Abstract
The use of extracellular vesicles (EV) in nano drug delivery has been demonstrated in many previous studies. In this study, we discuss the sources of extracellular vesicles, including plant, salivary and urinary sources which are easily available but less sought after compared with blood and tissue. Extensive research in the past decade has established that the breadth of EV applications is wide. However, the efforts on standardizing the isolation and purification methods have not brought us to a point that can match the potential of extracellular vesicles for clinical use. The standardization can open doors for many researchers and clinicians alike to experiment with the proposed clinical uses with lesser concerns regarding untraceable side effects. It can make it easier to identify the mechanism of therapeutic benefits and to track the mechanism of any unforeseen effects observed.
Keywords: extracellular vesicles, stem cells, isolation and purification methods, clinical application
1. Introduction
Cell development and maintenance of homeostasis are related to intracellular communication, both in a specific site or between various tissues. Cells communicate via cell junctions, secretions and electrical stimuli [1,2]. Similarly, EVs are another mode of communication between cells and tissues. They carry cargos containing proteins, lipids, receptors and genetic molecules. Based on their cellular origin, different types of EVs have been identified: apoptotic bodies, micro vesicles and exosomes, to name a few [3,4,5].
Nomenclature standardization efforts have been made since the first International society for Extracellular Vesicles meeting (ISEV), but the umbrella term “extracellular vesicles” remains [6,7].
In the past couple of decades, extracellular vesicles have attracted the attention of the scientific community as a source of versatile communication mediators. Numerous studies are being performed to study them. Their characteristics, including small size, less toxicity and immunogenicity and being modifiable, make them suitable biomarkers and drug delivery vehicles [8,9,10,11].
The main challenges for studying extracellular vesicles are their isolation and characterization. There are some conventional methods including ultracentrifugation and ultrafiltration, and several novel techniques including microfluidic chips and immunoaffinity precipitation kits for this purpose [12,13,14]. The clinical use of EV can be affected by the isolation method [14,15]. Therefore, trying to choose the best protocol and customize it based on the study seems necessary.
Moreover, to study EVs, it is crucial to determine their characteristics. Various properties of EVs should be identified, such as size, density, concentration, protein and nucleotide content, surface protein and lipid structure [12,16,17]. Extracellular vesicles inherit their characteristics and content from their parent cells. Therefore, clarifying their characteristics can be used for the diagnosis and prognosis of various conditions [18,19,20,21].
In this study, sources of the extracellular vesicle are described, and various isolation and characterization techniques have been discussed.
2. Different Methods for EV Isolation and Purification
In the past few decades, there has been considerable attention on using extracellular vesicles as biomarkers for various conditions and as drug delivery vehicles. One of the challenges encountered for wide application is choosing an optimum, efficient and reliable isolation method [11,22]. Filtration, ultracentrifugation and affinity separation are the most common isolation methods [23,24,25]. To isolate well-purified and healthy extracellular vesicles, a suitable combination of isolation and purification methods is necessary, Figure 1 [26,27,28].
Figure 1.
Schematic representation of different methods for extracellular isolation and purification.
Various peptides, proteins, lipids and cell debris contaminants are present in the source samples, some of which are similar to EVs in structure and composition, whereas some interact with EVs, preventing extraction [22,29,30].
In the following paragraphs, EV isolation methods are discussed in further detail, Figure 1.
2.1. Centrifugation-Ultracentrifugation-Density Gradient
A centrifuge is a device for separating particles from a solution according to their size, shape and density, and the viscosity of the medium. It causes denser elements (cells, particles, proteins) to separate at the bottom of a tube. The greater the difference in density, the faster they separate [31,32].
Ultracentrifugation is the gold standard method for extracellular vesicle isolation. The different types of ultracentrifugation are differential ultracentrifugation, density gradient centrifugation and rate-zonal centrifugation techniques [23,33].
Differential ultracentrifugation was the first technique that was used for extracellular vesicle isolation. This method is based on density, size and shape of the EVs [23,34,35,36,37,38]. The duration of centrifugation, temperature and sample dilution play pivotal role in separation efficiency [39,40]. It is easy to use and requires no or slight sample pretreatment, but it takes longer time, needs more labor and a large sample [23,41,42]. In addition, various types of EVs cannot be separated via this technique [38]. Separation via density gradient could be considered for reaching higher purification as described below [23].
Density gradient centrifugation (DGC) is another ultracentrifugation method. The difference between UC and DGC is, centrifugation occurs in a tube that contains preconstructed density gradient medium in case of DGC [23,31,35]. Sucrose and iodixanol (OptiPrep®) are the most used media. Through this technique, extracellular vesicles can be separated from proteins [23]. Furthermore, various kinds of EVs could be separated based on their density [14]. Longer cycle durations, low yield rate and requirement of larger volume of sample (in comparison with UC) are the drawbacks of this technique [43,44].
Gradient centrifugation is rate-zonal centrifugation based on density gradient and sedimentation rate. The sample is added to the top of the tube and through centrifugation, compounds with higher density go through the dense layer, easier that lighter compounds. The duration of centrifugation should be controlled to avoid pellet construction at the bottom of the tube [23,45]. In addition, via this technique, particles with the same density and different diameter (size) can be separated [46]. This technique causes more extracellular recovery in comparison with density gradient centrifugation [23].
2.2. Precipitation
This method works based on dispersibility alteration [23,31]. A water-excluding compound is first added to the sample. Polyethylene glycol (PEG), a polymer, is the commonly used compound for this purpose. After adding the polymer, centrifugation or filtration is needed for separation. The polymer dries the sample and leads to the precipitation of other molecules. [30,31,47].
Precipitation is considered a quick and easy method for EV isolation and can be used for small or large volume samples. In addition, this method requires little proficiency and not a specialized apparatus. The selectivity and quality of the isolate (unspecific co-precipitation) in this method is poor, and it must be combined with other method(s) [23,31,35,48,49]. To overcome this, filtration or ultracentrifugation can be carried out before treatment with PEG [23,31]. In addition, some commercial precipitation kits have been developed [23,50]. These kits are fast, easy to use and do not require a specific apparatus. However, they are expensive, not applicable for large samples and are not efficient to separate different types of EVs [51,52,53]. Other compounds that can be used for precipitation are Acetat salt and protamine [51,54,55,56].
Precipitation should always be followed by centrifugation and filtration to eliminate contaminants [51,54,56].
Lectin is another chemical precipitant. In this technique, the sample is pretreated via centrifugation for separating cell debris and lectin is added to the sample and incubated overnight. Lectin conjugates with the carbohydrate of the exosome membrane, changes its solubility and causes precipitation, following which exosomes/EVs will be separated via centrifugation [23,31,57]. Chemical precipitations methods are simple, cost less, and are suitable for different sample sizes [51,54,56,58,59,60].
2.3. Size Based Approaches
As the title suggests, EV isolation here is based on size differentiation. Various techniques purify EVs based on size, including ultrafiltration, isolation kits, sequential filtration, size-exclusion chromatography (SEC), field-flow fractionation (FFF) and hydrostatic filtration dialysis (HFD) [23].
Ultrafiltration is the most common size-based isolation method. In this technique, sample goes through membrane filters with different pore sizes. EVs are then separated based on size and molecular weight [23,31]. One of the limitations of ultrafiltration is EV clogging and trapping in the membrane. This can be prevented by initial filtration using large pore filters, followed by filtration through small pore filters [23,48,61]. The other drawbacks of ultrafiltration are poor efficiency and filter plugging [23,48,62,63]. Ultrafiltration also leads to deformation of EVs due to the pre membrane pressure (this disadvantage can be reduced by forcing lower pressure). However, the technique is still popular as it is less time-consuming and does not require expensive instruments [23,48,50,63].
In recent times, isolation kits based on size differentiation have been developed. One of them is the ExoMir Kit (Bioo Scientific; Austin, TX, USA) that contains two different membranes (upper membrane: 200 nm and downer: 20 nm) in a syringe [23].
In addition, ExoTIC (exosome total isolation chip) technology is the other kit that could purify EVs by passing the sample through different filters. These kits are easy to use, have a high yield rate and can be used for different types of bio-fluid samples [64,65]. Other methods are tangential flow filtration (TFF), direct filtration, and cyclic TFF [66].
Sequential filtration is another technique where the sample is passed through different filters. In each step particles with larger size than membrane pores are trapped, and smaller particles go through it. It is a semi-automated technique. Therefore, it is easy to use and less time-consuming [62]. Filter trapping is a limitation of sequential filtration that leads to membrane plugging and yield rate reduction [62,67,68].
Size-exclusion chromatography is another method that isolates EVs based on size. It consists of a column that allows penetration of smaller particles. This causes bigger particles to exit the column earlier. This protects the structure, integrity and biological function of EVs [23,62,69,70,71,72]. In this method, the sample does not rely on extensive pretreatment [62,70].
The first time that this method was developed, starch and water were used to form pores in the column, but through time, various other compounds such as dextran polymer (Sephadex), agarose (Sepharose) and polyacrylamide (Sephacryl or BioGel) have been used [23,62,73,74,75].
The other innovation for EV isolation based on the size differentiation is field-flow fractionation (FFF). In this method, sample is injected into a chamber that is affected via a cross flow, whereas bigger particles will be pushed to the walls of the chamber due to the cross flow, smaller particles elute earlier [23,76]. This method provides an opportunity to isolate various types of EVs and even very tiny compounds. It is faster, highly efficient, label-free and has higher sample recovery [77].
Another technique called hydrostatic dialysis isolation (HDI) uses hydrostatic forces for isolation. Small particles diffuse through the membrane and larger ones stay in the tube [23,78]. Via this method, the Tamm–Horsfall Protein, one of the abundant proteins in urinary EVs, is eliminated. After HDI, centrifugation is performed for further purification [79].
2.4. Affinity
Affinity based EV isolation is based on the antigens present on the EV surface. Antigens on the EV membrane are considered as markers to distinguish their sources [23,80,81,82]. These antigens are captured via specific antibodies [23,35,83]. This method provides highly purified EVs, but the harvest rate is low [23,49]. Pretreatment of samples, especially plasma, with ultracentrifugation or ultrafiltration is necessary [23,81]. In the study conducted by Tauro BJ et al., the efficacy and the results of three different methods including ultracentrifugation, density gradient isolation and immunoaffinity capture method indicate that immunoaffinity causes the highest purification of EV [80]. The limitation of this method is related to the availability of antibodies of the identified antigen. Masking of the antigens on the EV surface can prevent isolation via immunoaffinity capture methods [14]. Enzyme-linked immunosorbent assay (ELISA) is the most common immunoaffinity-based isolation and identification method [23,84]. Samples should be pretreated with ultracentrifugation before affinity capture [23,31].
One of the most effective methods to elevate EV harvest through immunoaffinity is increasing the surface area of presenting antibodies. Magneto-immunoprecipitation is a technique used for this. In this, a biotinylated antibody specific to the presenting antigen is attached to the surface of magnetic beads coated with streptavidin. Isolated EVs are then detached and used for other purposes while preserving the activity of EV protein [23,31,85]. This technique is easy and fast, but a high affinity between antigen and antibody can prevent the detachment of EVs [86].
In a study that was performed by Zhang J et.al, a combination of three methods was used to reach the optimum level of EV purification. Their protocol contains tangential flow filtration, centrifugation and immunomagnetic affinity technique; the first and second steps produce purified EVs and with immunomagnetic affinity; EVs that contain specific markers are isolated [87,88].
2.5. Micro-, Nano-Fluidics, Chips
Micro-, nano-fluidic chips isolate EVs based on their biochemical properties using acoustic, electrophoretic, and electromagnetic technology. This method is fast, inexpensive, efficient and can be used on small samples [23,31,89,90,91].
Microchips have been developed to isolate EVs with different approaches, including immunoaffinity, size and density-based separations. Through immunoaffinity capture, markers on the EV membrane bind to their specific antibody on the beads or inner surfaces modified by antibodies. The major limitation is the need for appearance of specific antigen on the EV surface. Developing size based microchips can surpass this limitation [91].
For size-based isolation of microchips, pressure and electrophoresis techniques are used. Electrophoresis is preferred in comparison with pressure as it prevents pore blockage [90,91].
In addition, nanowires, nano-sized deterministic lateral displacement (nano-DLD) and viscoelastic flow are the other techniques that can be used. The mechanism using nanowires is similar to SEC and contains micro-porous silicon. Nano-DLD is a pillar-array-based microfluidic method that categorizes elements in an incessant stream [91], whereas viscoelastic flow is a novel passive and label-free technique in this category that separate particles based on variance among elastic lift forces executed on compounds with different sizes in a viscoelastic medium [91,92].
Acoustic separation is one of the techniques that is used via micro-fluidic chips. In this technique, the sample is exposed to ultrasonic waves. The larger particles are affected via heavier radiation and transferred to the pressure node faster. The ultrasonic wave frequencies are controlled to separate specific particles based on the size range [23,89]. Furthermore, development of this technique produces highly purified EVs and can separate them from very low density lipoproteins with remarkable efficiency [93].
The other technique in this group is immuno-based microfluidic isolation. The mechanism is similar to ELISA. Compared with ELISA, smaller samples can be used in this method (microliter). The specificity of the method is related to the specificity of antibodies that are immobilized on the chip [23,94,95]. As mentioned before, antibodies can be loaded on the beads located on the inner surface of the microchannel [96]. To reach the mentioned specificity, Exochip has been developed recently and due to the anti-CD68 antibodies (conjugate with CD68 that is expressed on the exosomes are released via various cell types) that are fixed on the microfluidic chips, the specific EV are isolated [23,97]. ExoSearch is the other microfluidic chip for EV isolation that can be used for smaller samples and consumes lesser time [23,94]. The modified magnetic beads via specific antibodies identify CA-125, EpCAM and CD24 on the EVs of ovarian cancer [94,98].
3. Comparison of Different Methodological Isolation Procedures
The optimal isolation method is one of the greatest challenges for the clinical use of EVs. As mentioned before, various types of isolation methods have been developed. Each method had its own advantages and disadvantages. When considering different methods, “an ideal method for isolation of EVs should be relatively simple, inexpensive, should not require a complex or expensive equipment, should be relatively fast and allow for isolation of EVs from a large volume of samples” [31,35].
The pros and cons of each method have been described in summary in Table 1.
Table 1.
Comparison of EV isolation techniques in terms of source, recovery, purity, sample volume and time.
| Method | Sources | Time | Volume | Recovery | Purity | Ref |
|---|---|---|---|---|---|---|
| Ultracentrifugation | MCF-7 cell line | 4 h | 1 mL | - | Moderate | [44] |
| Ultracentrifugation | Non-Small-Cell Lung Cancer (NSCLC) SK-MES-1 cell line | 20 h | 500 µL | 70% | <UF | [63] |
| Ultracentrifugation | Human colon carcinoma LIM1863 cells | 2h | 500 μL | 5–25% | Low | [80] |
| OptiPrep™ density gradient centrifugation | Human colon carcinoma LIM1863 cells | >21 h | 500 μL | 5–25% | >UC | [80] |
| OptiPrep™ density gradient centrifugation | human breast cancer cell line MCF-7 | 20 h | 1 mL | - | Very high | [44] |
| Density Gradient centrifugation | Tca8113 human tongue squamous cell carcinoma cell line | 20 h | >1 mL | >UC | Similar to UC | [101] |
| ExoQuick-TC™ precipitation | human breast cancer cell line MCF-7 | 13 h | 1 mL | - | Low | [44] |
| ExoChip | Blood serum | <2 h | <400 μL | Low | - | [97] |
| TEI precipitation | human breast cancer cell line MCF-7 | 13 h | 1 mL | - | Low | [44] |
| Ultrafiltration | Non-Small-Cell Lung Cancer (NSCLC) SK-MES-1 cell line | 18 h | 500 µL | 90% | >UC | [63] |
| Sequential filtration | MDA231 breast cancer cells | - | 150 mL | <UC | High | [102] |
| heparin/polymer-coated microspheres | Plasma | 1 h | 2 mL | 81% | High | [103] |
| Heat Shock Protein (HSP)-binding peptide Vn96 | HT-29 cell | 32 min | 2 mL | Poor | Poor | [104] |
| Liquid biopsy chip + HSP-binding peptide Vn96 | MCF7 | 20 min | 0.2 mL | 99% | - | [105] |
| Enzyme-linked immunosorbent assay | LNCaP cell line HCT116 cell line |
2 h | 100 μL | 75–80% | - | [34] |
| Integrated microfluidic platform | Plasma | 2 h | 30 μL | >99.9% | - | [106] |
| anti-EpCAM coated magnetic beads | Human colon carcinoma LIM1863 cells | Overnight | >1 mL | 5–25% | >UC | [80] |
| Acoustic Nanofilter | Red blood cells | <30 min | 50 μL | >80% | - | [89] |
| Microfluidic ExoSearch chip | Blood | >40 min | 20 μL | 42–97.3% | - | [94] |
| Immune-microfluidic | Cell line (ovarian cancer C30) | ~100 min | 30 μL | >99.9% | - | [95] |
| Microfluidic affinity separation chip | Serum | 20–40 min | 20–100 μL | ~60% | - | [98] |
| Micro fluidic viscoelastic flows | Serum | <5 min | <100 μL | >80% | >90% | [92] |
| Microfluidic viscoelastic flow | Blood | ∼25 min | - | > 99% | ∼98.4% | [107] |
| Double-filtration microfluidic device | Urine | <10 min | <100 μL | 74.2% | - | [108] |
| Modified acoustic | Blood | 25 min | 100 μL | 82% | 98% | [109] |
| Crossflow microfiltration | Lipo246 cell line | 30 min | - | 32–76% | Low | [110] |
| Centrifugal microfluidic | Human breast adenocarcinoma cell line, MCF-7 Lung adenocarcinoma cell line, H1975 |
<4 min | <10 μL | 90% | 85% | [111] |
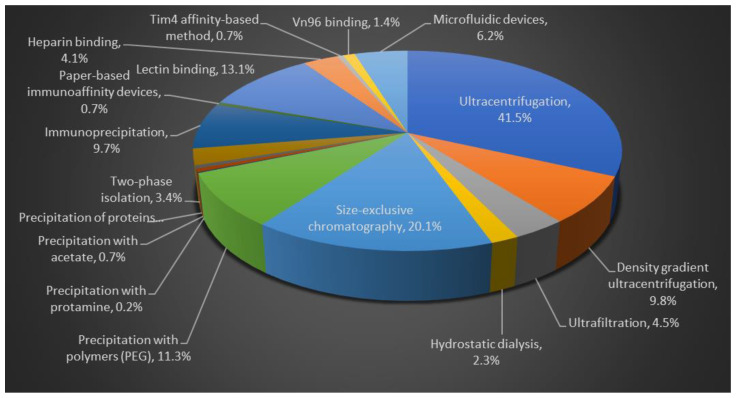
The percentages of research studies published describing each isolation method are described in pie chart, Figure 2. The comparison studies have stated that UC is the primary isolation method for 41.5% of the evaluations (for source volumes from <1 to >100 mL) [99,100].
Figure 2.
Comparison of different isolation methods for EVs purification. Flow chart data is based on published literature.
4. Clinical Application of EVs
4.1. EVs as Diagnosis Biomarkers
Extracellular vesicles are sacs that are secreted by almost all types of cells and are responsible for intracellular communication. They inherit their content and characteristics from their donor cells [10,112,113]. Pathological and physiological characteristics of donor cells are reflected in the appearance of specific nucleotide and proteins (on the EV surface or in their content) [10,114]. In addition, the rate of EV secretion can be changed in various conditions. Therefore, EVs are precise markers for the diagnosis, prognosis and monitoring of different pathologies [113,115].
Extracellular vesicles are desirable markers for Alzheimer’s disease (AD). For instance, increased levels of Aβ1-42, total tau, p-T181 tau and p-S396 tau have been determined in the exosomes that are isolated from the plasma of AD patients compared with healthy candidates [116,117]. Additionally, lysosomal protein such as cathepsin D and LAMP1 and synaptic proteins such as synaptophysin, synaptopodin, synaptotagmin-2 and neurogranin are increased and decreased in the exosome content of plasma in AD [118,119,120]. miRNA are the other molecules that can be searched in AD patient exosomes. miR-29b, miR-181c, miR-15b, miR-146a and miR-107 are examples of miRNA that increased in AD [120].
Cancers can also be diagnosed via EV markers. Increasing in the level of some miRNAs (let-7a, miR-1,229, miR-1,246, miR-150, miR-21, miR-223, and miR-23a) in exosomes have been found in early stage of colon cancer that can be considered as biomarkers [121,122,123]. In addition, high presentation of miRNA141 and miRNA195 have been detected in circulating exosomes of early stage breast cancer patients [124]. lncRNA content of exosomes can be the other candidate for cancer diagnosis, for instance lincRNA-p21 in the urine exosomes of prostate cancer patients [20,125,126]. The main challenge for considering a specific marker on the EV is overlapping markers of different conditions. For example, upregulation of CD95L on macro vesicles have been found to be related to oral squamous cell carcinoma; however, its elevated expression is also identified in pregnancy [114,127,128]. Changes that occurred in several protein expression including psoriasin, kertain-14, galectin-7, epidermal fatty acid binding protein (E-FABP), migration inhibitor factor-related protein (MRP8) and 14 and stratifin can be considered as bladder cancer biomarkers [129,130].
Extracellular can be used for cardiovascular disease diagnosis. miRNA-133a, miRNA-143/145, miRNA-150, miRNA-155, miRNA-214, miRNA-223, and miRNA320b in exosomes are considered as markers of arthrosclerosis (AS) [131,132]. Several proteins are suggested as biomarkers of AS, including integrins derived from exosomes secreted via macrophage foam cell [133], PSMA6 (prostate-specific membrane antigen), PSMA7, and annexin A2 in blood exosomes originated from innate immune system cells [21,134] and VCAM-1 (vascular cell adhesion molecule 1) and eNOS (endothelial constitutive nitric oxide synthase) identified in plasma endothelial cell-derived exosome [131,135].
miRNA-1 and miRNA-133a alter in circulation of Acute Coronary Syndromes (ACS) and Myocardial Infarction (MI) patients [136,137,138]. Extracellular vesicle miRNA content such as miRNA-133b, miRNA-208b, and miRNA-499 are considered as MI biomarkers [138,139]. In addition, miRNA-233 is the other exosomal miRNA that can be used for diagnosis of ischemic stroke [138,140]. The other examples of exosomal miRNA with diagnostic potential are miRNA-34a, miRNA-146a, MiRNA-92, miRNA-192, and miRNA-194 are associated with heart failure [138,141,142,143].
Furthermore, proteins of exosomes can be changed in cardiovascular disease that can be considered as biomarkers. TNF-a increases in hypoxia condition [144,145], angiotensin II (AngII) type 1 receptor is overexpressed in high pressure [145], polygenic immunoglobulin receptor (pIgR), complement factor C5a (C5a) and cystatin C are upregulated in Acute Coronary syndrome [146] and Complement C1q subcomponent subunit A (C1QA) and Complement C5 (C5), Apolipoprotein D (APOD) and Apolipoprotein C-III (APOCC3) and Platelet glycoprotein Ib alpha chain (GP1BA) and platelet basic protein (PPBP) are associated with MI [138,144,147].
Moreover, extracellular vesicles can be used for diagnosis and prognosis of some infectious diseases. Some examples are as follows: Akt and CD9 in exosomes increase during urinary tract infection and can be identified as biomarkers [148]. Exosomal lncRNA-HEIH increases in Chronic hepatitis C (CHC) related hepatocellular carcinoma and can be introduced as a marker [149]. Upregulation of neurofilament-light (NF-L), high mobility group box 1 (HMGB1) and amyloid β in circulating exosomes of HIV-infected patients can indicate neuronal damage via this virus [150,151].
Therefore, extracellular vesicles are potential biomarkers of various conditions. Their content and their release can be changed via a variety of diseases. to use EVs as biomarkers, optimal isolation methods and standardized characterization protocols are necessary for this purpose [120].
4.2. EVs as Therapeutic Vehicle
In addition to being markers, EVs are identified as biological vehicles. They can carry various kinds of compounds including peptides, proteins, lipids, nucleic acids (RNA, DNA) and carbohydrates. In addition, extracellular vesicles, especially exosomes, can be modified and loaded with various cargoes [152,153,154]. There are different types of extracellular vesicle loading methods that provide appropriate situation to load and deliver different compounds, these methods include, incubation, transfection, physical treatment (sonication, extrusion, freeze–thaw treatment, electroporation and surfactant treatment and dialysis), in situ association and synthesis [152,155].
Currently, the potential of different kinds of nucleic acids (including miRNA, siRNA, lncRNA, mRNA, DNA, etc.) against various conditions has been cleared [156,157,158]. Their clinical application is faced with various challenges such as degradability in blood that cause short half-life, immunogenicity, accumulation in kidney and live and disability to cross hydrophilic membrane [159,160,161,162,163,164]. Therefore, it seems crucial to develop an appropriate and efficient delivery vehicle [157]. A wide variety of studies have been performed using exosomes as delivery vehicle for nucleic acids. Among various types of EVs, micro vesicles are the most appropriate vehicle for them [165]. The summary of studies that deliver miRNA and siRNA via exosomes have been described in Table 2 and Table 3.
Table 2.
Therapy based on miRNA-loaded exosomes.
| Cargo | Donor Cell | Target Cell | Condition | Loading Method | Isolation Method | Route of Administration | Result | Ref |
|---|---|---|---|---|---|---|---|---|
| Cancer Therapy | ||||||||
| Let-7a | HEK293 cell expressing GE11 | EGFR-expressing breast cancer | Breast cancer | Pre-transfection | ultracentrifugation | i.v | Tumor growth inhibition | [166] |
| miR146-b | MSC | Glioma | Primery brain tumor | transfection using electroporation | ExoQuick-TC | intratumoral | Tumor growth inhibition | [167] |
| miR-143 | Human bone-marrow-derived | Osteosarcoma cell line 143B | Osteosarcoma | Transfection using lipofectamin | ultracentrifugation | Not applicable | Migration inhibition | [168] |
| miR-122 | MSC | Hepatocellular carcinoma cells | Hepatocellular carcinoma | Transfection by plasmid | ExoQuick-TC | intratumoral | Enhancing chemotherapeutic sensitivity, tumor growth inhibition | [169] |
| miR-134 | Hs578T and Hs578Ts(i)8 | Hs578T and Hs578Ts(i)8 | Breast cancer | Transfection | ultracentrifugation and ExoQuick, | Not applicable | Reducing migration and invasion, Enhancing chemotherapeutic sensitivity | [170] |
| Anti-miR-9 | MSC | Drug resistant glioblastoma multiforme (BT145and BT164) | Brain tumor | Pre-overexpression | Ultracentrifugation and Total Exosome Isolation kit from Invitrogen | Not applicable | Enhancing chemotherapeutic sensitivity | [171] |
| Neurodegenerative diseases | ||||||||
| miR-219 | Dendritic cells | oligodendrocytes | multiple sclerosis and dysmyelinating syndromes | - | ExoQuick and precipitation with centrifugation | Intra nasal | increase baseline myelination, reduce oxidative stress, and improve remyelination | [172] |
| miR-17-92 | MSC | Cerebral cells | post–middle cerebral artery occlusion (strock) | Transfection with a miR-17–92 cluster plasmid | multistep centrifugation | intravenous | improvement of neurological function and oligodendrogenesis, neurogenesis, and neuritis | [173] |
| miR-133b | MSC | Cerebral cells | middle cerebral artery occlusion (MCAO) (stroke) | infected with lentivirus constructed with the vectors of LentimiRaGFP-hsa-miR-133b Vector | multistep centrifugation | intra arterially | enhancing neurological recovery and plasticity post stroke | [174] |
| miR-124 | Bone marrow from adult male mice | Ischemic cells | stroke | electroporation | ultracentrifugation | intravenous injection | promoted cortical neural progenitors to obtain neuronal identity and protect against ischemic injury | [175] |
| miR-30d-5p | adiposederived stem cells (ADSCs) | Cerebral cells | stroke | Transfection by Lipofectamine® 2000 | ultracentrifugation | injection through the tail vein | suppressing the inflammatory response and preventing cerebral injury by inhibiting outophagy-mediated microglia polarization to M1 | [176] |
| Cardiovascular diseases | ||||||||
| miR-19a, miR-451 | mesenchymal stem cells (MSC) overexpressing GATA-4 | neonatal rat ventricles cardiomyocytes | MI | Naturally exist in exosomes of donor cells | Precipitation (CBI) | intramyocardial injection | reduced apoptosis of cardyomyioyte and enhanced resistance to cardyomyocyte hypoxia | [177,178] |
| miR-146a | human CDCs (or normal human dermal fibroblasts [NHDFs] | Neonatal rat cardiomyoctes (NRCMs) | MI | Naturally exist in exosomes of donor cells | Exoquick ExosomePrecipitation Solution (System Biosciences) | intramyocardial injection | redevelop injured heart muscle | [177,179] |
| miR-21 | cardiac progenitor cell (CPC) | mouse cardiac endothelial cells | MI | Naturally exist in exosomes of CPC | precipitated with ExoQuick TC (System Biosciences) | In vitro | inhibiting role in the apoptosis pathway via downregulating programmed cell death 4 | [177,180] |
Table 3.
Summary of studies conducted on siRNA as a cargo of exosomes.
| Cargo | Donor Cell | Isolation Method | Loading Method | Condition | Biological Effect | Ref |
|---|---|---|---|---|---|---|
| PLK1 siRNA | human embryonic kidney (HEK) cell | ultracentrifugation | electroporation | Bladder cancer | Effective delivery of PLK1 siRNA | [181] |
| GAPDH siRNA | Engeneered self-dendritic cells to express Lamp2b | ultracentrifugation | electroporation | Alzheimer’s disease | knockdown of BACE1 | [182] |
| Alexa flour 488 labeled siRNA | HeLa and ascites with the presence of exosomal marker proteins HLA-ABC and CD63 on the membrane of these exosomes by dot blot analysis. | ultracentrifugation | chemical treatment (lipofectamin) | cancer | Silencing of RAB51 | [183,184] |
| siRNA | NIH3T3 cells | ultracentrifugation | electroporation | lymphoma | Silencing of c-Myc and stimulation of caspase-3 | [184,185] |
| siRNA | HEK293T cell (transduced by a lentiviral vector bearing-LAMP2b-DARPin G3 chimeric gene) | sequential centrifugation | electroporation | HER2-positive breast cancer (SKBR3 cells) | Down regulating the TPD52 gene | [186] |
| BCR-ABL siRNA | HEK293T (transfected with of IL3-Lamp2b plasmid DNA) | ultracentrifugation | Transfection with Lipofectamin | Chronic Myeloid Leukemia | inhibit Bcr-Abl and cancer cell growth | [187] |
| siRNA or shRNA targeting KRAS | normal fibroblast-like mesenchymal cells | ultracentrifugation | electroporation | Pancreatic cancer | suppressed cancer in multiple mouse models | [188] |
| siRNA | human induced pluripotent stem cells (huiPSCs) | centrifugation | electroporation | Pulmonary inflammation | Efficient delivery of the target siRNA into HMVECs, inhibiting the ICAM-1 protein expression | [189] |
| opioid receptor mu (MOR) siRNA | human embryonic kidney 293T cotransfected with siRNA and the RVG-Lamp2b plasmid using Lipofectamine 2000) | using an exosome isolation kit (Invitrogen) | transfection | morphine addiction | reduces MOR mRNA and protein levels in Neuro2A cells and the mouse brain | [190] |
| hydrophobically modified small interfering RNAs(hsiRNAs) | U87 glioblastoma cells | ultracentrifugation | co-incubation | Huntington’s disease | bilateral silencing of Huntingtin mRNA | [191] |
| SiRNA | embryonic cortical neuronal culture | ExoQuick (based on the production company’s instruction) | electroporation | Spinal cord-injury | knockdown of ASC protein transformation and significant decrease in caspase 1 activation | [192] |
| siRNA | Human hepatoma cells HuH7 | ultracentrifugation | Tranfection (Lentiviral vectors LV-shCD81 and LV-shNS5b were constructed. LV-shNS5b contains expression cassettes of shRNA and targets the viral NS5b region) | Hepatitis C virus infection and other liver disease | suppression of CD81 expression in target hepatocytes | [193] |
The evaluation of effects of exosomes derived from embryonic stem cells reveals that exosomes can transfer their mRNA content to the target cells and protect their functional activities [194,195]. In addition, exosomes derived from mesenchymal stem cells contain IGF-1 m-RNA that causes expression of IGF-1 and leads to renoprotective effects [196].
In addition, some studies have evaluated EVs to deliver peptides and proteins. For instance, exosomes loaded with Survivin-T34A cause growth limitation of prostate tumors via apoptosis induction [197]. Catalase, a redox enzyme, was loaded in macrophage-derived exosomes and injected intravenously to the mouse model of acute brain injury that lead to neuroprotective effects [198]. In the other study, human MUC1 (hMUC1) has been expressed in two MHC type-distinct mouse cell lines. The result of the study indicate that the engineered exosomes can stimulate immune system and suppress hMUC1-expressing tumor growth, specifically and efficiently [199]. In the other study, performed by Admyre C, a combination of 23 immunogenic peptides from EBV, CMV and influenza virus were loaded into monocyte-derived DC exosomes and exhibited immune system induction via stimulation of CD8+ T cell [200].
Furthermore, extracellular vesicles can carry some other compounds including synthetic and natural molecules. Doxorubicin [201,202], paclitaxel [203] and rhodamine 123 [204] delivery to the cancer cells and dopamine transferring into the brain [205] are the examples of the synthetic molecules. Curcumin and celasterol are the compounds that, when divided from plant extracts, have also been loaded into exosomes and used against inflammation and cancer, respectively [57,206,207,208].
Overall, it seems that extracellular vesicles are successful in embracing different types of cargoes. Furthermore, Extracellular vesicles can be modified for target therapy. Different approaches have been investigated for this purpose that was mentioned before [209,210]. The strategy for EV modification is related to the condition or characteristics of target cells [155]. The most common modification approach is designing exosomes via specific ligands to bind to target receptors. For this aim, there are two different techniques: 1. Direct assembling, 2. Transfection. Her2 and EGFR are located using these techniques and targeted in breast cancer and colorectal cancer, respectively [155,211,212]. The other approach for EV engineering is using pH-sensitive peptides on their surface, which in acidic environments causes deformation releasing the cargo [155,213]. In addition, magnetic directing can be used to direct therapy towards the target. EVs that are designed by magnetic compounds are directed by outer magnetic force to the target area [155,214]. This revealed the potential of EVs to load various cargoes and then modify as an appropriate vehicle for different molecules [155].
5. Conclusions
Isolation and characterization of EVs needs more attention to reach a high efficacy and specificity. For this, the characteristics of sample and study should be considered. In addition, advantages and disadvantages of methods as well as their properties and conditions help us to choose the most effective method [215,216]. Standardization of purification methods would be a great step to understand the requirements. Clear classification of these methods based on the characteristics of EVs, prognostic, diagnostic and clinical requirements, cost-effectiveness would be prudent. The main areas to focus on with regard to isolation and purification are high selectivity and greater efficiency [114,217,218]. This will increase the reach of EVs for clinicians and scientists to try the EVs as the need arises. This in turn would increase the knowledge regarding their behavior in clinically relevant scenarios.
Author Contributions
A.A., F.M., N.B., S.P.K., M.U. wrote, edited, and approved the published manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970;170:404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- 2.Krutovskikh V.A., Piccoli C., Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene. 2002;21:1989–1999. doi: 10.1038/sj.onc.1205187. [DOI] [PubMed] [Google Scholar]
- 3.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 4.Paolicelli R.C., Bergamini G., Rajendran L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Ratajczak J., Wysoczynski M., Hayek F., Janowskawieczorek A., Ratajczak M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 6.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant M.L., Rood I.M., Deegens J.K.J., Klein J.B. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017;13:731–749. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andaloussi S.E., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 9.Nawaz M., Camussi G., Valadi H., Nazarenko I., Ekström K., Wang X., Principe S., Shah N., Ashraf N.M., Fatima F., et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014;11:688–701. doi: 10.1038/nrurol.2014.301. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi S., Balbi C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biology. 2020;9:258. doi: 10.3390/biology9090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodam S.P., Ullah M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/15330338211041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R., Greening D.W., Zhu H.-J., Takahashi N., Simpson R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furi I., Momen-Heravi F., Szabo G. Extracellular vesicle isolation: Present and future. Ann. Transl. Med. 2017;5:263. doi: 10.21037/atm.2017.03.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Skovronova R., Grange C., Dimuccio V., Deregibus M.C., Camussi G., Bussolati B. Surface Marker Expression in Small and Medium/Large Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Naive or Apoptotic Condition Using Orthogonal Techniques. Cells. 2021;10:2948. doi: 10.3390/cells10112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartjes T.A., Mytnyk S., Jenster G.W., Van Steijn V., Van Royen M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering. 2019;6:7. doi: 10.3390/bioengineering6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corso G., Heusermann W., Trojer D., Görgens A., Steib E., Voshol J., Graff A., Genoud C., Lee Y., Hean J., et al. Systematic characterization of extracellular vesicle sorting domains and quantification at the single molecule–single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J. Extracell. Vesicles. 2019;8:1663043. doi: 10.1080/20013078.2019.1663043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., Zambirinis C.P., Rodrigues G., Molina H., Heissel S., et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–1061.e18. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yekula A., Muralidharan K., Kang K.M., Wang L., Balaj L., Carter B.S. From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers. Methods. 2020;177:58–66. doi: 10.1016/j.ymeth.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousafzai N.A., Jin H., Ullah M., Wang X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021;11:5233–5248. [PMC free article] [PubMed] [Google Scholar]
- 21.Ullah A., Mabood N., Maqbool M., Khan L., Ullah M. Cytidine deamination-induced perpetual immunity to SAR-CoV-2 infection is a potential new therapeutic target. Int. J. Med. Sci. 2021;18:3788–3793. doi: 10.7150/ijms.61779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monguió-Tortajada M., Gálvez-Montón C., Bayes-Genis A., Roura S., Borràs F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Experientia. 2019;76:2369–2382. doi: 10.1007/s00018-019-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullah M., Qian N.P.M., Yannarelli G., Akbar A. Heat shock protein 20 promotes sirtuin 1-dependent cell proliferation in induced pluripotent stem cells. World J. Stem Cells. 2021;13:659–669. doi: 10.4252/wjsc.v13.i6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbar A., Pillalamarri N., Jonnakuti S., Ullah M. Artificial intelligence and guidance of medicine in the bubble. Cell Biosci. 2021;11:108. doi: 10.1186/s13578-021-00623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian Y., Gong M., Hu Y., Liu H., Zhang W., Zhang M., Hu X., Aubert D., Zhu S., Wu L., et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles. 2019;9:1697028. doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullah A., Mabood N., Maqbool M., Khan L., Khan M., Ullah M. SAR-CoV-2 infection, emerging new variants and the role of activation induced cytidine deaminase (AID) in lasting immunity. Saudi Pharm. J. 2021;29:1181–1184. doi: 10.1016/j.jsps.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullah M., Qian N.P.M., Yannarelli G. Advances in innovative exosome-technology for real time monitoring of viable drugs in clinical translation, prognosis and treatment response. Oncotarget. 2021;12:1029–1031. doi: 10.18632/oncotarget.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pillalamarri N., Abdullah, Ren G., Khan L., Ullah A., Jonnakuti S., Ullah M. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl. Oncol. 2021;14:101095. doi: 10.1016/j.tranon.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarovni N., Corrado A., Guazzi P., Zocco D., Lari E., Radano G., Muhhina J., Fondelli C., Gavrilova J., Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M., Jin K., Gao L., Zhang Z., Li F., Zhou F., Zhang L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods. 2018;2:1800021. doi: 10.1002/smtd.201800021. [DOI] [Google Scholar]
- 36.Muller L., Hong C.-S., Stolz D.B., Watkins S.C., Whiteside T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods. 2014;411:55–65. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiemstra T.F., Charles P.D., Gracia T., Hester S.S., Gatto L., Al-Lamki R., Floto R.A., Su Y., Skepper J.N., Lilley K.S., et al. Human Urinary Exosomes as Innate Immune Effectors. J. Am. Soc. Nephrol. 2014;25:2017–2027. doi: 10.1681/ASN.2013101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livshits M.A., Khomyakova E., Evtushenko E., Lazarev V.N., Kulemin N., Semina S.E., Generozov E., Govorun V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015;5:17319. doi: 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baranyai T., Herczeg K., Onódi Z., Voszka I., Módos K., Marton N., Nagy G., Mäger I., Wood M.J., El Andaloussi S., et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE. 2015;10:e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullah M., Kodam S.P., Mu Q., Akbar A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano. 2021;15:3612–3620. doi: 10.1021/acsnano.0c10689. [DOI] [PubMed] [Google Scholar]
- 41.Soares Martins T., Catita J., Martins Rosa I., AB da Cruz e Silva O., Henriques A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE. 2018;13:e0198820. doi: 10.1371/journal.pone.0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano-Pertierra E., Oliveira-Rodríguez M., Rivas M., Oliva P., Villafani J., Navarro A., Blanco-López M.C., Cernuda-Morollón E. Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering. 2019;6:8. doi: 10.3390/bioengineering6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salih M., Zietse R., Hoorn E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol.-Ren. Physiol. 2014;306:F1251–F1259. doi: 10.1152/ajprenal.00128.2014. [DOI] [PubMed] [Google Scholar]
- 44.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3:24858. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson N.G. An introduction to particle separations in zonal centrifuges. Natl. Cancer Inst. Monogr. 1966;21:9–39. [PubMed] [Google Scholar]
- 46.Rikkert L.G., Engelaer M., Hau C.M., Terstappen L.W.M.M., Nieuwland R., Coumans F.A. Rate zonal centrifugation can partially separate platelets from platelet-derived vesicles. Res. Pract. Thromb. Haemost. 2020;4:1053–1059. doi: 10.1002/rth2.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D.-K., Nishida H., An S.Y., Shetty A.K., Bartosh T.J., Prockop D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA. 2015;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeringer E., Barta T., Li M., Vlassov A.V. Strategies for Isolation of Exosomes. Cold Spring Harb. Protoc. 2015;2015:319–323. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 49.Batrakova E.V., Kim M. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amarnath S., Foley J.E., Farthing D.E., Gress R.E., Laurence A., Eckhaus M.A., Métais J., Rose J.J., Hakim F.T., Felizardo T.C., et al. Bone Marrow-Derived Mesenchymal Stromal Cells Harness Purinergenic Signaling to Tolerize Human T h1 Cells In Vivo. Stem Cells. 2014;33:1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carnino J.M., Lee H., Jin Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019;20:240. doi: 10.1186/s12931-019-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunkara V., Woo H.-K., Cho Y.-K. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst. 2015;141:371–381. doi: 10.1039/C5AN01775K. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K.R., Alberts B.M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970;40:734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- 54.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller S., König A.-K., Marmé F., Runz S., Wolterink S., Koensgen D., Mustea A., Sehouli J., Altevogt P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 56.Deregibus M.C., Figliolini F., D’Antico S., Manzini P.M., Pasquino C., De Lena M., Tetta C., Brizzi M.F., Camussi G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ullah M. Need for Specialized Therapeutic Stem Cells Banks Equipped with Tumor Regression Enzymes and Anti-Tumor Genes. J. Biomed. Allied Res. 2020;2:13. doi: 10.37191/mapsci-2582-4937-2(1)-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brownlee Z., Lynn K.D., Thorpe P.E., Schroit A.J. A novel “salting-out” procedure for the isolation of tumor-derived exosomes. J. Immunol. Methods. 2014;407:120–126. doi: 10.1016/j.jim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rider M.A., Hurwitz S.N., Meckes D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crescitelli R., Lässer C., Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021;16:1548–1580. doi: 10.1038/s41596-020-00466-1. [DOI] [PubMed] [Google Scholar]
- 61.Liga A., Vliegenthart A.D.B., Oosthuyzen W., Dear J.W., Kersaudy-Kerhoas M. Exosome isolation: A microfluidic road-map. Lab Chip. 2015;15:2388–2394. doi: 10.1039/C5LC00240K. [DOI] [PubMed] [Google Scholar]
- 62.Yang D., Zhang W., Zhang H., Zhang F., Chen L., Ma L., Larcher L.M., Chen S., Liu N., Zhao Q. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lobb R.J., Becker M., Wen S.W., Wong C.S.F., Wiegmans A.P., Leimgruber A., Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeppesen D., Hvam M.L., Primdahl-Bengtson B., Boysen A.T., Whitehead B., Dyrskjøt L., Ørntoft T.F., Howard K.A., Ostenfeld M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu F., Vermesh O., Mani V., Ge T.J., Madsen S.J., Sabour A., Hsu E.-C., Gowrishankar G., Kanada M., Jokerst J.V., et al. The Exosome Total Isolation Chip. ACS Nano. 2017;11:10712–10723. doi: 10.1021/acsnano.7b04878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim K., Park J., Jung J.-H., Lee R., Park J.-H., Yuk J.M., Hwang H., Yeon J.H. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021;5:016103. doi: 10.1063/5.0037768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soda N., Rehm B.H.A., Sonar P., Nguyen N.-T., Shiddiky M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J. Mater. Chem. B. 2019;7:6670–6704. doi: 10.1039/C9TB01490J. [DOI] [PubMed] [Google Scholar]
- 68.Peterson M.F., Otoc N., Sethi J.K., Gupta A., Antes T.J. Integrated systems for exosome investigation. Methods. 2015;87:31–45. doi: 10.1016/j.ymeth.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Gheinani A.H., Vögeli M., Baumgartner U., Vassella E., Draeger A., Burkhard F.C., Monastyrskaya K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018;8:3945. doi: 10.1038/s41598-018-22142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L., Franquesa M., Beyer K., Borràs F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016;6:srep33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navajas R., Corrales F.J., Paradela A. Proteomics for Biomarker Discovery. Humana Press; New York, NY, USA: 2019. Serum Exosome Isolation by Size-Exclusion Chromatography for the Discovery and Validation of Preeclampsia-Associated Biomarkers; pp. 39–50. [DOI] [PubMed] [Google Scholar]
- 72.Stranska R., Gysbrechts L., Wouters J., Vermeersch P., Bloch K., Dierickx D., Andrei G., Snoeck R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018;16:1. doi: 10.1186/s12967-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.GH L., CR R. The separation of substances on the basis of their molecular weights, using columns of starch and water. Biochem. J. 1955;60:xxxiv. [PubMed] [Google Scholar]
- 74.Ruysschaert T., Marque A., Duteyrat J.-L., Lesieur S., Winterhalter M., Fournier D. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005;5:11. doi: 10.1186/1472-6750-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogel R., Coumans F.A.W., Maltesen R.G., Böing A.N., Bonnington K.E., Broekman M.L., Broom M.F., Buzás E.I., Christiansen G., Hajji N., et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles. 2016;5:31242. doi: 10.3402/jev.v5.31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang D., Oh S., Ahn S.-M., Lee B.-H., Moon M.H. Proteomic Analysis of Exosomes from Human Neural Stem Cells by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography−Tandem Mass Spectrometry. J. Proteome Res. 2008;7:3475–3480. doi: 10.1021/pr800225z. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019;14:1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Musante L., Tataruch D., Gu D., Martin A.B., Calzaferri G., Aherne S., Holthofer H. A Simplified Method to Recover Urinary Vesicles for Clinical Applications and Sample Banking. Sci. Rep. 2014;4:7532. doi: 10.1038/srep07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musante L., Tataruch D.E., Holthofer H. Use and Isolation of Urinary Exosomes as Biomarkers for Diabetic Nephropathy. Front. Endocrinol. 2014;5:149. doi: 10.3389/fendo.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tauro B.J., Greening D.W., Mathias R.A., Ji H., Mathivanan S., Scott A.M., Simpson R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Mathivanan S., Lim J.W.E., Tauro B.J., Ji H., Moritz R.L., Simpson R.J. Proteomics Analysis of A33 Immunoaffinity-purified Exosomes Released from the Human Colon Tumor Cell Line LIM1215 Reveals a Tissue-specific Protein Signature. Mol. Cell. Proteom. 2010;9:197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2010;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. Erratum in 2010, 116, 153. [DOI] [PubMed] [Google Scholar]
- 83.Cui Y., Gao J., He Y., Jiang L. Plant extracellular vesicles. Protoplasma. 2019;257:3–12. doi: 10.1007/s00709-019-01435-6. [DOI] [PubMed] [Google Scholar]
- 84.Hosseini S., Vázquez-Villegas P., Rito-Palomares M., Martinez-Chapa S.O. Enzyme-Linked Immunosorbent Assay (ELISA): From A to Z. Springer; Singapore: 2018. [Google Scholar]
- 85.Hong C.S., Muller L., Boyiadzis M., Whiteside T.L. Isolation and Characterization of CD34+ Blast-Derived Exosomes in Acute Myeloid Leukemia. PLoS ONE. 2014;9:e103310. doi: 10.1371/journal.pone.0103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shao H., Im H., Castro C.M., Breakefield X., Weissleder R., Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J., Nguyen L.T.H., Hickey R., Walters N., Wang X., Kwak K.J., Lee L.J., Palmer A.F., Reátegui E. Immunomagnetic sequential ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. Sci. Rep. 2021;11:8034. doi: 10.1038/s41598-021-86910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shih C.-L., Chong K.-Y., Hsu S.-C., Chien H.-J., Ma C.-T., Chang J.W.-C., Yu C.-J., Chiou C.-C. Development of a magnetic bead-based method for the collection of circulating extracellular vesicles. New Biotechnol. 2016;33:116–122. doi: 10.1016/j.nbt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Lee K., Shao H., Weissleder R., Lee H. Acoustic Purification of Extracellular Microvesicles. ACS Nano. 2015;9:2321–2327. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davies R.T., Kim J., Jang S.C., Choi E.-J., Gho Y.S., Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 91.Guo S.-C., Tao S.-C., Dawn H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J. Extracell. Vesicles. 2018;7:1508271. doi: 10.1080/20013078.2018.1508271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C., Guo J., Tian F., Yang N., Yan F., Ding Y., Wei J., Hu G., Nie G., Sun J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 93.Wu M., Chen C., Wang Z., Bachman H., Ouyang Y., Huang P.-H., Sadovsky Y., Huang T.J. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab Chip. 2019;19:1174–1182. doi: 10.1039/C8LC01134F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Z., Yang Y., Zeng Y., He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–496. doi: 10.1039/C5LC01117E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang C., Fu Y., Liu G., Shu B., Davis J., Tofaris G.K. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nanomicro Lett. 2021;14:3. doi: 10.1007/s40820-021-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talebjedi B., Tasnim N., Hoorfar M., Mastromonaco G.F., De Almeida Monteiro Melo Ferraz M. Exploiting Microfluidics for Extracellular Vesicle Isolation and Characterization: Potential Use for Standardized Embryo Quality Assessment. Front. Vet. Sci. 2021;7:1139. doi: 10.3389/fvets.2020.620809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanwar S.S., Dunlay C.J., Simeone D.M., Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–1900. doi: 10.1039/C4LC00136B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hisey C.L., Dorayappan K.D.P., Cohn D.E., Selvendiran K., Hansford D.J. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip. 2018;18:3144–3153. doi: 10.1039/c8lc00834e. [DOI] [PubMed] [Google Scholar]
- 99.Guerreiro E.M., Vestad B., Steffensen L.A., Aass H.C.D., Saeed M., Øvstebø R., Costea D.-E., Galtung H.K., Søland T.M. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS ONE. 2018;13:e0204276. doi: 10.1371/journal.pone.0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deville S., Berckmans P., Van Hoof R., Lambrichts I., Salvati A., Nelissen I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE. 2021;16:e0245835. doi: 10.1371/journal.pone.0245835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Z., Wang C., Li T., Liu Z., Li L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol. Lett. 2014;8:1701–1706. doi: 10.3892/ol.2014.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinemann M.L., Ilmer M., Silva L.P., Hawke D.H., Recio A., Vorontsova M.A., Alt E., Vykoukal J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A. 2014;1371:125–135. doi: 10.1016/j.chroma.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 103.Mao W., Wen Y., Lei H., Lu R., Wang S., Wang Y., Chen R., Gu Y., Zhu L., Abhange K.K., et al. Isolation and Retrieval of Extracellular Vesicles for Liquid Biopsy of Malignant Ground-Glass Opacity. Anal. Chem. 2019;91:13729–13736. doi: 10.1021/acs.analchem.9b03064. [DOI] [PubMed] [Google Scholar]
- 104.Knol J.C., de Reus I., Schelfhorst T., Beekhof R., de Wit M., Piersma S.R., Pham T.V., Smit E.F., Verheul H.M., Jiménez C.R. Peptide-mediated ‘miniprep’ isolation of extracellular vesicles is suitable for high-throughput proteomics. EuPA Open Proteom. 2016;11:11–15. doi: 10.1016/j.euprot.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bathini S., Pakkiriswami S., Ouellette R.J., Ghosh A., Packirisamy M. Magnetic particle based liquid biopsy chip for isolation of extracellular vesicles and characterization by gene amplification. Biosens. Bioelectron. 2021;194:113585. doi: 10.1016/j.bios.2021.113585. [DOI] [PubMed] [Google Scholar]
- 106.He M., Crow J., Roth M., Zeng Y., Godwin A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14:3773–3780. doi: 10.1039/C4LC00662C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sancho-Albero M., Sebastián V., Sesé J., Pazo-Cid R., Mendoza G., Arruebo M., Martín-Duque P., Santamaría J. Isolation of exosomes from whole blood by a new microfluidic device: Proof of concept application in the diagnosis and monitoring of pancreatic cancer. J. Nanobiotechnol. 2020;18:150. doi: 10.1186/s12951-020-00701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang L.-G., Kong M.-Q., Zhou S., Sheng Y.-F., Wang P., Yu T., Inci F., Kuo W.P., Li L.-J., Demirci U., et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017;7:srep46224. doi: 10.1038/srep46224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu M., Ouyang Y., Wang Z., Zhang R., Huang P.-H., Chen C., Li H., Li P., Quinn D., Dao M., et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA. 2017;114:10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casadei L., Choudhury A., Sarchet P., Sundaram P.M., Lopez G., Braggio D., Balakirsky G., Pollock R., Prakash S. Cross-flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12062. doi: 10.1002/jev2.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeo J.C., Kenry, Zhao Z., Zhang P., Wang Z., Lim C.T. Label-free extraction of extracellular vesicles using centrifugal microfluidics. Biomicrofluidics. 2018;12:024103. doi: 10.1063/1.5019983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schey K.L., Luther J., Rose K.L. Proteomics characterization of exosome cargo. Methods. 2015;87:75–82. doi: 10.1016/j.ymeth.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rikkert L.G., Beekman P., Caro J., Coumans F.A.W., Enciso-Martinez A., Jenster G., Le Gac S., Lee W., Van Leeuwen T.G., Loozen G.B., et al. Cancer-ID: Toward Identification of Cancer by Tumor-Derived Extracellular Vesicles in Blood. Front. Oncol. 2020;10:608. doi: 10.3389/fonc.2020.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Julich H., Willms A., Lukacs-Kornek V., Kornek M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front. Immunol. 2014;5:413. doi: 10.3389/fimmu.2014.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castellani C., Burrello J., Fedrigo M., Burrello A., Bolis S., Di Silvestre D., Tona F., Bottio T., Biemmi V., Toscano G., et al. Circulating extracellular vesicles as non-invasive biomarker of rejection in heart transplant. J. Heart Lung Transplant. 2020;39:1136–1148. doi: 10.1016/j.healun.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 116.Eren E., Hunt J.F., Shardell M., Chawla S., Tran J., Gu J., Vogt N.M., Johnson S.C., Bendlin B.B., Kapogiannis D. Extracellular vesicle biomarkers of Alzheimer’s disease associated with sub-clinical cognitive decline in late middle age. Alzheimer’s Dement. 2020;16:1293–1304. doi: 10.1002/alz.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fiandaca M.S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J.B., Abner E.L., Petersen R.C., Federoff H.J., Miller B.L. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015;11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goetzl E.J., Boxer A., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L., Kapogiannis D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goetzl E.J., Kapogiannis D., Schwartz J.B., Lobach I.V., Goetzl L., Abner E.L., Jicha G.A., Karydas A.M., Boxer A., Miller B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee S., Mankhong S., Kang J.-H. Extracellular Vesicle as a Source of Alzheimer’s Biomarkers: Opportunities and Challenges. Int. J. Mol. Sci. 2019;20:1728. doi: 10.3390/ijms20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogata-Kawata H., Izumiya M., Kurioka D., Honma Y., Yamada Y., Furuta K., Gunji T., Ohta H., Okamoto H., Sonoda H., et al. Circulating Exosomal microRNAs as Biomarkers of Colon Cancer. PLoS ONE. 2014;9:e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohammadi S., Yousefi F., Shabaninejad Z., Movahedpour A., Mahjoubin-Tehran M., Shafiee A., Moradizarmehri S., Hajighadimi S., Savardashtaki A., Mirzaei H. Exosomes and cancer: From oncogenic roles to therapeutic applications. IUBMB Life. 2019;72:724–748. doi: 10.1002/iub.2182. [DOI] [PubMed] [Google Scholar]
- 123.Ullah M., Akbar A. Clinical Relevance of RNA Editing to Early Detection of Cancer in Human. Int. J. Stem Cell Res. Ther. 2020;7:66. doi: 10.23937/2469-570x/1410066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Corcoran C., Friel A., Duffy M.J., Crown J., O’Driscoll L. Intracellular and Extracellular MicroRNAs in Breast Cancer. Clin. Chem. 2011;57:18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 125.Işın M., Uysaler E., Özgür E., Köseoğlu H., Şanlı Ö., Yücel Ö.B., Gezer U., Dalay N. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front. Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ullah M., Akbar A., Yannarelli G. Clinical Applications of RNA Editing Technology for the Early Detection of Cancer and Future Directions. Technol. Cancer Res. Treat. 2020;19:1533033820964194. doi: 10.1177/1533033820964194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abrahams V.M., Chavez S., Guller S., Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol. Hum. Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 128.Kim J.W., Wieckowski E., Taylor D.D., Reichert T.E., Watkins S., Whiteside T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 129.Pisitkun T., Johnstone R., Knepper M.A. Discovery of Urinary Biomarkers. Mol. Cell. Proteom. 2006;5:1760–1771. doi: 10.1074/mcp.R600004-MCP200. [DOI] [PubMed] [Google Scholar]
- 130.Schorey J.S., Bhatnagar S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu M., Yuan S., Li S., Li L., Liu M., Wan S. The Exosome-Derived Biomarker in Atherosclerosis and Its Clinical Application. J. Cardiovasc. Transl. Res. 2018;12:68–74. doi: 10.1007/s12265-018-9796-y. [DOI] [PubMed] [Google Scholar]
- 132.Cervio E., Barile L., Moccetti T., Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:482171. doi: 10.1155/2015/482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niu C., Wang X., Zhao M., Cai T., Liu P., Li J., Willard B., Zu L., Zhou E., Li Y., et al. Macrophage Foam Cell–Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016;5:e004099. doi: 10.1161/JAHA.116.004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C., Li Z., Liu Y., Yuan L. Exosomes in atherosclerosis: Performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11:3996–4010. doi: 10.7150/thno.56035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goetzl E.J., Schwartz J.B., Mustapic M., Lobach I.V., Daneman R., Abner E.L., Jicha G.A. Altered cargo proteins of human plasma endothelial cell–derived exosomes in atherosclerotic cerebrovascular disease. FASEB J. 2017;31:3689–3694. doi: 10.1096/fj.201700149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C., Pei F., Zhu X., Duan D.D., Zeng C. Circulating microRNAs as novel and sensitive biomarkers of acute myocardial Infarction. Clin. Biochem. 2012;45:727–732. doi: 10.1016/j.clinbiochem.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuwabara Y., Ono K., Horie T., Nishi H., Watanabe S., Kinoshita M., Baba O., Chujo Y., Nagao K., Kojima Y., et al. MicroRNA-1 and MicroRNA-133a Elevations in Serum of Patients with Cardiovascular Disease Indicate the Existence of Myocardial Damage. Am. Heart Assoc. 2011;124:A11460. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 138.Zamani P., Fereydouni N., Butler A.E., Navashenaq J.G., Sahebkar A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2018;29:313–323. doi: 10.1016/j.tcm.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 139.Deddens J.C., Vrijsen K.R., Colijn J.M., Oerlemans M.I., Metz C.H.G., Van Der Vlist E.J., Nolte-’t Hoen E.N.M., den Ouden K., Jansen Of Lorkeers S.J., Van Der Spoel T.I.G., et al. Circulating Extracellular Vesicles Contain miRNAs and are Released as Early Biomarkers for Cardiac Injury. J. Cardiovasc. Transl. Res. 2016;9:291–301. doi: 10.1007/s12265-016-9705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Y., Song Y., Huang J., Qu M., Zhang Y., Geng J., Zhang Z., Liu J., Yang G.-Y. Increased Circulating Exosomal miRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017;8:57. doi: 10.3389/fneur.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsumoto S., Sakata Y., Suna S., Nakatani D., Usami M., Hara M., Kitamura T., Hamasaki T., Nanto S., Kawahara Y., et al. Circulating p53-Responsive MicroRNAs Are Predictive Indicators of Heart Failure After Acute Myocardial Infarction. Circ. Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 142.Goren Y., Kushnir M., Zafrir B., Tabak S., Lewis B.S., Amir O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 143.Halkein J., Tabruyn S.P., Ricke-Hoch M., Haghikia A., Nguyen N.-Q., Scherr M., Castermans K., Malvaux L., Lambert V., Thiry M., et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu X., Deng L., Wang D., Li N., Chen X., Cheng X., Yuan J., Gao X., Liao M., Wang M., et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: Initiated by hypoxia inducible factor 1α, presented by exosomes. J. Mol. Cell. Cardiol. 2012;53:848–857. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 145.Pironti G., Strachan R.T., Abraham D., Yu S.M.-W., Chen M., Chen W., Hanada K., Mao L., Watson L.J., Rockman H.A. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Hoog V.C., Timmers L., Schoneveld A.H., Wang J.W., van de Weg S.M., Sze S.K., van Keulen J.K., Hoes A.W., den Ruijter H.M., de Kleijn D.P., et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care. 2013;2:53–60. doi: 10.1177/2048872612471212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheow E.S.H., Cheng W.C., Lee C.N., de Kleijn D., Sorokin V., Sze S.K. Plasma-derived Extracellular Vesicles Contain Predictive Biomarkers and Potential Therapeutic Targets for Myocardial Ischemic (MI) Injury. Mol. Cell. Proteom. 2016;15:2628–2640. doi: 10.1074/mcp.M115.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mizutani K., Kawakami K., Horie K., Fujita Y., Kameyama K., Kato T., Nakane K., Tsuchiya T., Yasuda M., Masunaga K., et al. Urinary exosome as a potential biomarker for urinary tract infection. Cell. Microbiol. 2019;21:e13020. doi: 10.1111/cmi.13020. [DOI] [PubMed] [Google Scholar]
- 149.Zhang C., Yang X., Qi Q., Gao Y., Wei Q., Han S. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 2018;21:651–659. doi: 10.3233/CBM-170727. [DOI] [PubMed] [Google Scholar]
- 150.Sun B., Dalvi P., Abadjian L., Tang N., Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS. 2017;31:F9–F17. doi: 10.1097/QAD.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ullah M. Novel Coronavirus (COVID-19) Treatment Options. Biomed. J. Sci. Tech. Res. 2020;27:20872–20874. doi: 10.26717/BJSTR.2020.27.004518. [DOI] [Google Scholar]
- 152.Donoso-Quezada J., Ayala-Mar S., González-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: A review. Crit. Rev. Biotechnol. 2020;40:804–820. doi: 10.1080/07388551.2020.1785385. [DOI] [PubMed] [Google Scholar]
- 153.Li S.-P., Lin Z.-X., Jiang X.-Y., Yu X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018;39:542–551. doi: 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keerthikumar S., Chisanga D., Ariyaratne D., Al Saffar H., Anand S., Zhao K., Samuel M., Pathan M., Jois M., Chilamkurti N., et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2015;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fu S., Wang Y., Xia X., Zheng J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi: 10.1016/j.impact.2020.100261. [DOI] [Google Scholar]
- 156.Wang F., Zuroske T., Watts J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020;19:441–442. doi: 10.1038/d41573-020-00078-0. [DOI] [PubMed] [Google Scholar]
- 157.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 158.Patil S.D., Rhodes D.G., Burgess D.J. DNA-based therapeutics and DNA delivery systems: A comprehensive review. AAPS J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lässer C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin. Biol. Ther. 2012;12:S189–S197. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- 160.Chen Y., Gao D.-Y., Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2014;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garzon R., Marcucci G., Croce C.M. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y., Wang Z., Gemeinhart R.A. Progress in microRNA delivery. J. Control. Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aryani A., Denecke B. Exosomes as a Nanodelivery System: A Key to the Future of Neuromedicine? Mol Neurobiol. 2016;53:818–834. doi: 10.1007/s12035-014-9054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Van den Boorn J.G., Schlee M., Coch C., Hartmann G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 165.Kanada M., Bachmann M., Hardy J.W., Frimannson D.O., Bronsart L., Wang A., Sylvester M.D., Schmidt T.L., Kaspar R.L., Butte M.J., et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohno S.-I., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shimbo K., Miyaki S., Ishitobi H., Kato Y., Kubo T., Shimose S., Ochi M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014;445:381–387. doi: 10.1016/j.bbrc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 169.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O’Brien K., Lowry M.C., Corcoran C., Martinez V.G., Daly M., Rani S., Gallagher W., Radomski M.W., MacLeod R.A., O’Driscoll L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6:32774–32789. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Munoz J.L., Bliss S.A., Greco S.J., Ramkissoon S.H., Ligon K.L., Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther.-Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pusic A.D., Pusic K.M., Clayton B., Kraig R.P. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol. 2013;266:12–23. doi: 10.1016/j.jneuroim.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xin H., Katakowski M., Wang F., Qian J.-Y., Liu X.S., Ali M.M., Buller B., Zhang Z.G., Chopp M. MicroRNA-17–92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., Chopp M. Exosome-Mediated Transfer of miR-133b from Multipotent Mesenchymal Stromal Cells to Neural Cells Contributes to Neurite Outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang J., Zhang X., Chen X., Wang L., Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther.-Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang M., Wang H., Jin M., Yang X., Ji H., Jiang Y., Zhang H., Wu F., Wu G., Lai X., et al. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell. Physiol. Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 177.Gartz M., Strande J.L. Examining the Paracrine Effects of Exosomes in Cardiovascular Disease and Repair. J. Am. Heart Assoc. 2018;7:e007954. doi: 10.1161/JAHA.117.007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu B., Kim H.W., Gong M., Wang J., Millard R.W., Wang Y., Ashraf M., Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2014;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ibrahim A.G.-E., Cheng K., Marbán E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao J., Pan Y., Li X.H., Yang X.Y., Feng Y.L., Tan H.H., Jiang L., Feng J., Yu X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Greco K.A., Franzen C.A., Foreman K.E., Flanigan R.C., Kuo P.C., Gupta G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes. Urology. 2016;91:241.e1–241.e7. doi: 10.1016/j.urology.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 182.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 183.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang J., Zheng Y., Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017;7:533. doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lunavat T.R., Jang S.C., Nilsson L., Park H.T., Repiska G., Lässer C., Nilsson J.A., Gho Y.S., Lötvall J. RNAi delivery by exosome-mimetic nanovesicles–Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–238. doi: 10.1016/j.biomaterials.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 186.Limoni S.K., Moghadam M.F., Moazzeni S.M., Gomari H., Salimi F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2018;187:352–364. doi: 10.1007/s12010-018-2813-4. [DOI] [PubMed] [Google Scholar]
- 187.Bellavia D., Raimondo S., Calabrese G., Forte S., Cristaldi M., Patinella A., Memeo L., Manno M., Raccosta S., Diana P., et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7:1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C., Melo S., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ju Z., Ma J., Wang C., Yu J., Qiao Y., Hei F. Exosomes from iPSCs Delivering siRNA Attenuate Intracellular Adhesion Molecule-1 Expression and Neutrophils Adhesion in Pulmonary Microvascular Endothelial Cells. Inflammation. 2016;40:486–496. doi: 10.1007/s10753-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 190.Liu Y., Li D., Liu Z., Zhou Y., Chu D., Li X., Jiang X., Hou D., Chen X., Chen Y., et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015;5:17543. doi: 10.1038/srep17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Didiot M.-C., Hall L.M., Coles A.H., Haraszti R.A., Godinho B.M., Chase K., Sapp E., Ly S., Alterman J.F., Hassler M.R., et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016;24:1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vaccari J.P.D.R., Brand F., Adamczak S., Lee S.W., Perez-Barcena J., Wang M.Y., Bullock M.R., Dietrich W.D., Keane R.W. Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 2015;136:39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pan Q., Ramakrishnaiah V., Henry S., Fouraschen S., de Ruiter P.E., Kwekkeboom J., Tilanus H.W., Janssen H.L.A., van der Laan L.J. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2011;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 194.Ratajczak J., Miękus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 195.Ullah M., Liu D.D., Rai S., Razavi M., Concepcion W., Thakor A.S. Pulsed focused ultrasound enhances the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles in acute kidney injury. Stem Cell Res. Ther. 2020;11:398. doi: 10.1186/s13287-020-01922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tomasoni S., Longaretti L., Rota C., Morigi M., Conti S., Gotti E., Capelli C., Introna M., Remuzzi G., Benigni A. Transfer of Growth Factor Receptor mRNA Via Exosomes Unravels the Regenerative Effect of Mesenchymal Stem Cells. Stem Cells Dev. 2013;22:772–780. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aspe J.R., Osterman C.D., Jutzy J., Deshields S., Whang S., Wall N.R. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles. 2014;3:23244. doi: 10.3402/jev.v3.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cho J.-A., Yeo D.-J., Son H.-Y., Kim H.-W., Jung D.-S., Ko J.-K., Koh J.S., Kim Y.-N., Kim C.-W. Exosomes: A new delivery system for tumor antigens in cancer immunotherapy. Int. J. Cancer. 2004;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 200.Admyre C., Johansson S.M., Paulie S., Gabrielsson S. Direct exosome stimulation of peripheral humanT cells detected by ELISPOT. Eur. J. Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 201.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 202.Schindler C., Collinson A., Matthews C., Pointon A., Jenkinson L., Minter R.R., Vaughan T.J., Tigue N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE. 2019;14:e0214545. doi: 10.1371/journal.pone.0214545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O., et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R., Yin V.P., Lockman P., Bai S. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qu M., Lin Q., Huang L., Fu Y., Wang L., He S., Fu Y., Yang S., Zhang Z., Zhang L., et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 206.Aqil F., Kausar H., Agrawal A., Jeyabalan J., Kyakulaga A.-H., Munagala R., Gupta R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016;101:12–21. doi: 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 207.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., Zhang H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aqil F., Munagala R., Jeyabalan J., Agrawal A., Gupta R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017;19:1691–1702. doi: 10.1208/s12248-017-0154-9. [DOI] [PubMed] [Google Scholar]
- 209.Das C.K., Jena B.C., Banerjee I., Das S., Parekh A., Bhutia S.K., Mandal M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol. Pharm. 2018;16:24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 210.Yim N., Ryu S.-W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun M.R.S.W., Park J.-H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kooijmans S.A.A., Gitz-Francois J.J.J.M., Schiffelers R.M., Vader P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale. 2018;10:2413–2426. doi: 10.1039/C7NR06966A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gomari H., Moghadam M.F., Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753–5762. doi: 10.2147/OTT.S173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee H., Park H., Noh G.J., Lee E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018;202:323–333. doi: 10.1016/j.carbpol.2018.08.141. [DOI] [PubMed] [Google Scholar]
- 214.Zhuang M., Chen X., Du D., Shi J., Deng M., Long Q., Yin X., Wang Y., Rao L. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale. 2019;12:173–188. doi: 10.1039/C9NR05865F. [DOI] [PubMed] [Google Scholar]
- 215.Sáenz-Cuesta M., Arbelaiz A., Oregi A., Irizar H., Osorio-Querejeta I., Muñoz-Culla M., Banales J., Falcón-Pérez J.M., Olascoaga J., Otaegui D. Methods for extracellular vesicles isolation in a hospital setting. Front. Immunol. 2015;6:50. doi: 10.3389/fimmu.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Momen-Heravi F., Balaj L., Alian S., Mantel P.-Y., Halleck A.E., Trachtenberg A.J., Soria C.E., Oquin S., Bonebreak C.M., Saracoglu E., et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013;394:1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ohno S.-I., Drummen G.P.C., Kuroda M. Focus on Extracellular Vesicles: Development of Extracellular Vesicle-Based Therapeutic Systems. Int. J. Mol. Sci. 2016;17:172. doi: 10.3390/ijms17020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ullah M., Akbar A., Yannarelli G. Applications of artificial intelligence in, early detection of cancer, clinical diagnosis and personalized medicine. Artif. Intell. Cancer. 2020;1:39–44. doi: 10.35713/aic.v1.i2.39. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.