Abstract
Simple Summary
Atezolizumab plus bevacizumab has been approved as the first-line systemic treatment for unresectable hepatocellular carcinoma (uHCC) patients. However, the real-world practice of this combination is limited. We reported 48 uHCC patients who received atezolizumab plus bevacizumab, the median progression-free survival (PFS) was 5.0 months, and the objective response rate and disease control rate were 27.1% and 68.8%, respectively. The severity of most adverse events was predominantly grade 1–2, and most patients tolerated the toxicities. We also used inflammatory biomarkers to predict PFS, including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Univariate and multivariate analyses revealed NLR and PLR were independent prognostic factors for superior PFS. The significance of our study is the first research to investigate the prognostic value of NLR and PLR among uHCC patients receiving atezolizumab plus bevacizumab. It would bring more information to physicians about the efficacy and safety of atezolizumab plus bevacizumab in real-world clinical practice.
Abstract
Atezolizumab plus bevacizumab has been approved as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma (uHCC). This study was designed to assess the clinical impact of atezolizumab plus bevacizumab in uHCC patients. A total of 48 uHCC patients receiving atezolizumab plus bevacizumab were identified, including first-line, second-line, third-line, and later-line settings. In these patients, the median progression-free survival (PFS) was 5.0 months, including 5.0 months for the first-line treatment, not reached for the second-line treatment, and 2.5 months for the third line and later line treatment. The objective response rate and disease control rate to atezolizumab plus bevacizumab were 27.1% and 68.8%, respectively. The severity of most adverse events was predominantly grade 1–2, and most patients tolerated the toxicities. The ratios of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte (PLR) were used to predict PFS in these patients. The optimal cutoff values of NLR and PLR were 3 and 230, and NLR and PLR were independent prognostic factors for superior PFS in the univariate and multivariate analyses. Our study confirms the efficacy and safety of atezolizumab plus bevacizumab in uHCC patients in clinical practice and demonstrates the prognostic role of NLR and PLR for PFS in these patients.
Keywords: atezolizumab, bevacizumab, hepatocellular carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
1. Introduction
Hepatocellular carcinoma (HCC) is globally one of the most common cancers and the second leading cause of cancer-related deaths in Taiwan [1]. Sorafenib and lenvatinib have been approved as first-line systemic treatment in patients with unresectable HCC (uHCC) who are not feasible for surgical intervention or other locoregional therapies, such as trans-arterial chemoembolization (TACE) or radiofrequency ablation (RFA) [2,3,4]. The SHARP and Asia-Pacific trials have confirmed the overall survival (OS) benefit of sorafenib in these uHCC patients compared to the placebo group [2,4]. According to the REFLECT trial, lenvatinib is non-inferior to sorafenib with respect to OS, and is better than sorafenib with respect to progression-free survival (PFS) and objective response rate (ORR) [3]. Recently, the IMbrave150 trial, a global, open-label, phase 3 study has shown that atezolizumab plus bevacizumab prolongs PFS and OS than does sorafenib in patients with uHCC [5]. In addition, the ORR of atezolizumab plus bevacizumab was approximately 30%, and the percentage of adverse events was comparable to that of sorafenib. Therefore, atezolizumab plus bevacizumab is becoming the preferred first-line systemic treatment against uHCC in clinical practice.
Increasing evidence has revealed that chronic inflammation plays a predominant role in the process of tumor progression, including cancer cell proliferation, angiogenesis, and metastasis [6,7]. Tumor-associated neutrophils (TANs) have two different phenotypes: N1 (anti-tumorigenic) and N2 (protumorigenic), and high infiltration with N2 TANs has contributed to tumor cell proliferation, distant metastasis and poor prognosis [8]. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are easy-to-obtain and cost-effective biomarkers that are widely used to predict treatment response and prognosis in several cancer types [9,10,11,12,13,14,15,16,17]. In addition, a review article summarizes the current evidence on the role of TANs in the pathogenesis and progression of HCC, and highlights the significance of NLR as a reliable biomarker with prognostic potential for HCC [8]. Several studies have confirmed the role of NLR and PLR in HCC [18]. NLR and PLR have been reported to be useful prognostic factors for predicting outcomesin patients with HCC who underwent hepatectomy [19,20]. On the other hand, NLR and PLR are regarded as independent markers of poor prognosis in HCC patients who received TACE or RFA [21,22,23]. In HCC patients with liver transplantation, elevated NLR and PLR are associated with early tumor recurrence [24,25]. Liu reported a meta-analysis that demonstrated the role of NLR and PLR in patients with HCC who were receiving sorafenib; patients with a lower baseline NLR and PLR had better response to sorafenib and superior OS compared to those with a higher NLR [26]. Another Japanese study revealed that low NLR was independently associated with better PFS, OS, and disease control in patients with HCC who received lenvatinib [27]; PLR could be used to predict OS in patients with uHCC who received lenvatinib [28]. Recently, NLR and PLR were reported to have strong predictive roles in patients with HCC who were treated with anti–PD-1 therapy, such as nivolumab [29].
Several studies have confirmed the efficacy and safety of sorafenib and lenvatinib in patients with HCC [30,31,32,33,34,35]. Nevertheless, information on atezolizumab plus bevacizumab in real-world practice is relatively limited. Recently, Iwamoto reported the first real-world outcomes of atezolizumab plus bevacizumab treatment in patients with uHCC in Japan [36]. However, to the best of our knowledge, information regarding the clinical impact of NLR in patients with uHCC receiving atezolizumab plus bevacizumab is unclear. The present study was designed to explore the efficacy and safety of atezolizumab plus bevacizumab and the prognostic significance of the NLR and PLR in patients with uHCC.
2. Materials and Methods
2.1. Study Population
We retrospectively reviewed patients with uHCC who received atezolizumab plus bevacizumab at Kaohsiung Chang Gung Memorial Hospital between January 2020 and October 2021. The eligibility criteria were as follows: (1) no evidence of a second malignancy or concurrent cholangiocarcinoma; (2) treatment with atezolizumab plus bevacizumab for more than 2 cycles; (3) follow-up duration > 4 weeks; (4) no esophageal or gastric varices detected by upper gastrointestinal endoscopy; and (5) precise collection of clinical data. Finally, 48 patients who were treated with atezolizumab plus bevacizumab were included in the study.
The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count measured in peripheral blood, and PLR was calculated as the ratio of absolute platelet count to absolute lymphocyte count. The receiver operating characteristic (ROC) curve analysis was used to identify the optimal cut-off values of NLR and PLR according to the Youden index (Youden Index = Sensitivity + Specificity − 1, range from 0 to 1), which is a commonly used measure of overall diagnostic effectiveness [37]. A cutoff value of 3 used for NLR, and a cutoff of 230 used for the PLR group were both reported by previous literature [29,38,39]. In addition, albumin-bilirubin (ALBI) score was calculated based on serum albumin and total bilirubin values using the following formula: ALBI score = (log10 bilirubin [μmol/L] × 0.66) + (albumin [g/L] × −0.085). The ALBI score was categorized into grade 1 (−2.60 or less), grade 2 (−2.59 to −1.39), or grade 3 (greater than −1.39).
2.2. Treatment Protocol and Safety Assessment
In our study, patients received atezolizumab at a dose of 1200 mg and bevacizumab at a dose of 5–7.5 mg/kg intravenously every 3 weeks. Treatment was continued until disease progression or the development of intolerable AEs. Patients were followed up at the outpatient clinic for assessment of AEs every 3 weeks, and the grade of AEs was assigned based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [40].
2.3. Staging and Evaluation of Response
HCC diagnosis was based on pathological findings or according to the non-invasive criteria of the American Association for the Study of Liver Disease (AASLD) guidelines [41,42]. HCC was staged using the Barcelona Clinic Liver Cancer (BCLC) staging classification at the time of atezolizumab plus bevacizumab initiation [43]. Each patient must have had at least one measurable target lesion to evaluate treatment response using dynamic computed tomography (CT) or magnetic resonance imaging (MRI) of the liver every 9 weeks after commencement of treatment. The response was independently determined by two radiologists without any clinical information, in accordance with the guidelines of the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [44].
2.4. Statistical Analysis
All data analyses were performed using the SPSS 19 software (IBM, Armonk, NY, USA). The chi-square test was used for categorical variables. PFS was determined from the date of atezolizumab plus bevacizumab initiation to disease progression or death due to any cause. Actuarial analysis of cumulative survival was performed using the Kaplan–Meier method, and the differences were assessed with the log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) and p-values were calculated to quantify the strength of the associations between the prognostic parameters and survival. Parameters significantly associated with PFS in the univariate analysis were selected as covariates for multivariate Cox proportional hazards models. Statistical significance was set at p < 0.05.
2.5. Ethics Statement
The present study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of Chang Gung Medical Foundation approved this study (202101199B0) and waived the requirement for written informed consent owing to the retrospective design of this study.
3. Results
3.1. Characteristics of Patients
Our study cohort consists of 48 patients with uHCC who received atezolizumab plus bevacizumab at Kaohsiung Chang Gung Memorial Hospital between January 2020 and October 2021, including 38 men and 10 women with a median age of 62 years (range: 31–80 years). The characteristics of these patients were documented at the time of atezolizumab plus bevacizumab administration. All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1, and all were classified as BCLC staging classification C. Most of these patients had Child–Pugh classification A (87.5%), and only 6 (12.5%) patients had Child–Pugh classification B. In terms of viral hepatitis, hepatitis B virus (HBV) infection was reported in 28 (58.3%) patients and hepatitis C virus (HCV) infection in 13 (27.1%) patients. The percentages of albumin-bilirubin (ALBI) 1 and 2 were similar (47.9% vs. 52.1%). At the time of analysis, the median follow-up period was 9.5 months for all 48 patients (range: 2.4–23.8). The demographic characteristics of the patients are presented in Table 1.
Table 1.
Characteristics of 48 patients with unresectable hepatocellular carcinoma who received atezolizumab plus bevacizumab.
| Variable | Patient Number (%) |
|---|---|
| Age (median, range) | 62 years old (31–80) |
| Sex | |
| Male | 38 (79.2%) |
| Female | 10 (20.8%) |
| ECOG PS | |
| 0 | 31 (64.6%) |
| 1 | 17 (35.4%) |
| Child–Pugh classification | |
| A | 42 (87.5%) |
| B | 6 (12.5%) |
| BCLC classification | |
| C | 48 (100.0%) |
| ALBI grade | |
| 1 | 23 (47.9%) |
| 2 | 25 (52.1%) |
| Viral hepatitis status | |
| Hepatitis B | 28 (58.3%) |
| Hepatitis C | 13 (27.1%) |
| No | 7 (14.6%) |
| Macrovascular invasion | |
| Yes | 26 (54.2%) |
| No | 22 (45.8%) |
| Main portal vein thrombosis | |
| Yes | 9 (18.8%) |
| No | 39 (81.2%) |
| Hepatectomy before atezolizumab plus bevacizumab | |
| Yes | 18 (37.5%) |
| No | 30 (62.5%) |
| Lymph node metastasis at the time of atezolizumab plus bevacizumab | |
| Yes | 12 (25.0%) |
| No | 36 (75.0%) |
| Extrahepatic spread at the time of atezolizumab plus bevacizumab | |
| Yes | 26 (54.2%) |
| No | 22 (45.8%) |
| AFP at the time of atezolizumab plus bevacizumab (median, range) ng/mL | 157.1 (2.1->80000) |
ECOG PS: Eastern Cooperative Oncology Group Performance Status; BCLC: Barcelona-Clinic Liver Cancer; ALBI: Albumin-Bilirubin; AFP: alpha-fetoprotein.
3.2. Assessment of the Cut-Off Value of NLR and PLR
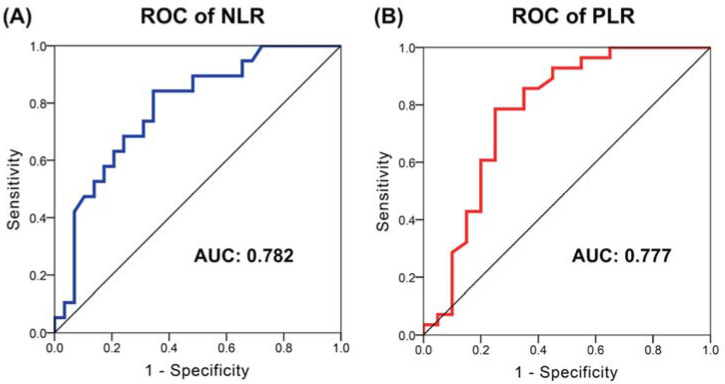
The optimal cutoff values of NLR and PLR were determined by ROC analysis; the ideal cutoff values for NLR and PLR were 3 and 230, respectively. According to the ROC curves, the area under the curve (AUC) for NLR and PLR was 0.782 (95% CI: 0.651–0.914, p = 0.001) and 0.777 (95% CI: 0.630–0.924, p = 0.001), respectively (Figure 1).
Figure 1.
The receiver operating characteristic (ROC) curves of the NLR and PLR in patients with unresectable HCC who received atezolizumab plus bevacizumab. The area under the curves (AUC) for NLR (A) and for PLR (B).
3.3. Efficacy Analyses of Atezolizumab plus Bevacizumab
The response to atezolizumab plus bevacizumab treatment was determined based on the RECIST criteria version 1.1, including 13 (27.1%) patients with partial response (PR), 20 (41.7%) with stable disease (SD), and 15 (31.2%) with progressive disease (PD), indicating a disease control rate (DCR) of 68.8%. The PFS was 9.6 months, 7.6 months, and 2.4 months in patients with PR, SD, and PD, respectively (p < 0.001).
In addition, atezolizumab/bevacizumab was used as first-line treatment in 27 (56.2%) patients, second-line treatment in 12 (25.0%) patients, and third-line and later line treatment in 9 (18.8%) patients. The median PFS was 5.0 months for the first-line treatment, NR for the second-line treatment, and 2.5 months for the third line and later line treatment groups (p = 0.042).
In the first-line setting, the ORR and DCR were 29.6% and 66.6%, respectively; there was an ORR of 25.0% and DCR of 83.3% in the second-line group; even in the third line and later lines, the PR was 22.2% and DCR was 55.5%. The results of the survival analyses and treatment effects of atezolizumab plus bevacizumab are presented in Table 2.
Table 2.
Analysis of progression-free survival (PFS) according to the response rates and treatment lines of atezolizumab plus bevacizumab.
| Variables | Number of Patients | PFS (Months) | p Value |
|---|---|---|---|
| Treatment response | |||
| Partial response | 13 (27.1%) | 9.6 | <0.001 * |
| Stable disease | 20 (41.7%) | 7.6 | |
| Progressive disease | 15 (31.2%) | 2.4 | |
| Treatment lines | |||
| First line | 27 (56.2%) | 5.0 | 0.042 * |
| Second line | 12 (25.0%) | NR | |
| Third line and later lines | 9 (18.8%) | 2.5 | |
| Treatment lines | Partial response | Stable disease | Disease control rate |
| First line (N = 27) | 8 (29.6%) | 10 (37.0%) | 18 (66.6%) |
| Second line (N = 12) | 3 (25.0%) | 7 (58.3%) | 10 (83.3%) |
| Third and later lines (N = 9) | 2 (22.2%) | 3 (33.3%) | 5 (55.5%) |
PFS: progression-free survival; NR: not reached. * Statistically significant.
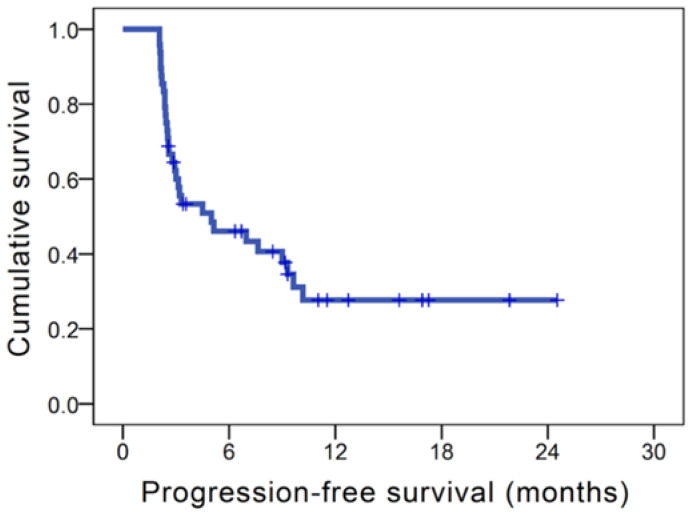
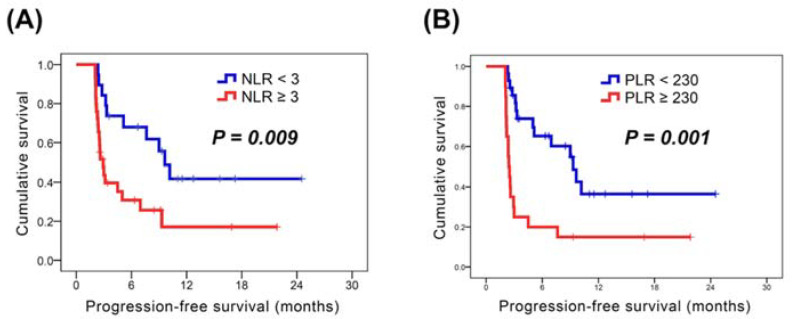
In our study, the median PFS of the whole population was 5.0 months (Figure 2). In the analysis of PFS, there were no significant differences in all parameters in the univariate analysis, except for ECOG PS, AFP > 400 ng/mL, NLR, and PLR. The 31 patients who had ECOG PS 0 had better PFS than those with ECOG PS 1 (9.6 months versus 2.6 months, p = 0.004); superior PFS was noted in 27 patients with AFP < 400 ng/mL compared to the remaining 21 patients with AFP ≥ 400 ng/mL (9.6 months versus 2.8 months, p = 0.002). The patients with NLR < 3 were found to have longer PFS in compassion with those with NLR ≥ 3 (9.6 months versus 2.9 months, p = 0.009, Figure 3A); patients who had a PLR < 230 had a longer PFS than those who had PLR ≥ 230 (9.3 months versus 2.4 months, p = 0.001, Figure 3B). Multivariate analysis showed that no hepatectomy before atezolizumab plus bevacizumab (p = 0.019; HR, 0.39; 95% CI, 0.18–0.86), AFP < 400 ng/mL (p = 0.001; HR, 0.24; 95% CI, 0.11–0.54), NLR < 3 (p = 0.019; HR, 0.34; 95% CI, 0.14–0.84), and PLR < 230 (p = 0.014; HR, 0.36; 95% CI, 0.16–0.81) were independent prognostic factors for superior PFS. The univariate and multivariate analyses of PFS were shown in Table 3.
Figure 2.
Kaplan–Meier survival curves of progression-free survival (PFS) in patients with unresectable hepatocellular carcinoma who received atezolizumab plus bevacizumab.
Figure 3.
Kaplan–Meier survival analyses (A) Kaplan–Meier survival curves for patients with NLR ≥ 3 vs. those with NLR < 3; (B) Kaplan–Meier survival curves for patients with PLR ≥ 230 vs. those with PLR < 230.
Table 3.
Univariate and multivariate analyses of progression-free survival (PFS) in 48 patients with unresectable hepatocellular carcinoma who received atezolizumab plus bevacizumab.
| Characteristics | No. of Patients | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| PFS (Months) | p Value | HR (95% CI) | p Value | ||
| Age | |||||
| <60 years | 21 (43.8%) | 5.0 | 0.45 | ||
| ≥60 years | 27 (56.2%) | 4.5 | |||
| Sex | |||||
| Male | 38 (79.2%) | 5.1 | 0.99 | ||
| Female | 10 (20.8%) | 3.0 | |||
| ECOG PS | |||||
| 0 | 31 (64.6%) | 9.6 | 0.004 * | ||
| 1 | 17 (35.4%) | 2.6 | |||
| Child–Pugh classification | |||||
| A | 42 (87.5%) | 5.0 | 0.63 | ||
| B | 6 (12.5%) | 4.5 | |||
| Treatment lines | |||||
| First line | 27 (56.2%) | 5.0 | 0.60 | ||
| Second and later lines | 21 (43.8%) | 3.2 | |||
| ALBI grade | |||||
| 1 | 23 (47.9%) | 5.0 | 0.40 | ||
| 2 | 25 (52.1%) | 4.5 | |||
| Hepatitis B | |||||
| Yes | 28 (58.3%) | 5.0 | 0.79 | ||
| No | 20 (41.7%) | 3.3 | |||
| Hepatitis C | |||||
| Yes | 13 (27.1%) | 5.1 | 0.46 | ||
| No | 35 (72.9%) | 5.0 | |||
| Macrovascular invasion | |||||
| Yes | 26 (54.2%) | 3.3 | 0.23 | ||
| No | 22 (45.8%) | 7.6 | |||
| Main portal vein thrombosis | |||||
| Yes | 9 (18.8%) | 5.1 | 0.24 | ||
| No | 39 (81.2%) | 3.2 | |||
| Hepatectomy before atezolizumab plus bevacizumab | |||||
| Yes | 18 (37.5%) | 2.8 | 0.06 | ||
| No | 30 (62.5%) | 7.6 | 0.39 (0.18–0.86) | 0.019 * | |
| Lymph node metastasis at the time of atezolizumab plus bevacizumab | |||||
| Yes | 12 (25.0%) | 3.1 | 0.30 | ||
| No | 36 (75.0%) | 5.1 | |||
| Extrahepatic spread at the time of atezolizumab plus bevacizumab | |||||
| Yes | 26 (54.2%) | 3.3 | 0.92 | ||
| No | 22 (45.8%) | 5.1 | |||
| AFP ≥ 400 at the time of atezolizumab plus bevacizumab | |||||
| Yes | 21 (43.8%) | 2.8 | 0.002 * | ||
| No | 27 (56.2%) | 9.6 | 0.24 (0.11–0.54) | 0.001 * | |
| NLR | |||||
| ≥3 | 29 (60.4%) | 2.9 | 0.009 * | ||
| <3 | 19 (39.6%) | 9.6 | 0.34 (0.14–0.84) | 0.019 * | |
| PLR | |||||
| ≥230 | 20 (41.7%) | 2.4 | 0.001 * | ||
| <230 | 28 (58.3%) | 9.3 | 0.36 (0.16–0.81) | 0.014 * | |
ECOG PS: Eastern Cooperative Oncology Group Performance Status; HR: hazard ratio; CI: confidence interval; ALBI: Albumin-Bilirubin; AFP: alpha-fetoprotein; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio. * Statistically significant, p < 0.05.
3.4. Safety Analyses
Most patients (93.8%) experienced AEs following atezolizumab plus bevacizumab treatment. The most common AEs were aspartate/alanine aminotransferase increase (85.4%), followed by proteinuria (35.4%), fatigue (25.0%), hypertension (22.9%), decreased appetite (22.9%), abdominal pain (18.8%), and nausea (14.6%). The severity of most AEs was grade 1–2; grade 3–4 toxicities were relatively rare, including aspartate/alanine aminotransferase increase (20.8%), hypertension (6.3%), proteinuria (4.2%), and diarrhea (2.0%). There were no drug-related grade 5 AEs. Most patients tolerated the AEs of atezolizumab plus bevacizumab, and none of the patients experienced treatment interruption or dose adjustment due to AEs. The frequencies of drug-related AEs are listed in Table 4.
Table 4.
The treatment-related adverse events in the 48 patients with unresectable hepatocellular carcinoma who received atezolizumab plus bevacizumab.
| Adverse Event | Any Grades | Grade 3/4 |
|---|---|---|
| Hypertension | 11 (22.9%) | 3 (6.3%) |
| Fatigue | 12 (25.0%) | 0 |
| Proteinuria | 17 (35.4%) | 2 (4.2%) |
| Aspartate/Alanine aminotransferase increase (baseline) | 34 (70.8%) | 3 (6.3%) |
| Aspartate/Alanine aminotransferase increase (after lenvatinib) | 41 (85.4%) | 10 (20.8%) |
| Diarrhea | 3 (6.3%) | 1 (2.0%) |
| Decreased appetite | 11 (22.9%) | 0 |
| Skin rash | 10 (20.8%) | 0 |
| Abdominal pain | 9 (18.8%) | 0 |
| Nausea | 7 (14.6%) | 0 |
| Palmar-Plantar erythrodysesthesia | 3 (6.3%) | 0 |
| Bleeding | 4 (8.3%) | 0 |
3.5. Case Presentation
Case 1: The 53-year-old man had past history of HBV and liver cirrhosis, and has been diagnosed with HCC in February 2021. MRI of liver revealed multiple liver tumors over S5/S7/S8 with left, right and main portal vein thrombosis. Then he received atezolizumab plus bevacizumab since February 2021; image was followed after completion of three cycles of this combination therapy. MRI of liver demonstrated that decreased in size of liver tumors and portal vein thrombosis, indicating PR (Figure S1).
Case 2: The 61-year-old woman has been HBV related liver cirrhosis and splenomegaly, and HCC was diagnosed in January 2021. MRI of liver showed liver tumors over S4/S7/S8 and enlarged lymph nodes over gastrohepatic and precaval regions. In addition, multiple bone metastasis was detected by bone scan. After that, she received atezolizumab plus bevacizumab for 5 cycles, and stable condition of liver tumors was mentioned by followed MRI of liver. Bone scan also revealed mild regressive change of bone metastasis. The treatment response was regarded as SD (Figure S2).
Case 3: The 31-year-old man has been diagnosed with HCC with lung metastasis in September 2020. CT of liver showed liver tumor over S7 and CT of chest revealed one small nodule over right upper lobe. Atezolizumab plus bevacizumab were given since September 2020 with a total of three cycles. Followed CT of liver demonstrated increased in size of liver tumor over S7, accompanied with multiple new liver nodules over both lobes, suggesting progression. On the other hand, markedly progressive change of metastatic lung nodule over right upper lobe was also mentioned on CT of chest. In conclusion, the response to atezolizumab plus bevacizumab was PD (Figure S3).
4. Discussion
Our study showed real-world evidence of the efficacy and safety of atezolizumab plus bevacizumab in patients with uHCC. Patients who used this combination therapy as first-line, second-line, or third-line therapy were enrolled. The ORR and DCR were 27.1% and 68.8%, respectively. The median PFS was 5.0 months for all patients, including 5.0 months for first-line use, NR for second-line use, and 2.5 months for third-line and later line use. In addition, we also reported the clinical predictors of NLR and PLR in these patients; higher NLR and PLR were independent prognostic factors of worse PFS in patients with uHCC who received atezolizumab plus bevacizumab. Most AEs of atezolizumab plus bevacizumab were grade 1–2, and most patients tolerated the toxicities. In conclusion, we showed the clinical efficacy and safety of atezolizumab plus bevacizumab and reveal the prognostic value of NLR and PLR for patients with uHCC in real-world clinical practice.
In our study, the dose of bevacizumab was different from that in the IMbrave150 trial. In the IMbrave150 trial, bevacizumab was administered at a dose of 15 mg/kg every 3 weeks; however, we only prescribed bevacizumab at a dose of 5 or 7.5 mg/Kg in our study. Bevacizumab is an anti-angiogenic agent with additional immunomodulatory effects, including normalizing tumor vasculature, increasing T-cell infiltration, decreasing the activity of immunosuppressive cells, and promoting the maturation of dendritic cells [45,46]. Bevacizumab may enhance the efficacy of atezolizumab by reversing vascular endothelial growth factor (VEGF)-mediated immunosuppression. Therefore, bevacizumab dose may not be fixed. Bevacizumab was prescribed with irinotecan/5-fluouracil at a dose of 5 mg/kg or 10 mg/kg every 2 weeks for patients with colorectal cancer (CRC) in a randomized phase III EAGLE study [47]. The median PFS was similar for both doses of bevacizumab, and no clear clinical benefit was noted in the high-dose bevacizumab group. In addition, Gordon et al. reported that free serum concentrations of VEGF could drop below detectable limits even when the dose of bevacizumab was as low as 0.3 mg/kg, indicating that a higher dose of bevacizumab may not be necessary for optimal activity in cancer treatment [48].
NLR and PLR have been proven to be associated with disease progression, tumor recurrence, and clinical outcome in several cancer types. Recently, a review article summarizes the current evidence on the significance of NLR in the pathogenesis and progression of HCC, and highlights the role of NLR as a reliable biomarker in the potential involvement in tumor therapy for HCC, such as immunotherapy, chemotherapy or liver transplantation [8]. Sorafenib and lenvatinib have been approved for first-line systemic treatment in patients with uHCC, and several studies have focused on the clinical utility of NLR and PLR in tracking treatment response in patients with HCC who received sorafenib or lenvatinib [26,27,28]. However, the prognostic value of NLR and PLR in patients receiving atezolizumab plus bevacizumab remained unclear. Our study is the first to report an association between NLR/PLR and clinical outcomes in this group. Patients with NLR < 3 had better PFS than those with NLR ≥ 3 (9.6 months versus 2.9 months); superior PFS was also mentioned in patients with PLR < 230 than in those with PLR ≥ 230 (9.3 months versus 2.4 months). Therefore, the results of the current study establish the clinical utility of NLR and PLR as biomarkers for tracking PFS in patients with uHCC receiving atezolizumab plus bevacizumab.
Recently, Iwamoto et al. reported the first real-world outcomes of atezolizumab plus bevacizumab treatment in 60 patients with HCC in Japan [36]. In our study, there were 27 patients who received atezolizumab plus bevacizumab as first-line treatment with a PR of 29.6% and DCR of 66.6%; these data were similar to the results of the IMbrave150 study. However, information about this combination therapy in the second-line or later line setting was unclear. Our study enrolled 20 and 15 patients with uHCC who received atezolizumab plus bevacizumab as second-line, or third-line and later lines, respectively. In the second-line setting, the ORR and DCR were 25.0% and 83.3%, respectively; in the third line and later lines, ORR and DCR were 22.2% and 55.5%, respectively. The median PFS was NR in the second-line group and 2.5 months in the third-line and later line groups, respectively. The clinical outcomes of atezolizumab plus bevacizumab in the second-line and later line settings were acceptable. To the best of our knowledge, this is the first study to present the clinical efficacy of atezolizumab plus bevacizumab in second-line and later line settings.
Our study enrolled 20 and 15 patients with uHCC who received atezolizumab plus bevacizumab as second-line, or third-line and later lines, respectively. In the second-line setting, the ORR and DCR were 25.0% and 83.3%, respectively; in the third line and later lines ORR and DCR were 22.2% and 55.5%, respectively. The median PFS was NR in the second-line group and 2.5 months in the third-line and later line groups, respectively. The clinical outcomes of atezolizumab plus bevacizumab in the second-line and later line settings were acceptable. To the best of our knowledge, this is the first study to present the clinical efficacy of atezolizumab plus bevacizumab in second-line and later line settings.
Our study has some limitations. First, the sample size of patients with receiving atezolizumab plus bevacizumab was relatively small; therefore, some prognostic factors of PFS may be difficult to identify. Second, the duration of the follow-up period may not have been long enough, therefore OS could not be evaluated. Third, there were different treatment strategies for atezolizumab plus bevacizumab, including first-line, second-line, third-line, and later lines, resulting in the relatively small population in the different groups. Therefore, there may be biases in the results of PFS. However, to the best of our knowledge, this is one of the limited studies designed to assess the clinical efficacy and safety, and the first cohort study designed to evaluate the prognostic role of NLR and PLR in patients who received atezolizumab plus bevacizumab in real-world practice.
5. Conclusions
Our study confirms the efficacy and safety of atezolizumab plus bevacizumab in patients with uHCC in clinical practice and identifies a prognostic value of NLR and PLR for predicting PFS in these patients.
Acknowledgments
We appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for statistics work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14020343/s1. Figure S1: The 53-year-old man had past history of hepatitis B and liver cirrhosis, and has been diagnosed with hepatocellular carcinoma in February 2021., Figure S2: The 61-year-old woman has been hepatitis B related liver cirrhosis and splenomegaly, and hepatocellular carcinoma was diagnosed in January 2021., Figure S3: The 31-year-old man has been diagnosed with hepatocellular carcinoma with lung metastasis in September 2020.
Author Contributions
Conceptualization: Y.-H.C.; methodology: J.-H.W.; formal analysis and investigation: Y.-Y.C.; writing—original draft: Y.-H.C.; writing—review and editing: Y.-H.C.; resources: K.-M.K., M.-C.T., Y.-H.K., C.-H.H., W.-F.L. and H.-L.L.; supervision: C.-C.W.; validation: Y.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (grant number: MOST 110-2321-B-182A-003-) and the Chang Gung Memorial Hospital (grant numbers: CIRPG8K0011, CORPG8L0371 and NZRPG8L0031).
Institutional Review Board Statement
The present study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of Chang Gung Medical Foundation approved this study (202101199B0).
Informed Consent Statement
The Institutional Review Board of Chang Gung Medical Foundation waived the requirement of written informed consent due to the retrospective design of this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ministry of Health and Welfare of Taiwan . Cancer Registry Annual Report 1972—2015. Health Promotion Administration, Ministry of Health and Welfare; Taipei City, Taiwan: 2015. [Google Scholar]
- 2.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Elinav E., Nowarski R., Thaiss C.A., Hu B., Jin C., Flavell R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 7.Greten F.R., Grivennikov S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvanitakis K., Mitroulis I., Germanidis G. Tumor-Associated Neutrophils in Hepatocellular Carcinoma Pathogenesis, Prognosis, and Therapy. Cancers. 2021;13:2899. doi: 10.3390/cancers13122899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannaga A., Arasaradnam R.P. Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: A systematic review. World J. Gastroenterol. 2020;26:5022–5049. doi: 10.3748/wjg.v26.i33.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cupp M.A., Cariolou M., Tzoulaki I., Aune D., Evangelou E., Berlanga-Taylor A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. doi: 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ethier J.L., Desautels D., Templeton A., Shah P.S., Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchioni M., Primiceri G., Ingrosso M., Filograna R., Castellan P., De Francesco P., Schips L. The Clinical Use of the Neutrophil to Lymphocyte Ratio (NLR) in Urothelial Cancer: A Systematic Review. Clin. Genitourin. Cancer. 2016;14:473–484. doi: 10.1016/j.clgc.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Ni L., Tao J., Xu J., Yuan X., Long Y., Yu N., Wu R., Zhang Y. Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020;301:251–261. doi: 10.1007/s00404-019-05372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai P.L., Su W.J., Leung W.H., Lai C.T., Liu C.K. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J. Cancer Res. Ther. 2016;12:582–589. doi: 10.4103/0973-1482.144356. [DOI] [PubMed] [Google Scholar]
- 15.Wu C.C., Li S.H., Lu H.I., Lo C.M., Wang Y.M., Chou S.Y., Chen Y.H. Inflammation-based prognostic scores predict the prognosis of locally advanced cervical esophageal squamous cell carcinoma patients receiving curative concurrent chemoradiotherapy: A propensity score-matched analysis. PeerJ. 2018;6:e5655. doi: 10.7717/peerj.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J.J., Hu Z.G., Shi W.X., Deng T., He S.Q., Yuan S.G. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2015;21:2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin X., Wu L., Yang H., Yang H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine. 2019;98:e17475. doi: 10.1097/MD.0000000000017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J., Cai J., Li H., Zeng K., He L., Fu H., Zhang J., Chen L., Yao J., Zhang Y., et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: A Meta-Analysis and Systematic Review. Cell. Physiol. Biochem. 2017;44:967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 19.Wang D., Bai N., Hu X., OuYang X.W., Yao L., Tao Y., Wang Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. doi: 10.7717/peerj.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T., Zhu J., Zhao L., Mai K., Ye J., Huang S., Zhao Y. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J. Surg. Oncol. 2017;115:718–728. doi: 10.1002/jso.24549. [DOI] [PubMed] [Google Scholar]
- 21.Chen K., Zhan M.X., Hu B.S., Li Y., He X., Fu S.R., Xin Y.J., Lu L.G. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma. Oncol. Lett. 2018;15:315–323. doi: 10.3892/ol.2017.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He C., Zhang Y., Cai Z., Lin X. The prognostic and predictive value of the combination of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma who receive transarterial chemoembolization therapy. Cancer Manag. Res. 2019;11:1391–1400. doi: 10.2147/CMAR.S190545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schobert I.T., Savic L.J., Chapiro J., Bousabarah K., Chen E., Laage-Gaupp F., Tefera J., Nezami N., Lin M., Pollak J., et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur. Radiol. 2020;30:5663–5673. doi: 10.1007/s00330-020-06931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W., Ye Y., Wang T., Zhang F., Geng L., Yu J., Zhou L., Yan S., Zheng S. Prognostic prediction of male recipients selected for liver transplantation: With special attention to neutrophil to lymphocyte ratio. Hepatol. Res. 2016;46:899–907. doi: 10.1111/hepr.12633. [DOI] [PubMed] [Google Scholar]
- 25.Xia W., Ke Q., Wang Y., Wang W., Zhang M., Shen Y., Wu J., Xu X., Zheng S. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J. Surg. Oncol. 2015;13:60. doi: 10.1186/s12957-015-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Gong Y., Zhang Q., Cai P., Feng L. Prognostic Roles of Blood Inflammatory Markers in Hepatocellular Carcinoma Patients Taking Sorafenib. A Systematic Review and Meta-Analysis. Front. Oncol. 2019;9:1557. doi: 10.3389/fonc.2019.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tada T., Kumada T., Hiraoka A., Michitaka K., Atsukawa M., Hirooka M., Tsuji K., Ishikawa T., Takaguchi K., Kariyama K., et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40:968–976. doi: 10.1111/liv.14405. [DOI] [PubMed] [Google Scholar]
- 28.Tada T., Kumada T., Hiraoka A., Michitaka K., Atsukawa M., Hirooka M., Tsuji K., Ishikawa T., Takaguchi K., Kariyama K., et al. Platelet-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma who receive lenvatinib: An inverse probability weighting analysis. Eur. J. Gastroenterol. Hepatol. 2021;32:261–268. doi: 10.1097/MEG.0000000000001734. [DOI] [PubMed] [Google Scholar]
- 29.Dharmapuri S., Ozbek U., Lin J.Y., Sung M., Schwartz M., Branch A.D., Ang C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020;9:4962–4970. doi: 10.1002/cam4.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.Y., Chen C.L., Lin C.C., Wang C.C., Liu Y.W., Li W.F., Chen Y.H. Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study. Cancers. 2021;13:4584. doi: 10.3390/cancers13184584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.Y., Wang C.C., Liu Y.W., Li W.F., Chen Y.H. Clinical impact of lenvatinib in patients with unresectable hepatocellular carcinoma who received sorafenib. PeerJ. 2020;8:e10382. doi: 10.7717/peerj.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu W., Tong Z. Sorafenib in the treatment of patients with hepatocellular carcinoma (HCC) and microvascular infiltration: A systematic review and meta-analysis. J. Int. Med. Res. 2020;48:300060520946872. doi: 10.1177/0300060520946872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraoka A., Kumada T., Atsukawa M., Hirooka M., Tsuji K., Ishikawa T., Takaguchi K., Kariyama K., Itobayashi E., Tajiri K., et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019;8:3719–3728. doi: 10.1002/cam4.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraoka A., Kumada T., Kariyama K., Takaguchi K., Atsukawa M., Itobayashi E., Tsuji K., Tajiri K., Hirooka M., Shimada N., et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137–146. doi: 10.1002/cam4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen A., Tang C., Wang Y., Chen Y., Yan X., Zhang C., Liu R., Wei X., Zhu Y., Zhang H., et al. A systematic review of sorafenib in Child-Pugh A patients with unresectable hepatocellular carcinoma. J. Clin. Gastroenterol. 2013;47:871–880. doi: 10.1097/MCG.0b013e3182a87cfd. [DOI] [PubMed] [Google Scholar]
- 36.Iwamoto H., Shimose S., Noda Y., Shirono T., Niizeki T., Nakano M., Okamura S., Kamachi N., Suzuki H., Sakai M., et al. Initial Experience of Atezolizumab Plus Bevacizumab for Unresectable Hepatocellular Carcinoma in Real-World Clinical Practice. Cancers. 2021;13:2786. doi: 10.3390/cancers13112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schisterman E.F., Perkins N.J., Liu A., Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 38.Personeni N., Giordano L., Abbadessa G., Porta C., Borbath I., Daniele B., Van Laethem J.L., Van Vlierberghe H., Trojan J., De Toni E.N., et al. Prognostic value of the neutrophil-to-lymphocyte ratio in the ARQ 197-215 second-line study for advanced hepatocellular carcinoma. Oncotarget. 2017;8:14408–14415. doi: 10.18632/oncotarget.14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun S., Wang X., Chen J. Using Pre-Treatment Neutrophil-to-Lymphocyte Ratio to Predict the Prognosis of Young Patients with Hepatocellular Carcinoma Implemented Minimally Invasive Treatment. J. Adolesc. Young Adult Oncol. 2020;9:85–89. doi: 10.1089/jayao.2019.0046. [DOI] [PubMed] [Google Scholar]
- 40.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. [(accessed on 1 September 2021)];2017 November 27; Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- 41.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 42.Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J., Tateishi R., Han K.H., Chawla Y.K., Shiina S., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Hegde P.S., Wallin J.J., Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Wallin J.J., Bendell J.C., Funke R., Sznol M., Korski K., Jones S., Hernandez G., Mier J., He X., Hodi F.S., et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwamoto S., Takahashi T., Tamagawa H., Nakamura M., Munemoto Y., Kato T., Hata T., Denda T., Morita Y., Inukai M., et al. FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: The randomized phase III EAGLE study. Ann. Oncol. 2015;26:1427–1433. doi: 10.1093/annonc/mdv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon M.S., Margolin K., Talpaz M., Sledge G.W., Jr., Holmgren E., Benjamin R., Stalter S., Shak S., Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.