Abstract
The effects of acute stress on memory encoding are complex. Recent work has suggested that both the delay between stress and encoding and the relevance of the information learned to the stressor may modulate the effects of stress on memory encoding, but the relative contribution of each of these two factors is unclear. Therefore, in the present study, we manipulated (1) acute stress, (2) the delay between stress and encoding, and (3) the relevance of the information learned to the stressor. The results indicated that stress during encoding led to better memory for study materials that were related to the stressor relative to memory for study materials that were unrelated to the stressor. This effect was numerically reduced for materials that were encoded 40 min after stressor onset (23 min after the stressor had ended) compared with items encoded at the time of the stressor, but this difference was not significant. These results suggest that the relevance of the information learned to the stressor may play a particularly important role in the effects of stress on memory encoding, which has important implications for theories of stress and memory.
The effects of stress on memory have been the subject of much research (Wolf et al. 2016; Shields et al. 2017, 2021; Quaedflieg and Schwabe 2018; Kalbe et al. 2020). Despite this, it is still somewhat unclear how acute stress influences memory encoding (i.e., the learning of new information); some prior studies have found that stress impairs memory encoding (Schwabe and Wolf 2010), whereas other studies have found that stress enhances encoding (Wiemers et al. 2013). Theories of stress and memory suggest that specific factors may determine whether stress at encoding enhances or impairs memory (e.g., Mather and Sutherland 2011; Schwabe et al. 2012; Mather et al. 2016). To date, however, little work has examined the relative contributions of these factors. We addressed this gap in the present study by experimentally manipulating two factors that are important to different theories of stress and memory in order to determine the effects of these factors in contributing to the effects of stress on memory.
Early theories of stress and memory were nonspecific in the enhancing effects of stress surrounding memory encoding, as these theories posited nonspecific enhancements of memory driven by conjoint effects of noradrenergic activity and glucocorticoids (e.g., McGaugh 2000). These early theories, however, are inconsistent with more recent work finding that stress prior to or during encoding may only enhance memory under some conditions (e.g., Maheu et al. 2005; Payne et al. 2007; Smeets et al. 2009; Herten et al. 2017; Wolf 2018; Kalbe et al. 2020). Two theories of stress and memory encoding attempt to explain these findings by positing that either the relevance of the information learned to the stressor (i.e., stress relevance) or the delay between stress onset and encoding (i.e., the stress-encoding delay) plays a central role in the effects of stress on memory encoding. For example, the arousal-biased competition theory of stress and memory suggests that highly arousing (e.g., stress-relevant) stimuli will bias perception and therefore memory encoding in favor of those highly arousing stimuli at the expense of nonarousing stimuli (e.g., stress-irrelevant) (Mather and Sutherland 2011) via noradrenergic direction of attention as well as direct beneficial effects of noradrenergic activity on emotional memory consolidation (Mather et al. 2016). Another theory of stress and memory, the dual-mode theory, proposes that the stress response is time dependent, such that the biological effects of stress on the hippocampus at a short stress-encoding delay facilitate memory encoding, whereas the effects at a longer stress-encoding delay impair memory encoding (Schwabe et al. 2012). This time-dependent effect of stress on memory encoding, according to the dual-mode model, is thought to be mediated by the different time courses of distinct stress-responsive hormones: Norepinephrine increases and decreases relatively quickly in the stress response and is thought to mediate some of the rapid effects of stress on memory, whereas cortisol increases and decreases at a slower pace and is thought to mediate slower time-dependent effects of stress on memory through nongenomic and subsequently genomic mechanisms (Joëls et al. 2006; Schwabe et al. 2012).
The above theories suggest that both the stress relevance of information and the stress-encoding delay should dictate whether stress enhances or impairs memory encoding. In support of this, the existing literature suggests that stress can lead to better memory encoding for stress-relevant than stress-irrelevant materials (Smeets et al. 2009; Wiemers et al. 2013; Wolf 2018; Kalbe et al. 2020), and that stress enhances memory encoding when the stressor occurs during or immediately prior to encoding compared with when the stressor occurs sometime prior to encoding (Vogel and Schwabe 2016; Shields et al. 2017; Zoladz et al. 2018; Goldfarb et al. 2019). Unfortunately, however, almost all of the studies that have found beneficial effects of stress on the encoding of stress-relevant materials versus stress-irrelevant materials have had very short or even no stress-encoding delay, rather than a relatively long stress-encoding delay (Wiemers et al. 2013; though see Smeets et al. 2009). Additionally, many studies have found that stress immediately prior to encoding enhances memory even for stress-irrelevant items or information (Payne et al. 2007; Henckens et al. 2009; Wolf 2012; Zoladz et al. 2013, 2014). Similarly, although a recent meta-analysis found support for both of these factors in modulating the effects of stress on encoding (Shields et al. 2017), this meta-analysis was unable to tease apart the relative contribution of these two factors. To date, only one study manipulated both the stress-encoding delay and item relevance (Smeets et al. 2009), but this study was relatively underpowered and thus may have obtained a null effect in one of these factors due to a lack of power. Thus, it is not clear whether stress generally improves memory encoding for stress-relevant materials or whether the beneficial effects of stress on encoding are limited to conditions in which encoding occurs with very little or no delay from the stressor itself.
We examined the contributions of stress relevance and the stress-encoding delay to the effects of stress on memory encoding in this experiment by randomly assigning 130 participants to a stress or control condition. Each participant encoded two lists of words—one during the stressor and one 40 min after its onset (which was 23 min after the stressor ended)—to determine the role of the stress-encoding delay. This second-list delay was chosen because of both prior experimental work (Zoladz et al. 2011) and a meta-analysis (Shields et al. 2017), both of which indicated that stress immediately prior to encoding enhanced memory, whereas when stress onset occurred more than 25 min prior to encoding, stress impaired memory. Each of the word lists contained a mix of 12 stress-relevant and 12 stress-irrelevant words. Drawing on theories of stress and memory, we expected both stress relevance and a short stress-encoding delay to enhance memory, with the greatest enhancement for stress-relevant words with a short stress-encoding delay.
Results
Effects of stress on cortisol
We first examined whether our stress manipulation successfully produced a cortisol response. As expected, the Stress × Time interaction was significant (F(1,129.0) = 25.32, P < 0.001). Decomposing this interaction, we found that participants in the stress condition (M = 6.41, SE = 0.70) did not differ from participants in the control condition at baseline (M = 5.80, SE = 0.74; t(218.0) = 0.60, P = 0.548), whereas postmanipulation, participants in the stress condition (M = 12.46, SE = 0.70) had significantly higher cortisol levels than participants in the control condition (M = 6.37, SE = 0.74; t(218.0) = 5.98, P < 0.001). The Sex × Stress × Time interaction was nonsignificant (F(1,129.0) = 0.11, P = 0.742), indicating that cortisol changes as a function of the manipulation did not differ by participant sex.
Effects of stress on memory
Free recall
Next, we examined how stress during and prior to encoding affected free recall for stress-relevant and stress-irrelevant information in a mixed-model ANOVA with Stress, Stress-Encoding Delay, Item Relevance, and Sex as factors.1 We found significant main effects of Stress-Encoding Delay (F(1,378.0) = 84.47, P < 0.001) and Relevance (F(1,378.0) = 7.24, P = 0.007), which were qualified by a significant Stress × Item Relevance interaction (F(1,378.0) = 4.02, P = 0.046). Sex did not moderate any of these effects (Ps > 0.053). Decomposing the significant Stress × Item Relevance interaction, we found that participants in the stress condition showed significantly better free recall of stress-relevant information (M = 1.50, SE = 0.12) than of stress-irrelevant information (M = 1.05, SE = 0.12; t(378.0) = 3.42, P < 0.001). Participants in the control condition, however, did not differ between free recall of stress-relevant information (which was relevant to the speech in the control task; M = 1.25, SE = 0.12) and free recall of stress-irrelevant information (M = 1.19, SE = 0.12; t(378.0) = 0.47, P = 0.638).
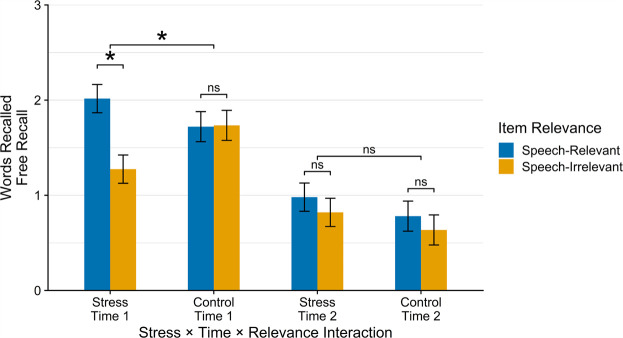
Although we did not find a significant Stress × Item Relevance × Stress-Encoding Delay interaction (F(1,378.0) = 3.71, P = 0.054) because the interaction was close to significant we followed this up with separate mixed-model ANOVAs at each stress-encoding delay (i.e., no delay and 40 min after stress/control onset) with Stress, Item Relevance, and Sex as factors. At no stress-encoding delay, we found a significant main effect of Relevance (F(1,126.0) = 6.97, P = 0.009), which was qualified by a significant Stress × Item Relevance interaction (F(1,126.0) = 7.49, P = 0.007). Decomposing this interaction, at no stress-encoding delay, we found that participants in the stress condition showed significantly better free recall of stress-relevant information (M = 2.02, SE = 0.15) than of stress-irrelevant information (M = 1.28, SE = 0.15; t(378.0) = 3.98, P < 0.001), whereas participants in the control condition did not (speech-relevant: M = 1.72, SE = 0.16; speech-irrelevant: M = 1.74, SE = 0.16; t(378.0) = −0.74, P = 0.463). Participants in the stress condition did not recall more stress-relevant words than participants in the control condition (t(114.0) = 1.19, P = 0.236) (see Fig. 1), indicating that stress biased the type of information recalled rather than improving free recall in this paradigm.
Figure 1.
Free recall of speech-relevant and speech-irrelevant words by experimental condition and manipulation-encoding delay. Participants in the stress condition showed significantly greater free recall of speech-relevant words than speech-irrelevant words when the words were encoded during the stress/control manipulation (after speech preparation time but before the speech was delivered), whereas participants in the control condition did not. Free recall did not differ by an experimental condition or speech relevance with a 40-min delay between stress/control manipulation onset and encoding.
At a 40-min delay between stress onset and encoding, we found no significant main effects of any of the predictors (i.e., Stress, Item Relevance, or Sex; Ps > 0.128), and no significant interactions between the predictors (Ps > 0.094). Neither stress nor control participants differed in recall (Ps > 0.390) between speech-relevant and speech-irrelevant words when those words were learned with a 40-min delay between the stress/control speech onset and encoding (see Fig. 1). Participants in the stress condition did not recall more speech-relevant (stress-relevant) words than participants in the control condition at a 40-min delay between speech onset and learning (t(222.0) = 1.08, P = 0.284).
Cued recall2
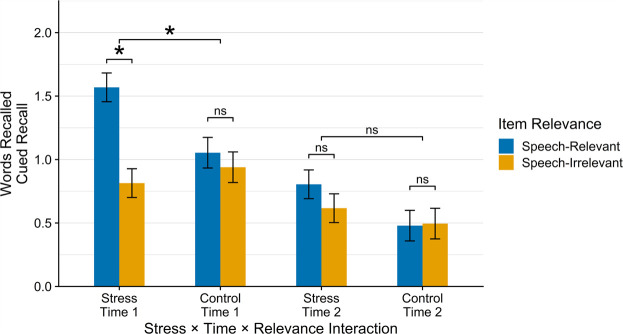
Next, we examined whether providing a cue would influences the effects of stress at or prior to encoding on memory in a mixed-model ANOVA with cued words as the outcome and Stress, Stress-Encoding Delay, Item Relevance, and Sex as factors. We found significant main effects of Stress-Encoding Delay (F(1,378.0) = 42.67, P < 0.001) and Relevance (F(1,378.0) = 11.80, P < 0.001), which were qualified by a significant Stress × Item Relevance interaction (F(1,378.0) = 7.76, P = 0.006) (Fig. 2). Decomposing this interaction, we found that participants in the stress condition showed significantly better cued recall of stress-relevant information (M = 1.19, SE = 0.09) than of stress-irrelevant information (M = 0.72, SE = 0.09; t(378.0) = 4.54, P < 0.001). For participants in the control condition, however, there was no significant difference between cued recall of stress-relevant information (M = 0.77, SE = 0.10) and cued recall of stress-irrelevant information (M = 0.72, SE = 0.10; t(378.0) = 0.45, P = 0.656). Sex was not a significant predictor of recall performance (P = 0.268), nor did sex moderate the effect of any other variable on recall (Ps > 0.242).
Figure 2.
Cued recall of speech-relevant and speech-irrelevant words by experimental condition and manipulation-encoding delay. Participants in the stress condition showed significantly greater cued recall of speech-relevant words than speech-irrelevant words when the words were encoded during the stress/control manipulation (after speech preparation time but before the speech was delivered), whereas participants in the control condition did not. Cued recall did not differ by experimental condition or speech relevance with a 40-min delay between stress/control manipulation onset and encoding.
Although we did not find a significant Stress × Item Relevance × Stress-Encoding Delay interaction (F(1,378.0) = 2.07, P = 0.152), we followed this up with separate mixed-model ANOVAs at each stress-encoding delay (i.e., no delay and a 40-min delay between speech onset and learning) with cued recall as the outcome and Stress, Item Relevance, and Sex as predictors. With no delay between speech onset (i.e., stress/control) and encoding, we found a significant main effect of Item Relevance (F(1,126.0) = 13.23, P < 0.001), which was qualified by a significant Stress × Item Relevance interaction (F(1,126.0) = 7.18, P = 0.008). Decomposing this interaction, at no delay between stress/control and encoding, we found that participants in the stress condition showed significantly better cued recall of stress-relevant information (M = 1.57, SE = 0.14) than of stress-irrelevant information (M = 0.81, SE = 0.14; t(126.0) = 4.60, P < 0.001), whereas participants in the control condition did not (speech-relevant: M = 1.07, SE = 0.14; speech-irrelevant: M = 0.95, SE = 0.14; t(126.0) = 0.66, P = 0.511). Additionally, participants in the stress condition showed significantly greater cued recall of speech-relevant (i.e., stress-relevant) words than participants in the control condition (t(108.0) = 2.53, P = 0.013), indicating that, relative to a control condition, stress-enhanced cued recall of stress-relevant information encoded with no delay between stress and encoding.
At a 40-min delay between stress onset and encoding, we found a significant main effect of Stress on cued recall (F(1,58.1) = 4.03, P = 0.049), but no other significant main effects (Ps > 0.115) or interactions (Ps > 0.214). Following up on the main effect of stress, relative to participants in the control condition, participants in the stress condition showed significantly greater cued recall of speech-relevant words (t(135.0) = 2.34, P = 0.020) when those words were encoded 40 min poststressor onset.3
Correlations between cortisol and memory
We also examined whether changes in cortisol were associated with the free or cued recall of information learned during stress or 40 min poststressor onset. We examined these potential associations both over all participants and in the stress group alone and we assessed possible quadratic (e.g., inverted-U) associations between changes in cortisol as well. In all of these analyses, changes in cortisol (neither Δ-cortisol nor residualized change scores) were not associated with any memory outcome (Ps > 0.120).
Discussion
We found that across the two recall tasks, stress during encoding led to greater recall of stress-relevant materials relative to stress-irrelevant material. Although this effect was numerically larger for materials encoded during the stressor than those encoded after a short delay, the stress-relevance effect was not significantly different across the delay conditions. However, the stress–relevance interaction was significant for materials encoded during the stressor but not for those encoded after a short delay, suggesting that delay might play a role in the effects of stress on memory encoding. As is often the case in studies of stress and memory (e.g., Schwabe and Wolf 2010), we did not observe any associations between cortisol responses to stress and memory performance. Altogether, our results suggest that the relevance of learned information to a stressor plays a larger role than the delay between stress onset and encoding in the effects of stress on memory encoding. These results are most consistent with predictions from the arousal-biased competition theory of memory and perception (Mather and Sutherland 2011), as stress led to a relative preference for stress-relevant words (vs. irrelevant words) whereas control participants did not differ in recall between those word types. In short, it seems that both item relevance and the delay between stress and encoding are important factors, but item relevance to the stressor may be the most important factor in contributing to enhancing the effects of stress on encoding.
Although both the stress-encoding delay and item relevance to the stressor were unable to be teased apart in the meta-analysis, we are not the first to manipulate these two variables in the same study. In particular, Smeets et al. (2009) examined the effects of stress at encoding of the same word list used in this study and had participants learn the words both immediately after stressor offset (15 min postonset) or 2 h poststressor offset before completing a free recall test 24 h later.4 It should be noted that the encoding task used by Smeets et al. (2009) differed from the task used here; Smeets et al.’s encoding task involved auditory presentation, it had an immediate recall test as soon as the full list was presented and the list was presented and tested three times during encoding. We chose not to use this same task due to work published after Smeets et al.’s study, which suggested that an immediate recall test may blunt the effects of stress on encoding (Wolf 2012). We believe that this difference in encoding tasks may explain the relatively poorer memory performance in our study compared with Smeets et al.’s study. Nonetheless, as in our study, Smeets et al. (2009) found that stress improved memory for stress-relevant information regardless of the delay between stress and encoding. However, their comparison (control) group was a group that learned the words 60 min prestressor and postlearning glucocorticoid administration significantly influences memory retention up to 3 h postlearning (Micheau et al. 1984), indicating that their control group may have been influenced by stress as well. Although Smeets et al. (2009) also present the results of a no-stress control comparison at the end of their results, they do not specify how their groups differ in recall relative to the no-stress control other than presenting a significant interaction in an ANOVA and it is therefore unclear how the stress-encoding delay or the stress-relevance of the items altered the effects of stress on memory encoding. Our results therefore clarify the effects of these factors on free recall as well as elucidating their contribution to cued recall.
Some studies (e.g., Schwabe et al. 2008; Zoladz et al. 2018) have found that stress enhances recall of stress-irrelevant (i.e., not related) information if that stressor occurs shortly prior to encoding, which differs from our results. Some notable factors may explain this difference. First, these studies did not have participants learn information under stress—as we did—but had participants learn the information shortly after stressor offset. It is possible that stress enhances the encoding of information—even stress-irrelevant information—if the stressor concludes shortly before that information is learned. Second, these studies did not include stress-relevant information and it is possible that the inclusion of this information leads to the preferential encoding of stress-relevant information at the expense of stress-irrelevant information. Similarly, it is possible that relatively underexplored factors, such as context, could modulate whether stress enhances or impairs memory for certain information (Shields et al. 2019a). Future work should examine each of these possibilities.
Although much animal work has found that glucocorticoids play a role in the effects of stress on memory (e.g., Joëls et al. 2006; Roozendaal and Mirone 2020), we did not observe an association between cortisol and memory in this study. This lack of association, however, is consistent with prior work in humans, which has found that the effects of stress on memory when stress occurs during the nongenomic effect window of glucocorticoids are related to interactions between cortisol and markers of noradrenergic activity, but not to cortisol in isolation (e.g., Smeets et al. 2009). Therefore, it is possible that we would have observed an association between memory and the conjunction of cortisol and noradrenergic activity if we had assessed noradrenergic activity.
Our results have intriguing implications for theories of stress and memory. In particular, our results are more in line with predictions from the arousal-biased competition theory of stress and memory (Mather and Sutherland 2011) than with predictions from the dual-mode model (Schwabe et al. 2012), as we found that stress benefited recall of stress-relevant information without any significant effects of the delay between stress and encoding, though we note that the relative support for the arousal-biased competition theory from our data is relatively weak. The dual-mode model places a primary emphasis on the stress-encoding delay, highlighting the time-dependent effects of norepinephrine and cortisol on memory as the primary mechanism through which the stress-encoding delay modulates the effects of stress on memory encoding (Schwabe et al. 2012). In contrast, the arousal-biased competition theory contends that stress acts to facilitate memory largely via noradrenergic direction of attentional resources toward stress-relevant or otherwise arousing information (Mather and Sutherland 2011), coupled with the general facilitation of emotional memory by noradrenergic activity (Mather et al. 2016). The significant modulation of stress by item relevance but not delay that we observed therefore provides relatively greater support for the arousal-biased competition theory given its tenet that modulation of attention toward stress-relevant information is a primary driver of stress effects on memory encoding. However, neither of these theories can easily account for why stress enhanced recall of stress-relevant information after stress-induced arousal had presumably dissipated (i.e., 23 min poststressor offset) (Hidalgo et al. 2015; Newton et al. 2017; Shields et al. 2019b). Alternatively, Roozendaal and Mirone (2020) recently observed an interesting dissociation between the effects of glucocorticoids and noradrenergic activity on memory, such that glucocorticoid activity increased memory generalization, whereas noradrenergic activity enhances memory accuracy. The time course of our experiment entails that noradrenergic activity would have been much more prominent during the encoding of list one than list two (noradrenergic activity increases rapidly following stress onset and fades shortly after stress offset) (Joëls et al. 2006), with the opposite being true for nongenomic glucocorticoid effects (glucocorticoids increase much slower than noradrenergic activity and fade over a much longer time) (Schwabe et al. 2012). Therefore, the observed results may be consistent with the idea that noradrenergic activity enhances memory accuracy whereas glucocorticoid activity impairs memory precision. A fourth theoretical explanation of our results comes from the contextual binding theory of stress and memory (Sazma et al. 2019), which suggests that stress benefits memory by creating a particularly salient memory for the context in which items are learned, thereby making recall of contextually related information (e.g., stress-relevant information learned in that context) easier. Although we did not experimentally manipulate context in this study, it is possible that the reason why stress enhanced recall of stress-relevant information of the list encoded under stress, but not of the list encoded 40 min poststressor onset, was that the list encoded under stress was closer in temporal context than the second list was to the highly contextually salient event that was the stressor. Although speculative, this possibility provides avenues for future research.
Some limitations of this study should be noted. First, this study examined the effects of stress on memory in a healthy young adult population of relatively high socioeconomic status. The effects of stress on memory sometimes differ in older adults (e.g., Pulopulos et al. 2013), so future research should attempt to determine whether our results generalize to other populations. Second, our memory paradigm resulted in fairly low recall rates. It is possible that stress may interact with the stress-encoding delay or item relevance in a different way when information is encoded more strongly, as the effects of stress on memory retrieval differ depending on memory strength (Smith et al. 2016). Third, because we only collected two saliva samples and only assayed for cortisol, we were unable to characterize the entire cortisol response, including the entirety of reactivity as well as recovery, or examine interactions with markers of noradrenergic activity, such as salivary α-amylase (sAA), all of which may have shown associations with memory (e.g., Smeets et al. 2009). Similarly, because we did not assess cortisol on the retrieval day, it is possible that the stress and control groups differed in baseline cortisol levels on the retrieval day; however, to our knowledge, all similar stress studies—including studies in our laboratory—that assessed cortisol on the retrieval day have found no differences in baseline cortisol between groups in cortisol at retrieval (e.g., McCullough and Yonelinas 2013). Fourth, we did not assess baseline or poststressor affect, and as such, the only manipulation check we had was the differential cortisol changes between groups. It is possible that negatively valenced or autonomic arousal may have persisted longer than we anticipated (into the second list) and we are unable to assess that possibility. Fifth, the filler task used, which asked participants to remember the video they watched, is atypical and may have influenced the behavioral results. We chose this filler task in order to limit rehearsal of the first list prior to learning the second list, but this filler task may be responsible in part for the relatively low recall rates we observed. Sixth, participants in this study were told to intentionally encode the word lists (along with the filler videos) for a potential memory task, which is somewhat unusual for studies of stress and memory. We made this decision because intentional encoding was used in the initial study from which our stimuli were obtained (Smeets et al. 2009) and because a meta-analysis did not find differences in the effects of stress on memory encoding depending on whether encoding was intentional or incidental (Shields et al. 2017). Seventh, because encoding of the second list always followed the first, it is possible that memory for the second list was affected by proactive interference from both the first list and the filler movie, which participants were told they may be tested on as well, and this interference may explain the relatively poor memory performance observed for the second list. Finally, although prior work has found that the items in the second list were relevant enough to the stressor to be remembered even when encoded long after stressor offset (Smeets et al. 2009), the first list was encoded in the presence of evaluators, entailing that stress relevance may have been higher for the first list.
In conclusion, we found that the effects of stress on encoding were modulated by the relevance of the information to the stressor but not significantly by the delay between stress and encoding. However, relevance of the information to the stressor modulated the effects of stress on memory for information encoded during the stressor, but not for information encoded after a short delay, suggesting that delay may still play some role in the interaction. In particular, we found that stress enhanced recall of stress-relevant information learned during—but not after—stress relative to stress-irrelevant information. These results therefore appear to suggest that although both factors may be important, the relevance of the information learned to a stressor may play a particularly important role in the effects of stress on encoding, which has important implications for theories of stress and memory.
Materials and Methods
Participants
One-hundred-thirty (67 female) young adults (Mage = 19.8, SDage = 1.9, range: 18–35) attending University of California at Davis participated in this study. These participants were randomly assigned to the acute stress induction (n = 69; 37 female) or control condition (n = 61; 30 female). A sample size of 126 participants was targeted because it provided 95% power to detect the effect of stress on memory encoding with our conditions according to a recent meta-analysis (i.e., a one-tailed test for an effect size of 0.592 with our study conditions; Shields et al. 2017), and we slightly oversampled in case of unexpected errors or data loss. We did not invite participants who had a current illness, diabetes, history of stroke, neurological disorders, current or former diagnosis of posttraumatic stress disorder, hospitalization for a psychiatric disorder within the past year, current injury or illness within the past week, major sleep disturbances within the past 6 wk, or consumption of more than eight caffeinated beverages a day. Similarly, individuals who were pregnant, nursing, on any form of medication (including hormonal birth control or asthma medication) or illegal drugs, had taken any mood-altering medications within the past two months, or had taken oral or injected corticosteroids within the past three months were not invited to participate. Participants were instructed not to eat, drink anything besides water, use tobacco, brush their teeth or floss, or engage in any exercise for 2 h prior to the start of the study. Compliance with these instructions and inclusion criteria (i.e., no drug or hormonal contraceptive use) was assessed using a questionnaire at the beginning of the study.
Materials and procedure
Participants arrived at the laboratory in groups of two to four. Upon arrival, each participant was greeted by an experimenter who instructed the participant to rinse their mouth out with a provided glass of purified water. The participant was then invited into the laboratory and seated at his/her assigned isolated computer, and then (if necessary) was told to wait to begin until the experimenter gave permission. The experimenter waited until all the participants had arrived to give each participant permission to begin. Once given permission, each participant provided informed consent and completed miscellaneous measures for ∼5 min to allow acclimation to the testing environment. Participants’ computers then reached a password-protected screen that instructed them to wait for instructions from the experimenters. Participants waited until all other participants for the session completed the initial measures, upon which time the first (baseline) saliva sample was taken.
Next, participants completed the laboratory-based stressor or control task, depending on their time slot's assigned condition. An experience of acute stress was induced using a modified version of the Trier Social Stress Test for Groups (TSST-G) (von Dawans et al. 2011). The modifications we made were consistent with those made by Smeets et al. (2007, 2009) to enable our assessment of learning of stress-relevant information. In particular, because participants learned 24 personality-related words (see the memory task description below), the speech task was modified so as to require participants to give a speech on their personality.
All participants were first informed that they should prepare a speech on their personality using a provided blank piece of paper. Participants in the stress condition were further informed that they would be giving this speech in front of a panel of trained evaluators. After 3 min of preparation, participants in the stress induction condition had their preparation paper taken from them, were brought to a room with the evaluators facing them and wall dividers to prevent the participants from interacting and then handed a piece of paper containing a mixed list of 12 personality-related (i.e., stress-relevant) words and 12 similar words that were not related to personality (i.e., stress-irrelevant). All of the words for the memory task are presented in the Supplemental Material. In the control condition, after 3 min of preparation, participants in the control condition simply brought the same paper containing the 24 words as was given to participants in the stress condition and asked to stand. All participants were then told to try to memorize the words on that list for the next 2 min, since their memory would be tested for those words (participants in the stress condition did this with the evaluators looking at them as they stood; participants in the control condition did this at their desks). After 2 min had elapsed, participants in the stress induction condition were conspicuously recorded while they spoke on their personality in front of a live panel of evaluators trained to maintain neutral facial expressions at all times. In the control condition, participants in the control condition stood at their desks and delivered their speeches quietly without social evaluation from any evaluators or other participants (i.e., out of earshot). The speech portion of the stressor/control task lasted a total of 12 min.
Participants were then returned to their desks and a password was entered to allow the study to continue on their computers. After completing a filler measure, participants then watched a filler video (on the history of manners) until a total of 15 min poststressor offset had elapsed. Participants were told to try to remember this video because their memory for it would be tested, though no memory test for video contents was given. We chose to instruct participants to try to remember this video in order to discourage participants from rehearsing the first list of words during the waiting period between the first and second lists. After a total of 15 min poststressor offset had elapsed, participants then provided the postmanipulation saliva sample. Participants then watched a second filler video (on traveling) for ∼8 min, or until a total of 23 min poststressor offset had elapsed; participants were again told that they should try to remember this video because their memory for it would be tested. After 23 min poststressor offset had elapsed, the experimenter provided participants with a second mixed list of 12 personality-related (i.e., stress-relevant) words and 12 similar words that were not related to personality (i.e., stress-irrelevant). These words were those used by Smeets et al. (2009)—Smeets et al. also had 24 additional words of the same kind from an unanalyzed recognition task, which we used for our other list (see below), counterbalanced in the order presented. All participants were again given 2 min to study the words and told to try to remember the words since their memory for them would be tested. The order of these lists was counterbalanced across participant condition and sex. Thus, participants learned a total of 24 stress-relevant words, 12 during the manipulation, and 12 ∼40 min later (as the first list was given at the beginning of the 17-min stressor), as well as 24 stress-irrelevant words on the same schedule. Therefore, we were able to assess memory for stress-relevant information that was presented both while and after the stressor occurred. After 2 min of study for the second list had elapsed, participants completed a filler personality questionnaire before being dismissed for that session.
All participants returned 48 h later and were greeted by the experimenter, seated at their same computers as before, and started on the study. Participants completed filler questionnaires for ∼10 min to allow acclimation to the study environment. Then, the experiment advanced to a screen summoning the experimenter, who advanced the participant to the free recall instructions. Participants were asked “to write down as many of the words that [they could] remember from either/both of the two lists [they] studied during the first part of the experiment,” and were given a full 5 min to write these words down. After a full 5 min had elapsed, the experiment automatically continued to the cued recall tasks. In the first of these, participants were cued to recall the stress-relevant words with the following prompt: “You learned 24 words related to your or someone else's personality. Please enter those words in the box below.” Participants were then given 3 min to recall as many words as they could remember. After 3 min had elapsed, the experiment automatically advanced to the second cued recall task. Participants were cued to recall the stress-irrelevant words with the following prompt: “You also learned 24 words unrelated to personality but that can describe people's or things’ characteristics (e.g., physical appearance). Please enter those words in the box below.” Participants were then given 3 min to recall as many of these words as they could remember. After completing this task, participants were debriefed and dismissed.
Saliva samples
Participants provided two saliva samples (baseline, which was provided immediately prestressor, and postmanipulation, which was provided 15 min poststressor offset, or 32 min poststressor onset) using a passive drool method. Immediately after collection, the saliva vials were placed in a freezer kept at −20°C until assayed.
Cortisol
Saliva samples were assayed in duplicate for cortisol using high-sensitivity salivary cortisol ELISA kits (Salimetrics LLC) according to the manufacturer's instructions. The interassay CV was 3.68% and the average intraassay CV was 3.11%. The sensitivity for these assays was 0.012 µg/dL. All controls were in the expected ranges. Participant sex, condition (i.e., stress vs. control), and list order were counterbalanced across plates to ensure balanced sample representation in each plate. Cortisol concentrations were converted from micrograms per deciliter to nanomoles per liter for consistency with most human stress literature.
Data analysis
All data were analyzed in R, version 4.0.2. Because the acute stress manipulation necessitated randomization of participant sessions to conditions (i.e., rather than participants), analyses required a multilevel model to account for shared variability within sessions. Thus, all analyses were linear mixed models with participants nested within sessions. Although not stated in the results, all mixed-models nested participants within time slots because of randomized time slots—rather than participants—to conditions (i.e., Stress vs. Control). Similarly, although not stated in the results, all memory analyses controlled for study list order counterbalance (see “Materials and Procedure”).
Supplementary Material
Acknowledgments
This research was supported by a University of California at Davis Provost Dissertation Year Fellowship to G.S.S., and National Institutes of Health EY025999 to A.P.Y.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053469.121.
To examine the data further, we separated words into low- and high-arousal words (we thank a helpful reviewer for suggesting this analysis). In this model with free recall as the outcome, high-arousal words were significantly more likely to be recalled than low-arousal words (F(1,882.0) = 28.09, P < 0.001). However, no significant interactions emerged between word arousal and condition (stress vs. control), with or without any other factors (e.g., there was also no significant Stress × Item Relevance × Stress-Encoding Delay × Arousal interaction). Interestingly, though, once the effect of word arousal was accounted for, the Stress × Item Relevance × Stress-Encoding Delay interaction in free recall, which was just outside of significance in our main analyses (P = 0.054) emerged as significant in this model (F(1,882.0) = 4.16, P = 0.042), which further justified our decomposition of this interaction within the main analyses.
Because cued recall was tested after free recall, in cued recall subjects may not have reported some of the items that they had already reported in free recall. To assess this possibility, we conducted exploratory analyses, where if a participant had recalled a word during free recall but not cued recall, we added it to their cued recall total. These exploratory analyses revealed the same findings as those presented here. Therefore, we retained participants’ original cued recall responses in analyses.
We also examined intrusions from the noncued items (e.g., stress-irrelevant items recalled during the cue for personality-related words) as a function of stress, sex, item relevance, and stress-encoding delay. Noncued item intrusions were greater for items learned during the first list (P = 0.020) and for stress-irrelevant items (P < 0.001), but no other main effect or interaction reached significance (Ps > 0.059). Noncued item intrusions did not differ by stress, nor did stress interact with item relevance or stress-encoding delay to predict noncued item intrusions (Ps > 0.210).
Smeets et al. (2009) further examined word arousal as a moderating factor, but the different encoding task we used, which differed from theirs, led us to decide not to examine this factor in primary analyses due to low recall rates.
References
- Goldfarb EV, Tompary A, Davachi L, Phelps EA. 2019. Acute stress throughout the memory cycle: diverging effects on associative and item memory. J Exp Psychol Gen 148: 13–29. 10.1037/xge0000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Hermans EJ, Pu Z, Joëls M, Fernández G. 2009. Stressed memories: how acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. 10.1523/JNEUROSCI.1184-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herten N, Otto T, Wolf OT. 2017. The role of eye fixation in memory enhancement under stress—an eye tracking study. Neurobiol Learn Mem 140: 134–144. 10.1016/j.nlm.2017.02.016 [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Pulopulos MM, Puig-Perez S, Espin L, Gomez-Amor J, Salvador A. 2015. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behav Brain Res 292: 393–402. 10.1016/j.bbr.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. 2006. Learning under stress: how does it work? Trends Cogn Sci 10: 152–158. 10.1016/j.tics.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kalbe F, Bange S, Lutz A, Schwabe L. 2020. Expectancy violation drives memory boost for stressful events. Psychol Sci 31: 1409–1421. 10.1177/0956797620958650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. 2005. The perfect time to be stressed: a differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry 29: 1281–1288. 10.1016/j.pnpbp.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. 2011. Arousal-biased competition in perception and memory. Perspect Psychol Sci 6: 114–133. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. 2016. Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci 39: e200. 10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Yonelinas AP. 2013. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiol Learn Mem 106: 11–17. 10.1016/j.nlm.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. 2000. Memory—a century of consolidation. Science 287: 248–251. 10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- Micheau J, Destrade C, Soumireu-Mourat B. 1984. Time-dependent effects of posttraining intrahippocampal injections of corticosterone on retention of appetitive learning tasks in mice. Eur J Pharmacol 106: 39–46. 10.1016/0014-2999(84)90675-7 [DOI] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Lyle KB, Szabo YZ, Miller JJ, Warnecke AJ. 2017. Salivary cytokine response in the aftermath of stress: an emotion regulation perspective. Emotion 17: 1007–1020. 10.1037/emo0000156 [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. 2007. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem 14: 861–868. 10.1101/lm.743507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulopulos MM, Almela M, Hidalgo V, Villada C, Puig-Perez S, Salvador A. 2013. Acute stress does not impair long-term memory retrieval in older people. Neurobiol Learn Mem 104: 16–24. 10.1016/j.nlm.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Schwabe L. 2018. Memory dynamics under stress. Memory 26: 364–376. 10.1080/09658211.2017.1338299 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Mirone G. 2020. Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology 114: 104588. 10.1016/j.psyneuen.2020.104588 [DOI] [PubMed] [Google Scholar]
- Sazma MA, McCullough AM, Shields GS, Yonelinas AP. 2019. Using acute stress to improve episodic memory: the critical role of contextual binding. Neurobiol Learn Mem 158: 1–8. 10.1016/j.nlm.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. 2010. Learning under stress impairs memory formation. Neurobiol Learn Mem 93: 183–188. 10.1016/j.nlm.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. 2008. Effects of pre-learning stress on memory for neutral, positive and negative words: different roles of cortisol and autonomic arousal. Neurobiol Learn Mem 90: 44–53. 10.1016/j.nlm.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. 2012. Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36: 1740–1749. 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, Yonelinas AP. 2017. The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol Bull 143: 636–675. 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Dunn TM, Trainor BC, Yonelinas AP. 2019a. Determining the biological associates of acute cold pressor post-encoding stress effects on human memory: the role of salivary interleukin-1β. Brain Behav Immun 81: 178–187. 10.1016/j.bbi.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, McCullough AM, Ritchey M, Ranganath C, Yonelinas AP. 2019b. Stress and the medial temporal lobe at rest: functional connectivity is associated with both memory and cortisol. Psychoneuroendocrinology 106: 138–146. 10.1016/j.psyneuen.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Hostinar CE, Vilgis V, Forbes EE, Hipwell AE, Keenan K, Guyer AE. 2021. Hypothalamic–pituitary–adrenal axis activity in childhood predicts emotional memory effects and related neural circuitry in adolescent girls. J Cogn Neurosci 33: 872–886. 10.1162/jocn_a_01687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. 2007. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biol Psychol 76: 116–123. 10.1016/j.biopsycho.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Smeets T, Wolf OT, Giesbrecht T, Sijstermans K, Telgen S, Joëls M. 2009. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology 34: 1152–1161. 10.1016/j.psyneuen.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Smith AM, Floerke VA, Thomas AK. 2016. Retrieval practice protects memory against acute stress. Science 354: 1046–1048. 10.1126/science.aah5067 [DOI] [PubMed] [Google Scholar]
- Vogel S, Schwabe L. 2016. Stress in the zoo: tracking the impact of stress on memory formation over time. Psychoneuroendocrinology 71: 64–72. 10.1016/j.psyneuen.2016.04.027 [DOI] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, Heinrichs M. 2011. The Trier Social Stress Test for Groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 36: 514–522. 10.1016/j.psyneuen.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT. 2013. What we remember from a stressful episode. Psychoneuroendocrinology 38: 2268–2277. 10.1016/j.psyneuen.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Wolf OT. 2012. Immediate recall influences the effects of pre-encoding stress on emotional episodic long-term memory consolidation in healthy young men. Stress 15: 272–280. 10.3109/10253890.2011.622012 [DOI] [PubMed] [Google Scholar]
- Wolf OT. 2018. Memories of and influenced by the Trier Social Stress Test. Psychoneuroendocrinology 105: 98–104. 10.1016/j.psyneuen.2018.10.031 [DOI] [PubMed] [Google Scholar]
- Wolf OT, Atsak P, de Quervain DJ, Roozendaal B, Wingenfeld K. 2016. Stress and memory: a selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. J Neuroendocrinol 28. 10.1111/jne.12353 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, Talbot JN. 2011. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiol Behav 103: 467–476. 10.1016/j.physbeh.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, Lyle SM, Talbot JN. 2013. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiol Learn Mem 100: 77–87. 10.1016/j.nlm.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Peters DM, Kalchik AE, Hoffman MM, Aufdenkampe RL, Woelke SA, Wolters NE, Talbot JN. 2014. Brief, pre-learning stress reduces false memory production and enhances true memory selectively in females. Physiol Behav 128: 270–276. 10.1016/j.physbeh.2014.02.028 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Duffy TJ, Mosley BE, Fiely MK, Nagle HE, Scharf AR, Brown CM, Earley MB, Rorabaugh BR, Dailey AM. 2018. Interactive influence of sex, stressor timing, and the BclI glucocorticoid receptor polymorphism on stress-induced alterations of long-term memory. Brain Cogn 133: 72–83. 10.1016/j.bandc.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.