Abstract
Phytochrome A (phyA) is the far-red (FR) light photoreceptor in plants that is essential for seedling de-etiolation under FR-rich environments, such as canopy shade. TANDEM ZINC-FINGER/PLUS3 (TZP) was recently identified as a key component of phyA signal transduction in Arabidopsis thaliana; however, how TZP is integrated into the phyA signaling networks remains largely obscure. Here, we demonstrate that ELONGATED HYPOCOTYL5 (HY5), a well-characterized transcription factor promoting photomorphogenesis, mediates FR light induction of TZP expression by directly binding to a G-box motif in the TZP promoter. Furthermore, TZP physically interacts with CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1), an E3 ubiquitin ligase targeting HY5 for 26S proteasome-mediated degradation, and this interaction inhibits COP1 interaction with HY5. Consistent with those results, TZP post-translationally promotes HY5 protein stability in FR light, and in turn, TZP protein itself is destabilized by COP1 in both dark and FR light conditions. Moreover, tzp hy5 double mutants display an additive phenotype relative to their respective single mutants under high FR light intensities, indicating that TZP and HY5 also function in largely independent pathways. Together, our data demonstrate that HY5 and TZP mutually upregulate each other in transmitting the FR light signal, thus providing insights into the complicated but delicate control of phyA signaling networks.
HY5 mediates FR light induction of TZP expression by directly binding to a G-box motif in the TZP promoter, while TZP competes with HY5 for binding to COP1 as another substrate, and post-translationally promotes HY5 protein abundance in FR light.
Introduction
Plant canopy shade is enriched in far-red (FR) light because the red (R; 600–700 nm) and blue (B; 400–500 nm) wavelengths of sunlight are absorbed by chlorophyll and carotenoid pigments of upper leaves, while FR wavelengths (700–750 nm) are transmitted or reflected by plant tissues (Casal, 2013; Fiorucci and Fankhauser, 2017; Legris et al., 2019). Plants use the phytochrome family of photoreceptors to perceive the R and FR components of their light environment, and phytochromes are widely conserved in seed plants and nonseed plants including ferns, mosses, and charophyte algae (Li et al., 2011, 2015; Rensing et al., 2016; Paik and Huq, 2019; Yang et al., 2020; Cheng et al., 2021). The Arabidopsis thaliana genome encodes five phytochrome proteins, named phytochromes A to E (phyA–phyE). PhyB through phyE are relatively stable in the light, while phyA is highly light-labile (Sharrock and Clack, 2002). Phytochromes exist in vivo in two distinct but interconvertible forms: the R-absorbing Pr form and the FR-absorbing Pfr form, and it has long been believed that only the Pfr form of phytochrome is biologically active (Bae and Choi, 2008; Li et al., 2011; Legris et al., 2019). However, it was recently proposed that the nucleus-localized Pr form of phytochrome may also have non-negligible biological activity (Li and Hiltbrunner, 2021). Moreover, although all five phytochromes have virtually identical photophysical properties, they display distinct response profiles: phyB–E have an action peak in R light, which conforms to the understanding that R light triggers the conversion of the Pr form to the active Pfr form; however, the response profile of phyA exhibits a peak in the FR range of the spectrum although only ∼3% of phyA is in the Pfr form in FR light (Mancinelli, 1994; Rausenberger et al., 2011; Sheerin and Hiltbrunner, 2017). A mathematical model developed a decade ago suggested that the mechanism responsible for shifting the action peak of phyA from R to FR light relies on specific molecular interactions rather than intrinsic changes to the spectral properties of phyA (Rausenberger et al., 2011).
Genetic screens have been extensively conducted in the past three decades to uncover the components of the FR light signaling pathway. The far-red elongated hypocotyl (fhy1) and fhy3 mutants were first identified in a pioneering screen for mutants defective in FR light responses (Whitelam et al., 1993). The FHY1 and FHY3 loci were cloned later by separate studies (Desnos et al., 2001; Wang and Deng, 2002), and subsequent genetic and biochemical characterization of their functions revealed that FHY1, and its homolog FHY1-LIKE (FHL; Zhou et al., 2005), are two small plant-specific proteins required for nuclear import of the phyA photoreceptor (Hiltbrunner et al., 2005, 2006). FHY3, and its homolog FAR-RED IMPAIRED RESPONSE1 (FAR1; Hudson et al., 1999), are transposase-derived transcription factors that activate the expression of FHY1 and FHL by directly binding to their promoters (Lin et al., 2007, 2008), thus indirectly regulating phyA nuclear accumulation. LONG HYPOCOTYL IN FAR-RED1 (HFR1), an atypical basic helix-loop-helix (bHLH) protein, and LONG AFTER FAR-RED LIGHT1 (LAF1), an R2R3-MYB transcription factor, were identified by forward genetic screening for mutants showing long hypocotyls in FR light (Fairchild et al., 2000; Fankhauser and Chory, 2000; Soh et al., 2000; Ballesteros et al., 2001). PHYTOCHROME INTERACTING FACTORS (PIFs) are a group of bHLH transcription factors that physically interact with phytochromes (Leivar and Quail, 2011; Lee and Choi, 2017; Pham et al., 2018). Numerous studies have demonstrated that PIFs play important roles in mediating FR light signaling and shade avoidance response (Kim et al., 2003; Lorrain et al., 2008, 2009; Li et al., 2012; de Wit et al., 2016; Paulisic et al., 2021) and that PIFs target thousands of genes in vivo by directly binding to either G-box (CACGTG) or E-box (CANNTG) motifs in their promoters (Martínez-García et al., 2000; Huq et al., 2002, 2004; Hornitschek et al., 2009; Zhang et al., 2013; Pfeiffer et al., 2014; Qi et al., 2020). Recently, we identified two mutant alleles of TANDEM ZINC-FINGER/PLUS3 (TZP) by screening a new library of Arabidopsis mutants and demonstrated that TZP acts as a key positive regulator of FR light signaling that interacts with phyA and regulates phyA phosphorylation and protein abundance in FR light (Zhang et al., 2018). Interestingly, it was also shown that TZP interacts with phyB to regulate photoperiodic flowering (KaiserLi et al., 2015), and that TZP plays a negative role in mediating blue light signaling (Loudet et al., 2008; Perrella et al., 2018).
ELONGATED HYPOCOTYL5 (HY5) and CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) are two central regulators of photomorphogenesis, and their interaction defines a key regulatory hub for light control of Arabidopsis seedling development (Ang et al., 1998; Yi and Deng, 2005; Lau and Deng, 2012; Gangappa and Botto, 2016; Xu, 2020; Han et al., 2020). HY5, a bZIP-family transcription factor, is involved in regulating photomorphogenic development under a wide range of wavelengths, including FR, R, B, and ultraviolet-B light (Koornneef et al., 1980; Oyama et al., 1997; Ulm et al., 2004; Li et al., 2011; Gangappa and Botto, 2016; Xu, 2020). The abundance of HY5 protein correlates well with the extent of seedling photomorphogenic development (Osterlund et al., 2000), showing the pivotal function of HY5 in regulating photomorphogenesis. Consistent with its hierarchical role, HY5 was shown to target 3,195 to 11,797 Arabidopsis genes in several independent studies using different plant materials, growth conditions, and approaches (Lee et al., 2007; Zhang et al., 2011; Kurihara et al., 2014; Hajdu et al., 2018). However, two recent studies identified much smaller numbers (297 and 422 genes), but likely high-confidence sets of direct HY5 target genes (Burko et al., 2020; Canibano et al., 2021). HY5 and its homolog, HY5 HOMOLOG (HYH), directly bind to ACGT-containing elements (ACEs) or G-boxes present in the promoters of their direct target genes (Chattopadhyay et al., 1998; Lee et al., 2007; Shin et al., 2007; Song et al., 2008; Li et al., 2010; Zhang et al., 2011; Gangappa and Botto, 2016; Burko et al., 2020; Canibano et al., 2021).
COP1 is an evolutionarily highly conserved E3 ubiquitin ligase that contains a RING-finger domain in its N-terminus and a WD40-repeat domain in its C-terminus, which are connected by a coiled-coil domain in the middle (Deng et al., 1992; Yi and Deng, 2005; Han et al., 2020). COP1 forms complexes with SUPPRESSOR OF phyA-105 (SPA) proteins that target several photomorphogenesis-promoting factors, such as HY5, HYH, LAF1, HFR1, and even phytochromes, for degradation via the 26S proteasome (Osterlund et al., 2000; Holm et al., 2002; Seo et al., 2003, 2004; Duek et al., 2004; Jang et al., 2005; Yang et al., 2005; Jang et al., 2010). Interestingly, SPA proteins contain an N-terminal serine/threonine kinase domain, which was recently found to phosphorylate PIF1, PIF4, and HY5 (Paik et al., 2019; Lee et al., 2020; Wang et al., 2021), indicating that the COP1/SPA complexes contain both kinase and E3 ubiquitin ligase activities that selectively trigger rapid phosphorylation and degradation of their substrates. Upon light exposure, however, photo-activated phytochromes and cryptochromes disrupt the interactions between COP1 and SPA proteins and thus inactivate the COP1/SPA E3 ubiquitin ligase complexes, thereby allowing accumulation of photomorphogenesis-promoting factors and initiation of photomorphogenesis (Lian et al., 2011; Liu et al., 2011; Zuo et al., 2011; Lu et al., 2015; Sheerin et al., 2015).
Here, we report a mutual relationship between HY5 and TZP in transducing the FR light signal. We show that HY5 positively regulates TZP expression in response to FR light by directly binding to the G-box motif in the TZP promoter. In addition, TZP physically interacts with COP1 and competes with HY5 for binding to COP1 as another substrate. Thus, TZP post-translationally promotes HY5 protein stability in FR light. Moreover, the additive phenotype of tzp hy5 double mutants relative to their single mutants under high FR light intensities indicates that TZP and HY5 also function in largely independent pathways. Together, our data demonstrate that, in addition to their independent functions in phyA signaling, HY5 and TZP mutually upregulate each other by distinct mechanisms, thus enhancing FR light signaling.
Results
HY5 directly binds to the G-box motif in the TZP promoter
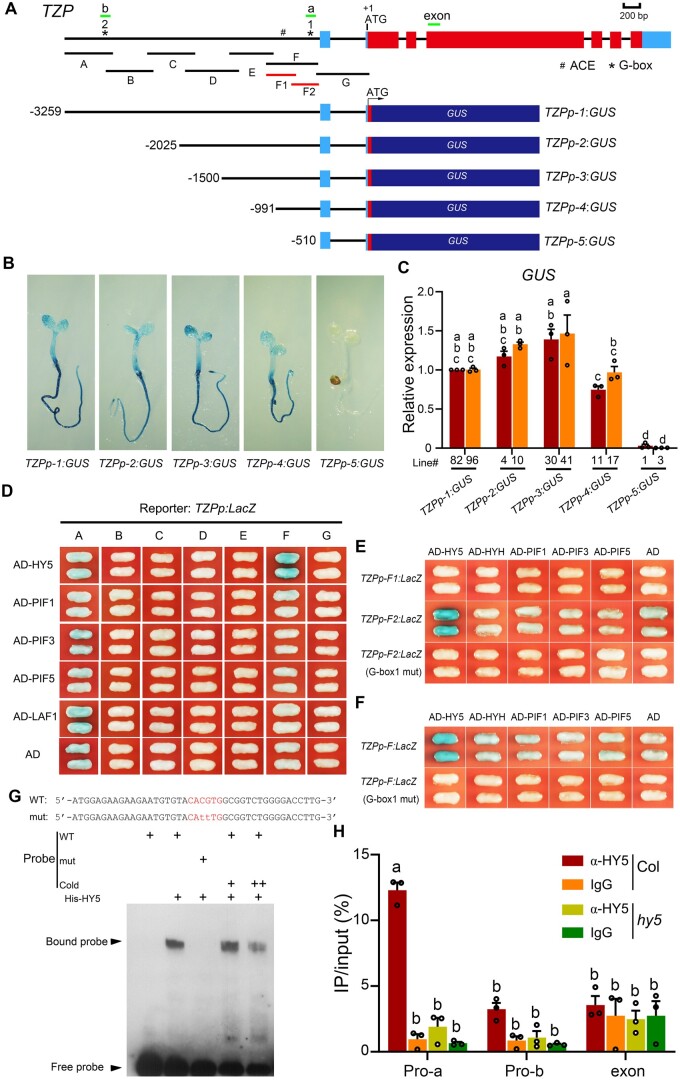
Our previous study showed that the TZP transcript level was dramatically induced by light (Zhang et al., 2018). To define the promoter fragment responsible for TZP light induction, we generated a series of 5′ deletions of the TZP promoter, and fused them with the β-glucuronidase (GUS) reporter (Figure 1A). At least 8–10 independent homozygous lines were obtained for each construct and were grown in FR light for 4 days and then analyzed by histochemical staining. We detected GUS activity in transgenic lines harboring TZPp-1:GUS, TZPp-2:GUS, TZPp-3:GUS, and TZPp-4:GUS reporters, but not in those transformed with TZPp-5:GUS (Figure 1B). We then performed reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays to compare the relative expression levels of GUS driven by different fragments of the TZP promoter, and our data indicated that TZPp-1 to TZPp-4 displayed similar levels of GUS expression in FR light; in contrast, TZPp-5 totally lost the ability to activate GUS expression (Figure 1C). Together, these data indicated that a 482-bp region of the TZP promoter (from −991 to −510; Figure 1A) was critical for TZP to respond to FR light.
Figure 1.
HY5 directly binds to a G-box motif in the TZP promoter. A, Illustration of TZP promoter fragments used to drive GUS gene expression. The adenine residue of the translational start codon (ATG) was assigned position +1, and the length of promoter fragments used to drive GUS expression was determined based on this number. “A” through “G” indicate the corresponding promoter fragments used in the yeast one-hybrid assays shown in (D). “F1” and “F2” indicate the corresponding promoter subfragments used in the yeast one-hybrid assays shown in (E). The short lines (named “a”, “b”, and “exon”) depict the location of amplicons used for the ChIP-qPCR assays shown in (H). Asterisks indicate the positions of two G-box motifs in the TZP promoter. B, Representative GUS staining results of TZPp:GUS transgenic lines in which GUS gene expression was driven by various TZP promoter fragments. Homozygous transgenic seedlings were grown in FR light (1 μmol m−2 s−1) for 4 days, and then treated with GUS staining buffer for 6 h, and representative histochemical staining results are shown. C, RT-qPCR assays showing the relative expression of GUS in two independent homozygous lines of TZPp-1:GUS, TZPp-2:GUS, TZPp-3:GUS, TZPp-4:GUS and TZPp-5:GUS, respectively. Four-day-old seedlings grown in FR light were subjected to RT-qPCR assays. Error bars represent se of three different pools of seedlings. Data are normalized to TUBULIN3. Different letters represent significant differences determined by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). D, Yeast one-hybrid assays showing that HY5 displayed strong binding to the “F” fragment of the TZP promoter. Empty vector expressing the AD domain alone was used as the negative control. E, Yeast one-hybrid assays showing that HY5 only bound to the “F2”, but not to the “F1” fragment of the TZP promoter, while mutating G-box1 in the “F2” fragment to CAaaTG abolished HY5 binding. F, Yeast one-hybrid assays showing that mutating G-box1 in the “F” fragment to CAaaTG abolished HY5 binding. G, EMSAs showing that HY5 specifically bound to G-box1 of the TZP promoter in vitro. H, ChIP-qPCR assays showing that HY5 bound to the TZP promoter in vivo. Four-d-old Col and hy5 mutant seedlings grown in FR light (1 μmol m−2 s−1) were harvested and subjected to ChIP analysis using anti-rabbit IgG and anti-HY5 antibodies, respectively, and the precipitated DNA was recovered and analyzed by qPCR assays. Data are means ± se of three pools of seedlings. Different letters represent significant differences determined by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3).
To investigate whether FR-induced TZP expression was mediated by the key transcription factors acting in the phyA signaling pathway, we performed yeast one-hybrid assays to test whether HY5, PIFs (including PIF1, PIF3, and PIF5) and LAF1 could directly bind to the TZP promoter. We divided the TZP promoter into seven overlapping fragments, designated “A” to “G”, in which the “F” fragment included the 482-bp region essential for TZP to respond to FR (Figure 1A). These TZP promoter fragments were used, respectively, to drive LacZ reporter gene expression in yeast cells, and intriguingly, our yeast one-hybrid assay data indicated that HY5 displayed strong binding to the “F” fragment of the TZP promoter (Figure 1D), suggesting that HY5 may play a role in regulating TZP expression.
HY5 was previously shown to directly bind ACEs or G-boxes (Lee et al., 2007; Zhang et al., 2011; Gangappa and Botto, 2016; Burko et al., 2020; Canibano et al., 2021). Analysis of the “F” fragment of the TZP promoter revealed the presence of only two ACEs, one of which conforms to the consensus G-box motif (CACGTG) and is therefore named G-box1. G-box1 is in close proximity to the transcriptional start site of TZP. The TZP promoter also contains another G-box motif in the upstream “A” fragment that we named G-box2 (Figure 1A). We then divided the “F” fragment of the TZP promoter further into “F1” (containing the ACE) and “F2” (containing G-box1), and our yeast one-hybrid assay data showed that HY5 bound to “F2”, but not to “F1” (Figure 1E). Mutating G-box1 to CAaaTG in both the “F2” and “F” fragments totally abolished HY5 binding in yeast cells (Figure 1, E and F). In addition, our electrophoretic mobility shift assay (EMSA) data further showed that His-tagged HY5 directly bound in vitro to a subfragment of the TZP promoter containing the wild-type G-box1, but not if G-box1 was mutated (Figure 1G). These data demonstrate that HY5 directly binds to G-box1 of the TZP promoter. In contrast, HYH and PIFs (including PIF1, PIF3, and PIF5) were unable to bind to either the F2 or F fragment of the TZP promoter in yeast cells (Figure 1, D–F).
To further explore HY5 binding to the TZP promoter in vivo, we performed chromatin immunoprecipitation (ChIP) assays using anti-HY5 antibodies and 4-day-old Col and hy5 mutant seedlings grown in FR light. ChIP assays using anti-rabbit IgG served as negative controls. Our qPCR data indicated that the “a” amplicon of the TZP promoter (containing G-box1) was highly enriched in the anti-HY5 ChIP sample from Col, but not in that from hy5 mutant seedlings (Figure 1H). In contrast, the “b” amplicon (containing G-box2) and an exon fragment of TZP (Figure 1A) were not enriched in the anti-HY5 ChIP sample from Col seedlings (Figure 1H). Together, our data demonstrate that HY5 binds to the fragment of the TZP promoter containing G-box1 in vivo.
HY5 and the G-box1 motif mediate TZP expression in FR light
To investigate whether HY5 was involved in regulating TZP expression, we first grew Col, hy5-215 and UBQ10p:HA-HY5 (HA-HY5; Li et al., 2020) seedlings in darkness (D) or continuous FR, R, B, and white (W) light for 4 days, and then compared their TZP transcript levels by RT-qPCR assays. TZP expression was evidently lower in hy5 mutant seedlings, but elevated in HY5-overexpression seedlings compared with Col in FR light (Figure 2A), indicating that HY5 positively regulates TZP expression in FR light. TZP expression was not significantly altered in hy5 mutants in darkness or other light conditions (Figure 2A), implying that HY5 modulates TZP expression predominantly in FR light.
Figure 2.
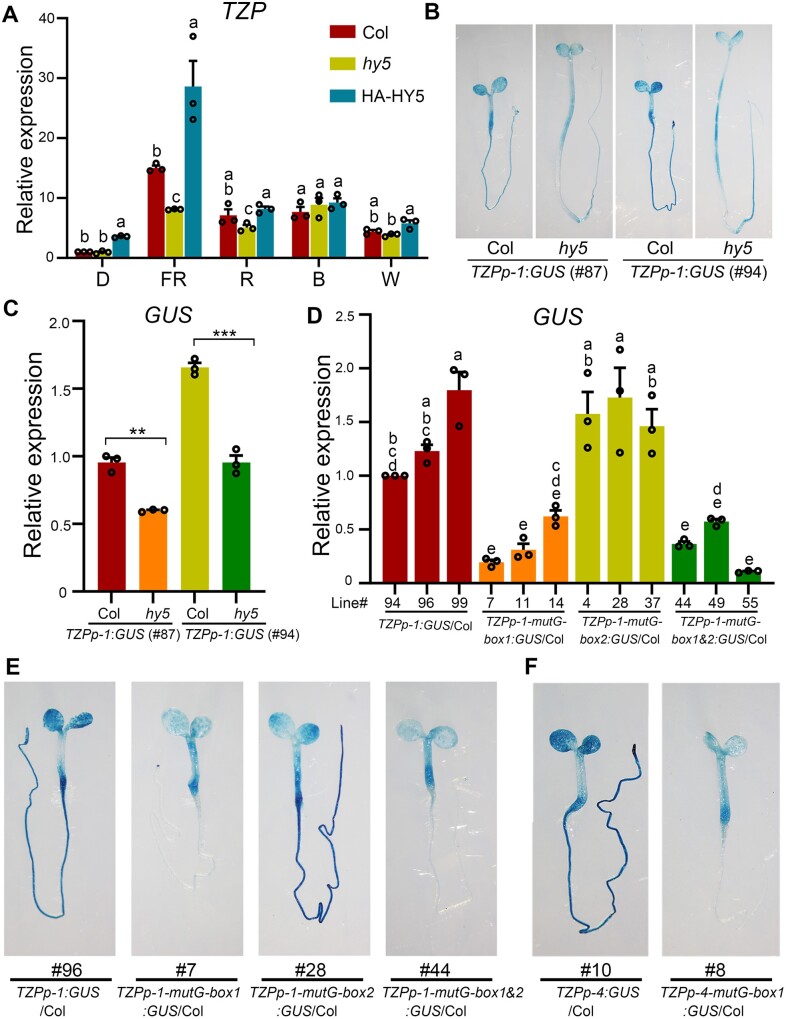
HY5 and the G-box1 motif mediate TZP expression in FR light. A, RT-qPCR assays showing the relative expression of TZP in 4-day-old Col, hy5-215 and HY5-overexpression (HA-HY5) seedlings in darkness (D) or continuous FR light (1 μmol m−2 s−1), R light (10 μmol m−2 s−1), B light (3 μmol m−2 s−1), or W light (50 μmol m−2 s−1). Error bars represent se of three different pools of seedlings. Data are normalized to TUBULIN3. Different letters represent significant differences determined by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). B, Histochemical staining of two homozygous TZPp-1:GUS lines (#87 and #94) in Col and hy5 backgrounds, respectively. Seedlings were grown in FR light (1 μmol m−2 s−1) for 4 days, and then treated with GUS staining buffer, and representative histochemical staining results are shown. C, RT-qPCR assays showing that GUS expression was significantly decreased in the absence of HY5. Seedlings were grown in FR light (1 μmol m−2 s−1) for 4 days, and then subjected to RT-qPCR assays. Error bars represent se of three different pools of seedlings. Data are normalized to TUBULIN3. **P < 0.01 and ***P < 0.001 (Student’s t test; Supplemental Data Set S3) for the indicated pair of samples. D, RT-qPCR assays showing the relative expression of GUS in three independent homozygous lines of TZPp-1:GUS, TZPp-1-mutG-box1:GUS, TZPp-1-mutG-box2:GUS and TZPp-1-mutG-box1&2:GUS seedlings. Error bars represent se of three different pools of seedlings. Data are normalized to TUBULIN3. Different letters represent significant differences determined by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). E, Representative histochemical staining of 4-day-old homozygous TZPp-1:GUS, TZPp-1-mutG-box1:GUS, TZPp-1-mutG-box2:GUS and TZPp-1-mutG-box1&2:GUS seedlings grown in FR light (1 μmol m−2 s−1). F, Representative histochemical staining of 4-day-old homozygous TZPp-4:GUS and TZPp-4-mutG-box1:GUS seedlings grown in FR light (1 μmol m−2 s−1).
To further investigate whether HY5 regulates the spatial expression pattern of TZP, we crossed two independent alleles of TZPp-1:GUS with the hy5-215 mutant. Four-day-old TZPp-1:GUS and TZPp-1:GUS hy5 seedlings grown in continuous FR light were analyzed by histochemical staining. Our results showed that, whereas TZP was widely expressed in cotyledons, hypocotyls and roots, disruption of HY5 resulted in lower TZP expression in all seedling tissues in FR light (Figure 2B). We performed RT-qPCR assays to compare the GUS transcript levels in TZPp-1:GUS and TZPp-1:GUS hy5 seedlings grown in continuous FR light, and our data confirmed that GUS expression was significantly lower in the absence of HY5 (Figure 2C). Notably, TZP tended to be expressed at the bottom of the hypocotyls in wild-type seedlings, whereas this tendency was disrupted by the absence of HY5 (Figure 2B). Together, our data demonstrate that HY5 regulates spatial expression patterns of TZP in FR light.
To investigate the role of the G-box1 motif in FR-induced TZP expression, we mutated it to CAaaTG in the TZPp-1:GUS reporter (generating TZPp-1-mutG-box1:GUS), and then transformed the mutated reporter into the Col background. As a control, the upstream G-box2 motif was mutated alone (TZPp-1-mutG-box2:GUS), or together with G-box1 (TZPp-1-mutG-box1&2:GUS), and transformed into Col as well. Multiple homozygous transgenic lines were obtained for each construct. Notably, our RT-qPCR data showed that mutation of G-box2 alone did not obviously alter GUS expression; however, mutation of G-box1 alone or together with G-box2 greatly decreased GUS expression level in FR light (Figure 2D). Intriguingly, whereas TZP was expressed in cotyledons, hypocotyls and roots in FR-grown TZPp-1:GUS transgenic seedlings, mutation of G-box1 totally abolished TZP expression in roots (Figure 2E;Supplemental Figure S1A). To further verify the importance of G-box1 in mediating TZP expression, we mutated it in the TZPp-4:GUS reporter that contained a short TZP promoter fragment (∼500-bp upstream of the transcriptional start site), and our histochemical staining results showed that mutation of G-box1 greatly decreased TZP expression in cotyledons and hypocotyls, and completely abolished TZP expression in roots (Figure 2F;Supplemental Figure S1B). Collectively, our data demonstrate that HY5 and the G-box1 motif serve as two key determinants for TZP expression in FR light.
HY5 mediates light induction of TZP protein accumulation in the light
We recently used a tzp complementation line (TZPp:TZP-3flag tzp-1) to show that TZP-3flag fusion proteins accumulated in different patterns under different light conditions (Zhang et al., 2018). To investigate whether the endogenous TZP proteins accumulated in similar patterns, we first generated monoclonal anti-TZP antibodies, and immunoblot data showed that our anti-TZP antibodies recognized multiple bands (between 100 and 150 kDa) in protein extracts of 4-day-old Col grown in continuous FR, R, and B light, but not in those of tzp-1 mutant seedlings, suggesting that these bands represent endogenous TZP proteins (Supplemental Figure S2). We then grew Col and TZPp:TZP-3flag tzp-1 seedlings in darkness or different light conditions for 4 days, and performed immunoblots using anti-Flag and anti-TZP antibodies, respectively. TZP-3flag fusion proteins displayed identical accumulation patterns in both anti-Flag and anti-TZP blots in all tested light conditions (Figure 3A). Notably, the endogenous TZP proteins in Col seedlings showed the same patterns of differential accumulation as those of TZP-3flag fusion proteins in FR, R, B, and W light (Figure 3A), suggesting that endogenous TZP proteins were indeed differentially modified in different light conditions.
Figure 3.
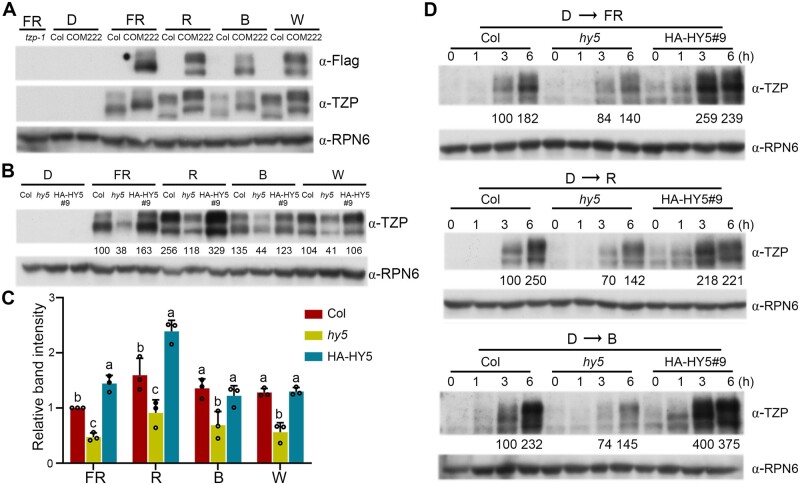
HY5 mediates light induction of TZP protein accumulation in the light. A, Immunoblots showing TZP protein levels (as shown by anti-Flag and anti-TZP immunoblots, respectively) in 4-day-old Col and homozygous tzp complementation (COM) seedlings (TZPp:TZP-3flag tzp-1) grown in D or continuous FR light (1 μmol m−2 s−1), R light (10 μmol m−2 s−1), B light (3 μmol m−2 s−1), or W light (50 μmol m−2 s−1). Anti-RPN6 was used as a sample loading control. B, Immunoblots showing TZP protein levels in 4-day-old Col, hy5-215 and HY5-overexpression (HA-HY5) seedlings grown in darkness (D) or continuous FR light (1 μmol m−2 s−1), R light (10 μmol m−2 s−1), B light (3 μmol m−2 s−1), or W light (50 μmol m−2 s−1). Anti-RPN6 was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of TZP normalized to those of RPN6 for each panel. The ratio of the first lane was set to 100. C, Relative intensities of the bands in (B). Data are means ± SD from three independent biological assays. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). D, Immunoblots showing TZP protein levels in Col, hy5-215 and HY5-overexpression (HA-HY5) seedlings grown in darkness (D) for 4 days and then transferred to FR light (50 μmol m−2 s−1), R light (10 μmol m−2 s−1), or B light (3 μmol m−2 s−1) for the indicated times. Anti-RPN6 was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of TZP normalized to those of RPN6 for each panel. The ratio of the first clear band was set to 100 for each blot.
To investigate whether HY5 mediates the accumulation of TZP proteins in the light, we grew Col, hy5-215, and HA-HY5 seedlings in D or continuous FR, R, B, and W light for 4 days, and our immunoblot data indicated that the levels of endogenous TZP proteins were much lower in hy5 mutant seedlings in all tested light conditions (Figure 3, B and C). In contrast, TZP protein levels were significantly higher in HA-HY5 seedlings in FR and R light (Figure 3, B and C). These data demonstrate that HY5 acts to promote TZP protein accumulation in the light.
To further test for a role of HY5 in modulating TZP transcript and protein accumulation upon light exposure, Col, hy5-215 and HA-HY5 seedlings were first grown in D for 4 days, and then transferred to FR, R, and B light for the indicated times ranging from 1 to 6 h. Our RT-qPCR assays indicated that TZP transcript levels were rapidly induced in Col seedlings upon FR, R, and B light exposure; however, this induction was significantly weakened in hy5-215 mutants, but strengthened in HA-HY5 seedlings (Supplemental Figure S3). Our immunoblot data showed that the relative levels of TZP proteins in Col, hy5-215, and HA-HY5 seedlings were consistent with those of TZP transcripts in response to FR, R, and B light (Figure 3D). Together, our data demonstrate that HY5 is required for rapid induction of TZP transcript and protein levels upon light exposure.
HY5- and TZP-regulated gene expression and hypocotyl growth in FR light
To investigate the genetic relationship between HY5 and TZP, we first introduced the tzp-1 and tzp-2 mutations into the HA-HY5 background, respectively, by genetic crossing. Interestingly, we observed that although HA-HY5 seedlings developed shorter hypocotyls in FR light, the hypocotyl lengths of HA-HY5 tzp-1 and HA-HY5 tzp-2 seedlings were longer than those of Col, but shorter than those of tzp-1 and tzp-2 mutants, respectively (Figure 4, A and B). We further generated homozygous tzp-1 hy5 and tzp-2 hy5 mutants (Supplemental Figure S4) and grew them in different fluence rates of continuous FR light for 4 days together with Col and their respective single mutants. Interestingly, the hypocotyl lengths of tzp hy5 double mutants were almost indistinguishable from those of hy5 mutant seedlings under 0.2 μmol m−2 s−1 of FR light (Supplemental Figure S5), indicating that hy5 is epistatic to tzp under low fluence rates of FR light. In contrast, the tzp hy5 double mutants developed significantly longer hypocotyls than tzp or hy5 single mutant seedlings under 10 or 50 μmol m−2 s−1 of FR light (Figure 4, A and B;Supplemental Figure S5), indicating that HY5 and TZP additively regulate hypocotyl growth under high fluence rates of FR light. Together, these data reveal both epistatic and additive effects of hy5 and tzp mutations on hypocotyl growth that are dependent on FR light intensities.
Figure 4.
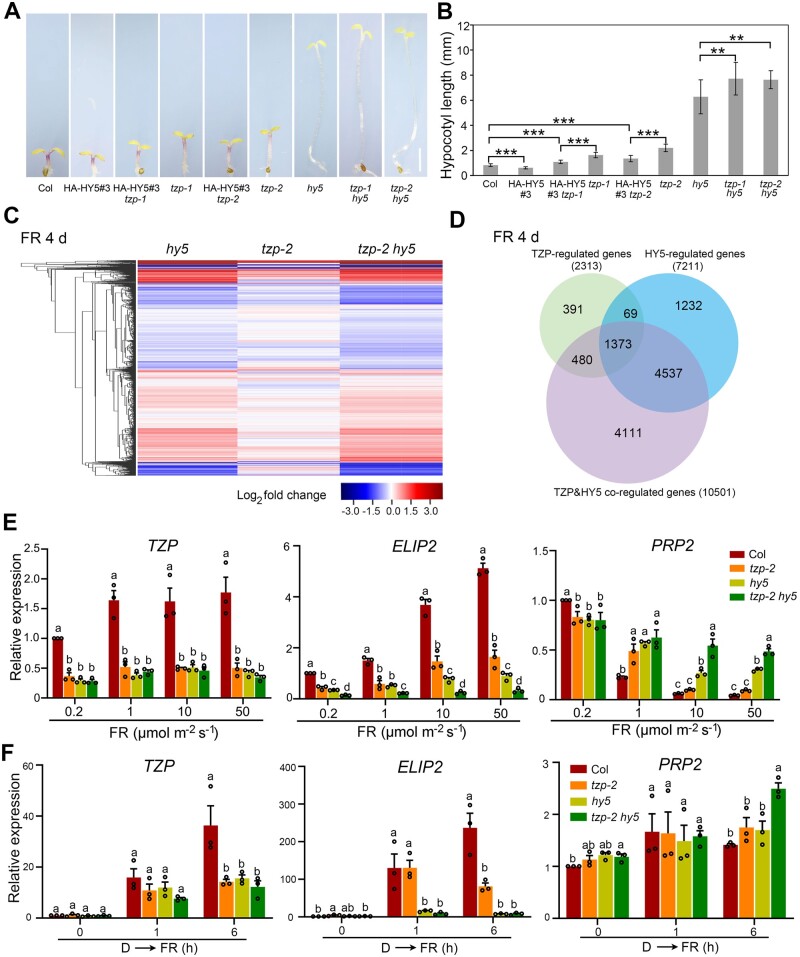
HY5 and TZP coordinately regulate FR light signaling. A, Phenotypes of 4-day-old Col, tzp-1, tzp-2, hy5, tzp-1 hy5, tzp-2 hy5, HA-HY5#3, HA-HY5#3 tzp-1, and HA-HY5#3 tzp-2 seedlings grown in FR light (10 μmol m−2 s−1). Scale bar = 1 mm. B, Hypocotyl lengths of the respective seedlings shown in (A). Data are means ± sd from 15 seedlings. **P < 0.01 and ***P < 0.001 (Student’s t test; Supplemental Data Set S3) for the indicated pair of samples. C, Cluster analysis of genes whose expression was changed in 4-day-old FR-grown (10 μmol m−2 s−1) hy5, tzp-2, and tzp-2 hy5 seedlings compared with those in Col. The bar represents the log2 of the ratio. D, Venn diagram showing the numbers and overlaps of genes whose expression was changed in hy5, tzp-2, and tzp-2 hy5 seedlings compared with those in Col. E, RT-qPCR data showing the expression levels of TZP, ELIP2, and PRP2 in Col, tzp-2, hy5, tzp-2 hy5 mutant seedlings grown under different rates of FR light for 4 days. Error bars represent SE of three different pools of seedlings. Data are normalized to TUBULIN3. Different letters represent significant differences by one-way ANOVA with Duncan’s post hoc test (P < 0.05; Supplemental Data Set S3). F, RT-qPCR assays showing the relative expression of TZP, ELIP2, and PRP2 in 4-day-old Col, hy5, tzp-2, and tzp-2 hy5 mutant seedlings grown in the dark, and then transferred to FR light (50 μmol m−2 s−1) for 1 h and 6 h. Error bars represent se of three different pools of seedlings. Data are normalized to TUBULIN3. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3).
To compare the transcriptomes regulated by TZP and HY5, Col, hy5, tzp-2, and tzp-2 hy5 mutant seedlings were grown under 10 μmol m−2 s−1 of FR light for 4 days, and then subjected to RNA-sequencing (RNA-seq) analyses. The RNA-seq data were collected from three independent biological samples (each sample with >2.0 G clean data), and differential gene expression analysis was performed using Cufflinks (Trapnell et al., 2013; cufflinks.cbcb.umd.edu). Our analysis revealed that 7,211, 2,313, and 10,501 genes displayed statistically significant changes (with the threshold of false discovery rate < 0.05) in hy5, tzp-2, and tzp-2 hy5 mutants, respectively, compared with FR-grown Col seedlings (Figure 4, C and D). These were thus defined as HY5-regulated, TZP-regulated, and TZP&HY5 co-regulated genes, respectively (Figure 4D).
Notably, a total of 1,373 genes were shown to be differentially expressed in hy5, tzp-2, and tzp-2 hy5 mutant seedlings (Figure 4D;Supplemental Data Set S1), including several previously reported light-responsive genes, such as EARLY LIGHT-INDUCED PROTEIN2 (ELIP2) regulating chlorophyll synthesis (Tzvetkova-Chevolleau et al., 2007), PRO-RICH PROTEIN2 (PRP2) shown to be coregulated by HY5 and a B-box (BBX) protein BBX23 (Zhang et al., 2017), and TZP itself. The mutation of tzp-2 was a single-base substitution in the coding region of TZP that changed Lys-355 (AAG) to a premature stop codon (TAG; Zhang et al., 2018); thus, it was interesting to notice that TZP expression was coregulated by HY5 and TZP itself. We then performed RT-qPCR assays to further validate the expression of these light-responsive genes in hy5, tzp-2, and tzp-2 hy5 mutant seedlings. Our data indicated that under both low and high fluence rates of FR light, TZP was similarly downregulated in hy5, tzp-2, and tzp-2 hy5 mutant seedlings, whereas ELIP2 was downregulated in hy5 and tzp-2 mutants and further decreased in tzp-2 hy5 double mutants (Figure 4E). However, PRP2 was upregulated in hy5 and tzp-2 mutants and further increased in tzp-2 hy5 double mutants only under high fluence rates (10 and 50 μmol m−2 s−1) of FR light (Figure 4E), indicating that HY5 and TZP-coregulated expression of PRP2 is dependent on FR light intensities. These data thus provide molecular evidence supporting the notion that both HY5 and TZP play important roles in mediating FR-responsive gene expression.
To further dissect the distinct roles of TZP and HY5 in mediating transcriptomic changes upon FR light exposure, Col, hy5, tzp-2, and tzp-2 hy5 mutant seedlings were first grown in darkness for 4 days, then transferred to FR for 1 and 6 h, respectively, and subjected to RNA-seq analyses. Our results showed that 6,918, 6,526, and 8,773 genes were shown to be differentially expressed in tzp-2, hy5, and tzp-2 hy5 mutant seedlings after 1 h of FR exposure compared with those in Col seedlings (Supplemental Figure S6, A and B). The fact that similar numbers of genes were differentially expressed in tzp-2 and hy5 mutant seedlings suggests that TZP may play a similarly important role as HY5 in mediating rapid expression changes of early FR-responsive genes. However, after 6 h of FR exposure, 9,355, 11,037, and 11,351 genes were differentially expressed in tzp-2, hy5, and tzp-2 hy5 mutant seedlings, respectively (Supplemental Figure S6B). The larger number of genes that were differentially expressed in hy5 than in tzp-2 mutants after 6 h (Supplemental Figure S6B) or 4 days of FR light (Figure 4D) suggests that HY5 plays a more important role than TZP in modulating gene expression after prolonged FR light exposure. Our RT-qPCR data showed that whereas both TZP and HY5 were similarly important for upregulating TZP expression in response to FR light, HY5 plays a more important role than TZP in mediating FR-induced ELIP2 expression (Figure 4F). In addition, while PRP2 was not obviously regulated by either HY5 or TZP after 1 h of FR exposure, it was additively upregulated by TZP and HY5 after 6 h of FR irradiation (Figure 4F). Collectively, our data demonstrate both epistatic and additive effects of hy5 and tzp mutations on gene expression and hypocotyl growth, and the effects are dependent on FR light intensity and duration.
TZP promotes HY5 protein abundance post-translationally
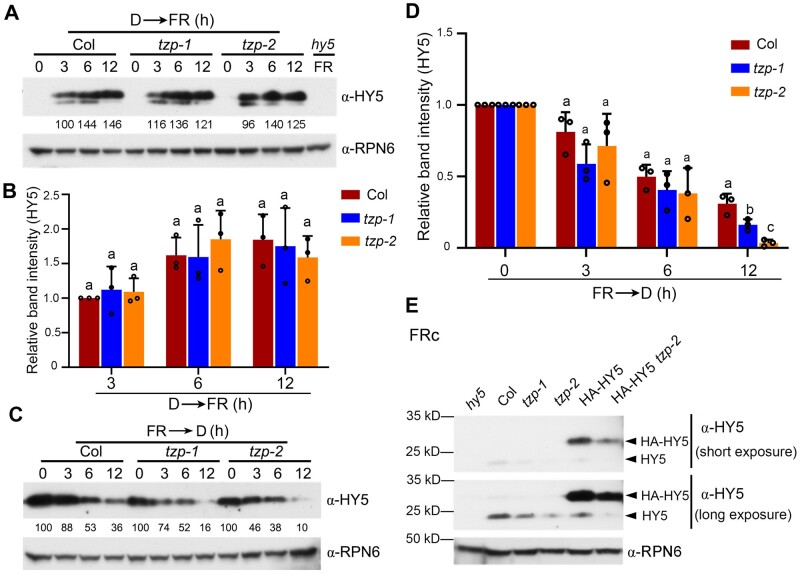
We recently showed that HY5 protein abundance was notably decreased in seedlings of two tzp mutants grown in continuous FR light (Zhang et al., 2018). To further investigate the molecular mechanisms underlying TZP regulation of HY5 protein abundance, Col and the two tzp mutants were first grown in darkness for 4 days, then transferred to FR light for the indicated times ranging from 3 to 12 h, and then harvested and subjected to immunoblotting. We observed that HY5 proteins accumulated rapidly and similarly upon FR light exposure in Col and the tzp mutants (Figure 5, A and B). These observations suggested that TZP may not be involved in regulating HY5 protein accumulation after short exposure to FR light. However, after we transferred 4-day-old FR-grown Col and tzp mutants to darkness for different times, we observed that HY5 was degraded faster in the tzp mutants than in Col (Figure 5, C and D), indicating that TZP enhances HY5 protein stability in the dark.
Figure 5.
TZP promotes HY5 protein abundance. A, Immunoblots showing HY5 protein levels in seedlings of Col and two tzp mutant lines grown in darkness (D) for 4 days and then transferred to FR light (50 μmol m−2 s−1) for the indicated times. Anti-RPN6 was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of HY5 normalized to those of RPN6 for each panel. The ratio of the first clear band was set to 100. B, Relative intensities of the bands in (A). Data are means ± sd from three independent biological assays. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). C, Immunoblots showing HY5 protein levels in seedlings of Col and two tzp mutant lines grown in FR (10 μmol m−2 s−1) for 4 days and then transferred to darkness (D) for the indicated times. Anti-RPN6 was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of HY5 normalized to those of RPN6 for each panel. The ratio of the first band was set to 100 in Col, tzp-1, and tzp-2, respectively. D, Relative intensities of the bands in (C). Data are means ± sd from three independent biological assays. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3). E, Immunoblots showing the HY5 protein levels in 4-day-old Col, tzp-1, tzp-2, HA-HY5#3, and HA-HY5#3 tzp-2 seedlings grown in FR light (10 μmol m−2 s−1). Anti-RPN6 was used as a sample loading control.
To investigate whether TZP regulates HY5 protein stability post-translationally, we grew HA-HY5 tzp-2 and HA-HY5 seedlings in continuous FR light for 4 days, and then compared the levels of HA-HY5 proteins by immunoblotting. Interestingly, we observed that HA-HY5 accumulated to a lower level in FR light in the absence of TZP (Figure 5E). The decreased levels of HA-HY5 proteins in the tzp mutants were not due to decreased expression of the transgene after genetic crossing (Supplemental Figure S7). These data suggest that TZP may promote HY5 protein abundance in FR light through post-translational mechanisms.
TZP physically interacts with COP1 but not with HY5
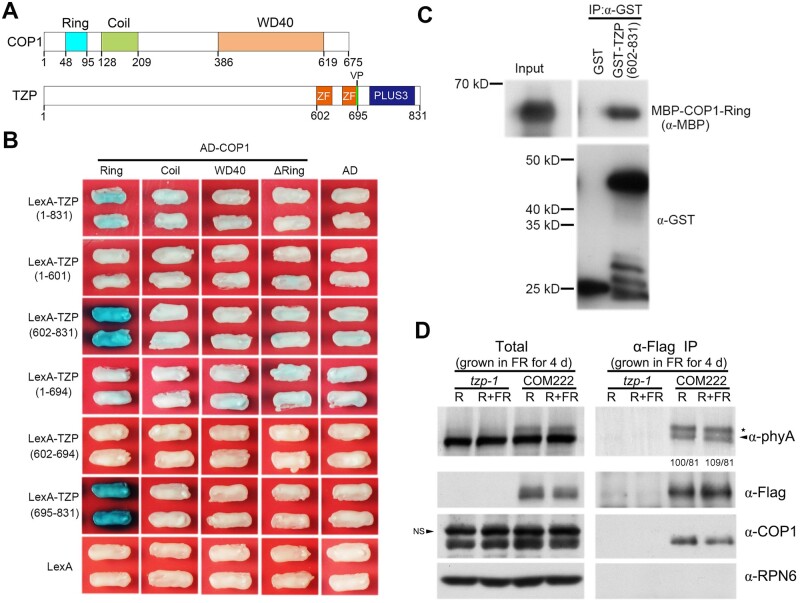
HY5 is targeted by COP1 for degradation via the 26S proteasome (Osterlund et al., 2000; Holm et al., 2002). Therefore, we asked whether TZP might be involved in regulating COP1-mediated HY5 degradation. To this end, we first performed yeast two-hybrid (Y2H) assays to examine whether TZP could physically interact with COP1. Bait vectors expressing various fragments of TZP fused to the LexA DNA binding domain, and prey vectors expressing various domains of COP1 fused to the activation domain (AD), were used in the assays. Intriguingly, we observed that the C-terminal domain of TANDEM ZINC-FINGER/PLUS3 (TZP) (a.a. 602–831) could directly interact with the RING-finger domain of COP1 (Figure 6, A and B). Pull-down assays showed that, indeed, the GST-tagged C-terminal domain of TZP, but not GST alone, was able to pull down the RING-finger domain of COP1 in vitro (Figure 6C). The C-terminal domain of TZP contains the tandem zinc-finger and PLUS3 domains, and our Y2H assays further showed that the PLUS3 domain was responsible for interacting with the RING-finger domain of COP1 (Figure 6B).
Figure 6.
TZP physically interacts with COP1. A, Domain structures of COP1 and TZP proteins. The numbers indicate the positions of each domain. B, Yeast two-hybrid assays showing that the C-terminal domain of TZP (both a.a. 602–831 and a.a. 695–831) interacts with the RING-finger domain of COP1 in yeast cells. C, Pull-down assays showing that the GST-tagged C-terminal domain of TZP (both a.a. 602–831), but not GST alone, could pull down the RING-finger domain of COP1 in vitro. MBP-tagged RING-finger domain of COP1 was incubated with immobilized GST or the GST-tagged C-terminal domain of TZP, and then the precipitated fractions were analyzed with anti-GST and anti-MBP antibodies, respectively. D, Co-IP assays showing that TZP interacted with COP1 in vivo. The tzp-1 and homozygous tzp complementation (COM) seedlings (TZPp:TZP-3flag tzp-1) were first grown in FR light (1 μmol m−2 s−1) for 4 days, and then the total proteins were extracted and treated with 5-min R light (20 μmol m−2 s−1) alone (R), or with 5-min R followed by 5-min FR light (20 μmol m−2 s−1; R+FR) before immunoprecipitation. After light treatments, total proteins were incubated with anti-Flag M2 Affinity Gel (Sigma-Aldrich). The total and precipitated proteins were subjected to immunoblot analyses with antibodies against Flag, COP1, phyA, and RPN6, respectively. The asterisk and arrowhead represent the phosphorylated and unphosphorylated phyA forms, respectively (Saijo et al., 2008). The numbers below anti-phyA IP blot indicate relative band intensities for the phosphorylated and unphosphorylated phyA, with the band intensity of phosphorylated phyA in the first lane set to 100. NS, non-specific bands.
Many COP1-interacting proteins, such as HY5, HYH, UV RESISTANCE LOCUS8 (UVR8), and cryptochromes, possess valine-proline (VP)-containing motifs that are essential for interacting with the WD40 domain of COP1 (Holm et al., 2001, 2002; Datta et al., 2006; Yin et al., 2015; Uljon et al., 2016; Ponnu et al., 2019). Interestingly, TZP contains only one instance of neighboring VP residues (VP693–694), which is located between the tandem zinc-finger and PLUS3 domains (Figure 6A). To investigate whether this VP motif is involved in mediating TZP interaction with COP1, we mutated both valine and proline to alanine residues (VP-AA), and tested the interaction of this mutated TZP with COP1 using Y2H assays. However, our data showed that the interactions between COP1 and full-length or C-terminal domain (a.a. 602–831) of TZP were not obviously affected by the VP-AA mutations (Supplemental Figure S8). Moreover, the PLUS3 domain of TZP (a.a. 695–831) lacking the VP motif strongly interacted with COP1 in yeast cells (Figure 6B), further indicating that TZP does not interact with COP1 via the VP motif.
To test for physical interaction between TZP and COP1 in vivo, we conducted coimmunoprecipitation (co-IP) assays using 4-day-old homozygous seedlings of TZPp:TZP-3flag tzp-1 complementation line #222 (COM222) grown in FR light. The tzp-1 mutant seedlings grown in the same conditions were used as a control. Our data showed that COP1 was coimmunoprecipitated by the anti-Flag antibody in TZPp:TZP-3flag tzp-1, but not in tzp-1 mutant seedlings (Figure 6D), indicating that COP1 associates with TZP in vivo. The in vivo association of TZP with phyA (Zhang et al., 2018) was used as a positive control. Interestingly, we observed that TZP preferentially associated with the phosphorylated form of phyA in vivo (Figure 6D), consistent with our finding that TZP plays a critical role in regulating phyA phosphorylation (Zhang et al., 2018).
We also performed Y2H assays to investigate whether TZP could physically interact with HY5. Our data showed that there was no obvious interaction between HY5 and TZP using various fragments of HY5 as bait and various fragments of TZP as prey, or using various fragments of TZP as bait and various fragments of HY5 as prey (Supplemental Figure S9, A–C). We also performed co-IP assays using 4-day-old 35S:HY5-GFP seedlings grown in FR light, and our data indicated that COP1, but not TZP, was coimmunoprecipitated by HY5-GFP (Supplemental Figure S9D). This conclusion was strengthened by another set of co-IP assays using two independent HA-HY5 lines expressing different levels of HA-HY5 proteins, which showed that larger amounts of COP1 were coimmunoprecipitated by higher levels of HA-HY5, whereas TZP was never shown to be coimmunoprecipitated by HA-HY5 (Supplemental Figure S9E). Collectively, our data suggest that TZP may not directly interact with HY5 in yeast cells and in vivo.
TZP inhibits the interaction between COP1 and HY5
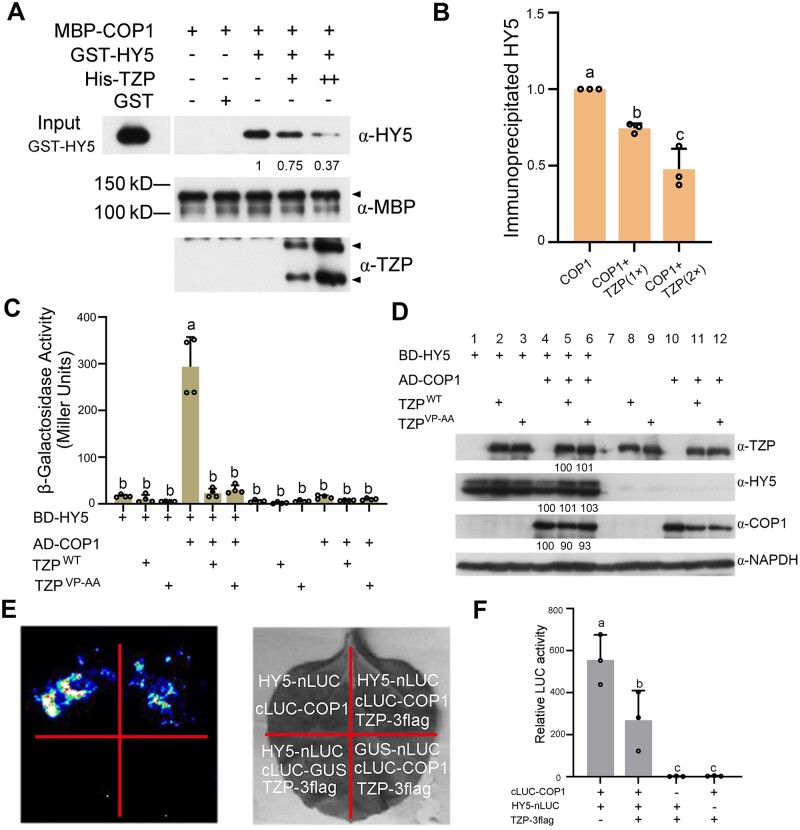
Considering that TZP directly interacts with COP1 (Figure 6) and that TZP promotes HY5 protein abundance post-translationally (Figure 5E), we asked whether TZP could regulate the interaction between COP1 and HY5. We first performed in vitro pull-down assays using MBP-COP1, GST-HY5, and His-TZP proteins expressed and purified from Escherichia coli. Our data showed that GST-HY5 proteins were pulled down by MBP-COP1 in the absence of His-TZP in vitro; however, addition of increasing amounts of His-TZP fusion proteins progressively reduced the amounts of GST-HY5 pulled down by MBP-COP1 (Figure 7, A and B).
Figure 7.
TZP inhibits the interaction between COP1 and HY5. A, TZP competes with HY5 to interact with COP1 in vitro. GST-HY5 was precipitated by MBP-COP1 using maltose agarose beads in the presence and absence of increasing concentrations of His-TZP. The pellet fraction was eluted and analyzed by immunoblotting using anti-HY5, anti-TZP, and anti-MBP antibodies, respectively. Numbers below the anti-HY5 blots indicate the relative band intensities of co-precipitated GST-HY5 normalized to that of precipitated MBP-COP1, respectively. The ratio of the first band was set to 1 for the blot. B, Relative intensities of pulled-down GST-HY5 bands in (A). Error bars represent se of three independent assays (n = 3; Supplemental Data Set S3). C, Yeast three-hybrid assays showing that TZP represses the interaction of COP1 with HY5 in yeast cells. AD-COP1, BD-HY5, TZP, and TZPVP-AA proteins were expressed in the yeast strain EGY48 as indicated. The β-galactosidase activities were measured by liquid culture assays using o-nitrophenyl-β-D-galactopyranoside as the substrate. Data are means ± sd of four independent yeast clones. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05). D, Immunoblots showing the levels of AD-COP1, BD-HY5, and TZP proteins in yeast cultures co-expressing the indicated combinations of proteins. Anti-GAPDH was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of AD-COP1, BD-HY5, and TZP normalized to those of loading control, respectively. The ratio of the first band was set to 100 for each blot. E, F, LCI assays showing that TZP inhibits COP1 interaction with HY5 in plant cells. A representative picture is shown in (E), and relative luciferase activity is shown in (F). Data are the means ± sd of three independent assays. Different letters represent significant differences by one-way ANOVA with Tukey’s post hoc test (P < 0.05; Supplemental Data Set S3)
We then performed yeast three-hybrid assays by expressing LexA-HY5, AD-COP1, and TZP proteins in yeast cells. Our data indicated that LexA-HY5 interacted robustly with AD-COP1 in yeast cells; however, the coexpression of TZP dramatically decreased their interaction (Figure 7C). Immunoblot data excluded the possibility that the decreased interaction was due to lower levels of LexA-HY5 or AD-COP1 proteins caused by the coexpression of TZP in yeast cells (Figure 7D). Although it was recently shown that CRY2 competes with VP-containing COP1 substrates (such as PRODUCTION OF ANTHOCYANIN PIGMENT2) via its VP motif (Ponnu et al., 2019), our data showed that both wild-type and mutated (VP-AA) TZP proteins totally abolished the interaction of HY5 with COP1 in yeast cells (Figure 7, C and D).
To further explore whether TZP acts to disrupt the interaction between HY5 and COP1 in planta, we performed luciferase complementation imaging (LCI) assays to compare the interactions between HY5-nLUC and cLUC-COP1 in Nicotiana benthamiana leaves in the presence and absence of coexpressed TZP. Our data showed that HY5-nLUC interacted strongly with cLUC-COP1 in N. benthamiana leaves in the absence of TZP; however, the coexpression of TZP-3flag led to a significant decrease in the interaction between HY5-nLUC and cLUC-COP1 (Figure 7, E and F). Collectively, our data demonstrate that TZP inhibits the interaction between COP1 and HY5, which may lead to enhanced stability of HY5 proteins in darkness (Figure 5, C and D) and FR light (Figure 5E).
COP1 negatively regulates TZP protein stability in darkness and FR light
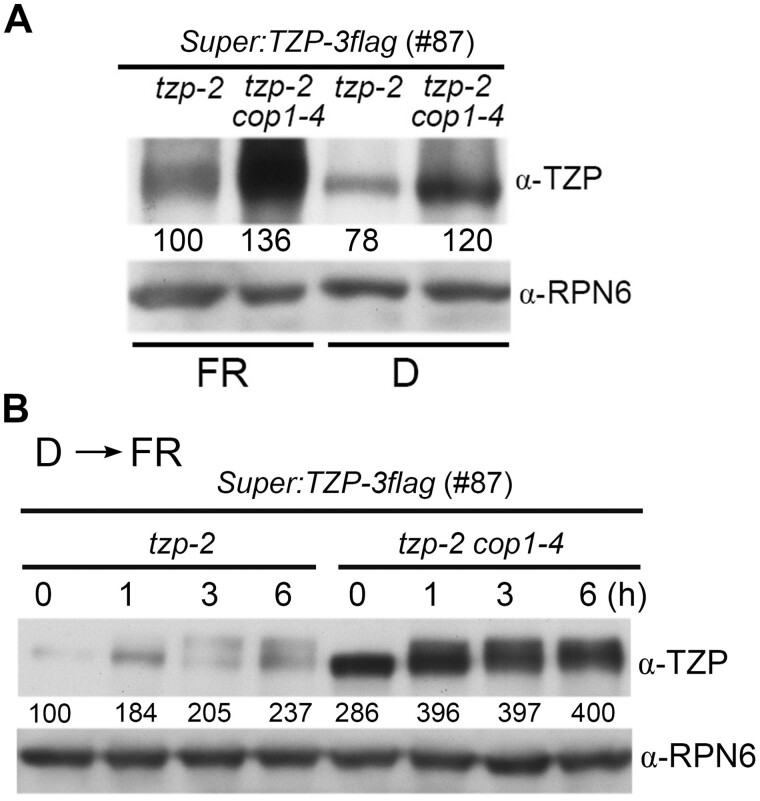
Finally, we asked whether TZP protein stability could be regulated by COP1 since TZP physically interacts with COP1. We generated the homozygous Super:TZP-3flag tzp-2 line, and crossed it with the cop1-4 mutant to obtain homozygous Super:TZP-3flag tzp-2 cop1-4 seedlings. We then grew them together in darkness and FR light for 4 days, and then compared the levels of TZP-3flag fusion proteins by immunoblotting. Our results showed that TZP-3flag protein levels were drastically increased in the absence of COP1 in both dark and FR light conditions (Figure 8A), and that the increase in protein abundance was not due to increased expression of the transgene in the cop1-4 background after genetic crossing (Supplemental Figure S10). In addition, after we transferred 4-day-old etiolated Super:TZP-3flag tzp-2 and Super:TZP-3flag tzp-2 cop1-4 seedlings to FR light, we observed that the levels of TZP-3flag proteins were indeed higher in the absence of COP1 at all times (Figure 8B). Moreover, our data indicated that TZP-3flag proteins were modified in response to FR light regardless of the presence or absence of COP1 (Figure 8B). Together, we conclude that TZP protein is destabilized by COP1 in darkness and FR light, and that TZP protein modification is dependent on light.
Figure 8.
COP1 negatively regulates the stability of TZP proteins in darkness and FR light. A, Immunoblots showing the levels of TZP proteins in 4-day-old Super:TZP-3flag tzp-2 (#87) and Super:TZP-3flag tzp-2 (#87) cop1-4 seedlings grown in darkness (D) and FR light (10 μmol m−2 s−1). B, Immunoblots showing the levels of TZP proteins in Super:TZP-3flag tzp-2 (#87) and Super:TZP-3flag tzp-2 (#87) cop1-4 seedlings grown in darkness for 4 days, and then transferred to FR light (50 μmol m−2 s−1) for the indicated times. In both (A) and (B), Anti-RPN6 was used as a sample loading control. Numbers below the immunoblots indicate the relative band intensities of TZP-3flag normalized to those of RPN6 for each panel. The ratio of the first band was set to 100 for each blot.
Discussion
TZP was recently identified as a key component of phyA signaling that can physically interact with the phyA photoreceptor (Zhang et al., 2018). Notably, our previous data showed that TZP transcript and protein levels were dramatically induced by light (Zhang et al., 2018). In this study, we demonstrated that HY5 mediates light induction of TZP expression by directly binding to a G-box motif in the TZP promoter (Figures 1 and 2). Moreover, our data indicated that TZP in turn promotes HY5 protein abundance post-translationally in FR light, and the mechanism may be explained, at least in part, by the fact that TZP physically interacts with COP1 and competes with HY5 for binding to COP1 as another substrate (Figures 5–8). Thus, our study uncovers a mutual relationship between TZP and HY5 in propagating the FR light signal: HY5 transcriptionally activates TZP expression, while TZP post-translationally promotes HY5 protein stability (Figure 9). Moreover, our genetic analyses showed that tzp hy5 double mutants developed longer hypocotyls than their respective single mutants under high fluence rates of FR light (Figure 4, A and B;Supplemental Figure S5), indicating that TZP and HY5 also function in largely independent pathways under high FR light intensities. Notably, our main findings, including TZP regulation of hypocotyl growth, coordinated regulation of hypocotyl growth by TZP and HY5, and TZP regulation of HY5 protein stability in FR light, are well reproduced using sucrose-free medium (Supplemental Figure S11), indicating that the functions of TZP uncovered in this study are not impacted by sucrose or sucrose metabolism.
Figure 9.
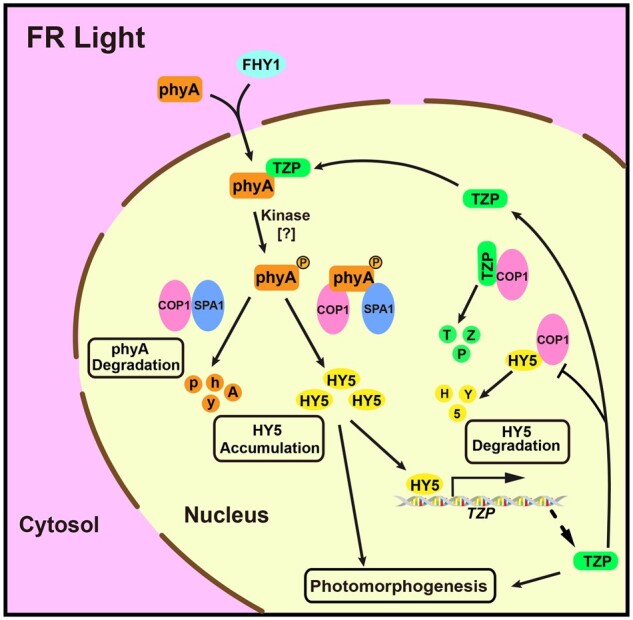
Working model depicting both interdependent and independent functions of HY5 and TZP in transmitting the FR light signal. HY5 transcriptionally activates TZP expression in FR light by directly binding to a G-box motif in the TZP promoter. TZP competes with HY5 for binding to COP1, and post-translationally promotes HY5 protein abundance in FR light. As a result, TZP protein itself is destabilized by COP1. In addition to their interdependent functions, TZP and HY5 also play independent roles in regulating photomorphogenesis under FR light. In addition, TZP was shown to be required for the production of the phosphorylated phyA form in the nucleus in FR light (Zhang et al., 2018).
TZP post-translationally promotes HY5 protein abundance in FR light
HY5 acts as a master regulator of seedling photomorphogenesis, as reflected by the fact that the abundance of HY5 protein directly correlates with the extent of photomorphogenesis (Osterlund et al., 2000). In this study, we showed that HA-HY5 driven by the UBQ10 promoter accumulated to a lower level in FR light in the absence of TZP (Figure 5E), and the decrease in HA-HY5 protein abundance was not due to lower expression of transgene after crossing (Supplemental Figure S7), thus suggesting that TZP may post-translationally promote HY5 protein abundance in FR light. Indeed, our data demonstrated that TZP can physically interact with COP1 (Figure 6), and this interaction inhibits COP1 interaction with HY5 (Figure 7). Thus, reduced interaction between COP1 and HY5 may lead to enhanced HY5 protein stability in FR light. In addition, we previously showed that TZP is required for the production of the phosphorylated phyA form in the nucleus in FR light, and that the phosphorylated phyA may represent a more active phyA form in vivo (Zhang et al., 2018; Zhou et al., 2018). Thus, the lack of active phosphorylated phyA in tzp mutants may result in insufficient inactivation of the COP1/SPA complexes, leading to reduced accumulation of HY5 proteins in FR light (Zhang et al., 2018). Therefore, TZP may promote HY5 protein accumulation in FR light through at least two distinct post-translational mechanisms, i.e. facilitating the production of active phosphorylated phyA (thus inactivating the COP1/SPA complexes more efficiently), and inhibiting COP1 interaction with HY5 (Figure 9). However, the relative contribution of each post-translational mechanism in promoting HY5 protein abundance in FR light needs further characterization. In addition, as HY5 was shown to activate its own expression (Abbas et al., 2014), decreased HY5 protein stability in the absence of TZP should lead to reduced levels of HY5 transcripts in tzp mutants. Consistent with this prediction, our recent study showed that HY5 transcript levels were slightly decreased in two tzp mutant lines in FR light (Zhang et al., 2018).
Interdependent and independent roles of HY5 and TZP in mediating phyA signaling
It was previously shown by ChIP-chip assays that HY5 could target approximately 9,600 Arabidopsis genes in white light (Zhang et al., 2011). In this study, our RNA-seq analyses indicated that the expression of over 7,200 genes was significantly changed in hy5 mutants in continuous FR light (Figure 4D). However, despite the hierarchical role of HY5 in mediating light signaling, our RNA-seq data revealed that 871 genes were differentially expressed in tzp but not in hy5 mutants, and that over 4,100 genes were differentially expressed only in tzp hy5 double mutants in continuous FR light (Figure 4D). In addition, our RNA-seq analyses revealed that TZP may play a similarly important role as HY5 in mediating rapid changes in the expression of early FR-responsive genes, although HY5 may play a more dominant role than TZP in modulating gene expression after prolonged FR light exposure (Supplemental Figure S6B). These transcriptome analyses demonstrate that TZP plays a critical role in modulating gene expression in FR light. Moreover, our genetic data showed that tzp hy5 double mutants were almost indistinguishable from hy5 mutant seedlings under low fluence rates of FR light, but developed longer hypocotyls than their respective single mutant seedlings under high fluence rates of FR light (Figure 4, A and B;Supplemental Figure S5), indicating that hy5 and tzp mutations had both epistatic and additive effects on hypocotyl growth that are dependent on FR light intensities. Consistent with these effects, RT-qPCR data revealed epistatic effects of hy5 and tzp mutations on PRP2 expression at low fluence rates of FR light, but additive effects at high fluence rates of FR light (Figure 4E). The occurrence of epistatic effects for hypocotyl growth and gene expression is consistent with the existence of mutual upregulation of TZP and HY5 demonstrated in this study, and this epistasis appears to be important at limiting levels of FR light. However, the additive effects of hy5 and tzp mutations on hypocotyl growth and gene expression indicate that HY5 and TZP also play independent functions in mediating phyA signaling. Collectively, our study uncovers both interdependent and independent roles of HY5 and TZP in propagating the FR light signal, thus providing new insights into the action mechanisms of these two key positive regulators of phyA signaling.
TZP is destabilized by COP1 in both darkness and FR light
Many COP1-interacting proteins, including HY5, HYH, UVR8, and CRY1/CRY2, possess the VP motifs that are essential for interacting with COP1 (Holm et al., 2001, 2002; Datta et al., 2006; Yin et al., 2015; Uljon et al., 2016; Ponnu et al., 2019). Recently, CRY2 was shown to promote the stability of COP1 substrates by competing for binding to COP1 via the VP motif in CRY2 (Ponnu et al., 2019). TZP also contains one such potential VP motif (Figure 6A); however, our results showed that this VP motif is not essential for TZP interaction with COP1 (Supplemental Figure S8) or for competing with HY5 to bind to COP1 (Figure 7, C and D). Moreover, both phytochromes and cryptochromes were shown to be degraded by COP1 (Shalitin et al., 2002; Seo et al., 2004; Jang et al., 2010; Weidler et al., 2012; Liu et al., 2016; Chen et al., 2021), and interestingly, our data showed that TZP protein is also destabilized by COP1 in both dark and FR light conditions (Figure 8). Therefore, it is evident that phytochromes, cryptochromes, and TZP promote the accumulation of HY5, but at the expense of being tagged for degradation by COP1 themselves. However, it should be noted that although TZP proteins accumulate in FR, R, B, and W light (Figure 3), TZP inhibition of COP1-mediated HY5 degradation may not be a general mechanism in all light conditions because the phenotypes of tzp mutants indicated that TZP acts as a negative regulator of B light signaling although TZP positively regulates FR light responses (Zhang et al., 2018). Thus, the relationship between TZP and HY5/COP1 in other light conditions (especially under B light) remains to be investigated in future research.
The biochemical properties of TZP?
Accumulating evidence shows that TZP plays pleiotropic roles in light signaling: the PLUS3 domain of TZP is able to bind single-stranded DNA (KaiserLi et al., 2015); TZP could act as a transcriptional regulator and associate with its target gene promoters by directly interacting with transcription factors (KaiserLi et al., 2015; Perrella et al., 2018); TZP is required for the production of the phosphorylated phyA form in vivo (Zhang et al., 2018). However, the biochemical properties of TZP still remain obscure. TZP does not contain a protein kinase domain, and its action mechanism in regulating phyA phosphorylation needs to be investigated in future studies. Nevertheless, our results, together with those from our previous study (Zhang et al., 2018), indicate that TZP plays a key role in transmitting the FR light signal and works in close concert with the central components of the phyA signaling pathway, including HY5, COP1 and the phyA photoreceptor itself. Our study thus provides critical insights into the molecular mechanisms of the enigmatic phyA signaling networks.
Materials and methods
Plant materials and growth conditions
The wild-type A. thaliana plants used in this study were the Columbia (Col) ecotype, unless otherwise indicated. The following mutants and transgenic plants have been previously described: tzp-1, tzp-2, and TZPp:TZP-3flag tzp-1 (#222; COM222; Zhang et al., 2018), hy5-215 (Oyama et al., 1997), and UBQ10p:HA-HY5 (HA-HY5) (#3 and #9; Li et al., 2020). The tzp-1 hy5, tzp-2 hy5, HA-HY5 (#3) tzp-1, HA-HY5 (#3) tzp-2, Super:TZP-3flag tzp-2 (#87) cop1-4 and TZPp-1:GUS hy5 were generated by genetic crossing. The light sources and growth conditions were as described previously (Yan et al., 2020). W light was provided by F17T8/TL841 bulb (Philips), while FR, R and B lights were provided by Snap-Lite LED modules (Quantum Devices). The fluence rates of the light growth chambers (Percival Scientific) were 50 μmol m−2 s−1 for continuous W light, 10 μmol m−2 s−1 for continuous R light, 3 μmol m−2 s−1 for continuous B light, and the indicated intensities for FR light. To grow Arabidopsis seedlings, the seeds were first surface sterilized, stratified in the dark at 4°C for 2–4 days, and then grown on Murashige and Skoog medium (pH 5.7) supplemented with 0.8% (w/v) agar (cat. no. A1296; Sigma-Aldrich) with 1% sucrose or without sucrose.
Plasmid construction and generation of transgenic Arabidopsis plants
To generate the GUS reporter gene driven by various fragments of the TZP promoter, a series of TZP promoter fragments upstream of the ATG start codon plus the first 45-bp region of the TZP coding sequence were amplified by PCR using genomic Col DNA as template and the respective pairs of primers listed in Supplemental Data Set S2, respectively, and then cloned into the XbaI–BamHI sites of the pBI101 vector. To mutate the G-box motifs in the TZP promoter, site-directed mutagenesis was performed using the indicated primer pairs (see Supplemental Data Set S2) to mutate G-box1 and G-box2 separately or together.
To generate the TZPp-A: LacZ to TZPp-G: LacZ, TZPp-F1: LacZ, and TZPp-F2: LacZ reporter constructs, the respective promoter fragments were amplified by PCR using genomic Col DNA as template and the corresponding pairs of primers (see Supplemental Data Set S2), and then cloned into the EcoRI–XhoI sites of the pLacZi2μ vector (Lin et al., 2007), respectively. To generate mutant TZPp-F-mutG-box1:LacZ and TZPp-F2-mutG-box1:LacZ reporter constructs, the promoter fragments were amplified by PCR using TZPp-1-mutGbox1:GUS as template, and then cloned into the EcoRI–XhoI sites of the pLacZi2μ vector, respectively.
The AD-HY5, AD-HYH, AD-PIF3, AD-LAF1, AD-PIF1, AD-PIF5, AD-COP1, AD-COP1-WD40, AD-COP1-ΔRing, and LexA-HY5, LexA-HY5 (1–77), LexA-HY5 (78–168), and LexA-HY5 (1–115) constructs were described previously (Ang et al., 1998; Dong et al., 2014; Li et al., 2010). To generate the prey vectors expressing various truncated forms of TZP, COP1 and HY5, the corresponding fragments were amplified by PCR using the primer pairs shown in Supplemental Data Set S2, and then cloned into the EcoRI–XhoI sites of the pB42AD vector (Clontech), respectively. To generate the bait vectors expressing various truncated forms of TZP, the corresponding fragments were amplified by PCR with the respective pairs of primers (see Supplemental Data Set S2), and then cloned into the EcoRI–XhoI sites of the pLexA vector (Clontech), respectively. To generate the pGADT7-TZP construct for expressing TZP proteins in yeast cells, the full-length coding sequence of TZP was amplified by PCR using the primer pairs shown in Supplemental Data Set S2, and then cloned into the KpnI–XhoI sites of the pGADT7 vector (Clontech). To generate the LexA-TZPVP-AA, LexA-TZP (602–831)VP-AA, and pGADT7-TZPVP-AA constructs, site-directed mutagenesis was performed using the indicated primer pairs (see Supplemental Data Set S2) to mutate the valine VP-AA in the TZP protein, respectively.
The MBP-COP1 and His-HY5 constructs were described previously (Chen et al., 2012; Lian et al., 2017). To generate His-TZP and His-TZP (350–550) constructs, the corresponding PCR fragments of TZP were cloned into the EcoRI–XhoI and BamHI–EcoRI sites of the pET-28a vector (Novagen), respectively. To generate the GST-TZP (602-831) construct, the corresponding PCR fragments of TZP were cloned into the EcoRI–XhoI sites of the pGEX-4T-1 vector (Amersham Biosciences), respectively. To generate the MBP-COP1-Ring construct, the corresponding PCR fragment of COP1 was cloned into the EcoRI–SalI sites of the pMAL-c2X vector (New England BioLabs). The primers used are listed in Supplemental Data Set S2.
The HY5-nLUC, GUS-nLUC, and cLUC-GUS constructs were described previously (Li et al., 2010; Wang et al., 2019a). To generate cLUC-COP1, the corresponding PCR fragment of COP1 was cloned into the KpnI–SalI sites of the 35S:cLUC vector (Chen et al., 2008).
To generate the Super:TZP-3flag construct, the full-length coding sequence of TZP was amplified by PCR using the pair of primers shown in Supplemental Data Set S2, and then cloned into the BamHI–KpnI sites of the pSuper1300-3flag vector (Qi et al., 2020).
To generate the HY5-GFP construct, a GFP fragment amplified from 35S:GFP-FHY1 (Shen et al., 2005) was inserted into the SalI–EcoRI sites of pRI101 (Takara) to obtain the pRI-GFP vector in which the original SalI and EcoRI sites were eliminated. Then, the EcoRI–XhoI fragment containing the full-length coding sequence of HY5 was ligated into the pRI-GFP vector digested by EcoRI and SalI, giving rise to the binary construct 35S:HY5-GFP.
To generate various transgenic plants, the corresponding constructs were transformed into Agrobacterium tumefaciens (strain GV3101), and then into Arabidopsis plants (Col for TZPp-1:GUS to TZPp-5:GUS, TZPp-1-mutG-box1:GUS, TZPp-1-mutG-box2:GUS, TZPp-1-mutG-box1&2:GUS, TZPp-4-mutG-box1:GUS, and 35S:HY5-GFP; tzp-2 for Super:TZP-3flag) by the floral dip method (Clough and Bent, 1998).
All of the primers used to generate the above-mentioned constructs are listed in Supplemental Data Set S2, and all constructs were confirmed by sequencing prior to usage in the various assays.
GUS staining
More than eight independent TZPp:GUS transgenic lines homozygous for a single copy of the reporter gene were obtained. GUS activity analysis was performed as described previously (Jefferson et al., 1987).
RNA extraction and RT-qPCR
Total RNA was extracted using the RNAprep Pure Plant Kit (Tiangen) from Arabidopsis seedlings subjected to the indicated light treatments. cDNA was synthesized from one microgram of total RNA using ReverAid First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. RT-qPCR was performed using the gene-specific primers listed in Supplemental Data Set S2 and PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) with a Step One Plus Real-Time PCR System (Applied Biosystems). PCR reactions were performed in triplicate for each sample, and the relative expression levels were normalized to that of TUBULIN3. Materials for RT-qPCR assays were collected from three biological replicates.
Yeast assays
Yeast one-hybrid and yeast two-hybrid assays were performed as described previously (Li et al., 2010; Zhang et al., 2018). For yeast three-hybrid assays, the constructs to express bait BD-HY5 (LexA-HY5), prey AD-COP1, and TZP proteins were co-transformed into the yeast strain EGY48. Yeast transformation was conducted as described in the Yeast Protocols Handbook (Clontech). Yeast transformants were then selected on SD/–Ura–Trp–Leu–His agar plates for 4 days at 30°C. The yeast cultures were cultivated overnight in 0.5 mL of SD/–Ura–Trp–Leu–His liquid medium supplemented with 2% glucose, then the overnight cultures were transferred into 0.5 mL SD/–Ura–Trp–Leu–His liquid medium supplemented with 1% Raffinose and 2% Galactose, and cultured with shaking in darkness for 3 h. The liquid assays to measure β-galactosidase activities using o-nitrophenyl-β-d-galactopyranoside as substrate were performed as described previously (Dong et al., 2020).
EMSA
EMSAs were performed using the biotin-labeled probes listed in Supplemental Data Set S2 and the LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions with minor modifications. Briefly, 2-μg purified His-HY5 protein was incubated together with unlabeled probes or biotin-labeled wild-type or mutant probes in 20-μL reaction mixtures containing 50-mM KCl, 10-mM Tris–HCl, 1-mM DTT, 2.5% (v/v) glycerol, and 50 ng·mL−1 poly(dI-dC). The binding reactions were incubated at 25°C for 20 min in a thermal cycler (Bio-Rad), and then separated on 6% native polyacrylamide gels in TBE buffer. The labeled probes were detected according to the manufacturer’s instructions provided with the EMSA kit.
ChIP
ChIP assays were performed as described previously (Wang et al., 2019b). Wild-type (Col) or hy5 seedlings grown under FR light for 4 days were used for ChIP assays. Briefly, 2 g of seedlings were first treated with 1% formaldehyde to cross-link the protein–DNA complexes under vacuum. After isolation, the resuspended chromatin was sonicated to 250- to 500-bp fragments at 4°C. The sheared chromatin was then incubated with anti-rabbit IgG (I5006; Sigma-Aldrich) or with polyclonal anti-HY5 antibodies (Li et al., 2010). The precipitated DNA samples were quantified by qPCR using the respective pair of primers listed in Supplemental Data Set S2. The ChIP values were normalized to their respective DNA input values.
Total protein extraction and immunoblotting
Total proteins were extracted as described previously (Qiu et al., 2017). Briefly, 100 mg 4-day-old seedlings grown under the indicated light conditions were harvested in the dark room and then homogenized in 300-µL extraction buffer consisting of 100-mM Tris–HCl, pH 7.5, 100-mM NaCl, 5-mM EDTA, pH 8.0, 5% SDS, 20% glycerol, 20-mM DTT, 40-mM β-mercaptoethanol, 2-mM PMSF, 1× EDTA-free protease inhibitor cocktail (Roche), 80-μM MG132 (Sigma), 80-μM MG115 (Sigma), 1% phosphatase inhibitor cocktail 3 (Sigma), and 10-mM N-ethylmaleimide under dim green light. Samples were immediately boiled for 10 min and then centrifuged at 16,000g for 10 min at room temperature. Proteins from the supernatant were used in the subsequent immunoblot assays. Immunoblotting was performed as previously described (Shen et al., 2005). Primary antibodies used in this study include anti-Flag (1:2,000 [v/v], catalog no. M20008; abmart), anti-MBP (1:2,000 [v/v], catalog no. abM59007-3-PU; BPI), anti-HY5 (1:1,000 [v/v]; Li et al, 2010), anti-COP1 (1:1,000 [v/v]; Zhang et al., 2018), anti-phyA (1:2,000 [v/v]; Zhang et al., 2018), anti-GST (1:3,000 [v/v], catalog no. G7781; Sigma-Aldrich), anti-RPN6 (1:3,000 [v/v]; Zhou et al., 2018), and anti-GAPDH (1:5,000 [v/v], catalog no. AC033; ABclonal).
The anti-TZP antibodies were made by Beijing Protein Innovation Co. Ltd (BPI). Briefly, His-TZP (350–550) proteins expressed in E. coli were used as antigens to immunize mice, and purified monoclonal antibodies were used in immunoblots (1:1,000 dilutions).
In vitro pull-down assays
Proteins with different tags were expressed in E. coli (BL21-codon plus) cells and purified as described previously (Lv et al, 2021). For in vitro pull-down assays to show the interaction between GST-TZP (602-831) and MBP-COP1-Ring, 2.5 μg of purified recombinant bait proteins (GST-TZP [602-831] or GST) were mixed with 2.5 μg of prey proteins (MBP-COP1-Ring) in 200 μL of binding buffer (50-mM Tris–HCl, pH 7.5, 100-mM NaCl, 0.6% Triton X-100, and 0.2% glycerol). After incubation at 4°C for 2 h, the mixture was incubated with Glutathione Sepharose 4B beads (GE Healthcare) and incubated for another 2 h. Then, the beads were washed six times with the binding buffer, and the precipitated proteins were eluted in 2× sodium dodecyl sulfate (SDS) loading buffer at 95°C for 15 min. The input and eluted proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) gels, and immunoblotted with anti-MBP or anti-GST antibodies, respectively.
The in vitro pull-down assays showing the competitive binding of HY5 and TZP to COP1 were performed as described previously (Xu et al., 2014). Briefly, MBP-COP1, GST-HY5, and His-TZP proteins were expressed and purified from E. coli. Then, the indicated combinations of recombinant proteins were incubated with 20 μL of amylose resin in 200 μL of binding buffer (50-mM Tris-HCl, pH 7.5, 150-mM NaCl, 0.6% Tween-20, and 1-mM DTT) for 3 h. The beads were collected and washed six times with 5 min of rotation each time with binding buffer, and the precipitated proteins were eluted in 2× SDS loading buffer at 95°C for 15 min. The eluted proteins were separated on SDS–PAGE gels, and probed with anti-HY5, anti-MBP or anti-TZP antibodies, respectively. The intensities of the GST-HY5 bands from three independent assays were quantified using ImageJ software. The sample without TZP was set to 1 from these ratios, and the relative values of the other samples were calculated based on this value. These relative values are shown as bar graphs.
Co-IP assays
For co-IP assays, the tzp-1 and homozygous TZPp:TZP-3flag tzp-1 #222 (COM222) transgenic seedlings were first grown in FR light for 4 days, and then harvested and homogenized in 2 mL of protein extraction buffer (50-mM Tris–HCl, pH 7.5, 150-mM NaCl, 10-mM MgCl2, 1-mM EDTA, 0.1% Nonidet P-40, 1-mM phenylmethanesulfonyl fluoride (PMSF), 1× MG132, and 1× EDTA-free protease inhibitor cocktail [Roche]) and centrifuged twice at 12,000g for 15 min at 4°C. Then, the extracts were divided into two equal parts, and treated with 5-min R light (R) or with 5-min R light followed by 5-min FR light (R+FR), respectively. After the light treatments, 100 μL of each sample was reserved as “total” and the remaining 900 μL were incubated with an anti-Flag M2 Affinity Gel (Sigma-Aldrich) for 2 h at 4°C. Then, the beads were washed three times with extraction buffer, and the immunoprecipitated proteins were analyzed by immunoblotting using anti-Flag, anti-phyA, anti-COP1, and anti-RPN6 antibodies, respectively.
LCI assays
Transient LCI assays in N. benthamiana were performed as described previously (Chen et al, 2008) with minor modifications. Briefly, the HY5-nLUC, cLUC-COP1, and Super:TZP-3flag constructs were transformed into Agrobacterium strains GV3101, then the Agrobacteria containing the indicated constructs were mixed and infiltrated into young but fully expanded leaves of 7-week-old N. benthamiana plants using a 2-mL needleless syringe. After infiltration, plants were grown under 16-h light/8-h dark for 2–3 days. Before imaging, the abaxial sides of leaves were sprayed with 1-mM luciferin, and then a CCD camera (1300B; Roper) was used to capture the LUC signal at −110°C with 10-min exposures.
Transcriptome analyses
For RNA-seq analysis, total RNA was extracted using the RNAprep Pure Plant Kit (TIANGEN) from Col, tzp-2, hy5, and tzp-2 hy5 mutant seedlings grown under continuous FR light for 4 days, and from Col, hy5, tzp-2, and tzp-2 hy5 mutant seedlings grown in darkness for 4 days and then transferred to FR light for 1 h and 6 h. Sequencing was carried out with the Illumina HiSeq 2000 platform and the resulting reads were mapped to the reference genome of A. thaliana (TAIR10) with TopHat (http://tophat.cbcb.umd.edu; Kim et al., 2013). Differentially expressed genes were analyzed using Cufflinks (Trapnell et al., 2013; http://cufflinks.cbcb.umd.edu), and transcript abundance was estimated by fragments per kilobase of exon model per million mapped fragments. Multiple testing was corrected via a false discovery rate (FDR) estimation and FDR values below 0.05 were considered to indicate differential expression.
Quantification and statistical analysis
Protein quantification was performed with ImageJ. One-way analysis of variance (ANOVA) and Student’s t test were performed with SPSS statistical software. Different letters above bars represent statistically significant differences at P < 0.05 for multiple comparisons, and levels that are not significantly different are indicated with the same letters. Values are represented as means ± sd.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: TZP (At5g43630), PHYA (At1g09570), HY5 (At5g11260), COP1 (At2g32950), ELIP2 (At4g14690), and PRP2 (At2g21140). The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Chen et al., 2021) in National Genomics Data Center (CNCB-NGDC Members and Partners, 2021), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number CRA004930 that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. G-box1 is essential for TZP expression in FR light.
Supplemental Figure S2. Specificity of anti-TZP antibodies.
Supplemental Figure S3. TZP expression is rapidly activated by HY5 upon exposure to FR, R, and B light.
Supplemental Figure S4. Genotyping of tzp and hy5 mutations by immunoblotting.
Supplemental Figure S5. Phenotypes of tzp, hy5, and tzp hy5 mutant seedlings grown under different fluence rates of FR light.
Supplemental Figure S6. RNA-seq analyses showing FR-regulated genes in Col, hy5, tzp-2, and tzp-2 hy5 mutant seedlings upon FR exposure.
Supplemental Figure S7. Expression levels of HA-HY5 in UBQ10p:HA-HY5 and UBQ10p:HA-HY5 tzp-2 seedlings grown in FR light.
Supplemental Figure S8. The VP motif of TZP is not essential for the interaction with COP1.
Supplemental Figure S9. TZP does not directly interact with HY5 in yeast cells and in vivo.
Supplemental Figure S10. Expression levels of TZP in Super:TZP-3flag tzp-2 and Super:TZP-3flag tzp-2 cop1-4 seedlings grown in FR light.
Supplemental Figure S11. TZP regulation of hypocotyl growth and HY5 accumulation in FR light in the absence of sucrose.
Supplemental Data Set S1. List of differentially expressed genes in hy5, tzp-2, and tzp-2 hy5 mutant seedlings in FR light compared with those in Col.
Supplemental Data Set S2. Primers used in this study.
Supplemental Data Set S3. Statistical analyses.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31970262, 31770321, and 32000187), China National Postdoctoral Program for Innovative Talents (BX20200371), China Postdoctoral Science Foundation (2020M670531 and 2021M693432), Chinese Universities Scientific Fund (2021TC064), and Beijing Outstanding University Discipline Program.
Conflict of interest statement. The authors declare no conflict of interests.
Supplementary Material
J.L., C.L., and L.Q. designed research. C.L., L.Q., S.Z., X.D., Y.J., Z.F., H.L., Y.Z., X.W., R.H., J.P., and J.D. performed research. J.C. analyzed the RNA-sequencing data. J.L. and R.L. discussed and interpreted the data. J.L., C.L., L.Q., and W.T. wrote the paper.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Jigang Li (jigangli@cau.edu.cn).
References
- Abbas N, Maurya JP, Senapati D, Gangappa SN, Chattopadhyay S (2014) Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 26: 1036–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Bae G, Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol 59: 281–311 [DOI] [PubMed] [Google Scholar]
- Ballesteros ML, Bolle C, Lois LM, Moore JM, Vielle-Calzada JP, Grossniklaus U, Chua NH (2001) LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev 15: 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burko Y, Seluzicki A, Zander M, Pedmale UV, Ecker JR, Chory J (2020) Chimeric activators and repressors define HY5 activity and reveal a light-regulated feedback mechanism. Plant Cell 32: 967–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canibano E, Bourbousse C, Garcia-Leon M, Garnelo Gomez B, Wolff L, Garcia-Baudino C, Lozano-Duran R, Barneche F, Rubio V, Fonseca S (2021) DET1-mediated COP1 regulation avoids HY5 activity over second-site gene targets to tune plant photomorphogenesis. Mol Plant 14: 963–982 [DOI] [PubMed] [Google Scholar]
- Casal JJ (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Shi X, Chen L, Dai M, Zhou Z, Shen Y, Li J, Li G, Wei N, Deng XW (2012) Phosphorylation of FAR-RED ELONGATED HYPOCOTYL1 is a key mechanism defining signaling dynamics of phytochrome A under red and far-red light in Arabidopsis. Plant Cell 24: 1907–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol 146: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, Dong L, Zhang Z, Yu C, Sun Y, et al. (2021). The genome sequence archive family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. DOI: 10.1016/j.gpb.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MC, Kathare PK, Paik I, Huq E (2021) Phytochrome signaling networks. Annu Rev Plant Biol 72: 217–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi GH, Deng XW, Holm M (2006) Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18: 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Galvao VC, Fankhauser C (2016) Light-mediated hormonal regulation of plant growth and development. Annu Rev Plant Biol 67: 513–537 [DOI] [PubMed] [Google Scholar]
- Deng XW, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH (1992) COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a Gβ homologous domain. Cell 71: 791–801 [DOI] [PubMed] [Google Scholar]
- Desnos T, Puente P, Whitelam GC, Harberd NP (2001) FHY1: a phytochrome A-specific signal transducer. Genes Dev 15: 2980–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Tang D, Gao Z, Yu R, Li K, He H, Terzaghi W, Deng XW, Chen H (2014) Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell 26: 3630–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Yan Y, Jiang B, Shi Y, Jia Y, Cheng J, Shi Y, Kang J, Li H, Zhang D, et al. (2020) The cold response regulator CBF1 promotes Arabidopsis hypocotyl growth at ambient temperatures. EMBO J 39: e103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek PD, Elmer MV, van Oosten VR, Fankhauser C (2004) The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr Biol 14: 2296–2301 [DOI] [PubMed] [Google Scholar]
- Fairchild CD, Schumaker MA, Quail PH (2000) HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev 14: 2377–2391 [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Chory J (2000) RSF1, an Arabidopsis locus implicated in phytochrome A signaling. Plant Physiol 124: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci AS, Fankhauser C (2017) Plant strategies for enhancing access to sunlight. Curr Biol 27: R931–R940 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9: 1353–1365 [DOI] [PubMed] [Google Scholar]
- Hajdu A, Dobos O, Domijan M, Balint B, Nagy I, Nagy F, Kozma-Bognar L (2018) ELONGATED HYPOCOTYL 5 mediates blue light signalling to the Arabidopsis circadian clock. Plant J 96: 1242–1254 [DOI] [PubMed] [Google Scholar]
- Han X, Huang X, Deng XW (2020) The photomorphogenic central repressor COP1: conservation and functional diversification during evolution. Plant Commun 1: 100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A, Tscheuschler A, Viczian A, Kunkel T, Kircher S, Schafer E (2006) FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol 47: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A, Viczian A, Bury E, Tscheuschler A, Kircher S, Toth R, Honsberger A, Nagy F, Fankhauser C, Schafer E (2005) Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol 15: 2125–2130 [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, Deng XW (2001) Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 20: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Lorrain S, Zoete V, Michielin O, Fankhauser C (2009) Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J 28: 3893–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M, Ringli C, Boylan MT, Quail PH (1999) The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev 13: 2017–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E, Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IC, Henriques R, Seo HS, Nagatani A, Chua NH (2010) Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22: 2370–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IC, Yang JY, Seo HS, Chua NH (2005) HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev 19: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserli E, Paldi K, O'Donnell L, Batalov O, Pedmale UV, Nusinow DA, Kay SA, Chory J (2015) Integration of light and photoperiodic signaling in transcriptional nuclear foci. Dev Cell 35: 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yi H, Choi G, Shin B, Song PS, Choi G (2003) Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15: 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP (1980) Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol 100: 147–160 [Google Scholar]
- Kurihara Y, Makita Y, Kawashima M, Hamasaki H, Yamamoto YY, Matsui M (2014) Next-generation sequencing of genomic DNA fragments bound to a transcription factor in vitro reveals its regulatory potential. Genes (Basel) 5: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Choi G (2017) Phytochrome-interacting factor from Arabidopsis to liverwort. Curr Opin Plant Biol 35: 54–60 [DOI] [PubMed] [Google Scholar]
- Lee S, Paik I, Huq E (2020) SPAs promote thermomorphogenesis by regulating the phyB-PIF4 module in Arabidopsis. Development 147: dev189233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M, Ince YC, Fankhauser C (2019) Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat Commun 10: 5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Melkonian M, Rothfels CJ, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Pryer KM, Mathews S (2015) Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun 6: 7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hiltbrunner A (2021) Is the Pr form of phytochrome biologically active in the nucleus? Mol Plant 14: 535–537 [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Gao S, Martinez C, He G, Zhou Z, Huang X, Lee JH, Zhang H, Shen Y, et al. (2010) Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell 22: 3634–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li G, Wang H, Deng XW (2011) Phytochrome signaling mechanisms. Arabidopsis Book 9: e0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Terzaghi W, Gong Y, Li C, Ling JJ, Fan Y, Qin N, Gong X, Zhu D, Deng XW (2020) Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat Commun 11: 1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung HS, et al. (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian HL, He SB, Zhang YC, Zhu DM, Zhang JY, Jia KP, Sun SX, Li L, Yang HQ (2011) Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev 25: 1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian N, Liu X, Wang X, Zhou Y, Li H, Li J, Mao T (2017) COP1 mediates dark-specific degradation of microtubule-associated protein WDL3 in regulating Arabidopsis hypocotyl elongation. Proc Natl Acad Sci USA 114: 12321–12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Teng Y, Park HJ, Ding L, Black C, Fang P, Wang H (2008) Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction. Plant Physiol 148: 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zuo Z, Liu H, Liu X, Lin C (2011) Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev 25: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang Q, Liu B, Wang W, Wang X, Park J, Yang Z, Du X, Bian M, Lin C (2016) The blue light-dependent polyubiquitination and degradation of Arabidopsis cryptochrome2 requires multiple E3 ubiquitin ligases. Plant Cell Physiol 57: 2175–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Trevisan M, Pradervand S, Fankhauser C (2009) Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J 60: 449–461 [DOI] [PubMed] [Google Scholar]
- Loudet O, Michael TP, Burger BT, Le Mette C, Mockler TC, Weigel D, Chory J (2008) A zinc knuckle protein that negatively controls morning-specific growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 105: 17193–17198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XD, Zhou CM, Xu PB, Luo Q, Lian HL, Yang HQ (2015) Red-light-dependent interaction of phyB with SPA1 promotes COP1-SPA1 dissociation and photomorphogenic development in Arabidopsis. Mol Plant 8: 467–478 [DOI] [PubMed] [Google Scholar]
- Lv J, Liu J, Ming Y, Shi Y, Song C, Gong Z, Yang S, Ding Y (2021) Reciprocal regulation between the negative regulator PP2CG1 phosphatase and the positive regulator OST1 kinase confers cold response in Arabidopsis. J Integr Plant Biol 63: 1568–1587 [DOI] [PubMed] [Google Scholar]
- Mancinelli AL (1994) The physiology of phytochrome action. InKendrick RE, Kronenberg GMH, eds, Photomorphogenesis in Plants. Kluwer Academic Publishers, Dordrecht, pp 211–269 [Google Scholar]
- Martínez-García JF, Huq E, Quail PH (2000) Direct targeting of light signals to a promoter element-bound transcription factor. Science 288: 859–863 [DOI] [PubMed] [Google Scholar]
- Members C-N, Partners (2021) Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2021. Nucleic Acids Res 49: D18–D28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Chen F, Ngoc Pham V, Zhu L, KimJl, , Huq E (2019) A phyB-PIF1-SPA1 kinase regulatory complex promotes photomorphogenesis in Arabidopsis. Nat Commun 10: 4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Huq E (2019) Plant photoreceptors: multi-functional sensory proteins and their signaling networks. Semin Cell Dev Biol 92: 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulisic S, Qin W, Arora Veraszto H, Then C, Alary B, Nogue F, Tsiantis M, Hothorn M, Martinez-Garcia JF (2021) Adjustment of the PIF7-HFR1 transcriptional module activity controls plant shade adaptation. EMBO J 40: e104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella G, Davidson MLH, O'Donnell L, Nastase AM, Herzyk P, Breton G, Pruneda-Paz JL, Kay SA, Chory J, Kaiserli E (2018) ZINC-FINGER interactions mediate transcriptional regulation of hypocotyl growth in Arabidopsis. Proc Natl Acad Sci USA 115: E4503–E4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Shi H, Tepperman JM, Zhang Y, Quail PH (2014) Combinatorial complexity in a transcriptionally centered signaling hub in Arabidopsis. Mol. Plant 7: 1598–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Kathare PK, Huq E (2018) Phytochromes and phytochrome interacting factors. Plant Physiol 176: 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnu J, Riedel T, Penner E, Schrader A, Hoecker U (2019) Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc Natl Acad Sci USA 116: 27133–27141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Liu S, Li C, Fu J, Jing Y, Cheng J, Li H, Zhang D, Wang X, Dong X, et al. (2020). PHYTOCHROME-INTERACTING FACTORS interact with the ABA receptors PYL8 and PYL9 to orchestrate ABA signaling in darkness. Mol Plant 13: 414–430 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Pasoreck EK, Reddy AK, Nagatani A, Ma W, Chory J, Chen M (2017) Mechanism of early light signaling by the carboxy-terminal output module of Arabidopsis phytochrome B. Nat Commun 8: 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausenberger J, Tscheuschler A, Nordmeier W, Wust F, Timmer J, Schafer E, Fleck C, Hiltbrunner A (2011) Photoconversion and nuclear trafficking cycles determine phytochrome A's response profile to far-red light. Cell 146: 813–825 [DOI] [PubMed] [Google Scholar]
- Rensing SA, Sheerin DJ, Hiltbrunner A (2016) Phytochromes: more than meets the eye. Trends Plant Sci 21: 543–546 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Zhu D, Li J, Rubio V, Zhou Z, Shen Y, Hoecker U, Wang H, Deng XW (2008) Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol Cell 31: 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH (2004) Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH (2003) LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423: 995–999 [DOI] [PubMed] [Google Scholar]
- Shalitin D, Yang H, Mockler TC, Maymon M, Guo H, Whitelam GC, Lin C (2002) Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417: 763–767 [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Clack T (2002) Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol 130: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin DJ, Hiltbrunner A (2017) Molecular mechanisms and ecological function of far-red light signaling. Plant Cell Environ 40: 2509–2529 [DOI] [PubMed] [Google Scholar]
- Sheerin DJ, Menon C, zur Oven-Krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof YD, Huq E, Hiltbrunner A (2015) Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Feng S, Ma L, Lin R, Qu LJ, Chen Z, Wang H, Deng XW (2005) Arabidopsis FHY1 protein stability is regulated by light via phytochrome A and 26S proteasome. Plant Physiol 139: 1234–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Park E, Choi G (2007) PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Soh MS, Kim YM, Han SJ, Song PS (2000) REP1, a basic helix-loop-helix protein, is required for a branch pathway of phytochrome A signaling in Arabidopsis. Plant Cell 12: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Yoo CM, Hong AP, Kim SH, Jeong HJ, Shin SY, Kim HJ, Yun DJ, Lim CO, Bahk JD, et al. (2008) DNA-binding study identifies C-box and hybrid C/G-box or C/A-box motifs as high-affinity binding sites for STF1 and LONG HYPOCOTYL5 proteins. Plant Physiol 146: 1862–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotech 31: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvetkova-Chevolleau T, Franck F, Alawady AE, Dall'Osto L, Carriere F, Bassi R, Grimm B, Nussaume L, Havaux M (2007) The light stress-induced protein ELIP2 is a regulator of chlorophyll synthesis in Arabidopsis thaliana. Plant J 50: 795–809 [DOI] [PubMed] [Google Scholar]
- Uljon S, Xu X, Durzynska I, Stein S, Adelmant G, Marto JA, Pear WS, Blacklow SC (2016) Structural basis for substrate selectivity of the E3 ligase COP1. Structure 24: 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, Schafer E, Nagy F (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci USA 101: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Deng XW (2002) Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J 21: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Paik I, Kim J, Hou X, Sung S, Huq E (2021) Direct phosphorylation of HY5 by SPA kinases to regulate photomorphogenesis in Arabidopsis. New Phytol 230: 2311–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ding Y, Li Z, Shi Y, Wang J, Hua J, Gong Z, Zhou JM, Yang S (2019a) PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev Cell 51: 222–235 [DOI] [PubMed] [Google Scholar]
- Wang X, Guo C, Peng J, Li C, Wan F, Zhang S, Zhou Y, Yan Y, Qi L, Sun K, et al. (2019b) ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol 221: 341–355 [DOI] [PubMed] [Google Scholar]
- Weidler G, Zur Oven-Krockhaus S, Heunemann M, Orth C, Schleifenbaum F, Harter K, Hoecker U, Batschauer A (2012) Degradation of Arabidopsis CRY2 is regulated by SPA proteins and phytochrome A. Plant Cell 24: 2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng JR, Carol P, Anderson ML, Cowl JS, Harberd NP (1993) Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D (2020) COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol 228: 1748–1753 [DOI] [PubMed] [Google Scholar]
- Xu X, Paik I, Zhu L, Bu Q, Huang X, Deng XW, Huq E (2014) PHYTOCHROME INTERACTING FACTOR1 enhances the E3 ligase activity of CONSTITUTIVE PHOTOMORPHOGENIC1 to synergistically repress photomorphogenesis in Arabidopsis. Plant Cell 26: 1992–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Li C, Dong X, Li H, Zhang D, Zhou Y, Jiang B, Peng J, Qin X, Cheng J, et al. (2020) MYB30 is a key negative regulator of Arabidopsis photomorphogenic development that promotes PIF4 and PIF5 protein accumulation in the light. Plant Cell 32: 2196–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lin R, Sullivan J, Hoecker U, Liu B, Xu L, Deng XW, Wang H (2005) Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17: 804–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu S, Lin R (2020) The role of light in regulating seed dormancy and germination. J Integr Plant Biol 62: 1310–1326 [DOI] [PubMed] [Google Scholar]
- Yi C, Deng XW (2005) COP1-from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol 15: 618–625 [DOI] [PubMed] [Google Scholar]
- Yin R, Arongaus AB, Binkert M, Ulm R (2015) Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell 27: 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, He H, Wang X, Wang X, Yang X, Li L, Deng XW (2011) Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J 65: 346–358 [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C, Zhou Y, Wang X, Li H, Feng Z, Chen H, Qin G, Jin D, Terzaghi W, et al. (2018) TANDEM ZINC-FINGER/PLUS3 is a key component of phytochrome A signaling. Plant Cell 30: 835–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huai J, Shang F, Xu G, Tang W, Jing Y, Lin R (2017) A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiol 174: 2487–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayba O, Pfeiffer A, Shi H, Tepperman JM, Speed TP, Quail PH (2013) A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet 9: e1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Hare PD, Yang SW, Zeidler M, Huang LF, Chua NH (2005) FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J 43: 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang L, Duan J, Cheng J, Shen Y, Wang X, Han R, Li H, Li Z, Wang L, et al. (2018) Hinge region of Arabidopsis phyA plays an important role in regulating phyA function. Proc Natl Acad Sci USA 115: E11864–E11873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, Lin C (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.