Abstract
In this study, we investigated medically or surgically actionable genes in inherited eye disease, based on clinical phenotype and genomic data. This retrospective consecutive case series included 149 patients with inherited eye diseases, seen by a single pediatric ophthalmologist, who underwent genetic testing between 1 March 2017 and 28 February 2018. Variants were detected using a target enrichment panel of 429 genes and known deep intronic variants associated with inherited eye disease. Among 149 patients, 38 (25.5%) had a family history, and this cohort includes heterogeneous phenotype including anterior segment dysgenesis, congenital cataract, infantile nystagmus syndrome, optic atrophy, and retinal dystrophy. Overall, 90 patients (60.4%) received a definite molecular diagnosis. Overall, NGS-guided precision care was provided to 8 patients (5.4%). The precision care included cryotherapy to prevent retinal detachment in COL2A1 Stickler syndrome, osteoporosis management in patients with LRP5-associated familial exudative vitreoretinopathy, and avoidance of unnecessary phlebotomy in hyperferritinemia-cataract syndrome. A revision of the initial clinical diagnosis was made in 22 patients (14.8%). Unexpected multi-gene deletions and dual diagnosis were noted in 4 patients (2.7%). We found that precision medical or surgical managements were provided for 8 of 149 patients (5.4%), and multiple locus variants were found in 2.7% of cases. These findings are important because individualized management of inherited eye diseases can be achieved through genetic testing.
Keywords: precision medicine, inherited eye disease, next-generation sequencing, genetic testing
1. Introduction
Clinical genetic testing with next-generation sequencing (NGS) has been gaining popularity of late [1,2]. Many inherited eye diseases, such as congenital cataracts or retinitis pigmentosa, exhibit considerable genetic heterogeneity; therefore, the use of NGS technology enables a larger number of patients to obtain a molecular diagnosis for their disease. Accurate molecular diagnosis is important for acquiring information regarding visual prognosis and guiding future family planning. However, genetic diagnosis generally has not led to a change in treatment or disease management. Since the US Food and Drug Administration approved gene therapy for Leber’s congenital amaurosis with biallelic RPE65 variants in December 2017, genetic testing has gained greater attention. In addition, clinical trials are currently underway on gene therapy for multiple inherited retinal diseases, such as achromatopsia or choroideremia, which were generally regarded as incurable in the past [3].
The American College of Medical Genetics and Genomics has recommended reporting of incidental findings in clinical exome sequencing for a total of 59 genes [4]. Most of these disorders are rare life-threatening conditions, and RB1 (retinoblastoma) was the sole vision-related gene. However, clinicians also should focus on genetic causes of less threatening, but treatable disorders, which require specific treatment [5]. These include Little’s syndrome, a genetic form of pseudo-aldosteronism, which can be treated with triamterene. Accurate diagnosis of syndromic ciliopathy through exome sequencing leads to early detection of renal disease before acute presentation [6]. In addition, dietary modification or preventive therapy can alter disease progression in patients with syndromic congenital cataracts [7]. As indicated by these examples, there is great potential for genetic testing to enhance patient care [8,9]. However, little is known regarding medically or surgically actionable genes in the field of ophthalmology.
Genetic testing is expensive, indeterminate in a large number of cases, and often not covered by health insurance. It remains controversial whether routine massive parallel sequencing in inherited eye diseases is warranted or not when clinical phenotype matches a single genetic cause [10]. Previous studies demonstrated the diagnostic accuracy of NGS in inherited eye diseases, but their usefulness regarding treatment has been not discussed much [11,12,13]. Here, we report the use of targeted-panel NGS to reach a clinical diagnosis and investigate whether it altered treatment in patients with inherited eye diseases.
2. Materials and Methods
Recruitment and Selection of Patients with Inherited Eye Diseases
The cohort for this study included 149 consecutive unrelated patients with inherited eye diseases, with or without systemic conditions, who agreed to undergo genetic testing between 1 March 2017 and 28 February 2019. All patients underwent ophthalmologic examinations, which included slit-lamp examination, determination of the presence and type of nystagmus, determination of the presence of other systemic symptoms, fundus examination, and measurement of visual acuity. If indicated, electroretinography (ERG, Reti-port, Roland Consult, or RetEval, LKC Technologies, Gaithersburg, MD, USA) was performed in accordance with the standards of the International Society for Clinical Electrophysiology of Vision. ERG used skin electrodes in children younger than 6 years; sedation with oral chloral hydrate was used in patients younger than 2 years. Spectral-domain optical coherence tomography (OCT, Heidelberg Engineering, Heidelberg, Germany) was performed when applicable. Informed written consent was provided by patients and/or parents, and peripheral blood samples were obtained for genetic analysis from all patients. This study was approved by Severance Hospital Institutional Review Board (4-2018-0436) and adhered to the tenets of the Declaration of Helsinki.
For the customized NGS panel, we selected 429 genes known to cause inherited eye diseases, based on our assessment of literature reviews, the RetNet database (https://sph.uth.edu/Retnet/, accessed on 10 January 2017), and the Online Mendelian Inheritance in Man database (https://www.ncbi.nlm.nih.gov/omim) (Table S1); we also included deep intronic or regulatory regions known to cause inherited eye diseases (Table S2). Target enrichment was performed with custom-designed RNA oligonucleotide probes and target enrichment kit (Celemics, Seoul, South Korea). Sequencing and bioinformatics analyses were performed as described previously [12]. Briefly, pooled libraries were sequenced using a NextSeq 550 sequencer (Illumina, San Diego, CA, USA) and the NextSeq Reagent Kit, version 2 (300 cycles). For each sample, quality metrics were calculated using the FastQC software and TEQC package. Sequences were aligned to the hg19 reference genome using BWA-aln. [14] Single nucleotide variants and small insertions or deletions were called and crosschecked using GATK version 3.8.0 with Haplotypecaller [15] and VarScan version 2.4.0. Each variant suspected to be pathogenic, likely pathogenic, or variant of uncertain significance (VUS) was confirmed by visual inspection of the bam file using the Integrated Genomics Viewer 2.3 software. Split-read based detection of large structural variations was conducted using Pindel [16] and Manta [17]. Read-depth based detection of copy number variation (CNV) was conducted using ExomeDepth version 1.1.10. [18], followed by visualization using a base-level read depth normalization algorithm designed by the authors. CopywriteR version 2.9.0 was used with a 1 Mb window option for off-target analysis and chromosomal CNV detection [19].
The pathogenicity of missense variants was predicted using 5 in silico prediction algorithms, including SIFT, PolyPhen2, FATHMM, and CADD [20]. Splice site analysis was performed using the MaxEntScan, NNSPLICE (Neural Network Splice Prediction), Human Splice Finder, GeneSplicer, and SpliceFinder-like algorithms implemented in the Alamut Visual software (Interactive Biosoftware, Rouen, France) and deep-learning-based SpliceAI [21,22]. The variant was filtered by genome aggregation database (gnomAD) and Korean Reference Genome database. The interpretation of variants followed the 5-tier classification system recommended by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [23]. For the purpose of this study, patients were grouped in the following 2 categories: (1) definite molecular diagnosis: patients with pathogenic or likely pathogenic disease-associated variant(s), for whom phenotypes were exactly matched to the genotypes; (2) unsolved: all other patients for whom no pathogenic or likely pathogenic variants existed.
For small nucleotide variations, pathogenic and likely pathogenic variants, as well as variants of uncertain significance (VUS), that required parental study were further examined by Sanger sequencing on a 3730 DNA Analyzer with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequencing data were aligned against appropriate reference sequences and analyzed using the Sequencher 5.3 software (Gene Codes Corp., Ann Arbor, MI, USA). Large exonic deletions and duplications were confirmed by using a multiplex ligation-dependent probe amplification kit (MRC Holland, The Netherlands) whenever necessary. Chromosomal CNVs detected by the off-target analysis were validated by Affymetrix Cytoscan 750K array (Genome build, hg19). Affymetrix Cytoscan 750K array contains 750,000 markers for copy number analysis, including 200,000 SNPs and 550,000 polymorphic probes. Data analysis was performed using Chromosomal Analysis Suite software version 1.2.2.
3. Results
3.1. Patient Demographics
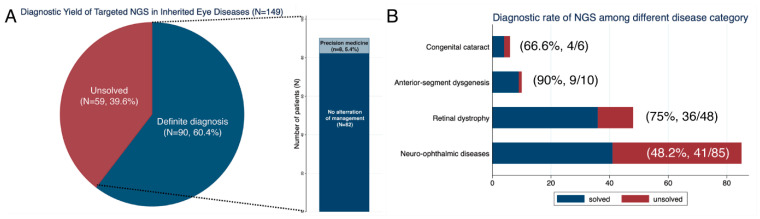
The systemic and ophthalmic features of the 149 unrelated are listed in online supplemental tables (Tables S3 and S4). Among 149 patients, 99 (66.4%) were male, and 38 (25.5%) had family histories of similar phenotypes (Figure S1). All patients were of a single ethnicity (Korean) except 1 Vietnamese; none of the patients were of consanguineous parentage. The average age at the time of genetic testing was 14.3 ± 16.5 years (Figure S2). Systemic phenotypes, such as developmental delay, cerebellar atrophy, cleft palate, or facial dysmorphism were noted in 26 patients (17.5%). Infantile nystagmus was presented in 103 patients (69.1%). The cohort was phenotypically heterogeneous. Six were congenital cataracts, 10 patients were anterior-segment dysgenesis including PAX6-related phenotype, 48 were retinal dystrophy, and 85 were neuro-ophthalmic condition such as optic atrophy, albinism, or FRMD7-related infantile nystagmus (Figure 1B).
Figure 1.
Diagnostic rate of targeted next-generation sequencing (NGS) in inherited eye diseases. (A) Overall diagnostic rate of targeted NGS and the proportion of patients who received precision medicine after genetic testing. (B) Diagnostic rate of targeted NGS among each disease category.
3.2. Molecular Diagnostic Rate and Personalized Medicine through Targeted Next-Generation Sequencing
Targeted NGS identified definite diagnostic variants in 90 patients (60.4%, 95% CI, 52.4–67.9%, Figure 1A and Table 1). The other 59 patients (39.6%) remained unsolved after targeted NGS. Among solved 90 patients, 25 patients were previously reported by our group [12,24,25,26,27,28,29,30]. The molecular diagnostic rate was higher in patients with family history than in singleton cases (86.8% vs. 51.4%, p < 0.001). The molecular diagnostic rate was also higher in patients with systemic phenotype than patients with a non-syndromic phenotype (73.9% vs. 57.9%, p < 0.001). The molecular diagnostic rates varied among disease groups. Sixty-six percent were solved in congenital cataracts, 90% (9/10) were solved in anterior segment dysgenesis, the causative variants were found in 75% (36/48) in the retinal dystrophy group, and 48.2% (41/85) were solved in the neuro-ophthalmic group (p < 0.004).
Table 1.
Disease-associated variants identified in 86 patients with definite diagnosis.
| No. | Initial Clinical Diagnosis |
Gene | Mutations | Zygosity | Segregation Analysis | gnomAD (MAF) | CADD | Previous Literature (PMID) |
ACMG Classification |
Accession ID for Transcript |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cone-rod dystrophy | ABCA4 | c.1958G>A:p.(Arg653His) c.3470T>G:p.(Leu1157*) |
Compound heterozygous | NA | 10/280000 None |
25.9 43 |
10711710 29975949 |
LP P |
NM_000350.2 |
| 2 d | LCA | AHI1 | c.2174G>A:p.(Trp725*) | Homozygous | NA | 4/248916 | 43 | 25445212 | P | NM_001134831.1 |
| 3 d | Laurence-Moon syndrome |
BBS1 | c.908delT:p.(Val303Glyfs*29) c.1285C>T:p.(Arg429*) |
Compound heterozygous | Paternal Maternal |
None 3/251446 |
34 40 |
32165824 e 12677556 |
P P |
NM_024649.4 |
| 4 d | IIN | CACNA1F | Exon 13-23 deletion | Hemizygous | NA | None | NA | 34064005 e | P | NM_005183.2 |
| 5 d | LCA | CACNA1F | c.2175_2179delins CATCATGTATGATGGTATCATGGCATT:p.(Gly726Ilefs*61) | Hemizygous | Maternal | None | 28.3 | 34064005 e | P | NM_005183.2 |
| 6 d | IIN | CACNA1F | c.1910+1G>A | Hemizygous | NA | None | 21 | 34064005 e | P | NM_005183.2 |
| LPR5 | c.3833G>A:p.(Trp1278*) | Heterozygous | NA | None | 45 | Novel | LP | NM_002335.2 | ||
| 7 d | IIN | CACNA1F | c.1301C>T:p.(Ala434Val) | Hemizygous | Maternal | None | 17.47 | 28002560 | LP | NM_005183.2 |
| 8 d | IIN | CACNA1F | c.1910+1G>A | Hemizygous | NA | None | 21 | 34064005 e | P | NM_005183.2 |
| 9 d | LCA | CEP290 | c.1666delA:p.(Ile556Phefs*17) c.3904C>T:p.(Gln1302*) |
Compound heterozygous |
Maternal Paternal |
245/174532 None |
31 37 |
16909394 25445212 |
P P |
NM_025114.3 |
| 10 | Achromatopsia | CNGA3 | c.1190G>T:p.(Glu397Val) a c.1279C>T:p.(Arg427Cys) a c.553C>G:p.(Leu185Val) |
Compound heterozygous |
Paternal Paternal Maternal |
None 110/281878 1/251394 |
25.5 33 20.5 |
18636117 11536077 31144483 |
LP LP LP |
NM_001298.2 |
| 11 d | Stickler syndrome | COL2A1 | c.3165+1G>A | Heterozygous | NA | None | 25.5 | 34680973 e | P | NM_001844.4 |
| 12 d | Pierre-Robin sequence |
COL2A1 | c.2680-3C>G | Heterozygous | NA | None | 23.7 | 34680973 e | P | NM_001844.4 |
| 13 d | LCA | CRX | c.101-1G>A b c.122G>A:p.(Arg41Gln) b |
Compound heterozygous |
Trans by IGV | None 1/31396 |
32 25.1 |
32165824 e 9427255 |
P P |
NM_000554.4 |
| 14 | Congenital cataract | CRYGC | c.173T>C:p.(Leu58Pro) | Heterozygous | NA | None | 26 | Novel | LP | NM_020989.3 |
| 15 | RP | EYS | c.8805C>G:p.(Tyr2935*) Exon 42-43 duplication |
Compound heterozygous |
NA | None None |
35 NA |
22363543 Novel |
LP US |
NM_001142800.1 |
| 16 | IIN | FRMD7 | c.368C>A:p.(Ser123Tyr) | Hemizygous | NA | None | 23.8 | Novel | LP | NM_194277.2 |
| 17 | IIN | FRMD7 | c.575A>C:p.(His192Pro) | Hemizygous | NA | 25/182271 | 25.6 | 30025138 | LP | NM_194277.2 |
| 18 | IIN | FRMD7 | c.637G>A:p.(Val213Met) | Heterozygous | NA | None | 32 | Novel | LP | NM_194277.2 |
| 19 | IIN | FRMD7 | c.685C>T:p.(Arg229Cys) | Hemizygous | NA | None | 34 | 17768376 | P | NM_194277.2 |
| 20 | IIN | FRMD7 | c.772A>G:p.(Lys241Arg) | Hemizygous | NA | None | 27.4 | 31106028 | LP | NM_194277.2 |
| 21 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Heterozygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 22 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 23 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Heterozygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 24 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Heterozygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 25 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 26 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 27 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 28 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 29 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 30 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 31 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 32 | IIN | FRMD7 | c.875T>C:p.(Leu292Pro) | Hemizygous | NA | 4/183318 | 27.5 | 25678693 | P | NM_194277.2 |
| 33 | IIN | FRMD7 | c.886G>T:p.(Gly296Cys) | Hemizygous | NA | None | 34 | 30015830 | LP | NM_194277.2 |
| 34 | IIN | FRMD7 | c.901T>C:p.(Tyr301His) | Hemizygous | NA | None | 26.7 | 29145603 e | LP | NM_194277.2 |
| 35 | IIN | FRMD7 | c.1016C>G:p.(Ser339Cys) | Hemizygous | NA | None | 34 | Novel | LP | NM_194277.2 |
| 36 | IIN | FRMD7 | c.1023_1030AGACCTCC: p.(Asp342Leufs*2) |
Hemizygous | NA | None | 35 | Novel | P | NM_194277.2 |
| 37 | IIN | FRMD7 | Exon 5 deletion | Hemizygous | NA | None | NA | Novel | P | NM_194277.2 |
| 38 | Congenital cataract | FTL | c.-168G>T | Heterozygous | Maternal | None | 20.8 | 9414300 | P | NM_000146.3 |
| 39 | FEVR | FZD4 | c.752C>G:p.(Pro251Arg) | Heterozygous | Maternal | None | 25.1 | Novel | LP | NM_012193.3 |
| 40 | FEVR | FZD4 | c.205C>T:p.(His69Tyr) | Heterozygous | NA | 138/277634 | 24.2 | 15370539 | P | NM_012193.3 |
| 41 | FEVR | FZD4 | c.470T>C:p.(Met157Thr) | Heterozygous | NA | None | 21.8 | 21097938 | P | NM_012193.3 |
| 42 | Congenital cataract | GJA3 | c.290T>G:p.(Leu97Arg) | Heterozygous | NA | None | 29 | Novel | US | NM_021954.3 |
| OPA1 | c.449-2A>C | Heterozygous | NA | None | 23.6 | Novel | P | NM_130832.2 | ||
| 43 | Ocular albinism | GPR143 | c.248T>C:p.(Leu83Pro) | Hemizygous | NA | None | 25.9 | 31106028 | LP | NM_000273.2 |
| 44 | Ocular albinism | GPR143 | c.360+2T>C | Hemizygous | NA | None | 23 | Novel | P | NM_000273.2 |
| 45 | Ocular albinism | GPR143 | c.925delG:p.(Ala309Profs*24) | Hemizygous | NA | None | 34 | Novel | P | NM_000273.2 |
| 46 | Ocular albinism | GPR143 | c.518C>G:p.(Ala173Asp) | Hemizygous | NA | None | 24.2 | Novel | LP | NM_000273.2 |
| 47 | Ocular albinism | GPR143 | Xp22.3 deletion | Hemizygous | NA | None | - | Novel | P | - |
| 48 | Ocular albinism | GPR143 | c.223_228dupGCTGCC: p.(Ala75_Ala76dup) |
Hemizygous | NA | None | 8.758 | 28339057 | LP | NM_000273.2 |
| 49 d | LCA | GUCY2D | c.1991A>C:p.(His664Pro) c.2984G>A:p.(Arg995Gln) |
Compound heterozygous | Maternal Paternal |
None 1/244998 |
27 35 |
28966547 32165824 e |
P LP |
NM_000180.3 |
| 50 d | LCA | GUCY2D | c.2649del:p.(Phe883Leufs*13) | Homozygous | NA | None | 33 | 28966547 | P | NM_000180.3 |
| 51 d | LCA | GUCY2D | c.1790G>A:p.(Gly597Glu) exon 4-5 duplication |
Compound heterozygous | Paternal De novo |
None None |
27.4 - |
29068479 32165824 e |
LP LP |
NM_000180.3 |
| 52 d | IIN | GUCY2D | c.1978C>T:p.(Arg660*) c.2947C>A:p.(Pro983Thr) a c.2960G>C:p.(Gly987Ala) a |
Compound heterozygous | Paternal Maternal |
1/251328 None None |
40 25.3 28.4 |
10766140 32165824 e 32165824 e |
P LP LP |
NM_000180.3 |
| 53 | CCDD | KIF21A | c.1067T>C:p.(Met356Thr) | Heterozygous | Maternal | None | 25.4 | 14595441 | LP | NM_001173464.1 |
| 54 | FEVR | LRP5 | c.607G>A:p.(Asp203Asn) | Heterozygous | Paternal | None | 32 | 16252235 | P | NM_002335.2 |
| 55 | Congenital cataract | NHS | c.1117C>T:p.(Arg373*) | Heterozygous | NA | None | 38 | 14564667 | P | NM_198270.2 |
| 56 d | LCA | NMNAT1 | c.275G>A:p.(Trp92*) c.709C>T:p.(Arg237Cys) |
Compound heterozygous | Paternal Maternal |
None 14/282780 |
38 35 |
32165824 e 22842227 |
LP P |
NM_022787.3 |
| 57 d | LCA | NMNAT1 | c.196C>T:p.(Arg66Trp) c.709C>T:p.(Arg237Cys) |
Compound heterozygous | Paternal Maternal |
22/282832 14/282780 |
35 35 |
22842227 22842227 |
P P |
NM_022787.3 |
| 58 d | Optic atrophy | NR2F1 | c.91_93dupCGC:p.(Arg31dup) | Heterozygous | NA | None | 19.92 | 34466801 e | LP | NM_005654.4 |
| 59 d | Optic atrophy | NR2F1 | c.513C>G:p.(Tyr171*) | Heterozygous | NA | None | 37 | 34466801 e | LP | NM_005654.4 |
| 60 d | IIN | NYX | c.182_183insT: p.(Cys62Valfs*53) |
Hemizygous | Maternal | None | 32 | 34064005 e | P | NM_022567.2 |
| 61 | Unexplained visual loss | NYX | c.38-1_38delGCinsTT: p.(Ala13Vafs*102) |
Hemizygous | Maternal | None | 14.49 | ClinVar | P | NM_022567.2 |
| 62 | Optic atrophy | OPA1 | c.1240A>C:p.(Thr414Pro) | Heterozygous | NA | None | 26.5 | 26905822 | LP | NM_015560.2 |
| 63 | Optic atrophy | OPA1 | c.795_798delTGAC: p.(Asp266Cysfs*41) |
Heterozygous | NA | None | 35 | Novel | P | NM_015560.2 |
| 64 | PAX6 phenotype | PAX6 | c.383G>A:p.(Arg128His) | Heterozygous | NA | None | 34 | 30167917 | LP | NM_000280.4 |
| 65 | PAX6 phenotype | PAX6 | c.607C>T:p.(Arg203*) | Heterozygous | NA | None | 36 | 7550230 | P | NM_000280.4 |
| 66 | PAX6 phenotype | PAX6 | c.397G>T:p.(Glu133*) | Heterozygous | NA | None | 38 | 16712695 | P | NM_000280.4 |
| 67 | PAX6 phenotype | PAX6 | c.702T>A:p.(Tyr234*) | Heterozygous | NA | None | 36 | Novel | P | NM_000280.4 |
| 68 | PAX6 phenotype | PAX6 | c.362C>T:p.(Ser121Leu) | Heterozygous | NA | None | 25.9 | 23734086 | LP | NM_000280.4 |
| 69 | PAX6 phenotype | PAX6 | c.607C>T:p.(Arg203*) | Heterozygous | NA | None | 37 | 7550230 | P | NM_000280.4 |
| 70 | PAX6 phenotype | PAX6 | c.702T>A:p.(Tyr234*) | Heterozygous | NA | None | 36 | Novel | P | NM_000280.4 |
| 71 | Achromatopsia | PDE6C | c.1771G>A:p.(Glu591Lys) c.2269C>T:p.(Gln757*) |
Compound heterozygous | Paternal Maternal |
2/251120 None |
33 49 |
26992781 Novel |
P P |
NM_006204.3 |
| 72 | Achromatopsia | PDE6C | c.85C>T:p.(Arg29Trp) c.712C>T:p.(Arg238*) |
Compound heterozygous | Maternal Paternal |
6/282886 2/251376 |
29.3 38 |
19615668 27124789 |
P P |
NM_006204.3 |
| 73 | Optic atrophy | PDHA1 | c.232G>A:p.(Ala78Thr) | Hemizygous | Maternal | None | 24.8 | Novel | LP | NM_000284.3 |
| 74 | RP | PRPH2 | c.708C>G:p.(Tyr236*) | Heterozygous | Paternal | None | 37 | 22863181 | P | NM_000322.4 |
| 75 | CSNB | RHO | c.302G>A:p.(Gly101Glu) | Heterozygous | NA | 2/250994 | 25.5 | 26161267 | LP | NM_000539.3 |
| 76 | Cone dystrophy | RP1 | c.4196delG:p.(Cys1399Leufs*5) c.6181delA:p.(Ile2061Serfs*12) |
Compound heterozygous | NA | None None |
23.7 23 |
25097241 29425069 |
P P |
NM_006269.1 |
| 77 | LCA | RPGRIP1 | c.3565_3571delCGAAGGC: p.(Arg1189Glyfs*7) |
Homozygous | NA | 4/249114 | 35 | 18682808 | P | NM_020366.3 |
| 78 d | PAX6 phenotype | SLC38A8 | c.692G>A:p.(Cys231Tyr) c.964C>T:p.(Gln322*) |
Compound heterozygous | Paternal Maternal |
None 2/248656 |
27.2 37 |
32744312 e 32744312 e |
LP P |
NM_001080442.1 |
| 79 d | Ocular albinism | SLC38A8 | c.995dupG:p.(Trp333Metfs*35) c.1214+5G>C |
Compound heterozygous | Paternal Maternal |
None None |
22.9 23.2 |
32744312 e 32744312 e |
P LP |
NM_001080442.1 |
| 80 d | PAX6 phenotype | SLC38A8 | c.558C>A:p.(Tyr186*) c.1078_1104del: p.(Ala360_leu368del) |
Compound heterozygous | Maternal Paternal |
None None |
58 16.29 |
32744312 e 32744312 e |
P LP |
NM_001080442.1 |
| 81 | Oculocutaneous albinism |
SLC45A2 | c.220T>C:p.(Trp74Arg) | Heterozygous | Maternal | None | 26.7 | Novel | US | NM_016180.3 |
| 82 d | LCA | SPATA7 | c.388C>T:p.(Gln130*) c.1160+1G>A |
Compound heterozygous | Maternal Paternal |
2/249908 None |
35 33 |
32165824 e 32165824 e |
P P |
NM_018418.4 |
| 83 d | LCA | TUBB3 | c.967A>G:p.(Met323Val) | Heterozygous | De novo | None | 25.3 | 33921132 e | LP | NM_006086.4 |
| 84 | Oculocutaneous albinism |
TYR | c.929dupC:p.(Arg311Lysfs*7) c.1037-7T>A |
Compound heterozygous | NA | 11/251178 242/280983 |
22.9 18.81 |
2511845 8217557 |
P P |
NM_000372.4 |
| 85 | Usher syndrome | USH2A | c.2802T>G:p.(Cys934Trp) c.11389+3A>T |
Compound heterozygous | Son c | 57/282482 2/250762 |
26.6 22.7 |
21686329 28714225 |
P LP |
NM_206933.2 |
| 86 | Usher syndrome | USH2A | c.8559-2A>G | Homozygous | NA | 8/251134 | 34 | 32093671 | P | NM_206933.2 |
| 87 | X-linked retinoschisis |
VPS13B | c.6200T>A:p.(Leu2067*) c.9530_9531del: p.(Ala3177Valfs*18) |
Compound heterozygous | NA | 3/250746 None |
39 27.5 |
15141358 Novel |
P P |
NM_017890.4 |
| 88 | Corneal dystrophy | ZEB1 | c.2034_2035delAA: p.(Pro680Phefs*5) |
Heterozygous | NA | None | 23.8 | Novel | P | NM_030751.5 |
| 89 | Corneal dystrophy | ZEB1 | c.1576delG:p.(Val526*) | Heterozygous | NA | None | 23.9 | Novel | P | NM_030751.5 |
| 90 | Optic atrophy | SOX5 | 12p12.2p12.1 deletion | Heterozygous | De novo | None | NA | Novel | P | NM_006940.4 |
ACMG: American College of Medical Genetics, CADD: combined annotation-dependent depletion, CCDD: congenital cranial dys-innervational disorder, FEVR: familial exudative vitreoretinopathy, IGV: integrative genomic viewer, IIN: idiopathic infantile nystagmus, gnomAD: genome aggregation dataset, LCA: Leber congenital amaurosis, LP: likely pathogenic, MAF: minor allele frequency, NA: not available, P: pathogenic, RP: retinitis pigmentosa, US: uncertain significance. a These two variants were existed in cis, confirmed by manual inspection through integrative genomic viewer. b These two variants were existed in trans, confirmed by manual inspection through integrative genomic viewer. c Segregation was confirmed by proband’s children. d Previously reported patients by the authors. e Novel, but previously reported by the authors.
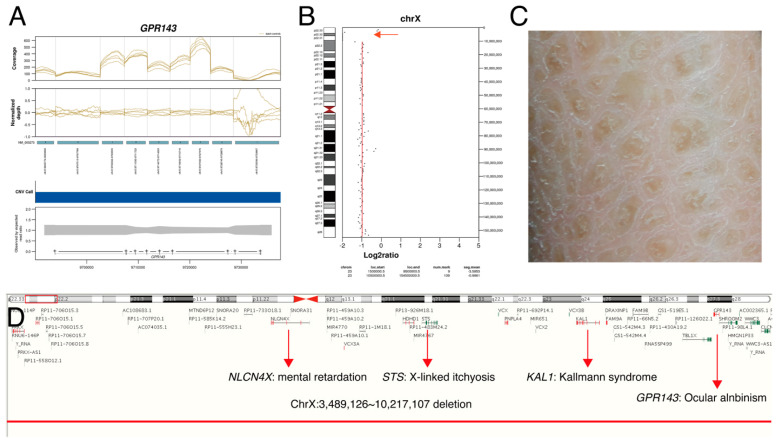
We identified a total of 118 disease-associated variants in 90 patients. FRMD7, PAX6, and GPR143 variants appeared to be frequently mutated in our cohort. Among 118 variants, 47 (39.8%) were novel variants. A total of 7 CNVs were detected, and 1 non-coding pathogenic variant was found. Overall, medically or surgically actionable genes were found in 8 patients (5.4%), and they received NGS-guided precision care after molecular diagnosis (Table 2 and Figure 1). The initial clinical diagnosis was revised in 22 patients (14.8%). Among 149 patients, unexpected chromosomal large CNVs (n = 2) and multi-locus variations (n = 2) were noted in 4 patients (2.7%) (Figure 2 and Table S3).
Table 2.
Eight patients who were received precision care after targeted next-generation sequencing.
| Patient No. | Sex/Age | Clinical Diagnosis before NGS Testing | Diagnosis after NGS Testing | Genes (Mode of Inheritance) |
Nucleotide Changes |
Amino Acid Changes | Management | Accession ID for Transcript |
|---|---|---|---|---|---|---|---|---|
| 6 | M/4.3 | IIN | CSNB | CACNA11 (XL) | c.1910+1G>A | - | Dual diagnosis | NM_005183.2 |
| FEVR | LRP51 (AD) | c.3833G>A | p.(Trp1278*) | Bone densitometry monitoring | NM_002335.2 | |||
| 11 | M/0.8 | Stickler syndrome | Stickler syndrome | COL2A11 (AD) | c.3165+1G>A | - | Prophylactic cryotherapy to prevent retinal detachment | NM_001844.4 |
| 12 | F/10.9 | Pierre-Robin sequence |
Stickler syndrome | COL2A11 (AD) | c.2680-3C>G | - | Prophylactic cryotherapy to prevent retinal detachment | NM_001844.4 |
| 38 | M/7.8 | Congenital cataract |
Hyperferritinemia-cataract syndrome | FTL1 (AD) | c.-168G>T | - | Avoid unnecessary phlebotomy or medical investigation | NM_000146.3 |
| 47 | M/5.1 | Ocular albinism | Xp22.33p22.2 deletion |
GPR1431 CLCN4, KAL1 NLGN4X, STS (XL) |
- | - | Hypotropic hypogonadism investigation | NM_000273.2 |
| 54 | F/4.8 | FEVR | FEVR | LRP51 (AD) | c.607G>A | p.(Asp203Asn) | Bone densitometry monitoring | NM_002335.2 |
| 73 | M/3.8 | Optic atrophy | Pyruvate dehydrogenase E1-α deficiency | PDHA11 (XL) | c.232G>A | p.(Ala78Thr) | Ketogenic diet Thiamine treatment |
NM_000322.4 |
| 90 | F/8.1 | Optic atrophy | 12p12.2p.12.1 Xp22.2p22.13 deletion |
SOX51 (AD) ABCC9 (AD) |
- | - | Regular monitoring of cardiac function |
NM_006940.4 NM_005691.3 |
AD, autosomal dominant; CSNB, congenital stationary night blindness; F, female; FEVR, familial exudative vitreoretinopathy; IIN, idiopathic infantile nystagmus; M, male; XL, X-linked. 1 Genes were associated with ophthalmological phenotypes.
Figure 2.
Copy number variations from targeted next-generation sequencing in a patient with ocular albinism (P47). A 5-year-old male had nystagmus since early infant. His past medical history was significant for congenital hypothyroidism, developmental delay, intellectual disability, and ichthyosis. Dilated fundus examination showed depigmented fundi and multichannel visual evoked potential showed chiasmal misrouting: (A) Normalized depth analysis on GPR143 region showed a whole gene deletion of GPR143; (B) chromosomal copy number variations called by off-target analysis using CopywriteR version 2.9.0 showed absence of read depth on Xp22.3 (red arrow); (C) dry, thickened, scaly skin was noted; (D) ChrX:3,489,126_10,217,107 deletion was confirmed by array comparative genomic hybridization.
3.3. Case Examples
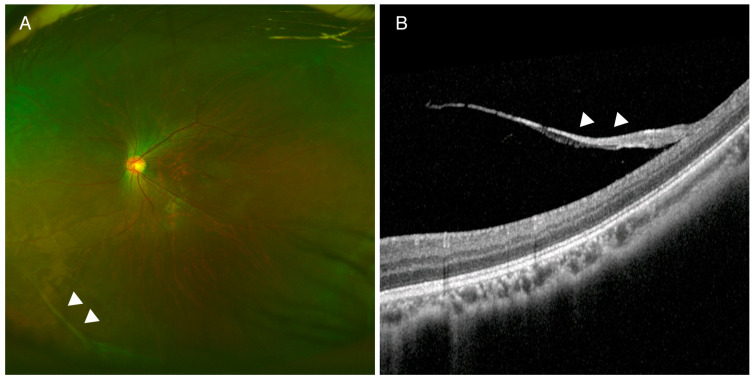
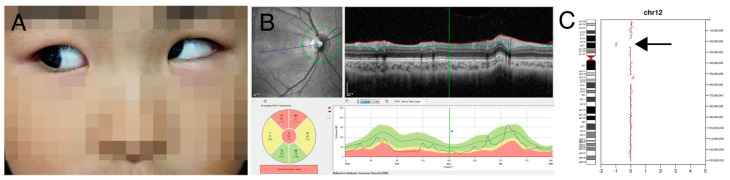
Case study 1 is important with respect to patient-specific treatment. The mother of the proband (P12) had a history of bilateral retinal detachment and had undergone vitrectomy by her twenties. The proband had exhibited high myopia since a young age, and she had micrognathia and a cleft palate. Dilated fundus examination showed bilateral thick vitreous strands at the retinal periphery (Figure 3). Targeted-panel NGS identified a COL2A1 c.2680-3C>G non-canonical splice site variant. The variant was not predicted by CADD to be deleterious (CADD score: 8.353), but it was predicted by Human Splice Funder 3.1 to cause a new splice site acceptor (wild type 63.92 vs. mutant 92.86: +45.28% variation); this was supported by the results from 2 other splice site prediction programs and SpliceAI (Figure S3). Prophylactic cryotherapy was applied to prevent future risk of retinal detachment.
Figure 3.
Fundus photography and optical coherence tomography (OCT) in a patient with Stickler syndrome (P12). (A) Peripheral vitreous degeneration was noted in wide fundus photography. (B) Spectralis OCT showed a thick vitreous band (arrowhead) attached to the retina. Targeted next-generation sequencing revealed a COL2A1 c.2680-3C>G variant, predicted to cause a new splicing acceptor site in intron 40. Prophylactic cryotherapy was applied to prevent retinal detachment.
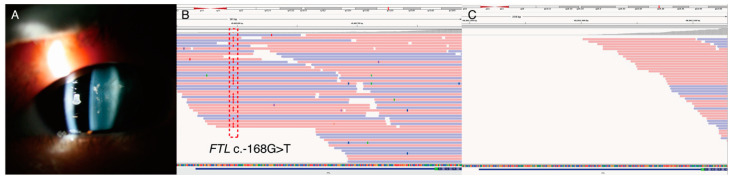
Case study 2 is an example of avoiding unnecessary treatment by using targeted NGS testing. The proband (P38) had congenital cataracts in early childhood. The mother and grandmother also had histories of congenital cataracts and had undergone bilateral cataract surgeries during their twenties. His mother had elevated ferritin levels, and repeated phlebotomies had been performed to reduce the burden of iron overload. Targeted-panel NGS revealed c.-168G>T in the FTL gene (Figure 4). This upstream regulatory variant was previously reported as pathogenic in hyperferritinemia-cataract syndrome [31], therefore, we included this region in the design of the targeted panel (Table S2). Individuals with hyperferritinemia-cataract syndrome do not have an excess of iron; repeated phlebotomies in these patients may cause anemia. Correct diagnosis of hyperferritinemia-cataract syndrome is important to avoid unnecessary treatments or invasive procedures, such as repeated phlebotomies or liver biopsies.
Figure 4.
(P38) A 7-year-old male had congenital cataracts in both eyes. Laboratory examination showed elevated ferritin level 1205.3 ng/mL (reference range: 23.9~336.2 ng/mL) without iron overload. The inheritance pattern was consistent with autosomal dominant. (A) Slit-lamp examination showed congenital cataracts since early childhood. (B) Integrative genomic viewer showed FTL c.-168G>T variant in upstream to starting codon. This region was included in our panel, and the variant was previously reported as pathogenic. (C) The 5′ untranslated region of exon 1 in FTL gene was not covered in commercially available exome sequencing. Molecular diagnosis of hyperferritinemia-cataract syndrome enables us to avoid unnecessary investigations or treatment such as repeated phlebotomy or liver biopsy.
Case study 3 illustrates that chromosomal CNV was identified after targeted NGS. A 4-year-old girl (P86) presented at our clinic for strabismus. She had micrognathia, developmental delay, and hirsutism (Figure 5A). Dilated fundus examination showed optic atrophy in both eyes (Figure 5B). At the age of 8 years, best-corrected visual acuity was 20/50 in both eyes. Targeted NGS showed 12p12 microdeletion (Figure 5C), and this chromosomal CNV was confirmed by microarray. This region included the SOX5 gene, and it was known to cause non-progressive optic atrophy and strabismus [32]. Regular monitoring of cardiac function is recommended because ABCC9 gene deletion (Table S5) is known to cause pericardial effusion and cardiomegaly [33].
Figure 5.
(P90) Chromosomal copy number variations analysis using off-target reads led to precision medicine (A) 8-year-old female showed strabismus and hirsutism was noted in philtrum area. (B) Spectral-domain optical coherence tomography showed optic nerve atrophy in the right eye. (C) Chromosomal 12p12 deletion was suspected by bioinformatics analysis using off-target reads (arrow). Array comparative genomic hybridization confirmed 12p.12.2p12.1 deletion. Optic nerve atrophy was thought to be related to a whole deletion of SOX5 gene. Because a whole deletion of the ABCC9 gene located nearby to the SOX5 gene was detected, monitoring of regular cardiac function was recommended.
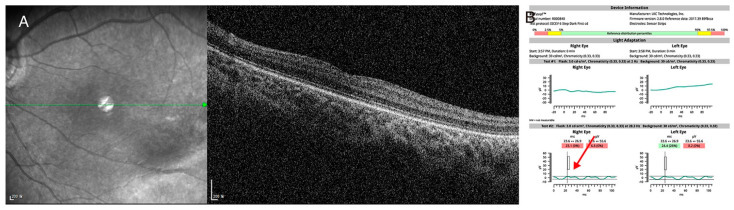
Case study 4 is an example of dual diagnosis in a single patient. A 4-year-old male came to our hospital for the evaluation of upbeat nystagmus. His best-corrected visual acuity was 20/150 in the right eye and 20/100 in the left eye, and mild myopia was noted. Targeted next-generation sequencing showed a novel heterozygous c.1910+1G>A variant in the CACNA1F gene and a novel heterozygousc.3833G>A:p.(Trp1278*) variant in the LRP5 gene. Detailed phenotyping was consistent with the phenotype, and a dual diagnosis was made (Figure 6).
Figure 6.
(P6) A case of dual diagnosis identified by targeted next-generation sequencing (A) Spectralis optical coherence tomography showed mild temporal retinal dragging with shallow fovea pit. (B) Hand-held electroretinogram showed severely attenuated light-adapted 3.0 response and reduced double peak in light-adapted 30 Hz flicker response (red arrow), which was consistent with incomplete congenital stationary night blindness. Targeted next-generation sequencing showed the hemizygous c.1910+1G>A canonical splice site variant in CACNA1F gene and c.3833G>A:p.(Trp1278*) nonsense variant in LRP5 gene. Dual diagnosis of familial exudative vitreoretinopathy and incomplete congenital stationary night blindness was made in this patient, and regular monitoring of bone densitometry was recommended.
4. Discussion
Molecular genetic tests based on NGS technology are widely used these days. However, patient-specific treatment is not available in most inherited genetic diseases. Our study revealed that the diagnostic yield of targeted NGS in inherited eye diseases was approximately 60%. Among patients who underwent genetic testing, about 5% of patients received precision care through genetic testing, and about 2.7% of patients had diagnoses that involved two or more diseases loci. Our result was consistent with a previous study, which reported that multiple molecular diagnoses could be made in 4.9% of cases in exome sequencing [34]. It is likely that many ophthalmologists do not have knowledge regarding which genetic profiles are medically or surgically actionable, and some may believe that genetic testing does not lead to a change in treatment. Our study showed that accurate molecular diagnosis has an impact on the understanding of molecular mechanisms, and it will help us to provide genotype-driven tailored investigation, prevent secondary complications or associated medical conditions, and avoid unnecessary treatment.
It has been reported that the molecular diagnostic rate varied among different disease groups [35]. Generally, the molecular pickup rate of NGS is higher in inherited eye diseases than other pediatric neurological diseases. In our study, anterior segment dysgenesis had the highest diagnostic rate. This high diagnostic rate is inconsistent with a previous study [36]. This could be related that most of the anterior segment dysgenesis were PAX6-related phenotypes except for one patient with Peter’s anomaly. In congenital cataracts, we identified causative variants in two-thirds of patients. This finding was consistent with a previous study [1]. In unsolved patients, one patient (P96) with oculocutaneous albinism had a heterozygous c.1025C>T:p.(Ala342Val) variant in the SLC24A5 gene. Because the discovery of regulatory or deep intronic variants receives more attention [37], further studies with genome sequencing are needed to find non-coding variants in this gene.
Some patients with rare inherited eye diseases may receive an incorrect diagnosis or require many years to reach a definitive diagnosis [11,38,39]. Young children are typically not sufficiently cooperative to complete eye examinations or other diagnostic tests [12]. Children with inherited eye diseases may harbor serious medical illnesses associated with ocular phenotypes. Occasionally, they may undergo unnecessary brain imaging or numerous investigations before a correct diagnosis [12]. For example, FEVR is known to be caused by six different genes: FZD4, TSPAN12, LRP5, NDP, ZNF408, or KIF11 [40]. Defects in one of these genes cause impaired development of the retinal vasculature by impacts on Norrin signaling pathways. Among these six genes, only the LRP5 variant causes osteoporosis by impacting Wnt signaling pathways [41]. Bone densitometry and calcium supplements are helpful in patients with LRP5-associated FEVR.
Previous studies reported that molecular diagnostic rates of inherited retinal diseases varied between 50% and 76% [2,11,13]. Our results were consistent with these results. Stone et al. demonstrated that a tier-based approach in genetic testing increased the molecular diagnostic rate [13]. A tier-based approach can detect variants in RPGR exon 15, which is poorly covered by exome or genome sequencing. We agree that a tier-based approach is more cost-effective and has greater diagnostic yields, but it requires a high degree of clinical expertise [42]. Moreover, Posey et al. demonstrated that about 5% of patients with inherited genetic diseases had multi-locus genomic variations [34]. Even when the clinical context indicates a single genetic etiology, targeted NGS can be considered first tier because patients may harbor other ophthalmic conditions, which are not recognized by clinical examination. Consugar et al. also reported that panel-based NGS is more sensitive than exome sequencing for variant detection in known genes [43]. Targeted-panel NGS can also detect known deep intronic or regulatory variants, as shown in our case (P38). Initial analysis of genes well known to be associated with a particular phenotype will improve the positive predictive value and reduce the likelihood of false ascertainment [44]. However, it is necessary to update the target panel regularly.
Some inherited eye diseases can be treatable and actionable (Table 3). For example, nystagmus associated with episodic ataxia type 2 caused by CACNA1A pathogenic variant can be ameliorated with acetazolamide, and Leber congenital amaurosis caused by bi-allelic RPE65 variants can be treated with gene therapy [45,46]. Retinal detachment in Stickler syndrome type 1, which is caused by COL2A1 pathogenic variants, can be prevented by prophylactic cryotherapy [47,48]. Prophylactic cryotherapy is not indicated in Stickler syndrome type II and III. High myopia and vitreous abnormalities are not specific to Stickler syndrome, and similar phenotypes are also noted in Knobloch syndrome [49]. The phenotypic complexity of rare genetic diseases may present a challenge to the clinician. Some inherited eye diseases are very rare and even experienced ophthalmologists who specialize in genetic eye diseases may not have seen them before. The suggested medically or surgically actionable genes in ophthalmology (Table 3) will help ophthalmologists or laboratory physicians to gain awareness of actionable genes in the field of ophthalmology.
Table 3.
Medically or surgically actionable genes in inherited eye diseases.
| Phenotype | Phenotypic MIM |
MIM Gene | Genes | Inheritance | Ophthalmic Phenotype |
Medical or Surgical Action |
|---|---|---|---|---|---|---|
| Abetalipoproteinemia | 200100 | 157147 | MTTP | AR | Pigmentary retinal degeneration | Vitamin A and E supplement |
| Ataxia with isolated vitamin E deficiency | 277460 | 600415 | TTPA | AR | Pigmentary retinal degeneration | Treatment with vitamin E |
| Blepharophimosis-Ptosis-Epichantus Inversus syndrome | 110100 | 605597 | FOXL2 | AD | Blepharophimosis, ptosis, epicanthus inversus | Refer to endocrinologist for premature ovarian failure |
| Cerebrotendinous xanthomatosis | 213700 | 606530 | CYP27A1 | AR | Congenital cataract | Chenodeoxycholic acid and statins |
| Congenital Cataracts | 613763 | 123590 | CRYAB | AD | Congenital cataract | Dilated cardiomyopathy screening |
| Congenital Cataracts | 607330 | 601637 | CYP51A1 | Congenital cataract | Check sterol profiling | |
| Episodic ataxia type 2 | 108500 | 601011 | CACNA1A | AD | Episodic nystagmus | Acetazolamide for ameliorating nystagmus |
| Familial exudative vitreoretinopathy | 133780 | 603506 | LRP5 | AD | Temporal retinal dragging | Refer to endocrinologist to monitor bone mineral density |
| Galactokinase deficiency | 230200 | 604313 | GALK1 | AR | Congenital cataract | Restriction of lactose and galactose intake |
| Hyperferritinemia-cataract syndrome | 600886 | 134790 | FTL | AD | Congenital cataract | Avoid unnecessary repeated phlebotomy |
| Knobloch syndrome | 267750 | 120328 | COL18A1 | AD | High myopia | Brain MRI to detect occipital encephalocele |
| Lathosterolosis | NA | 602286 | SCD5 | AR | Congenital cataract | Cholesterol reducing agent Ultrasound monitoring of the liver Liver transplant may be required |
| Leber congenital amaurosis | 204100 | 180069 | RPE65 | AR | Nystagmus Retinal degeneration |
Gene therapy (voretigene neparvovec-rzyl) |
| Pyruvate dehydrogenase E1-α deficiency |
312170 | 300502 | PDHA1 | XL | Optic atrophy Strabismus |
Ketogenic diet Thiamine treatment |
| Refsum Disease | 266500 | 602026 601757 |
PHYH, PEX7 |
AR | Pigmentary retinal degeneration | Diet free of phytol, phytanic acid, or their precursor, or plasmapheresis |
| Retinoblastoma | 180200 | 614041 | RB1 | AD | Intraocular tumor | Serial detail examination of fundus |
| Stickler syndrome type I |
108300 | 120140 | COL2A1 | AD | High myopia Vitreoretinal degeneration |
Prophylactic cryotherapy to prevent retinal detachment |
| Stomatin-deficient Cryohydrocytosis |
608885 | 138140 | SLC2A1 | AD | Congenital cataract | Ketogenic diet |
AD, autosomal dominant; AR, autosomal recessive; MIM, mendelian inheritance in man; NA, not available; XL, X-linked.
We could not determine the pathogenicity of variants in several cases. For example, we found a CASK c.2147A>C:p.(His716Pro) variant in a patient (P91) with nystagmus and no intellectual disability. This variant was predicted to be deleterious by in silico prediction (CADD:27.6, PoplyPhen2: 0.983, SIFT: 0.01). A null variant in CASK (calcium/calmodulin-dependent serine protein) induced variable intellectual disability, with or without nystagmus [50]. CASK promotes FRMD7 co-localization at the plasma membrane, where it enhances CASK-induced neurite length [51]. Although the CASK c.2147A>C variant was absent in a population database, a functional analysis should be conducted to confirm the pathogenicity of this missense variant. Because of efforts to construct large population datasets, such as the gnomAD, many variants that had been considered pathogenic are now reclassified as benign or likely benign [52]. For precision medicine to succeed, it also needs to be more accurate [9]. Comprehensive phenotyping, accurate bioinformatics analysis including CNV detection, and cautious interpretations are essential parts of genetic diagnosis. Physicians should also know the limitations of NGS that it cannot reliably detect variants in high GC-rich regions, segmental duplication, or short tandem repeats. We agreed that variants should be considered uncertain until proven otherwise [44].
Our study has several limitations. First, the design of the study was retrospective, but our cases were collected consecutively by a single experienced pediatric ophthalmologist. Second, the majority of our patients were of a single ethnicity (Korean). Third, although our panel included 429 genes and known deep intronic variants associated with inherited eye diseases, including congenital cataracts, new recently discovered genes may have been missed in this panel-based sequencing approach. We assessed medically or surgically actionable genes in the field of ophthalmology based on literature searches, Genetic Home References, and the authors’ clinical experiences. More thorough investigations or reviews of systematically curated databases are needed.
5. Conclusions
In conclusion, our study demonstrated that NGS can help make an accurate diagnosis, provide individual-specific treatment, and perform targeted medical investigations. Our approach helps to avoid numerous medical investigations or unnecessary treatments. Meticulous eye examinations are not easy for young patients, and some patients may harbor multi-locus genetic variations in two unrelated genes. Therefore, targeted NGS can be considered a first-line diagnostic tool in inherited eye diseases, even if a single genetic cause is suspected. With an understanding of genetic mechanisms in inherited eye diseases, it would be easier to provide more specific treatments. Until now, most inherited eye diseases lack interventions that can prevent or modify the progressive course. In the near future, more patients will receive precision care if new gene therapy or pharmacogenetics studies are undertaken and proven to be effective.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes13010027/s1: Table S1: 429 target genes associated with inherited eye diseases. Table S2: Non-coding deep intronic or regulatory variants covered by the panel. Table S3: The clinical features of 90 patients who receive definite diagnosis or possible diagnosis. Table S4: The clinical features of 59 patients with unsolved cases. Table S5: The results of chromosomal comparative genomic hybridization microarray analysis. Figure S1: Pedigree of 33 patients who had family history of nystagmus. Figure S2: The distribution of age at referral for genetic testing. Figure S3: Splice site prediction analysis in 4 patients with non-canonical splice site variants.
Author Contributions
Conceptualization, J.H.; formal analysis and investigation, D.S., D.W., S.-T.L., S.S. and J.R.C.; writing—original draft preparation, J.H., H.W.P. and D.M.; writing—review and editing, J.H., H.W.P. and D.M.; supervision, J.H.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Dongwha” Faculty Research Assistance Program of Yonsei University College of Medicine (6-2017-0169), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1007965) and a fund (#2018-ER6902-02) by Research of Korea Disease Control and Prevention Agency.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Severance Hospital (4-2018-0436).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gillespie R.L., O’Sullivan J., Ashworth J., Bhaskar S., Williams S., Biswas S., Kehdi E., Ramsden S.C., Clayton-Smith J., Black G.C., et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. 2014;121:2124–2137.e2. doi: 10.1016/j.ophtha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Carss K.J., Arno G., Erwood M., Stephens J., Sanchis-Juan A., Hull S., Megy K., Grozeva D., Dewhurst E., Malka S., et al. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am. J. Hum. Genet. 2017;100:75–90. doi: 10.1016/j.ajhg.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Clark K.R., During M.J., Cremers F.P., Black G.C., et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia S.S., Adelman K., Bale S.J., Chung W.K., Eng C., Evans J.P., Herman G.E., Hufnagel S.B., Klein T.E., Korf B.R., et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (acmg sf v2.0): A policy statement of the american college of medical genetics and genomics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017;19:249–255. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- 5.Westerink J., Visseren F.L., Spiering W. Diagnostic clinical genome and exome sequencing. N. Engl. J. Med. 2014;371:1169. doi: 10.1056/NEJMc1408914. [DOI] [PubMed] [Google Scholar]
- 6.Ellingford J.M., Sergouniotis P.I., Lennon R., Bhaskar S., Williams S.G., Hillman K.A., O’Sullivan J., Hall G., Ramsden S.C., Lloyd I.C., et al. Pinpointing clinical diagnosis through whole exome sequencing to direct patient care: A case of senior-loken syndrome. Lancet. 2015;385:1916. doi: 10.1016/S0140-6736(15)60496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillespie R.L., Urquhart J., Anderson B., Williams S., Waller S., Ashworth J., Biswas S., Jones S., Stewart F., Lloyd I.C., et al. Next-generation sequencing in the diagnosis of metabolic disease marked by pediatric cataract. Ophthalmology. 2016;123:217–220. doi: 10.1016/j.ophtha.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Manrai A.K., Ioannidis J.P., Kohane I.S. Clinical genomics: From pathogenicity claims to quantitative risk estimates. JAMA. 2016;315:1233–1234. doi: 10.1001/jama.2016.1519. [DOI] [PubMed] [Google Scholar]
- 9.Ashley E.A. Towards precision medicine. Nat. Rev. Genet. 2016;17:507–522. doi: 10.1038/nrg.2016.86. [DOI] [PubMed] [Google Scholar]
- 10.Stone E.M., Aldave A.J., Drack A.V., Maccumber M.W., Sheffield V.C., Traboulsi E., Weleber R.G. Recommendations for genetic testing of inherited eye diseases: Report of the american academy of ophthalmology task force on genetic testing. Ophthalmology. 2012;119:2408–2410. doi: 10.1016/j.ophtha.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R.L., Parry N.R.A., Barton S.J., Campbell C., Delaney C.M., Ellingford J.M., Hall G., Hardcastle C., Morarji J., Nichol E.J., et al. Panel-based clinical genetic testing in 85 children with inherited retinal disease. Ophthalmology. 2017;124:985–991. doi: 10.1016/j.ophtha.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Rim J.H., Lee S.T., Gee H.Y., Lee B.J., Choi J.R., Park H.W., Han S.H., Han J. Accuracy of next-generation sequencing for molecular diagnosis in patients with infantile nystagmus syndrome. JAMA Ophthalmol. 2017;135:1376–1385. doi: 10.1001/jamaophthalmol.2017.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone E.M., Andorf J.L., Whitmore S.S., DeLuca A.P., Giacalone J.C., Streb L.M., Braun T.A., Mullins R.F., Scheetz T.E., Sheffield V.C., et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124:1314–1331. doi: 10.1016/j.ophtha.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The genome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye K., Schulz M.H., Long Q., Apweiler R., Ning Z. Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Schulz-Trieglaff O., Shaw R., Barnes B., Schlesinger F., Kallberg M., Cox A.J., Kruglyak S., Saunders C.T. Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 18.Plagnol V., Curtis J., Epstein M., Mok K.Y., Stebbings E., Grigoriadou S., Wood N.W., Hambleton S., Burns S.O., Thrasher A.J., et al. A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics. 2012;28:2747–2754. doi: 10.1093/bioinformatics/bts526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuilman T., Velds A., Kemper K., Ranzani M., Bombardelli L., Hoogstraat M., Nevedomskaya E., Xu G., de Ruiter J., Lolkema M.P., et al. Copywriter: DNA copy number detection from off-target sequence data. Genome Biol. 2015;16:49. doi: 10.1186/s13059-015-0617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houdayer C. In silico prediction of splice-affecting nucleotide variants. Methods Mol. Biol. (Clifton N. J.) 2011;760:269–281. doi: 10.1007/978-1-61779-176-5_17. [DOI] [PubMed] [Google Scholar]
- 22.Jaganathan K., Kyriazopoulou Panagiotopoulou S., McRae J.F., Darbandi S.F., Knowles D., Li Y.I., Kosmicki J.A., Arbelaez J., Cui W., Schwartz G.B., et al. Predicting splicing from primary sequence with deep learning. Cell. 2019;176:535–548.e24. doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S.I., Woo S.J., Oh B.L., Han J., Lim H.T., Lee B.J., Joo K., Park J.Y., Jang J.H., So M.K., et al. Genetic characteristics and phenotype of korean patients with stickler syndrome: A korean multicenter analysis report no. 1. Genes. 2021;12:1578. doi: 10.3390/genes12101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurkute N., Bertacchi M., Arno G., Tocco C., Kim U.S., Kruszewski A.M., Avery R.A., Bedoukian E.C., Han J., Ahn S.J., et al. Pathogenic nr2f1 variants cause a developmental ocular phenotype recapitulated in a mutant mouse model. Brain Commun. 2021;3:fcab162. doi: 10.1093/braincomms/fcab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.M., Joo K., Han J., Woo S.J. Clinical and genetic characteristics of korean congenital stationary night blindness patients. Genes. 2021;12:789. doi: 10.3390/genes12060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S., Park S.E., Won D., Lee S.T., Han S.H., Han J. Tubb3 m323v syndrome presents with infantile nystagmus. Genes. 2021;12:575. doi: 10.3390/genes12040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuht H.J., Han J., Maconachie G.D.E., Park S.E., Lee S.T., McLean R., Sheth V., Hisaund M., Dawar B., Sylvius N., et al. Slc38a8 mutations result in arrested retinal development with loss of cone photoreceptor specialization. Hum. Mol. Genet. 2020;29:2989–3002. doi: 10.1093/hmg/ddaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surl D., Shin S., Lee S.T., Choi J.R., Lee J., Byeon S.H., Han S.H., Lim H.T., Han J. Copy number variations and multiallelic variants in korean patients with leber congenital amaurosis. Mol. Vis. 2020;26:26–35. [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.E., Lee J.S., Lee S.T., Kim H.Y., Han S.H., Han J. Targeted panel sequencing identifies a novel nr2f1 mutations in a patient with bosch-boonstra-schaaf optic atrophy syndrome. Ophthalmic Genet. 2019;40:359–361. doi: 10.1080/13816810.2019.1650074. [DOI] [PubMed] [Google Scholar]
- 31.Bennett T.M., Maraini G., Jin C., Sun W., Hejtmancik J.F., Shiels A. Noncoding variation of the gene for ferritin light chain in hereditary and age-related cataract. Mol. Vis. 2013;19:835–844. [PMC free article] [PubMed] [Google Scholar]
- 32.Nesbitt A., Bhoj E.J., McDonald Gibson K., Yu Z., Denenberg E., Sarmady M., Tischler T., Cao K., Dubbs H., Zackai E.H., et al. Exome sequencing expands the mechanism of sox5-associated intellectual disability: A case presentation with review of sox-related disorders. Am. J. Med. Genet. Part A. 2015;167:2548–2554. doi: 10.1002/ajmg.a.37221. [DOI] [PubMed] [Google Scholar]
- 33.Nichols C.G., Singh G.K., Grange D.K. Katp channels and cardiovascular disease: Suddenly a syndrome. Circ. Res. 2013;112:1059–1072. doi: 10.1161/CIRCRESAHA.112.300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posey J.E., Harel T., Liu P., Rosenfeld J.A., James R.A., Coban Akdemir Z.H., Walkiewicz M., Bi W., Xiao R., Ding Y., et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N. Engl. J. Med. 2017;376:21–31. doi: 10.1056/NEJMoa1516767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smedley D., Smith K.R., Martin A., Thomas E.A., McDonagh E.M., Cipriani V., Ellingford J.M., Arno G., Tucci A., Vandrovcova J., et al. 100,000 genomes pilot on rare-disease diagnosis in health care—Preliminary report. N. Engl. J. Med. 2021;385:1868–1880. doi: 10.1056/NEJMoa2035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A., Hayward J.D., Tailor V., Nyanhete R., Ahlfors H., Gabriel C., Jannini T.B., Abbou-Rayyah Y., Henderson R., Nischal K.K., et al. The oculome panel test: Next-generation sequencing to diagnose a diverse range of genetic developmental eye disorders. Ophthalmology. 2019;126:888–907. doi: 10.1016/j.ophtha.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 37.Khan M., Cornelis S.S., Pozo-Valero M.D., Whelan L., Runhart E.H., Mishra K., Bults F., AlSwaiti Y., AlTalbishi A., De Baere E., et al. Resolving the dark matter of abca4 for 1054 stargardt disease probands through integrated genomics and transcriptomics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020;22:1235–1246. doi: 10.1038/s41436-020-0787-4. [DOI] [PubMed] [Google Scholar]
- 38.Miraldi Utz V., Pfeifer W., Longmuir S.Q., Olson R.J., Wang K., Drack A.V. Presentation of trpm1-associated congenital stationary night blindness in children. JAMA Ophthalmol. 2018;136:389–398. doi: 10.1001/jamaophthalmol.2018.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Men C.J., Bujakowska K.M., Comander J., Place E., Bedoukian E.C., Zhu X., Leroy B.P., Fulton A.B., Pierce E.A. The importance of genetic testing as demonstrated by two cases of cacna1f-associated retinal generation misdiagnosed as lca. Mol. Vis. 2017;23:695–706. [PMC free article] [PubMed] [Google Scholar]
- 40.Gilmour D.F. Familial exudative vitreoretinopathy and related retinopathies. Eye. 2015;29:1–14. doi: 10.1038/eye.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., et al. Ldl receptor-related protein 5 (lrp5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 42.Moore A.T. Genetic testing for inherited retinal disease. Ophthalmology. 2017;124:1254–1255. doi: 10.1016/j.ophtha.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Consugar M.B., Navarro-Gomez D., Place E.M., Bujakowska K.M., Sousa M.E., Fonseca-Kelly Z.D., Taub D.G., Janessian M., Wang D.Y., Au E.D., et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015;17:253–261. doi: 10.1038/gim.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weck K.E. Interpretation of genomic sequencing: Variants should be considered uncertain until proven guilty. Genet. Med. Off. J. Am. Coll. Med. Genet. 2018;20:291–293. doi: 10.1038/gim.2017.269. [DOI] [PubMed] [Google Scholar]
- 45.Battistini S., Stenirri S., Piatti M., Gelfi C., Righetti P.G., Rocchi R., Giannini F., Battistini N., Guazzi G.C., Ferrari M., et al. A new cacna1a gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology. 1999;53:38–43. doi: 10.1212/WNL.53.1.38. [DOI] [PubMed] [Google Scholar]
- 46.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S., et al. Efficacy and safety of voretigene neparvovec (aav2-hrpe65v2) in patients with rpe65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fincham G.S., Pasea L., Carroll C., McNinch A.M., Poulson A.V., Richards A.J., Scott J.D., Snead M.P. Prevention of retinal detachment in stickler syndrome: The cambridge prophylactic cryotherapy protocol. Ophthalmology. 2014;121:1588–1597. doi: 10.1016/j.ophtha.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Ang A., Poulson A.V., Goodburn S.F., Richards A.J., Scott J.D., Snead M.P. Retinal detachment and prophylaxis in type 1 stickler syndrome. Ophthalmology. 2008;115:164–168. doi: 10.1016/j.ophtha.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 49.Hull S., Arno G., Ku C.A., Ge Z., Waseem N., Chandra A., Webster A.R., Robson A.G., Michaelides M., Weleber R.G., et al. Molecular and clinical findings in patients with knobloch syndrome. JAMA Ophthalmol. 2016;134:753–762. doi: 10.1001/jamaophthalmol.2016.1073. [DOI] [PubMed] [Google Scholar]
- 50.Moog U., Bierhals T., Brand K., Bautsch J., Biskup S., Brune T., Denecke J., de Die-Smulders C.E., Evers C., Hempel M., et al. Phenotypic and molecular insights into cask-related disorders in males. Orphanet J. Rare Dis. 2015;10:44. doi: 10.1186/s13023-015-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watkins R.J., Patil R., Goult B.T., Thomas M.G., Gottlob I., Shackleton S. A novel interaction between frmd7 and cask: Evidence for a causal role in idiopathic infantile nystagmus. Hum. Mol. Genet. 2013;22:2105–2118. doi: 10.1093/hmg/ddt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiffin N., Minikel E., Walsh R., O’Donnell-Luria A.H., Karczewski K., Ing A.Y., Barton P.J.R., Funke B., Cook S.A., MacArthur D., et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017;19:1151–1158. doi: 10.1038/gim.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.