Abstract
Aspergilus flavus is the main pathogenic fungus that causes food mold. Effective control of A. flavus contamination is essential to ensure food safety. The lipopeptides (LPs) produced by Bacillus strains have been shown to have an obvious antifungal effect on molds. In this study, an antagonist strain of Bacillus velezensis with obvious antifungal activity against A. flavus was isolated from the surface of healthy rice. Using HPLC-MS analysis, the main components of LPs produced by strain E2 were identified as fengycin and iturins. Further investigations showed that LPs could inhibit the spore germination, and even cause abnormal expansion of hyphae and cell rupture. Transcriptomic analyses showed that some genes, involved in ribosome biogenesis in eukaryotes (NOG1, KRE33) and aflatoxin biosynthesis (aflK, aflR, veA, omtA) pathways in A. flavus were significantly down-regulated by LPs. In conclusion, this study provides novel insights into the cellular and molecular antifungal mechanisms of LPs against grain A. flavus contamination.
Keywords: Aspergilus flavus, lipopeptides, antifungal activity, aflatoxin biosynthesis
1. Introduction
Aspergilus flavus is a common saprophytic fungus, which can colonize a range of economically important crops [1]. In addition to causing great economic losses, the contamination of food and feed by A. flavus also causes public health concerns because this fungi can produce mycotoxins (aflatoxin) [2,3]. Aflatoxins have been shown to possess carcinogenic, teratogenic, and mutagenic effects that are harmful to the human body [4]. Therefore, an efficient mechanism for controlling A. flavus contamination is urgently needed. Currently, the common method for controlling A. flavus contamination is using synthetic chemical fungicides. However, this method is limited because it can lead to the presence of fungicide residues in food and fungal resistance, as well as posing a threat to people’s health [5,6]. In contrast, biological control provides a safe and eco-friendly method for inhibiting the growth of fungal pathogens [7].
To date, many microbial strains, such as yeast, bacteria, and filamentous fungi, have been used for the control of mold contamination. The biological control mechanism mainly involves the production of direct antifungal substances, such as non-volatile metabolites [8], volatile organic compounds (VOCs) [9,10] and extracellular lytic enzymes [11]. Among the microbial strains associated with the biological control mechanism, the broad-spectrum antagonistic Bacillus has gradually become one of the most valuable, because of its good biological activity, high inhibitory activity to a variety of pathogenic microorganisms, and convenient mass production [12,13]. Other strains that have also been reported to have good antifungal activity include Bacillus subtilis fmbJ [14], Paenibacillus polymyxa APEC136 [12], Bacillus atrophaeus strain B44 [15], and Bacillus amyloliquefaciens MG3 [16].
Antifungal LPs are important metabolites produced by some strains of the genus Bacillus and they play an important role in disease suppression [17]. Recent studies have shown that LPs can be produced by various Bacillus strains, such as Bacillus subtilis [18], Bacillus amyloliquefaciens [19], Bacillus thuringiensis [20], and Bacillus cereus [21]. The antimicrobial LPs mainly include surfactin, fengycin, and iturin [22].
So far, molecular mechanisms associated with the antifungal activity of LPs against A. flavus are still to be elucidated. This study aimed to isolate and identify antagonistic bacterial against A. flavus, characterize the substance responsible for its antifungal activity, and explore the possible mode of action of the identified antifungal substance against pathogenic fungi.
2. Materials and Methods
2.1. Fungal Pathogen
A. flavus was isolated from the surface of moldy rice and identified by morphological characteristics and DNA sequencing. It was streaked on the surface of potato dextrose agar (PDA) plates, incubated at 28 °C for 5 days, and temporarily stored at 4 °C for later use [23]. A small quantity of sterile distilled water (SDW) was added to the plate to wash off the spores. The mold suspension was filtered with three layers of lens cleaning paper to remove the hyphae and a hemocytometer was used to adjust it to the desired concentration [10].
2.2. Isolation and Identification of Antagonistic Bacterial Strains
The isolation source of antagonistic bacteria was healthy rice, paddy leaves, and rhizosphere soil collected from the rice fields in the city of Zhenjiang City, China. Isolation and purification was carried out according to the method of Yang [8]. The isolated and purified strains were inoculated in LB medium at 28 °C in a rotatory shaker (180 rpm), for 24 h. Samples were collected and stored at 4 °C as for later use. The assessment of the inhibitory effects of the isolated bacterial strains was performed through cup–disc methods [24]. Holes with a diameter of 6 mm were punched in the center of the PDA plate (90 mm) with 4 holes equally spaced about 20 mm away from the center hole, and the agar in the holes was removed. 40 μL 1 × 105 CFU/mL of A. flavus spore suspension and 40 μL 1 × 108 CFU/mL of antagonistic bacterial suspension were selected for the test. These were incubated at 28 °C for 4 days. It was then observed and the size of the mycelial diameter of the pathogenic bacteria was measured. Three replicates were analyzed per treatment.
The bacteria with the best antagonistic effect were analyzed as follows. The morphological traits of strain E2 were observed and recorded after incubation on LB agar at 28 °C for 24 h. Physiological and biochemical tests of strain E2 for bacterial identification were performed as described in Bergey’s Manual of Determinative Bacteriology [25].
Total DNA was extracted according to the methods of Linlin [26]. Universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′ACGGCTACTGTACGACT- -T-3′) were used to amplify the 16S rDNA gene. PCR products were sequenced by Sangon Biotech Co. Ltd. The results of sequencing were compared using NCBI. Related sequences were downloaded and used to construct a phylogenetic tree with the Mega 6.0 software [27].
2.3. Extraction of Antifungal Substances
A single bacterial colony was transferred into LB medium and incubated in a rotatory shaker (180 rpm) for 24 h, as seed liquid. The seed liquid (1%) was transferred to the LB medium and incubated at 28 °C in a rotatory shaker (180 rpm) for 72 h. The fermentation broth was centrifuged at 10,000 rpm for 15 min at 4 °C. The pH of the collected supernatant was adjusted to 2.0 using 6 moL/L concentrated HCl, and it was placed at 4 °C for 24 h. The samples were centrifuged again at 10,000 rpm for 15 min at 4 °C, after 24 h [18]. The crude LPs were collected and then extracted by methanol for 6–8 h, and filtered through syringe-type 0.22 μm microbial filters. A vacuum concentrator was used to remove methanol from the mixture to obtain the yellowish-brown substance [28]. The extracted substance was dissolved in SDW and adjusted to neutral with 1 moL/L NaOH. After pre-freezing, it was freeze-dried in a vacuum freeze dryer for 48 h. The light brown loose powder solid was LPs.
2.4. LPs Minimal Inhibitory Concentration
A defined concentration (100 μL) of A. flavus spore suspension (1 × 105 CFU/mL) was spread on PDA plates (90 mm), and 5 holes (6 mm) were punched following the method described in Section 2.2. Different concentrations 0.25, 0.5, 1, 2.5, 5, 10, and 15 mg/mL of LPs and a 109 CFU/mL bacterial suspension were into each hole with a respective volume of 40 μL. An equivalent amount of SDW was added in place of the LPs suspension in one of the holes and was used as a non-treated control. The inhibitory diameter of hyphae was measured under different LPs treatment after 5 days. Three replicates were analyzed per treatment.
2.5. Effect of LPs on A. flavus Spore Germination
LPs were added into erlenmeyer flasks containing 20 mL of PDB medium to make the final concentration to 12.5, 25, 50, 100, 200, and 400 µg/mL. 2 mL of 1 × 107 A. flavus spore suspension was inoculated into each, and the PDB without LPs was used as non-treated control. After incubating at 28 °C and 75 rpm, for 10 h, the spore germination rate of A. flavus was observed and calculated. When the length of the germ tubes was equal to the maximum size of the swollen spore, the spores were considered to have germinated [29]. At least 100 spores were observed each time using a light microscope. The spore germination rates were calculated as follows: spore germination rate (%) = (number of germinated spores/total number of observed spores) × 100% [16]. Three replicates were analyzed per treatment.
2.6. Effect of LPs on A. flavus Mycelium Growth
LPs concentrations of 0, 12.5, 25, 50, 100, 200, 500, and 1000 µg/mL were prepared in PDA medium and poured into plates. A single hole was punched at the center of the plates with a sterile punch (6 mm), and the agar in the hole was removed. 40 µL 1 × 106 CFU/mL of A. flavus spore suspension was added to each hole. The PDA medium without LPs was used as the control. After incubating at 28 °C for 5 days, the diameter of the mycelium was observed and calculated. Three replicates were analyzed per treatment.
2.7. Microscope and Scanning Electron Microscopy (SEM)
A total of 1 × 106 CFU/mL A. flavus spore suspension was added to the PDB medium amended with an LPs concentration of 500 µg/mL. The PDB medium without LPs was used as the control. After incubating at 28 °C for 38 h, samples were prepared according to Ye [30]. The collected A. flavus hyphae were fixed in glutaraldehyde solution at 4 °C overnight. The specimens were washed 3 times with 0.1% moL/L PBS for 15 min each time. Then, 30, 50, 70, 90, and 95% ethanol were used to dehydrate the specimens in gradients for 15 min each. The specimens were dehydrated with 100% ethanol three times for 20 min each time. Next, the alcohol was replaced with pure tertiary alcohol three times, and the standing time for each session was 15 min. Finally, the mixed mycelial pellet and tert-butanol suspension were sucked and dropped on the sample table covered with a cover glass. They were vacuum dried in a freeze dryer that was pre-cooled for 1 h. Specimens were taken out after the air pressure dropped below 10 Pa. The dehydrated specimens were coated with gold-palladium and observed under the thermal field emission scanning electron microscope (JSM-7001F).
2.8. Analysis of LPs by HPLC-MS
LPs were qualitatively analyzed by a liquid Chromatograph Mass Spectrometer (*thermo LXQ LC/MS). The HPLC conditions were as follows: C18 column, 5 μm, 4.6 mm × 250 mm; mobile phase A [distilled water containing 0.08% (v/v) formic acid]; mobile phase B [acetonitrile containing 0.08% (v/v) formic acid]; and flow speed, 0.6 mL/min; Linear gradient programmes were set from 40% of phase B to 60% of phase B during a 35 min period; 30 °C and UV detection at 210 nm. Mass spectrometry conditions: ion source, ESI; ion spray voltage, 3500 V; temperature, 325 °C; mass spectra, 100–1500 m/z. Data acquisition and processing were performed using Xcalibur 4.2.
2.9. Transcriptomics Study on the Effect of LPs on A. flavus
2.9.1. RNA Extraction and Transcriptome Sequencing
According to the inoculation amount of 2%, 1 × 106 CFU/mL A. flavus spore suspension was inoculated into PDB medium with an LPs concentration of 1 mg/mL. The PDB medium without LPs was used as the control. A. flavus spores were incubated at 28 °C, 180 rpm, and protected from light for 38 h. Mycelium was collected and washed three times with SDW and frozen with liquid nitrogen. A total RNA of the sample was extracted according to the instructions of Trizol reagent kit (Invitrogen, Carlsbad, CA, USA). The concentration, purity, and integrity of RNA in the sample were detected with Agilent 2100, and the qualified samples were sent to Gene Denovo Biotechnology Co. (Guangzhou, China) for the construction and sequencing of the cDNA libraries. Ensembl_release51 was selected as the reference genome.
2.9.2. Transcriptome Sequencing
In order to ensure data quality, fastp was used to analyze the original raw reads. Low-quality data were filtered out and clean reads were obtained. DESeq2 software was used to screen differentially expressed genes. Genetic parameter of false discovery rate (FDR) ≤ 0.05 and absolute fold change (FC) ≥ 4 were considered to be differentially expressed gene [31]. The expressed genes (FDR ≤ 0.05 and absolute FC ≥ 4) were functionally annotated according to three databases, Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) [32]. Moreover, both upward and downward gene expression profiles were subjected to GO and KEGG enrichment analysis. In addition, GO and KEGG enrichment analyses were performed on both the upward and downward gene expression profiles.
2.9.3. RT-qPCR
Genes with significant differences were selected from the resulting transcriptomic structure. Primer design was performed by the method of Xiong [33]. The actin gene was used as a reference gene for relative gene expression analysis. The primers used were listed in Table 1. Referring to the method of Redshaw [34] and using a 25 μL reaction system, the parameters in the real-time fluorescence quantitative PCR analyzer were set as follows: pre-denaturation 30 s at 95 °C, denaturation 5 s at 95 °C, then annealing 30 s at 62 °C, and finally elongation 20 at 72 °C, 40 cycles; The dissolution curve is: 95 °C, 15 s; 60 °C,1 min; 95 °C, 15 s.
Table 1.
Primers used for RT-qPCR.
| Gene ID | Symbol | Primers (5′ to 3′) |
|---|---|---|
| AFLA_029920 | KRE33 | F: GGGATTTACCAGTCACCGCA R: AGCACGATTCCACCTCCTTC |
| AFLA_134410 | NOG1 | F: TGCTCCAGAACCGTCAAACT R: CGGCTGAGAACGACATCCAA |
| AFLA_033290 | laeA | F: CTTGCTCCCATACAGCCCTC R: TCCCATCACACTTCCACACC |
| AFLA_066460 | veA | F: AAGGTTGTCGTGTGCGGATT R: TGGGGTAGAGATTCGGTCAG |
| AFLA_139190 | aflK | F: GTGATTGAGGCGGGAGGAT R: GCCGTGTTGTCGTTGAGAG |
| AFLA_139360 | aflR | F: CCCCACTACCACCGTTTCAG R: CTCATCCACACAATCCTCGC |
| AFLA_139210 | omtA | F: TAGTTCATGGCCCGGTTCC R: AGGTTTGCCTTTCGTCTGCT |
| AFLA_139220 | omtB | F: GAGAGCGACACGCCGATAA R: GAAGAATGCGACCAAGGAGT |
| AFLA_047410 | actin | F: GAAGTTGCTGCTCTCGTCA R: GACCGACAATGGAGGGGAAG |
2.10. Statistical Analyses
All test data were statistically analyzed by Excel 2010 and SPSS 22.0 software. Duncan’s Multiple Range Test was used to analyze the significance of the difference, and the level of significance was set to p < 0.05.
3. Results
3.1. Screening and Identification of Antagonistic Strains against A. flavus
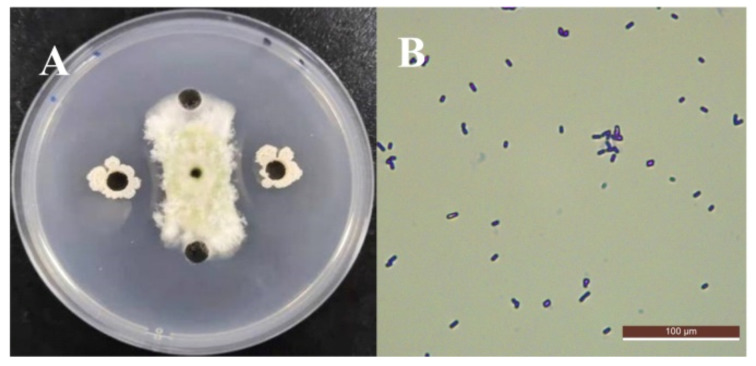
A total of 84 strains of microorganisms were isolated from the rice surface, soil, and paddy leaf surface. Through the cup–disc tests, strain E2 with the highest antifungal rate against A. flavus was selected as the antagonistic bacteria (Figure 1A). Cells of strain E2 were Gram-stain-positive and had a short rod shape (Figure 1B). The results of some physiological and biochemical tests are shown in Table 2. Except for one indicator of methyl red test which was negative, the results of the other 13 indicators, including the V-P test and the citrate utilization, were all positive. Under the guidance of these tests, the E2 strain was preliminarily speculated to belong to the Bacillus spp. [35].
Figure 1.
Inhibitory effect of strain E2 on A. flavus using cup–disc (A), Gram staining results of N2 and its morphology (B).
Table 2.
Physiological and biochemical properties of strain E2.
| Properties | Strain E2 | Properties | Strain E2 |
|---|---|---|---|
| Gram stain | + | Citrate utilization | + |
| Moveability | + | Glucose ferm entation | + |
| Catalase | + | Mannitol fermentation | + |
| V-P test | + | Carbon utilization–glucose | + |
| Methyl red test | − | Carbon utilization–mannitol | + |
| Starch hydrolusis | + | Carbon utilization–fructose | + |
| Gelaune liquefaction | + | Gelaune liquefaction | + |
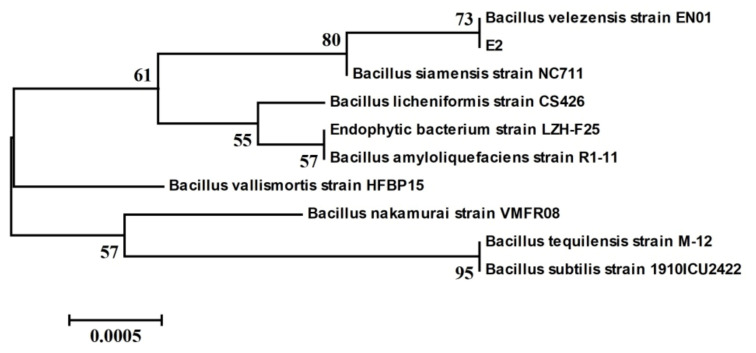
The Blast software on the NCBI website was used to analyze the obtained E2 16S rDNA gene sequence. The E2 strain had more than 99% homology with some Bacillus strains in the NCBI database. The phylogenetic tree was constructed as shown in Figure 2. Based on morphological characteristics and molecular biological identification, the antagonistic E2 strain was identified as Bacillus velezensis.
Figure 2.
Phylogenetic tree of strain E2 based on 16S rDNA gene sequences. The phylogenetic tree was constructed by the neighbor-joining method using MEGA 6.0 software. The bootstrap values are shown at the branch points.
3.2. The Minimal Inhibitory Concentration of LPs
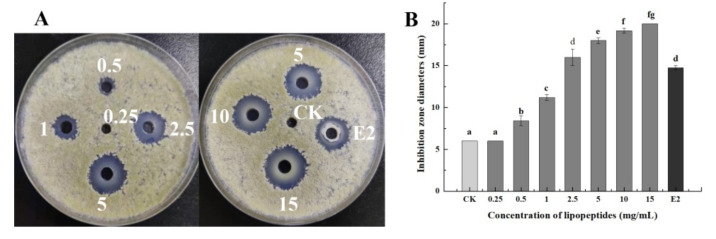
The inhibitory effect of LPs on A. flavus was obviously dependent on its concentration. With the increase in LPs concentration, the inhibitory effect became stronger (Figure 3). When the LPs concentration was 0.5 mg/mL, the inhibition zone produced by its inhibitory effect on A. flavus was 8.42 mm, which was the minimal inhibitory concentration of LPs. The diameter of the inhibition zone produced by the LPs concentration reaching 2.5 mg/mL was already higher than the diameter of inhibition produced by the 1 × 109 CFU/mL strain E2 suspension. This showed that the antifungal effect of high-concentration LPs was better than that of bacteria. There was no significant difference in the inhibitory effect of LPs at 10 mg/mL and 15 mg/mL (p < 0.05), so the maximum concentration of LPs was 15 mg/mL.
Figure 3.
Strain E2 and different concentrations of LPs produced different sized inhibitory zones on the plates containing A. flavus. (A): Inhibition of A. flavus treated with strain E2, LPs (0.25–15 mg/mL), and SDW (CK). (B): MIC inhibitory concentrations of LPs. The numbers of 0, 0.25, 0.5, 1, 2.5, 5, 10, 15 mg/mL and CK represent different concentrations of LPs and cell suspensions of strain E2 at the concentrations of 1 × 109 CFU/mL, respectively. Different letters above the bars indicate significant differences (LSD test, p < 0.05).
3.3. The Inhibitory Effect of LPs on the Germination of A. flavus Spores
When the concentration of LPs was 500 µg/mL, the spore germination rate of A. flavus was only 1.33%, and there was almost no germination after incubation for 10 h (Table 3). In the meantime, the spore germination rate of the control group was as high as 75.34%. As the concentration of LPs increased, the germination rate of spores was severely inhibited. This indicated that LPs at an appropriate concentration can effectively inhibit the germination of A. flavus spores.
Table 3.
Effects of different LPs concentrations on spore germination.
| Treatments | Lipopeptide (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| CK | 12.5 | 25 | 50 | 100 | 200 | 400 | |
| Spore germination rate (%) | 75.34 ±1.07 a |
67.41 ±1.82 b |
54.93 ±1.33 c |
35.49 ±3.14 d |
15.43 ±2.62 e |
5.80 ±1.31 f |
1.33 ±0.52 f |
Columns with different letters indicated significant differences (LSD test, p < 0.05).
3.4. Inhibitory Effect of LPs on Mycelial Growth
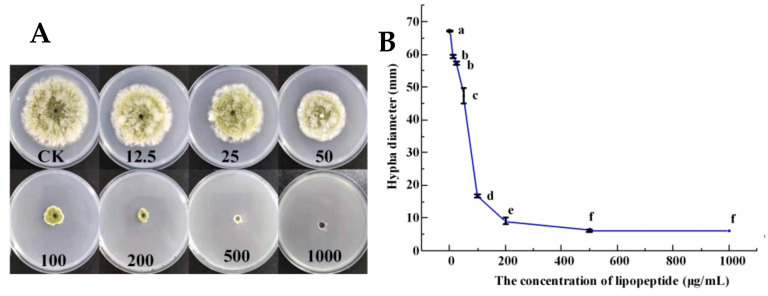
After culturing at 28 °C for 5 days, the diameter of the hyphae of A. flavus treated with LPs was much smaller than that of the control group (Figure 4). LPs could effectively inhibit the growth of A. flavus hyphae and had a very significant inhibitory effect on A. flavus. The hyphae of the pathogenic bacteria in the treatment group were thinner, and the hyphae at the edges were more chaotic. When the LPs concentration increased from 0 to 200 µg/mL, the mycelial diameter of A. flavus dropped sharply. The mycelial diameter did not decrease until the LPs concentration reached 1000 µg/mL, but a small number of hyphae could still be observed in the hole. This indicated that LPs could inhibit the mycelial growth of A. flavus, but could not completely kill it.
Figure 4.
The changes in growth diameter and inhibition rate of A. flavus under the effect of the control group (SDW) and test group (12.5–1000 µg/mL, LPs) were treated, respectively. (A) Colony morphology of A. flavus grown on media containing different concentrations of LPs. (B) Colony diameter of A. flavus after treating with LPs. Vertical bars represent standard errors of the mean. Different letters indicated significant differences (LSD test, p < 0.05).
3.5. Microscope and SEM Evaluation
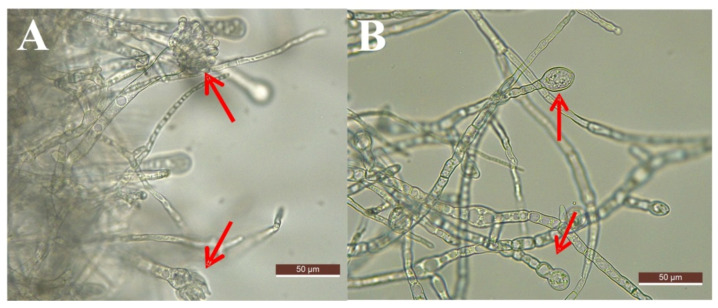
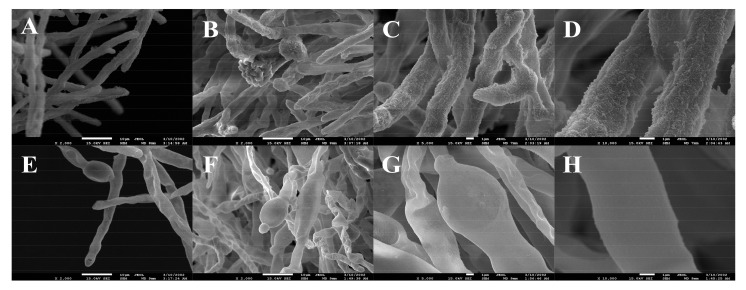
As shown in Figure 5 and Figure 6, the morphological changes in A. flavus hyphae were analyzed in detail with the use of an optical microscope and SEM. The pictures clearly displayed the inhibitory effect of LPs on the growth of A. flavus. In the control group, the hyphae of A. flavus were dense and produced conidia (Figure 5A). The morphology of the mycelium was regular and full, the structure was complete, and its surface had spikes (Figure 6A–D). In contrast, the hyphae of A. flavus treated with LPs were sparse and could not form conidia (Figure 5B). The hyphae were abnormally swollen, twisted, and collapsed. The top of the hyphae was damaged and their surface was smooth (Figure 6E–H).
Figure 5.
LPs released by Strain E2 exhibited an inhibitory effect on the growth of A. flavus (×400). (A): Control; (B): Treatment with 500 µg/mL LPs.
Figure 6.
SEM of A. flavus growth without the presence of antagonistic substances (control: (A–D)) and faced with LPs (E–H). The figures show: suppression of mycelial growth of A. flavus, the apex of the mycelium was broken (E); degenerative change in the morphology of the fungus hyphae (F); the mycelium was twisted, collapsed, and abnormally swollen (G); the mycelia was smooth and more swollen than the control group (H).
3.6. HPLC-MS Spectrometry Analysis
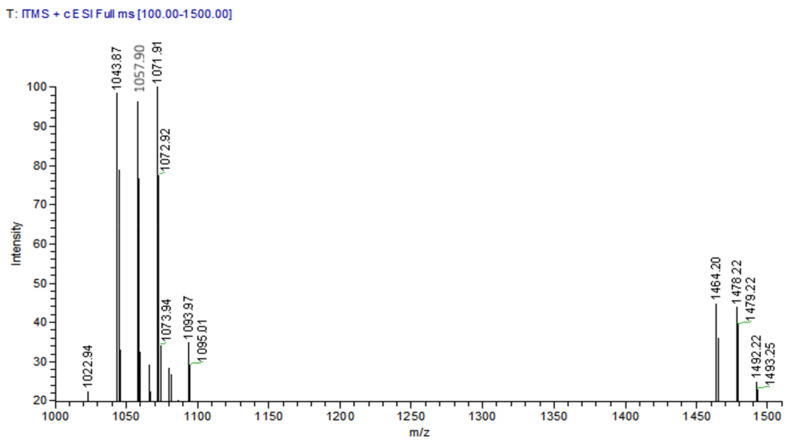
The results of mass spectrograms detected by LC-MS are shown in Figure 7. It could be seen that the relative molecular masses corresponding to the retention time of 13.25, 15.99, 16.52, 20.95, and 21.85 min were 1043.87, 1057.90, 1057.90 1071.91, and 1071.92, respectively (Figure 7). The mass spectrum peaks corresponded to the ion addition peaks of C14 Iturin A, C15 Iturin A, C15 Iturin A, C16 Iturin A, and C16 Iturin A (Table 4). The relative molecular masses corresponding to the retention time of 24.89, 27.20, and 30.61 min were 1464.20, 1478.20, and 1492.22, respectively (Figure 7). The mass spectrum peaks of them corresponded to the ion addition peaks of C16 Fengycin A, C16 Fengycin C, and C16 Fengycin D (Table 4). The results were similar to those of Youyou Wang [36] and Bo Zhang [37]. These results indicated that strain E2 could produce two LPs; Iturin and Fengycin.
Figure 7.
HPLC-MS for the LPs antibiotics isolated from strain E2.
Table 4.
LC-MS detection of purified LPs.
| Retention Time (min) | MS m/z | Identified Compounds | |
|---|---|---|---|
| [M + H]+ | [M + Na]+ | ||
| 13.25 | 1043.87 | 1065.97 | C14 Iturin A |
| 15.99 | 1057.90 | 1079.96 | C15 Iturin A |
| 16.52 | 1057.90 | 1079.97 | C15 Iturin A |
| 20.95 | 1071.91 | 1093.97 | C16 Iturin A |
| 21.85 | 1071.92 | 1093.97 | C16 Iturin A |
| 24.89 | 1464.20 | - | C16 FengycinA |
| 27.20 | 1478.20 | - | C16 FengycinC |
| 30.61 | 1492.22 | - | C16 FengycinD |
3.7. LPs Affected the Transcriptome of A. flavus
3.7.1. Analysis of Differentially Expression Genes
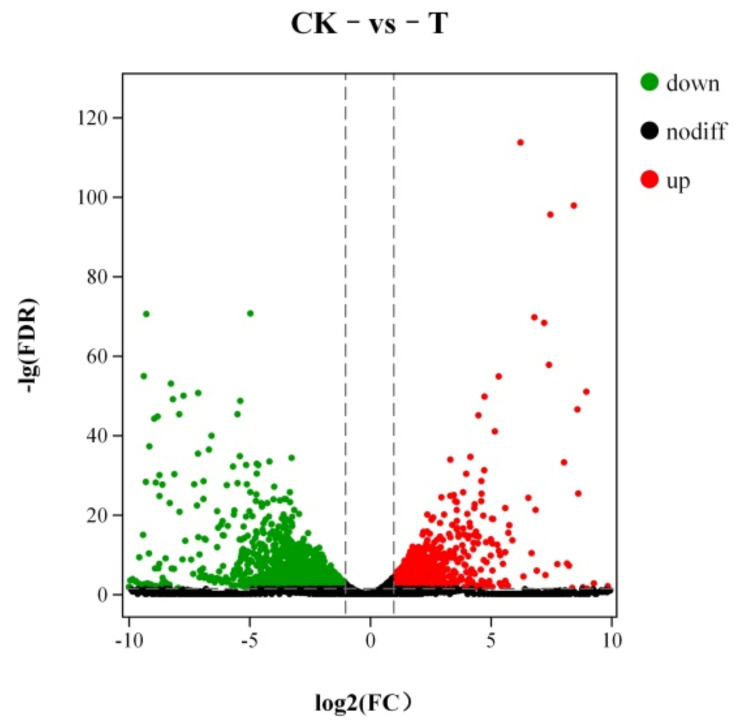
The results showed that under the biological control of LPs, numerous A. flavus genes were differentially expressed. The differentially expressed genes between the control group and the treatment group are shown in Figure 8. Each dot in the figure represents a gene. A total of 1500 differentially expressed genes were screened under the criteria of |log2 (Fold Change)| ≥ 2 and FDR < 0.05. There were 400 up-regulated expressions and 1100 down-regulated expressions.
Figure 8.
The volcano plot of differentially expressed genes.
3.7.2. GO Enrichment Analysis of Differentially Expressed Genes
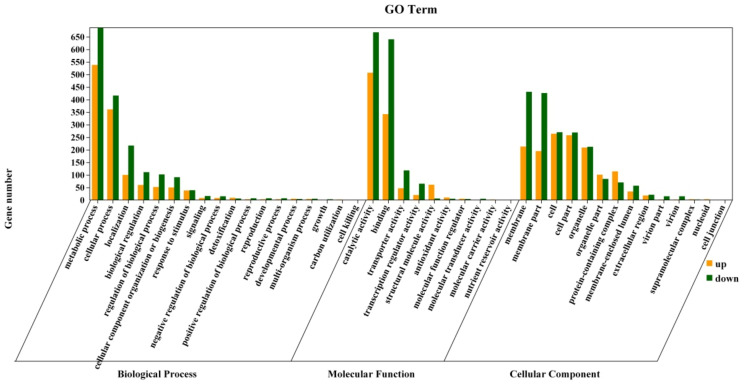
All genes and differential genes were classified by GO enrichment and classified into biological process, cell component, and molecular function. The most diverse genes in the biological process were found in the metabolic process (1174) and cellular process (777). Membrane (644) and membrane part (621) were the most diverse genes in the cell component. The most diverse genes in molecular function were catalytic activity (1175) and binding (982) (Figure 9). In most pathways, the number of down-regulated genes was higher than that of up-regulated genes. In contrast, LPs had a stronger inhibitory effect on the gene expression of A. flavus.
Figure 9.
GO Analysis of differential genes of A. flavus.
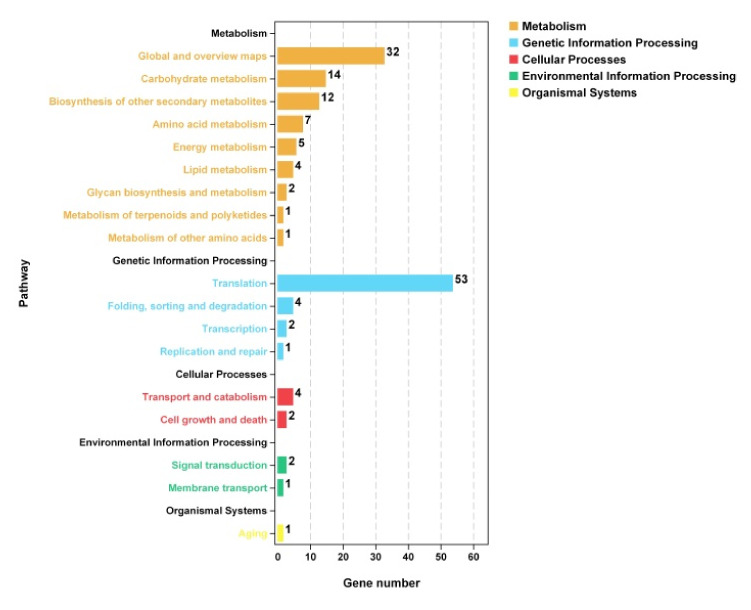
3.7.3. KEGG Enrichment Analysis of Differentially Expressed Genes
The KEGG enrichment classification results of the screened differential genes showed that a total of 212 differential genes were involved in 45 metabolic pathways (Figure 10). The pathways involving many of up-regulated genes mainly included biosynthesis of amino acids and oxidative phosphorylation. It was speculated that the active ingredients of LPs caused protein damage and the membrane structure was destroyed, thereby the protein synthesis of A. flavus was compensatively promoted to meet its growth needs. The pathways involving many of down-regulation of differential genes were mainly concentrated in ribosome biogenesis in eukaryotes and Aflatoxin biosynthesis.
Figure 10.
KEGG pathway classification of the screened differential genes.
Ribosomes are the cellular factories responsible for making proteins. In eukaryotes, ribosome biogenesis involves the production and correct assembly of four rRNAs and about 80 ribosomal proteins. Even under optimal growth conditions, the lack of these proteins will cause the biosynthesis of ribosomes to stall, and cell growth will stop. According to the results of transcriptome sequencing, the representative genes mpp10, SPAC57A7.06, KRE33, utp10, SPBC4F6.14, NOG2, and NOG1 related to ribosome synthesis in eukaryotes, were all down-regulated (Table 5), and the multiples of difference were exceeded twice.
Table 5.
Expression profiling of A. flavus genes involved in ribosome biogenesis in eukaryotes.
| Gene ID | Gene Description | Log2FC | Style |
|---|---|---|---|
| AFLA_012380 | U3 small nucleolar ribonucleoprotein protein Mpp10 | −2.22 | down |
| AFLA_029920 | nucleolar ATPase Kre33, putative | −2.48 | down |
| AFLA_033570 | SSU processome component Utp10, putative | −2.22 | down |
| AFLA_028940 | small nucleolar ribonucleoprotein complex subunit Utp14 | −2.88 | down |
| AFLA_112310 | small nucleolar ribonucleoprotein complex subunit, putative | −2.03 | down |
| AFLA_113720 | ribosome biogenesis (Nop4), putative | −2.30 | down |
| AFLA_134410 | nucleolar GTP-binding protein (Nog1), putative | −2.18 | down |
| AFLA_110550 | nucleolar GTPase, putative | −2.68 | down |
In order to explore the molecular mechanism of LPs on the synthesis of aflatoxin, the genes related to aflatoxin biosynthesis were screened based on the results of transcriptome sequencing. This demonstrated that the transcription level of most genes, whether it was regulatory genes or structural genes, was down-regulated to varying degrees (Table 6). Among them, aflQ, aflK, ordA, omtA, omtB, aflG, aflN, aflM, aflJ, aflD and aflT were significantly down-regulated.
Table 6.
Expression profiling of A. flavus genes involved in aflatoxin biosynthesis.
| Gene ID | Gene Description | Log2FC | Style |
|---|---|---|---|
| AFLA_138050 | flavonoid 3-hydroxylase, putative | −5.60 | down |
| AFLA_139140 | aflYa/nadA/NADH oxidase | −4.45 | down |
| AFLA_139190 | aflK/vbs/VERB synthase | −9.25 | down |
| AFLA_139200 | aflQ/ordA/ord-1/oxidoreductase/cytochrome P450 monooxigenase | −9.27 | down |
| AFLA_139210 | aflP/omtA/omt-1/O-methyltransferase A | −8.93 | down |
| AFLA_139220 | aflO/omtB/dmtA/O-methyltransferase B | −8.79 | down |
| AFLA_139260 | aflG/avnA/ord-1/cytochrome P450 monooxygenase | −9.13 | down |
| AFLA_139280 | aflN/verA/monooxygenase | −9.14 | down |
| AFLA_139300 | aflM/ver-1/dehydrogenase/ketoreductase | −7.10 | down |
| AFLA_139310 | aflE/norA/aad/adh-2/NOR reductase/dehydrogenase | −8.23 | down |
| AFLA_139320 | aflJ/estA/esterase | −8.15 | down |
| AFLA_139330 | aflH/adhA/short chain alcohol dehydrogenase | −3.34 | down |
| AFLA_139340 | aflS/pathway regulator | −2.77 | down |
| AFLA_139360 | aflR/apa-2/afl-2/transcription activator | −2.12 | down |
| AFLA_139370 | aflB/fas-1/fatty acid synthase beta subunit | −2.55 | down |
| AFLA_139390 | aflD/nor-1/reductase | −4.68 | down |
| AFLA_139410 | aflC/pksA/pksL1/polyketide synthase | −3.13 | down |
| AFLA_139420 | aflT/aflT/transmembrane protein | −5.48 | down |
| AFLA_066460 | developmental regulator AflYf/VeA | −0.40 | down |
| AFLA_033290 | regulator of secondary metabolism LaeA | −1.63 | down |
3.7.4. RT-qPCR Verification of Differentially Expressed Genes
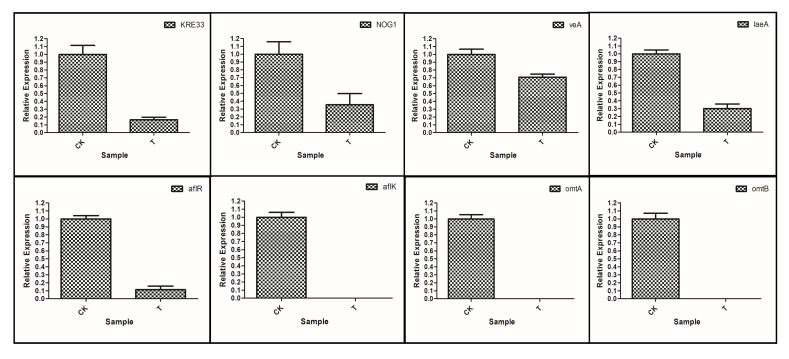
The 8 key genes were screened out and their expression levels were verified by RT-qPCR (Figure 11) KRE33 and NOG1 related to Ribosome biogenesis in eukaryotes were all down-regulated, and the aflR, aflK, omtA, and omtB involved in Aflatoxin biosynthesis were all down-regulated. A. flavus growth and secondary metabolism of global regulation of genes veA and laeA were also down-regulated. The results showed that the expression levels of those genes detected by the RT-qPCR were consistent with the transcriptome results. Therefore, the transcriptome results were valid and reliable.
Figure 11.
RT-qPCR verification results of some key differential genes. The actin gene was used as a reference gene. Vertical bars represent standard errors of the mean. (p < 0.05).
4. Discussion
As mentioned above, A. flavus can infect rice, peanuts, and corn and produce a mass of conidia [38]. Early prevention of A. flavus contamination is of great significance for global food security. Biological control is an effective means to reduce the use of chemicals. Biological control using microbes that were isolated from the environment was an effective and non-toxic approach for controlling disease. Studies have shown that some strains of the genus Bacillus have the ability to produce secondary metabolites with strong antifungal activity [39]. Among these antifungal substances, the LPs synthesized through non-ribosomal pathways (Fengycin, Iturin, and Surfactin) have the characteristics of strong antifungal activity, stable properties, and high safety [18,40]. However, their mode of action, especially their molecular mechanism of inhibiting A. flavus is not yet clearly understood. In the present study, we screened a batch of bacteria, and among them, strain E2 had the best inhibitory effect on A. flavus. The LPs were extracted according to the method of Zhang for follow-up studies [28].
In order to gain some insight into the mechanism of action of LPs on A. flavus, we undertook a more specific study on its antifungal effects at the cellular level. It was found that the minimal inhibitory concentration of LPs was 0.5 mg/mL, and its inhibitory effect with the concentration of 2.5 mg/mL was better than that of the bacterial suspension. In addition, the spore germination rate of LPs treatment was much lower than that of the control group. These results demonstrate that one of the mechanisms which enable strain E2 to have an inhibition effect on mold is the production of LPs, which is consistent with previous reports [41]. Combining the results of HPLC-MS and SEM experiments, it is believed that the antifungal effect of LPs depends on Fengycin and Iturin. Iturin has a high hemolytic function and have a strong inhibitory effect on a variety of pathogenic fungi. It mainly causes cell death by promoting the release of macromolecular substances, electrolytes, and the degradation of phospholipids. Fengycins caused the mycelium to be abnormally enlarged and twisted, damaged the cell wall, and formed the cavity [42]. These results indicate that the LPs produced by E2 have a destructive effect on the growth and reproduction of the hyphae of A. flavus. Additionally, the hypha of A. flavus was damaged when treated with LPs, suggesting that their antifungal activity might be related to the down-regulation of some key protein synthesis genes.
To address this, we analyzed the pathways related to the protein synthesis process. Ribosomal protein was closely related to the growth and development of A. flavus. Interestingly, we found that most of the genes related to Ribosome biogenesis in eukaryotes are down-regulated. Among them, KRE33 is essential for the pre-rRNA processing reaction of 18S rRNA synthesis and the assembly of 40S ribosomal subunits. The depletion of KRE33 led to defects in nucleolar assembly, cytokinesis, and cell cycle arrest. Combined with the research of Yanez-Mendizabal [43], it can be speculated that this key gene was down-regulated under the action of the Fengycins, which changed the structure and permeability of cytoplasmic membrane, leading to inhibition of mycelial growth and conidial germination. Nucleolar G protein 1 (NOG1) is a member of the ODN family of GTP-binding proteins, involved in the assembly of the 60S ribosomal subunit precursor [44]. The reduction in its expression led to a dramatic decrease in 60S ribosomal subunits, and over-accumulation of pre-rRNA processing. NOG2 encodes Nog2p. The transient nature of Nog2p and ribosomal precursors may be a GTP-dependent association. Nog2p is an essential protein required for the maintenance of normal rRNA levels [45]. Yan [16] reported that as the concentration of Iturin increased, the protein content of C. gloeosporioides gradually decreased. It can be speculated that Iturin inhibited the expression of these genes. The result of the action was the failure of protein synthesis due to the destruction organelle structures. The result of KEGG analysis showed that ribosome biogenesis was the most dysregulated pathway, and suggested that LPs depressed ribosome biogenesis and led to apoptosis of A. flavus [46].
Previous studies had confirmed that LPs can effectively inhibit the synthesis of aflatoxin [47], but its molecular mechanism is still unclear. To address this point, the influence of LPs on the pathway synthesizing aflatoxin at the molecular level was further investigated. Some genes involved in the synthesis of AFB1 and other aflatoxins were down-regulated during transcription after treatment with LPs. Among them, veA, as the core protein of the velvet complex (composed of veA, laeA, and velB) [48], regulates the growth and development of A. flavus. Additionally, the synthesis of aflatoxins is globally regulated by veA and laeA [49,50]. Additionally, veA is involved in conidiogenesis and the production of sclerotia. AflR and aflS are the biosynthetic regulatory genes of aflatoxin, which play a key role in the regulation of aflatoxin biosynthesis [51,52]. Most genes in the aflatoxin gene cluster are regulated by them [53]. AflK participates in the conversion of VAL to VERB, which is a key step in the formation of aflatoxin. It blocks the bifuran ring of aflatoxin, which is necessary for the binding of aflatoxin to DNA and confers the mode of action of aflatoxin as a mutagen [54]. AflP (omtA) protein is a key enzyme in the late stage of aflatoxin synthesis. These genes are all down-regulated under the action of LPs [55]. LPs reduced the production of aflatoxin by directly inhibiting the process of aflatoxin synthesis. In summary, LPs showed dual inhibitory activity on the growth and reproduction of fungi and the production of aflatoxin. Therefore, LPs produced by Bacillus velezensis E2 have the potential to prevent contamination by A. flavus in the food industry.
5. Conclusions
In this study, LPs produced by Bacillus velezensis E2 were shown to have the potential to control A. flavus contamination. The antifungal activity of LPs was associated with the destruction of cell membrane integrity and interference with the normal functioning of ribosomes. Under the treatment with LPs, the expression of genes related to aflatoxin synthesis and its growth was down-regulated, thereby affecting its normal physiological metabolism and the synthesis of secondary metabolites. These results provide a new strategy for preventing A. flavus contamination.
Acknowledgments
All authors are thankful to their representative universities/institutes for the support and services used in this study.
Author Contributions
S.L.; methodology, S.L.; software, X.X.; validation, S.L., T.Z. and J.M.; formal analysis, S.L.; investigation, S.L.; resources, L.Z.; data curation, X.X.; writing—original draft preparation, S.L.; writing—review and editing, W.S.; visualization, Q.S.; supervision, X.X.; project administration, W.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Research & Development projects of Jiangsu Province (BE2021338), Modern Agricultural Machinery Equipment and Technology Demonstration and Promotion of Jiangsu Province (NJ2020-10), State Key Laboratory of Crop Biology in Shandong Agricultural University (2020KF07), and Key R & D Plan of Zhenjiang Science and Technology Innovation Fund (NY2019010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data showed in this study are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jaibangyang S., Nasanit R., Limtong S. Effects of temperature and relative humidity on Aflatoxin B1 reduction in corn grains and antagonistic activities against Aflatoxin-producing Aspergillus flavus by a volatile organic compound-producing yeast, Kwoniella heveanensis DMKU-CE82. BioControl. 2021;66:433–443. doi: 10.1007/s10526-021-10082-x. [DOI] [Google Scholar]
- 2.Li H., Kang X., Wang S., Mo H., Hu L. Early detection and monitoring for Aspergillus flavus contamination in maize kernels. Food Control. 2021;121:107636. doi: 10.1016/j.foodcont.2020.107636. [DOI] [Google Scholar]
- 3.Xu D., Wei M., Peng S., Mo H., Huang L., Yao L., Hu L. Cuminaldehyde in cumin essential oils prevents the growth and aflatoxin B1 biosynthesis of Aspergillus flavus in peanuts. Food Control. 2021;125:107985. doi: 10.1016/j.foodcont.2021.107985. [DOI] [Google Scholar]
- 4.Li Q., Zhu X., Xie Y., Zhong Y. O-Vanillin, a promising antifungal agent, inhibits Aspergillus flavus by disrupting the integrity of cell walls and cell membranes. Appl. Microbiol. Biotechnol. 2021;105:5147–5158. doi: 10.1007/s00253-021-11371-2. [DOI] [PubMed] [Google Scholar]
- 5.Hua C., Kai K., Wang X., Shi W., Zhang D., Liu Y. Curcumin inhibits gray mold development in kiwifruit by targeting mitogen-activated protein kinase (MAPK) cascades in Botrytis cinerea. Postharvest Biol. Technol. 2019;151:152–159. doi: 10.1016/j.postharvbio.2019.02.006. [DOI] [Google Scholar]
- 6.Mohammadi S., Aminifard M.H. In vitro and in vivo antifungal activities of three essential oils against grey mould disease in Cucumber (Cucumis sativus) Asian J. Plant Sci. 2011;10:287–293. doi: 10.3923/ajps.2011.287.293. [DOI] [Google Scholar]
- 7.Massawe V.C., Hanif A., Farzand A., Mburu D.K., Ochola S.O., Wu L., Tahir H.A.S., Gu Q., Wu H., Gao X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology. 2018;108:1373–1385. doi: 10.1094/PHYTO-04-18-0118-R. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Quan X., Xue B., Goodwin P.H., Lu S., Wang J., Du W., Wu C. Isolation and identification of Bacillus subtilis strain YB-05 and its antifungal substances showing antagonism against Gaeumannomyces graminis var. tritici. Biol. Control. 2015;85:52–58. doi: 10.1016/j.biocontrol.2014.12.010. [DOI] [Google Scholar]
- 9.You W., Ge C., Jiang Z., Chen M., Li W., Shao Y. Screening of a broad-spectrum antagonist—Bacillus siamensis, and its possible mechanisms to control postharvest disease in tropical fruits. Biol. Control. 2021;157:104584. doi: 10.1016/j.biocontrol.2021.104584. [DOI] [Google Scholar]
- 10.Yang H., Wang L., Li S., Gao X., Wu N., Zhao Y., Sun W. Control of postharvest grey spot rot of loquat fruit with Metschnikowia pulcherrima E1 and potential mechanisms of action. Biol. Control. 2021;152:104406. doi: 10.1016/j.biocontrol.2020.104406. [DOI] [Google Scholar]
- 11.Melentiev A.I., Galimzianova N.F., Gilvanova E.A., Shchelchkova E.A., Kuzmina L.Y., Boyko T.F., Usanov N.G., Aktuganov G.E. Characterization of Novel Alkaliphilic Isolate of Bacillus mannanilyticus, Strain IB-OR17, Displaying Chitinolytic and Antifungal Activities. Adv. Appl. Microbiol. 2014;4:455–464. doi: 10.4236/aim.2014.48050. [DOI] [Google Scholar]
- 12.Kim Y.S., Balaraju K., Jeon Y. Effects of rhizobacteria Paenibacillus polymyxa APEC136 and Bacillus subtilis APEC170 on biocontrol of postharvest pathogens of apple fruits. J. Zhejiang Univ. Sci. B. 2016;17:931–940. doi: 10.1631/jzus.B1600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilming A., Begemann J., Kuhne S., Regestein L., Bongaerts J., Evers S., Maurer K.H., Büchs J. Metabolic studies of γ-polyglutamic acid production in bacillus licheniformis by small-scale continuous cultivations. Biochem. Eng. J. 2013;2013. 73:29–37. doi: 10.1016/j.bej.2013.01.008. [DOI] [Google Scholar]
- 14.Gong Q., Zhang C., Lu F., Zhao H., Bie X., Lu Z. Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control. 2014;36:8–14. doi: 10.1016/j.foodcont.2013.07.034. [DOI] [Google Scholar]
- 15.Chen L., Zhang H., Zhao S., Xiang B., Yao Z. Lipopeptide production by Bacillus atrophaeus strain B44 and its biocontrol efficacy against cotton rhizoctoniosis. Biotechnol. Lett. 2021;43:1183–1193. doi: 10.1007/s10529-021-03114-0. [DOI] [PubMed] [Google Scholar]
- 16.Yan F., Li C., Ye X., Lian Y., Wu Y., Wang X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Control. 2020;146:104281. doi: 10.1016/j.biocontrol.2020.104281. [DOI] [Google Scholar]
- 17.Touré Y., Ongena M., Jacques P., Guiro A., Thonart P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 2010;96:1151–1160. doi: 10.1111/j.1365-2672.2004.02252.x. [DOI] [PubMed] [Google Scholar]
- 18.Arroyave-Toro J.J., Mosquera S., Villegas-Escobar V. Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol. Control. 2017;114:195–200. doi: 10.1016/j.biocontrol.2017.08.014. [DOI] [Google Scholar]
- 19.Nanjundan J., Ramasamy R., Uthandi S., Ponnusamy M. Antimicrobial activity and spectroscopic characterization of surfactin class of lipopeptides from Bacillus amyloliquefaciens SR1. Microb. Pathog. 2019;128:374–380. doi: 10.1016/j.micpath.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Hathout Y., Ho Y.P., Ryzhov V., Demirev P., Fenselau C. Kurstakins: A new class of lipopeptides isolated from Bacillus thuringiensis. J. Nat. Prod. 2000;63:1492–1496. doi: 10.1021/np000169q. [DOI] [PubMed] [Google Scholar]
- 21.Ajesh K., Sudarslal S., Arunan C., Sreejith K. Kannurin, a novel lipopeptide from Bacillus cereus strain AK1: Isolation, structural evaluation and antifungal activities. J. Appl. Microbiol. 2013;115:1287–1296. doi: 10.1111/jam.12324. [DOI] [PubMed] [Google Scholar]
- 22.Yaseen Y., Diop A., Gancel F., Béchet M., Jacques P., Drider D. Polynucleotide phosphorylase is involved in the control of lipopeptide fengycin production in Bacillus subtilis. Arch. Microbiol. 2018;200:783–791. doi: 10.1007/s00203-018-1483-5. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Lin S., An S., Liu L., Hu Y., Wan L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019;131:420–434. doi: 10.1016/j.ijbiomac.2019.02.169. [DOI] [PubMed] [Google Scholar]
- 24.Dasari V.R.R.K., Nikku M.Y., Donthireddy S.R.R. Screening of antagonistic marine Actinomycetes: Optimization of process parameters for the production of novel antibiotic by Amycolatopsis alba var. nov. DVR D4. J. Microb. Biochem. Technol. 2011;3:92–98. [Google Scholar]
- 25.Brown J.H. Bergey’s manual of determinative bacteriology (5th ed.) Am. J. Public Health Nations Health. 1939;29:404. doi: 10.2105/AJPH.29.4.404. [DOI] [Google Scholar]
- 26.Shang L., Bai X., Chen C., Liu L., Li M., Xia X., Wang Y. Isolation and identification of a Bacillus megaterium strain with ochratoxin A removal ability and antifungal activity. Food Control. 2019;106:106743. doi: 10.1016/j.foodcont.2019.106743. [DOI] [Google Scholar]
- 27.Sudhir K., Glen S., Koichiro T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D., Yu S., Zhao D., Zhang J., Pan Y., Yang Y., Yang Z., Zhu J., Zhao Y., Li R. Inhibitory effects of non-volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezensis C16 against Alternaria solani. Biol. Control. 2021;152:104421. doi: 10.1016/j.biocontrol.2020.104421. [DOI] [Google Scholar]
- 29.Nanguy S.P.-M., Perrier-Cornet J.M., Bensoussan M., Dantigny P. Impact of water activity of diverse media on spore germination of Aspergillus and Penicillium species. Int. J. Food Microbiol. 2010;142:273–276. doi: 10.1016/j.ijfoodmicro.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 30.Ye W.-Q., Sun Y.-F., Tang Y.-J., Zhou W.-W. Biocontrol potential of a broad-spectrum antifungal strain Bacillus amyloliquefaciens B4 for postharvest loquat fruit storage. Postharvest Biol. Technol. 2021;174:111439. doi: 10.1016/j.postharvbio.2020.111439. [DOI] [Google Scholar]
- 31.Zhao L., Zhu H., Li B., Ngea G., Zhang H. The molecular mechanisms of disease-resistance in mandarins induced by Yarrowia lipolytica based on transcriptome technology. Biol. Control. 2021;14:104607. doi: 10.1016/j.biocontrol.2021.104607. [DOI] [Google Scholar]
- 32.Xu M., Yang Q., Boateng N.A.S., Ahima J., Dou Y., Zhang H. Ultrastructure observation and transcriptome analysis of Penicillium expansum invasion in postharvest pears. Postharvest Biol. Technol. 2020;165:111198. doi: 10.1016/j.postharvbio.2020.111198. [DOI] [Google Scholar]
- 33.Xiong J.S., Zhu H.Y., Bai Y.B., Hui L., Cheng Z.M. RNA sequencing-based transcriptome analysis of mature strawberry fruit infected by necrotrophic fungal pathogen Botrytis cinerea. Physiol. Mol. Plant Pathol. 2018;104:77–85. doi: 10.1016/j.pmpp.2018.08.005. [DOI] [Google Scholar]
- 34.Redshaw N., Wilkes T., Whale A., Cowen S., Huggett J., Foy C. A comparison of miRNA isolation and RT-qPCR technologies and their effects on quantification accuracy and repeatability. Biotechniques. 2013;54:155. doi: 10.2144/000114002. [DOI] [PubMed] [Google Scholar]
- 35.Bergey D.H., Krieg R.N., Holt G.J. Bergeys Manual of Systematic Bacteriology. Williams & Wilkins; Baltimore, MD, USA: 1984. [Google Scholar]
- 36.Wang Y., Liang J., Zhang C., Wang L., Gao W., Jiang J. Bacillus megaterium WL-3 lipopeptides collaborate against Phytophthora infestans to control potato late blight and promote potato plant growth. Front. Microbiol. 2020;11:1602. doi: 10.3389/fmicb.2020.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B., Li Y., Zhang Y., Qiao H., He J., Yuan Q., Chen X., Fan J. High-cell-density culture enhances the antimicrobial and freshness effects of Bacillus subtilis S1702 on table grapes (Vitis vinifera cv. Kyoho) Food Chem. 2019;286:541–549. doi: 10.1016/j.foodchem.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 38.Tian P.P., Lv Y.Y., Wei S., Zhang S.B., Hu Y.S. Antifungal properties of recombinant Puroindoline B protein against aflatoxigenic Aspergillus flavus. LWT Food Sci. Technol. 2021;144:111130. doi: 10.1016/j.lwt.2021.111130. [DOI] [Google Scholar]
- 39.Jiang C.-H., Liao M.-J., Wang H.-K., Zheng M.-Z., Xu J.-J., Guo J.-H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control. 2018;126:147–157. doi: 10.1016/j.biocontrol.2018.07.017. [DOI] [Google Scholar]
- 40.Laird M., Piccoli D., Weselowski B., McDowell T., Renaud J., MacDonald J., Yuan Z.-C. Surfactin-producing Bacillus velezensis 1B-23 and Bacillus sp. 1D-12 protect tomato against bacterial canker caused by Clavibacter michiganensis subsp. michiganensis. J. Plant Pathol. 2019;102:451–458. doi: 10.1007/s42161-019-00461-w. [DOI] [Google Scholar]
- 41.Liu Y., Teng K., Wang T., Dong E., Zhong J. Antimicrobial Bacillus velezensis HC6: Production of three kinds of lipopeptides and biocontrol potential in maize. J. Appl. Microbiol. 2019;128:242–254. doi: 10.1111/jam.14459. [DOI] [PubMed] [Google Scholar]
- 42.Kang B.R., Park J.S., Jung W.J. Antifungal evaluation of fengycin isoforms isolated from Bacillus amyloliquefaciens PPL against Fusarium oxysporum f. sp. lycopersici. Microb. Pathog. 2020;149:104509. doi: 10.1016/j.micpath.2020.104509. [DOI] [PubMed] [Google Scholar]
- 43.Yanez-Mendizabal V., Falconi C.E. Bacillus subtilis CtpxS2-1 induces systemic resistance against anthracnose in Andean lupin by lipopeptide production. Biotechnol Lett. 2021;43:719–728. doi: 10.1007/s10529-020-03066-x. [DOI] [PubMed] [Google Scholar]
- 44.Jensen B.C., Wang Q., Kifer C.T., Parsons M. The NOG1 GTP-binding protein is required for biogenesis of the 60 S ribosomal subunit. J. Biol. Chem. 2003;278:32204–32211. doi: 10.1074/jbc.M304198200. [DOI] [PubMed] [Google Scholar]
- 45.Saveanu C., Bienvenu D., Namane A., Gleizes P.E., Gas N., Jacquier A., Fromont-Racine M. Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps. EMBO J. 2014;20:6475–6484. doi: 10.1093/emboj/20.22.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cong L., Ping W., Ma L., Zheng M., Yang L., Xing F. Large-scale comparative analysis of eugenol-induced/repressed genes expression in Aspergillus flavus using RNA-seq. Front. Microbiol. 2018;9:1116. doi: 10.3389/fmicb.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farzaneh M., Shi Z.Q., Ahmadzadeh M., Hu L.B., Ghassempour A. Inhibition of the Aspergillus flavus growth and Aflatoxin B1 contamination on pistachio nut by Fengycin and Surfactin-producing Bacillus subtilis UTBSP1. Plant Pathol. J. 2016;32:209–215. doi: 10.5423/PPJ.OA.11.2015.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauscher S., Pacher S., Hedtke M., Kniemeyer O., Fischer R. A phosphorylation code of the Aspergillus nidulans global regulator VelvetA (VeA) determines specific functions. Mol. Microbiol. 2016;99:909–924. doi: 10.1111/mmi.13275. [DOI] [PubMed] [Google Scholar]
- 49.Cary J., Entwistle S., Satterlee T., Mack B.M., Gilbert M.K., Chang P.K., Scharfenstein L., Yin Y., Calvo A.M. The transcriptional regulator Hbx1 affects the expression of thousands of genes in the Aflatoxin-producing fungus Aspergillus flavus. G3 Genes Genomes Genet. 2019;9:167–178. doi: 10.1534/g3.118.200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghayum S.A., Shamsghahfarokhi M., Razzaghiabyaneh M. Expression of aflR, veA and laeA as regulators of Aflatoxins and cyclopiazonic acid biosynthesis pathway in Aspergillus flavus. Int. J. Mol. Clin. Microbiol. 2017;7:787–794. [Google Scholar]
- 51.Somashekar D., Rati E.R., Chandrashekar A. PCR-restriction fragment length analysis of aflR gene for differentiation and detection of Aspergillus flavus and Aspergillus parasiticus in maize. Int. J. Food Microbiol. 2004;93:101–107. doi: 10.1016/j.ijfoodmicro.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Liang Y., Kong Q., Yao Y., Xu S., Xie X. Fusion expression and anti-Aspergillus flavus activity of a novel inhibitory protein DN-AflR. Int. J. Food Microbiol. 2019;290:184–192. doi: 10.1016/j.ijfoodmicro.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Devi M.S., Sashidhar R.B. Antiaflatoxigenic effects of selected antifungal peptides. Peptides. 2019;115:15–26. doi: 10.1016/j.peptides.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Yu J., Fedorova N.D., Montalbano B.G., Bhatnagar D., Cleveland T.E., Bennett J.W., Nierman W.C. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol. Lett. 2011;322:145–149. doi: 10.1111/j.1574-6968.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu P., Li B., Yin R., Weng Q., Chen Q. Development and evaluation of ITS- and aflP-based LAMP assays for rapid detection of Aspergillus flavus in food samples. Can. J. Microbiol. 2014;60:579–584. doi: 10.1139/cjm-2014-0202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data showed in this study are contained within the article.