - An important regulator of cell wall growth and mechanics. Front Plant Sci 9: 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Chen Y, Kandemir B, Yi H, Wang JZ, Puri VM, Anderson CT (2018) Balancing strength and flexibility: how the synthesis, organization, and modification of guard cell walls govern stomatal development and dynamics. Front Plant Sci 9: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Dinneny JR (2020) A wall with integrity: surveillance and maintenance of the plant cell wall under stress. New Phytol 225: 1428–1439 [DOI] [PubMed] [Google Scholar]
- Sassi M, Traas J (2015) When biochemistry meets mechanics: a systems view of growth control in plants. Curr Opin Plant Biol 28: 137–143 [DOI] [PubMed] [Google Scholar]
- Schapire AL, Voigt B, Jásik J, Rosado A, Lopez-Cobollo R, Menzel D, Salinas J, Mancuso S, Valpuesta V, Baluska F, et al. (2008) Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell 20: 3374–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P (2010) Hemicelluloses. Ann Rev Plant Biol 61: 263–289 [DOI] [PubMed] [Google Scholar]
- Shigeyama T, Watanabe A, Tokuchi K, Toh S, Sakurai N, Shibuya N, Kawakami N (2016) α-Xylosidase plays essential roles in xyloglucan remodelling, maintenance of cell wall integrity, and seed germination in Arabidopsis thaliana. J Exp Bot 67: 5615–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Shimada T, Takagi J, Ichino T, Shirakawa M, Hara-Nishimura I (2018) Plant vacuoles. Annu Rev Plant Biol 69: 123–145 [DOI] [PubMed] [Google Scholar]
- Shinohara N, Sunagawa N, Tamura S, Yokoyama R, Ueda M, Igarashi K, Nishitani K (2017) The plant cell-wall enzyme AtXTH3 catalyses covalent cross-linking between cellulose and cello-oligosaccharide. Sci Rep 7: 46099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Ferraz R, Dupree P, Showalter AM, Coimbra S (2020) Three decades of advances in arabinogalactan-protein biosynthesis. Front Plant Sci 11: 610377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355: 287–289 [DOI] [PubMed] [Google Scholar]
- Stephan AB, Kunz HH, Yang E, Schroeder JI (2016) Rapid hyperosmotic-induced Ca 2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc Natl Acad Sci USA 113: E5242–E5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratilová B, Kozmon S, Stratilová E, Hrmova M (2020) Plant xyloglucan xyloglucosyl transferases and the cell wall structure: subtle but significant. Molecules 25: 5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Imai H, Kawamura Y, Uemura M (2016) Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 72: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Li K, Wang Z, Zhu K, Tan X, Cao J (2019) A review of plant vacuoles: formation, located proteins, and functions. Plants 8: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kutsuna N, Kanazawa Y, Kondo N, Hasezawa S, Sano T (2007) Intra-vacuolar reserves of membranes during stomatal closure: the possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol 48: 1159–1169 [DOI] [PubMed] [Google Scholar]
- Thor K, Jiang S, Michard E, George J, Scherzer S, Huang S, Dindas J, Derbyshire P, Leitão N, DeFalco TA, et al. (2020) The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 585: 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilsner J, Nicolas W, Rosado A, Bayer EM (2016) Staying tight: plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annu Rev Plant Biol 67: 337–364 [DOI] [PubMed] [Google Scholar]
- Toyota M, Gilroy S (2013) Gravitropism and mechanical signaling in plants. Am J Bot 100: 111–125 [DOI] [PubMed] [Google Scholar]
- Tran D, Galletti R, Neumann ED, Dubois A, Sharif-Naeini R, Geitmann A, Frachisse JM, Hamant O, Ingram GC (2017) A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat Commun 8: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Girault T, Guichard M, Thomine S, Leblanc-Fournier N, Moulia B, de Langre E, Allain JM, Frachisse JM (2021) Cellular transduction of mechanical oscillations in plants by the plasma-membrane mechanosensitive channel MSL10. Proc Natl Acad Sci USA 118: e1919402118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh DC, Alonso-Serra J, Asaoka M, Colin L, Cortes M, Malivert A, Takatani S, Zhao F, Traas J, Trehin C, et al. (2021) How mechanical forces shape plant organs. Curr Biol 31: R143–R159 [DOI] [PubMed] [Google Scholar]
- Tsabary G, Shani Z, Roiz L, Levy I, Riov J (2003) Abnormal ‘wrinkled’ cell walls and retarded development of transgenic Arabidopsis thaliana plants expressing endo-1,4-β-glucanase (cel1) antisense. Plant Mole Biol 51: 213–224 [DOI] [PubMed] [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS (2011) Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol 156: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T, Kondev J, Reeves D, Wiggins PA, Phillips R (2008) Role of lipid bilayer mechanics in mechanosensation. In Kamkin A, Kiseleva I, eds, Mechanosensitive Ion Channels: Mechanosensitivity in Cells and Tissues. Springer, Dordrecht, the Netherlands, pp 37–70 [Google Scholar]
- Vaahtera L, Schulz J, Hamann T (2019) Cell wall integrity maintenance during plant development and interaction with the environment. Nat Plants 5: 924–932 [DOI] [PubMed] [Google Scholar]
- Van der Does D, Boutrot F, Engelsdorf T, Rhodes J, McKenna JF, Vernhettes S, Koevoets K, Tintor N, Veerabagu M, Miedes E, et al. (2017) The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet 13: e1006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Luit AH, Olivari C, Haley A, Knight MR, Trewavas AJ (1999) Distinct calcium signaling pathways regulate calmodulin gene expression in tobacco. Plant Physiol 121: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veley KM, Maksaev G, Frick EM, January E, Kloepper SC, Haswell ES (2014) Arabidopsis MSL10 has a regulated cell death signaling activity that is separable from its mechanosensitive ion channel activity. Plant Cell 26: 3115–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veley KM, Marshburn S, Clure CE, Haswell ES (2012) Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol 22: 408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, Krüger F, Krebs M, Neubert C, Fink F, Lupanga U, Scheuring D, Boutté Y, Frescatada-Rosa M, Wolfenstetter S, et al. (2013) The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell 25: 3434–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V (2015) Quantitative description of ion transport via plasma membrane of yeast and small cells. Front Plant Sci 6: 927–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Park YB, Caporini MA, Rosay M, Zhong L, Cosgrove DJ, Hong M(2013) Sensitivity-enhanced solid-state NMR detection of expansin's target in plant cell walls. Proc Natl Acad Sci USA 110: 16444–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hawkins TJ, Richardson C, Cummins I, Deeks MJ, Sparkes I, Hawes C, Hussey PJ (2014) The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Curr Biol 24: 1397–1405 [DOI] [PubMed] [Google Scholar]
- Wang P, Richardson C, Hawkins TJ, Sparkes I, Hawes C, Hussey PJ (2016) Plant VAP27 proteins: domain characterization, intracellular localization and role in plant development. New Phytol 210: 1311–1326 [DOI] [PubMed] [Google Scholar]
- Wang X, Wilson L, Cosgrove DJ (2020) Pectin methylesterase selectively softens the onion epidermal wall yet reduces acid-induced creep. J Exp Bot 71: 2629–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li X, Fan B, Zhu C, Chen Z (2021) Regulation and function of defense-related callose deposition in plants. Int J Mol Sci 22: 2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Braybrook S, Huflejt M, Mosca G, Routier-Kierzkowska AL, Smith RS (2015) Measuring the mechanical properties of plant cells by combining micro-indentation with osmotic treatments. J Exp Bot 66: 3229–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J, Hube B, Fairn GD (2019) Integrity under stress: host membrane remodeling and damage by fungal pathogens. Cell Microbiol 21: e13016. [DOI] [PubMed] [Google Scholar]
- Wiggins P, Phillips R (2005) Membrane-protein interactions in mechanosensitive channels. Biophys J 88: 880–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Mixdorf M, Berg RH, Haswell ES (2016) Plastid osmotic stress influences cell differentiation at the plant shoot apex. Development 143: 3382–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, van der Does D, Ladwig F, Sticht C, Kolbeck A, Schürholz AK, Augustin S, Keinath N, Rausch T, Greiner S, et al. (2014) A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc Natl Acad Sci USA 111: 15261–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Anderson CT (2016) Interconnections between cell wall polymers, wall mechanics, and cortical microtubules: teasing out causes and consequences. Plant Signal Behav 11: e1215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang T, Zheng Y, Cosgrove DJ, Anderson CT (2016) Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol 170: 234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Stegmann M, Han Z, DeFalco TA, Parys K, Xu L, Belkhadir Y, Zipfel C, Chai J (2019) Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 572: 270–274 [DOI] [PubMed] [Google Scholar]
- Xiong TC, Jauneau A, Ranjeva R, Mazars C (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signalling. Plant J 40: 12–21 [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20: 3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, et al. (2010) MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol 152: 1284–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Kawamura Y, Uemura M (2008) Cryobehavior of the plasma membrane in protoplasts isolated from cold-acclimated Arabidopsis leaves is related to surface area regulation. Plant Cell Physiol 49: 944–957 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Ohtani M, Katagiri D, Hosokawa Y, Demura T (2020) Hechtian strands transmit cell wall integrity signals in plant cells. Plants 9: 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Iida K, Iida H (2021) MCAs in Arabidopsis are Ca2+-permeable mechanosensitive channels innately sensitive to membrane tension. Res Square doi: 10.21203/rs.3.rs-126545/v1 [DOI] [PMC free article] [PubMed]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilisky B, et al. (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Wen Z, Han Y, Zhuo W, Wang F, Xi C, Liu J, Gao P, Zhao H, Wang Y, et al. (2020) Heterogeneous expression of plasma-membrane-localised OsOSCA1.4 complements osmotic sensing based on hyperosmolality and salt stress in Arabidopsis osca1 mutant. Cell Calcium 91: 102261. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang D, Kang Y, Wu JX, Yao F, Pan C, Yan Z, Song C, Chen L (2018) Structure of the mechanosensitive OSCA channels. Nat Struct Mol Biol 25: 850–858 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu J, Wang X, Durachko DM, Zhang S, Cosgrove DJ (2021) Molecular insights into the complex mechanics of plant epidermal cell walls. Science 372: 706–711 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tong X, Liu SY, Chai LX, Zhu FF, Zhang XP, Zou JZ, Wang XB (2019b) Genetic analysis of a Piezo-like protein suppressing systemic movement of plant viruses in Arabidopsis thaliana. Sci Rep 9: 3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Han SW, Rodriguez-Welsh MF, Rojas-Pierce M (2014) Homotypic vacuole fusion requires VTI11 and is regulated by phosphoinositides. Mol Plant 7: 1026–1040 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Crespi VH, Kubicki JD, Cosgrove DJ, Zhong L (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21: 1025–1039 [Google Scholar]
- Zhou X, Lu J, Zhang Y, Guo J, Lin W, Van Norman JM, Qin Y, Zhu X, Yang Z (2021) Membrane receptor-mediated mechano-transduction maintains cell integrity during pollen tube growth within the pistil. Dev Cell 56: 1030–1042 [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2007) Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci 12: 90–97 [DOI] [PubMed] [Google Scholar]
- Zwiewka M, Nodzyński T, Robert S, Vanneste S, Friml J (2015) Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol. Plant 8: 1175–1187 [DOI] [PubMed] [Google Scholar]